Genome-Wide Association Study of Agronomic and Physiological Traits Related to Drought Tolerance in Potato
Abstract
1. Introduction
2. Results
2.1. Phenotypic Data Analysis
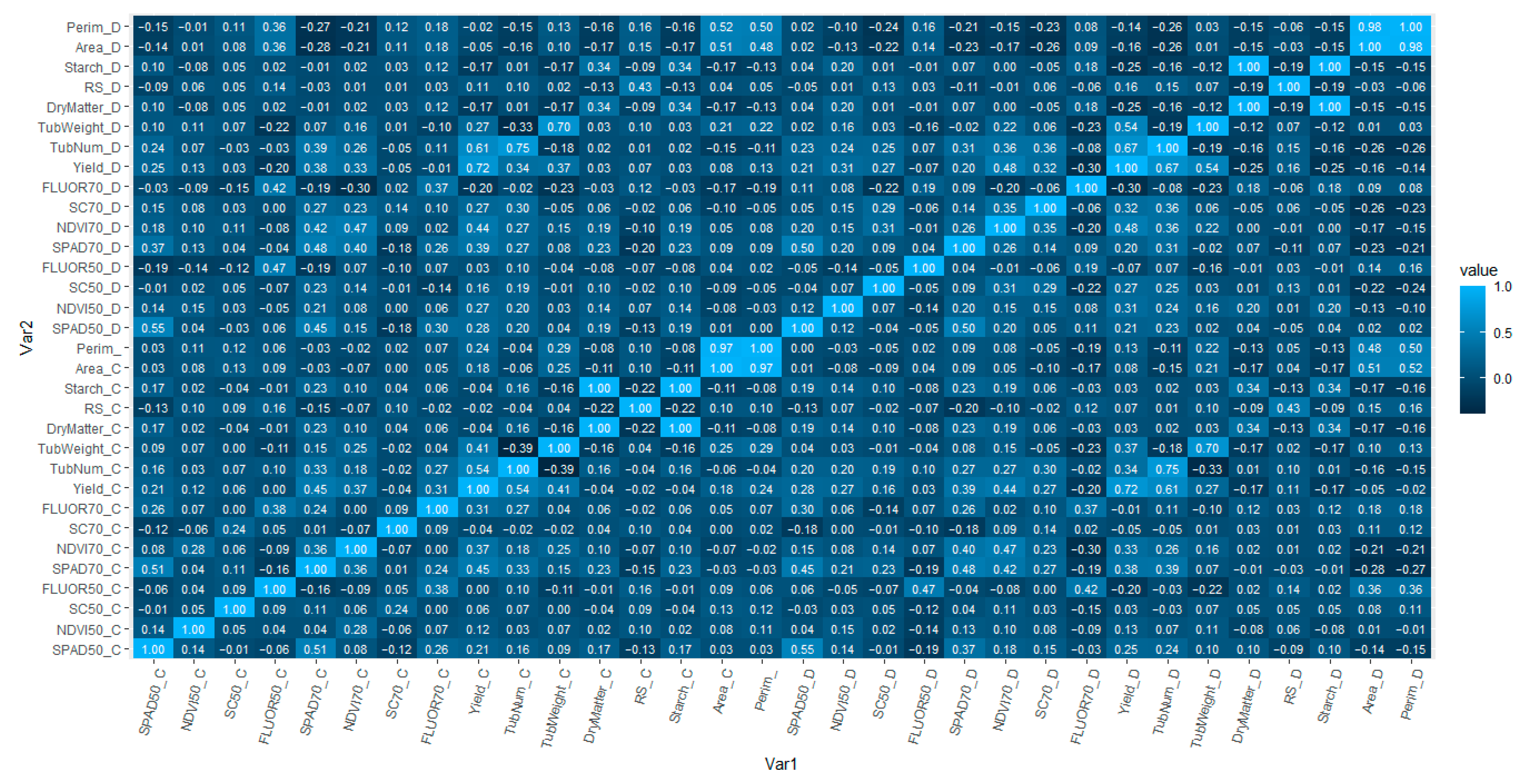
2.2. Population Structure Analysis and Linkage Disequilibrium
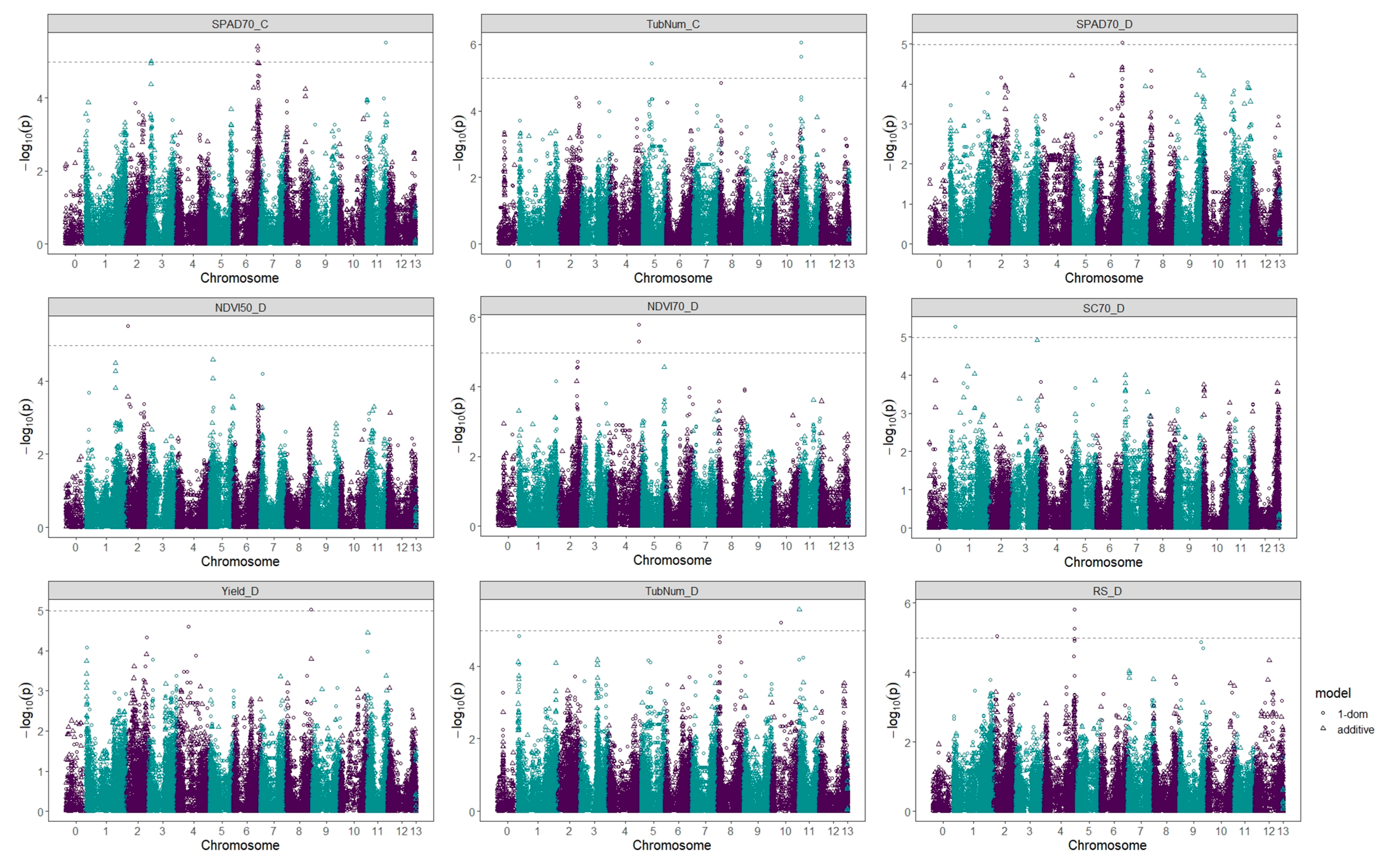
2.3. Genome-Wide Association Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Location
4.2. Experimental Design
4.3. Phenotypic Data Collection
4.4. DNA Extraction and Genotyping
4.5. Population Structure, Linkage Disequilibrium and GWAS Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pareek, A.; Dhankher, O.P.; Foyer, C.H. Mitigating the Impact of Climate Change on Plant Productivity and Ecosystem Sustainability. J. Exp. Bot. 2020, 71, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Malhi, G.S.; Kaur, M.; Kaushik, P. Impact of Climate Change on Agriculture and Its Mitigation Strategies: A Review. Sustainability 2021, 13, 1318. [Google Scholar] [CrossRef]
- Daccache, A.; Keay, C.; Jones, R.J.A.; Weatherhead, E.K.; Stalham, M.A.; Knox, J.W. Climate Change and Land Suitability for Potato Production in England and Wales: Impacts and Adaptation. J. Agric. Sci. 2012, 150, 161–177. [Google Scholar] [CrossRef]
- Nasir, M.W.; Toth, Z. Effect of Drought Stress on Potato Production: A Review. Agronomy 2022, 12, 635. [Google Scholar] [CrossRef]
- Lutaladio, N.B.; Castaldi, L. Potato: The Hidden Treasure. J. Food Compos. Anal. 2009, 22, 491–493. [Google Scholar] [CrossRef]
- Sun, S.; Wang, Y.; Wang, F.; Liu, J.; Luan, X.; Li, X.; Zhou, T.; Wu, P. Alleviating Pressure on Water Resources: A New Approach Could Be Attempted. Sci. Rep. 2015, 5, 14006. [Google Scholar] [CrossRef]
- Hill, D.; Nelson, D.; Hammond, J.; Bell, L. Morphophysiology of Potato (Solanum tuberosum) in Response to Drought Stress: Paving the Way Forward. Front. Plant Sci. 2021, 11, 597554. [Google Scholar] [CrossRef]
- Zarzyńska, K.; Boguszewska-Mańkowska, D.; Nosalewicz, A. Differences in Size and Architecture of the Potato Cultivars Root System and Their Tolerance to Drought Stress. Plant Soil Environ. 2017, 63, 159–164. [Google Scholar] [CrossRef]
- Hijmans, R.J. The Effect of Climate Change on Globar Potato Production. Am. J. Potato Res. 2003, 80, 271–279. [Google Scholar] [CrossRef]
- Aliche, E.B.; Oortwijn, M.; Theeuwen, T.P.J.M.; Bachem, C.W.B.; Visser, R.G.F.; van der Linden, C.G. Drought Response in Field Grown Potatoes and the Interactions between Canopy Growth and Yield. Agric. Water Manag. 2018, 206, 20–30. [Google Scholar] [CrossRef]
- Gervais, T.; Creelman, A.; Li, X.Q.; Bizimungu, B.; De Koeyer, D.; Dahal, K. Potato Response to Drought Stress: Physiological and Growth Basis. Front. Plant Sci. 2021, 12, 698060. [Google Scholar] [CrossRef]
- Obidiegwu, J.E.; Bryan, G.J.; Jones, H.G.; Prashar, A. Coping with Drought: Stress and Adaptive Responses in Potato and Perspectives for Improvement. Front. Plant Sci. 2015, 6, 542. [Google Scholar] [CrossRef]
- Pandey, J.; Scheuring, D.C.; Koym, J.W.; Vales, M.I. Genomic Regions Associated with Tuber Traits in Tetraploid Potatoes and Identification of Superior Clones for Breeding Purposes. Front. Plant Sci. 2022, 13, 952263. [Google Scholar] [CrossRef]
- Schreiber, L.; Nader-Nieto, A.C.; Schönhals, E.M.; Walkemeier, B.; Gebhardt, C. SNPs in Genes Functional in Starch-Sugar Interconversion Associate with Natural Variation of Tuber Starch and Sugar Content of Potato (Solanum tuberosum L.). G3 Genes Genomes Genet. 2014, 4, 1797–1811. [Google Scholar] [CrossRef]
- Li, Y.; Colleoni, C.; Zhang, J.; Liang, Q.; Hu, Y.; Ruess, H.; Simon, R.; Liu, Y.; Liu, H.; Yu, G.; et al. Genomic Analyses Yield Markers for Identifying Agronomically Important Genes in Potato. Mol. Plant 2018, 11, 473–484. [Google Scholar] [CrossRef]
- Zia, M.A.B.; Demirel, U.; Nadeem, M.A.; Çaliskan, M.E. Genome-Wide Association Study Identifies Various Loci Underlying Agronomic and Morphological Traits in Diversified Potato Panel. Physiol. Mol. Biol. Plants 2020, 26, 1003–1020. [Google Scholar] [CrossRef]
- Yousaf, M.F.; Demirel, U.; Naeem, M.; Çalışkan, M.E. Association Mapping Reveals Novel Genomic Regions Controlling Some Root and Stolon Traits in Tetraploid Potato (Solanum tuberosum L.). 3 Biotech 2021, 11, 174. [Google Scholar] [CrossRef]
- Nieto, C.A.O.; Van Bueren, E.T.L.; Allefs, S.; Vos, P.G.; Van Der Linden, G.; Maliepaard, C.A.; Struik, P.C. Association Mapping of Physiological and Morphological Traits Related to Crop Development under Contrasting Nitrogen Inputs in a Diverse Set of Potato Cultivars. Plants 2021, 10, 1727. [Google Scholar] [CrossRef]
- Koizumi, E.; Igarashi, T.; Tsuyama, M.; Ogawa, K.; Asano, K.; Kobayashi, A.; Sanetomo, R.; Hosaka, K. Association of Genome-Wide SNP Markers with Resistance to Common Scab of Potato. Am. J. Potato Res. 2021, 98, 149–156. [Google Scholar] [CrossRef]
- Yuan, J.; Bizimungu, B.; De Koeyer, D.; Rosyara, U.; Wen, Z.; Lagüe, M. Genome-Wide Association Study of Resistance to Potato Common Scab. Potato Res. 2020, 63, 253–266. [Google Scholar] [CrossRef]
- Mosquera, T.; Alvarez, M.F.; Jiménez-Gómez, J.M.; Muktar, M.S.; Paulo, M.J.; Steinemann, S.; Li, J.; Draffehn, A.; Hofmann, A.; Lübeck, J.; et al. Targeted and Untargeted Approaches Unravel Novel Candidate Genes and Diagnostic SNPs for Quantitative Resistance of the Potato (Solanum tuberosum L.) to Phytophthora Infestans Causing the Late Blight Disease. PLoS ONE 2016, 11, e0156254. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zou, M.; Zhao, L.; Li, H.; Xia, Z.; Wang, J. Genome-Wide Association Analysis of Late Blight Resistance Traits in Potato Germplasm Resources. Res. Sq. BMC Plant Biol. 2020, 12, 1–19. [Google Scholar]
- Plich, J.; Boguszewska-Mańkowska, D.; Marczewski, W. Relations Between Photosynthetic Parameters and Drought-Induced Tuber Yield Decrease in Katahdin-Derived Potato Cultivars. Potato Res. 2020, 63, 463–477. [Google Scholar] [CrossRef]
- Aliche, E.B.; Theeuwen, T.P.J.M.; Oortwijn, M.; Visser, R.G.F.; van der Linden, C.G. Carbon Partitioning Mechanisms in POTATO under Drought Stress. Plant Physiol. Biochem. 2020, 146, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Krannich, C.T.; Maletzki, L.; Kurowsky, C.; Horn, R. Network Candidate Genes in Breeding for Drought Tolerant Crops. Int. J. Mol. Sci. 2015, 16, 16378–16400. [Google Scholar] [CrossRef]
- Dahal, K.; Li, X.Q.; Tai, H.; Creelman, A.; Bizimungu, B. Improving Potato Stress Tolerance and Tuber Yield under a Climate Change Scenario—A Current Overview. Front. Plant Sci. 2019, 10, 563. [Google Scholar] [CrossRef]
- Pinheiro, C.; Chaves, M.M. Photosynthesis and Drought: Can We Make Metabolic Connections from Available Data? J. Exp. Bot. 2011, 62, 869–882. [Google Scholar] [CrossRef]
- Alter, S.; Bader, K.C.; Spannagl, M.; Wang, Y.; Bauer, E.; Schön, C.C.; Mayer, K.F.X. DroughtDB: An Expert-Curated Compilation of Plant Drought Stress Genes and Their Homologs in Nine Species. Database 2015, 2015, bav046. [Google Scholar] [CrossRef]
- Naeem, M.; Demirel, U.; Yousaf, M.F.; Caliskan, S.; Caliskan, M.E.; Wehling, P. Overview on Domestication, Breeding, Genetic Gain and Improvement of Tuber Quality Traits of Potato Using Fast Forwarding Technique (GWAS): A Review. Plant Breed. 2021, 140, 519–542. [Google Scholar] [CrossRef]
- Byrne, S.; Meade, F.; Mesiti, F.; Griffin, D.; Kennedy, C.; Milbourne, D. Genome-Wide Association and Genomic Prediction for Fry Color in Potato. Agronomy 2020, 10, 90. [Google Scholar] [CrossRef]
- Xu, X.; Pan, S.; Cheng, S.; Zhang, B.; Mu, D.; Ni, P.; Zhang, G.; Yang, S.; Li, R.; Wang, J.; et al. Genome Sequence and Analysis of the Tuber Crop Potato. Nature 2011, 475, 189–195. [Google Scholar] [CrossRef]
- Felcher, K.J.; Coombs, J.J.; Massa, A.N.; Hansey, C.N.; Hamilton, J.P.; Veilleux, R.E.; Buell, C.R.; Douches, D.S. Integration of Two Diploid Potato Linkage Maps with the Potato Genome Sequence. PLoS ONE 2012, 7, e36347. [Google Scholar] [CrossRef]
- Visser, R.G.F.; Bachem, C.W.B.; Borm, T.; de Boer, J.; van Eck, H.J.; Finkers, R.; van der Linden, G.; Maliepaard, C.A.; Uitdewilligen, J.G.A.M.L.; Voorrips, R.; et al. Possibilities and Challenges of the Potato Genome Sequence. Potato Res. 2014, 57, 327–330. [Google Scholar] [CrossRef]
- Baldwin, S.J.; Dodds, K.G.; Auvray, B.; Genet, R.A.; Macknight, R.C.; Jacobs, J.M.E. Association Mapping of Cold-Induced Sweetening in Potato Using Historical Phenotypic Data. Ann. Appl. Biol. 2011, 158, 248–256. [Google Scholar] [CrossRef]
- Zhu, C.; Gore, M.; Buckler, E.S.; Yu, J. Status and Prospects of Association Mapping in Plants. Plant Genome 2008, 1, 5–20. [Google Scholar] [CrossRef]
- Korte, A.; Farlow, A. The Advantages and Limitations of Trait Analysis with GWAS: A Review. Plant Methods 2013, 9, 1. [Google Scholar] [CrossRef]
- Myles, S.; Peiffer, J.; Brown, P.J.; Ersoz, E.S.; Zhang, Z.; Costich, D.E.; Buckler, E. Association Mapping: Critical Considerations Shift from Genotyping to Experimental Design. Plant Cell 2009, 21, 2194–2202. [Google Scholar] [CrossRef]
- Sharma, S.K.; MacKenzie, K.; McLean, K.; Dale, F.; Daniels, S.; Bryan, G.J. Linkage Disequilibrium and Evaluation of Genome-Wide Association Mapping Models in Tetraploid Potato. G3 Genes Genomes Genet. 2018, 8, 3185–3202. [Google Scholar] [CrossRef]
- Ozturk, G.; Yildirim, Z. Heritability Estimates of Some Quantitative Traits in Potatoes. Turkish J. F. Crops 2014, 19, 262–267. [Google Scholar] [CrossRef]
- Cabello, R.; Monneveux, P.; Bonierbale, M.; Khan, M.A. Heritability of Yield Components under Irrigated and Drought Conditions in Andigenum Potatoes. Am. J. Potato Res. 2015, 91, 492–499. [Google Scholar] [CrossRef]
- Rudack, K.; Seddig, S.; Sprenger, H.; Köhl, K.; Uptmoor, R.; Ordon, F. Drought Stress-Induced Changes in Starch Yield and Physiological Traits in Potato. J. Agron. Crop Sci. 2017, 203, 494–505. [Google Scholar] [CrossRef]
- Gupta, P.K.; Kulwal, P.L.; Jaiswal, V. Association Mapping in Crop Plants: Opportunities and Challenges; Elsevier: Amsterdam, The Netherlands, 2014; Volume 85, ISBN 9780128002711. [Google Scholar]
- Khlestkin, V.K.; Rozanova, I.V.; Efimov, V.M.; Khlestkina, E.K. Starch Phosphorylation Associated SNPs Found by Genome-Wide Association Studies in the Potato (Solanum tuberosum L.). BMC Genet. 2019, 20, 29. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, M.; Hakonarson, H. Analysing Biological Pathways in Genome-Wide Association Studies. Nat. Rev. Genet. 2010, 11, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Wani, S.H.; Suprasanna, P.; Tran, L.S.P. Salinity Responses and Tolerance in Plants. In Salinity Responses and Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2018; Volume 2, pp. 1–326. ISBN 9783319903187. [Google Scholar]
- Verslues, P.E.; Lasky, J.R.; Juenger, T.E.; Liu, T.W.; Nagaraj Kumar, M. Genome-Wide Association Mapping Combined with Reverse Genetics Identifies New Effectors of Low Water Potential-Induced Proline Accumulation in Arabidopsis. Plant Physiol. 2014, 164, 144–159. [Google Scholar] [CrossRef] [PubMed]
- Macovei, A.; Vaid, N.; Tula, S.; Tuteja, N. A New DEAD-Box Helicase ATP-Binding Protein (OsABP) from Rice Is Responsive to Abiotic Stress. Plant Signal. Behav. 2012, 7, 1138–1143. [Google Scholar] [CrossRef]
- Frey, P.A.; Hegeman, A.D.; Ruzicka, F.J. The Radical SAM Superfamily. Crit. Rev. Biochem. Mol. Biol. 2008, 43, 63–88. [Google Scholar] [CrossRef]
- Shen, Z.J.; Qin, Y.Y.; Luo, M.R.; Li, Z.; Ma, D.N.; Wang, W.H.; Zheng, H.L. Proteome Analysis Reveals a Systematic Response of Cold-Acclimated Seedlings of an Exotic Mangrove Plant Sonneratia Apetala to Chilling Stress. J. Proteom. 2021, 248, 104349. [Google Scholar] [CrossRef]
- Asada, K. Ascorbate Peroxidase—A Hydrogen Peroxide-scavenging Enzyme in Plants. Physiol. Plant. 1992, 85, 235–241. [Google Scholar] [CrossRef]
- Caverzan, A.; Passaia, G.; Barcellos Rosa, S.; Werner Ribeiro, C.; Lazzarotto, F.; Margis-Pinheiro, M. Plant Responses to Stresses: Role of Ascorbate Peroxidase in the Antioxidant Protection. Peroxidases Biochem. Charact. Funct. Potential Appl. 2013, 4, 142–158. [Google Scholar] [CrossRef]
- Sečenji, M.; Hideg, É.; Bebes, A.; Györgyey, J. Transcriptional Differences in Gene Families of the Ascorbate–Glutathione Cycle in Wheat during Mild Water Deficit. Plant Cell Rep. 2010, 29, 37–50. [Google Scholar] [CrossRef]
- D’Arcy-Lameta, A.; Ferrari-Iliou, R.; Contour-Ansel, D.; Pham-Thi, A.T.; Zuily-Fodil, Y. Isolation and Characterization of Four Ascorbate Peroxidase CDNAs Responsive to Water Deficit in Cowpea Leaves. Ann. Bot. 2006, 97, 133–140. [Google Scholar] [CrossRef]
- Demirel, U.; Morris, W.L.; Ducreux, L.J.M.; Yavuz, C.; Asim, A.; Tindas, I.; Campbell, R.; Morris, J.A.; Verrall, S.R.; Hedley, P.E.; et al. Physiological, Biochemical, and Transcriptional Responses to Single and Combined Abiotic Stress in Stress-Tolerant and Stress-Sensitive Potato Genotypes. Front. Plant Sci. 2020, 11, 169. [Google Scholar] [CrossRef]
- Alhoshan, M.; Zahedi, M.; Ramin, A.A.; Sabzalian, M.R. Effect of Soil Drought on Biomass Production, Physiological Attributes and Antioxidant Enzymes Activities of Potato Cultivars. Russ. J. Plant Physiol. 2019, 66, 265–277. [Google Scholar] [CrossRef]
- Bashir, M.A.; Silvestri, C.; Ahmad, T.; Hafiz, I.A.; Abbasi, N.A.; Manzoor, A.; Cristofori, V.; Rugini, E. Osmotin: A Cationic Protein Leads to Improve Biotic and Abiotic Stress Tolerance in Plants. Plants 2020, 9, 992. [Google Scholar] [CrossRef]
- Parkhi, V.; Kumar, V.; Sunilkumar, G.; Campbell, L.M.; Singh, N.K.; Rathore, K.S. Expression of Apoplastically Secreted Tobacco Osmotin in Cotton Confers Drought Tolerance. Mol. Breed. 2009, 23, 625–639. [Google Scholar] [CrossRef]
- Goel, D.; Singh, A.K.; Yadav, V.; Babbar, S.B.; Bansal, K.C. Overexpression of Osmotin Gene Confers Tolerance to Salt and Drought Stresses in Transgenic Tomato (Solanum lycopersicum L.). Protoplasma 2010, 245, 133–141. [Google Scholar] [CrossRef]
- Hakim; Ullah, A.; Hussain, A.; Shaban, M.; Khan, A.H.; Alariqi, M.; Gul, S.; Jun, Z.; Lin, S.; Li, J.; et al. Osmotin: A Plant Defense Tool against Biotic and Abiotic Stresses. Plant Physiol. Biochem. 2018, 123, 149–159. [Google Scholar] [CrossRef]
- Bartoli, C.G.; Gomez, F.; Gergoff, G.; Guiamét, J.J.; Puntarulo, S. Up-Regulation of the Mitochondrial Alternative Oxidase Pathway Enhances Photosynthetic Electron Transport under Drought Conditions. J. Exp. Bot. 2005, 56, 1269–1276. [Google Scholar] [CrossRef]
- Sunil, B.; Saini, D.; Bapatla, R.B.; Aswani, V.; Raghavendra, A.S. Photorespiration Is Complemented by Cyclic Electron Flow and the Alternative Oxidase Pathway to Optimize Photosynthesis and Protect against Abiotic Stress. Photosynth. Res. 2019, 139, 67–79. [Google Scholar] [CrossRef]
- Selinski, J.; Scheibe, R.; Day, D.A.; Whelan, J. Alternative Oxidase Is Positive for Plant Performance. Trends Plant Sci. 2018, 23, 588–597. [Google Scholar] [CrossRef]
- Chapagain, S.; Park, Y.C.; Kim, J.H.; Jang, C.S. Oryza Sativa Salt-Induced RING E3 Ligase 2 (OsSIRP2) Acts as a Positive Regulator of Transketolase in Plant Response to Salinity and Osmotic Stress. Planta 2018, 247, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sultan, M.A.R.F.; Liu, X.L.; Zhang, J.; Yu, F.; Zhao, H.X. Physiological and Comparative Proteomic Analysis Reveals Different Drought Responses in Roots and Leaves of Drought-Tolerant Wild Wheat (Triticum boeoticum). PLoS ONE 2015, 10, e0121852. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kondo, E.; Makino, A. Effects of Co-Overexpression of the Genes of Rubisco and Transketolase on Photosynthesis in Rice. Photosynth. Res. 2017, 131, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Manna, M.; Thakur, T.; Gautam, V.; Salvi, P. Imperative Role of Sugar Signaling and Transport during Drought Stress Responses in Plants. Physiol. Plant. 2021, 171, 833–848. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.; Yi, J.; Yang, Y.; Lei, C.; Gong, M. Transcriptome Response to Drought, Rehydration and Re-Dehydration in Potato. Int. J. Mol. Sci. 2020, 21, 159. [Google Scholar] [CrossRef]
- Kang, J.; Li, J.; Gao, S.; Tian, C.; Zha, X. Overexpression of the Leucine-Rich Receptor-like Kinase Gene LRK2 Increases Drought Tolerance and Tiller Number in Rice. Plant Biotechnol. J. 2017, 15, 1175–1185. [Google Scholar] [CrossRef]
- Ouyang, S.Q.; Liu, Y.F.; Liu, P.; Lei, G.; He, S.J.; Ma, B.; Zhang, W.K.; Zhang, J.S.; Chen, S.Y. Receptor-like Kinase OsSIK1 Improves Drought and Salt Stress Tolerance in Rice (Oryza sativa) Plants. Plant J. 2010, 62, 316–329. [Google Scholar] [CrossRef]
- Wu, T.; Tian, Z.; Liu, J.; Xie, C. A Novel Leucine-Rich Repeat Receptor-like Kinase Gene in Potato, StLRPK1, Is Involved in Response to Diverse Stresses. Mol. Biol. Rep. 2009, 36, 2365–2374. [Google Scholar] [CrossRef]
- Khan, M.A.; Saravia, D.; Munive, S.; Lozano, F.; Farfan, E.; Eyzaguirre, R.; Bonierbale, M. Multiple QTLs Linked to Agro-Morphological and Physiological Traits Related to Drought Tolerance in Potato. Plant Mol. Biol. Report. 2015, 33, 1286–1298. [Google Scholar] [CrossRef]
- Díaz, P.; Sarmiento, F.; Mathew, B.; Ballvora, A.; Vásquez, T.M. Genomic Regions Associated with Physiological, Biochemical and Yield-Related Responses under Water Deficit in Diploid Potato at the Tuber Initiation Stage Revealed by GWAS. PLoS ONE 2021, 16, e0259690. [Google Scholar] [CrossRef]
- Anithakumari, A.M.; Nataraja, K.N.; Visser, R.G.F.; van der Linden, C.G. Genetic Dissection of Drought Tolerance and Recovery Potential by Quantitative Trait Locus Mapping of a Diploid Potato Population. Mol. Breed. 2012, 30, 1413–1429. [Google Scholar] [CrossRef]
- Rak, K.; Bethke, P.C.; Palta, J.P. QTL Mapping of Potato Chip Color and Tuber Traits within an Autotetraploid Family. Mol. Breed. 2017, 37, 15. [Google Scholar] [CrossRef]
- Massa, A.N.; Manrique-Carpintero, N.C.; Coombs, J.J.; Zarka, D.G.; Boone, A.E.; Kirk, W.W.; Hackett, C.A.; Bryan, G.J.; Douches, D.S. Genetic Linkage Mapping of Economically Important Traits in Cultivated Tetraploid Potato (Solanum tuberosum L.). G3 Genes Genomes Genet. 2015, 5, 2357–2364. [Google Scholar] [CrossRef]
- Tagliotti, M.E.; Deperi, S.I.; Bedogni, M.C.; Huarte, M.A. Genome-Wide Association Analysis of Agronomical and Physiological Traits Linked to Drought Tolerance in a Diverse Potatoes (Solanum tuberosum) Panel. Plant Breed. 2021, 140, 654–664. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Smith, M.; Raes, D.; Wright, J.L. FAO-56 Dual Crop Coefficient Method for Estimating Evaporation from Soil and Application Extensions. J. Irrig. Drain. Eng. 2005, 131, 2–13. [Google Scholar] [CrossRef]
- Müller, K.; Cervenkova, I. Die Ermittlung Des Stärke- Und Trockensubstanzgehaltes in Kartoffelknollen Nach Bestimmung Des Unterwassergewichtes an Hand Modifizierter Tabellenwerte. Starch Stärke 1978, 30, 12–20. [Google Scholar] [CrossRef]
- Lindsay, H. A Colorimetric Estimation of Reducing Sugars in Potatoes with 3,5-Dinitrosalicylic Acid. Potato Res. 1973, 16, 176–179. [Google Scholar] [CrossRef]
- Pritchard, J.K. Documentation for Structure Software: Version 2.2; Department of Human Genetics University of Chicago: Chicago, IL, USA, 2007. [Google Scholar]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the Number of Clusters of Individuals Using the Software STRUCTURE: A Simulation Study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Earl, D.A.; vonHoldt, B.M. Structure Harvester: A Website and Program for Visualizing STRUCTURE Output and Implementing the Evanno Method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for Association Mapping of Complex Traits in Diverse Samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef]
- Remington, D.L.; Thornsberry, J.M.; Matsuoka, Y.; Wilson, L.M.; Whitt, S.R.; Doebley, J.; Kresovich, S.; Goodman, M.M.; Buckler, E.S., IV. Structure of Linkage Disequilibrium and Phenotypic Associations in the Maize Genome. Proc. Natl. Acad. Sci. USA 2001, 98, 11479–11484. [Google Scholar] [CrossRef] [PubMed]
- Rosyara, U.R.; De Jong, W.S.; Douches, D.S.; Endelman, J.B. Software for Genome-Wide Association Studies in Autopolyploids and Its Application to Potato. Plant Genome 2016, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
| Trait | F Value | |||||
|---|---|---|---|---|---|---|
| 2019 | 2020 | |||||
| Genotype(G) | Treatment(T) | G × T | Genotype(G) | Treatment(T) | G × T | |
| SPAD_50 | 4.83 *** | 15.17 *** | 1.39 ** | 8.57 *** | 5.51 * | 1.55 *** |
| NDVI_50 | 2.53 *** | 411.95 *** | 2.37 *** | 2.26 *** | 38.25 *** | 1.52 ** |
| SC_50 | 3.89 *** | 293.64 *** | 3.95 *** | 6.61 *** | 275.80 *** | 2.18 *** |
| FLUOR_50 | 7.72 *** | 148.46 *** | 2.79 *** | 3.81 *** | 3.81 ns | 3.56 *** |
| SPAD_70 | 6.58 *** | 62.22 *** | 2.20 *** | 8.63 *** | 7.04 ** | 1.56 *** |
| NDVI_70 | 4.30 *** | 360.85 *** | 2.58 *** | 2.89 *** | 61.99 *** | 1.75 *** |
| SC_70 | 4.49 *** | 322.74 *** | 3.76 *** | 4.23 *** | 140.85 *** | 1.72 *** |
| FLUOR_70 | 6.21 *** | 584.25 *** | 3.10 *** | 3.72 *** | 252.39 *** | 1.57 *** |
| Yield | 19.63 *** | 900.35 *** | 5.20 *** | 7.88 *** | 627.35 *** | 1.89 *** |
| TubNum | 11.63 *** | 352.54 *** | 1.98 *** | 6.25 *** | 17.57 *** | 1.54 *** |
| TubWeight | 8.97 *** | 859.01 *** | 2.62 *** | 6.24 *** | 541.72 *** | 1.95 *** |
| DryMatter | 1453.69 *** | 1200 *** | 802.88 *** | 1326.9 *** | 1200 *** | 530 *** |
| RS | 51.10 *** | 1200 *** | 30.74 *** | 32.97 *** | 72.53 *** | 18.85 *** |
| Starch | 1497.04 *** | 1200 *** | 853.63 *** | 1388.46 *** | 1200 *** | 558.55 *** |
| Area | 5.58 *** | 358.25 *** | 2.56 *** | 3.58 *** | 178.29 *** | 1.58 *** |
| Perim | 6.24 *** | 256.34 *** | 2.14 *** | 3.69 *** | 189.32 *** | 1.67 *** |
| Chromosome | Number of SNPs (Total) | Number of SNPs (Filtered) | Chromosome Length (bps) |
|---|---|---|---|
| CH00 | 464 | 156 | |
| CH01 | 3958 | 2486 | 88,663,952 |
| CH02 | 3335 | 1914 | 48,614,681 |
| CH03 | 2919 | 1637 | 62,190,286 |
| CH04 | 2798 | 1611 | 72,208,621 |
| CH05 | 2538 | 1520 | 52,070,158 |
| CH06 | 2390 | 1461 | 59,532,096 |
| CH07 | 2457 | 1407 | 56,760,843 |
| CH08 | 2043 | 1234 | 56,938,457 |
| CH09 | 2204 | 1296 | 61,540,751 |
| CH10 | 1865 | 1061 | 59,756,223 |
| CH11 | 2249 | 1361 | 45,475,667 |
| CH12 | 1942 | 1098 | 61,165,649 |
| CH13 | 28 | 17 | 155,312 |
| Total | 31,190 | 18,259 | 810,654,046 |
| Trait | Marker | Chrom. | Position | Ref | Alt | Effect | R2 | p-Value | FDR | Biological Function |
|---|---|---|---|---|---|---|---|---|---|---|
| SPAD70_C | PotVar0039950 | 6 | 53985614 | C | T | −2.07 | 0.0183 | 2.14 × 10−2 | 0.0361 | Radical SAM superfamily protein |
| SPAD70_C | solcap_snp_c2_15287 | 11 | 41743380 | A | G | −4.20 | 0.0681 | 7.05 × 10−6 | 0.0138 | P-loop containing nucleoside triphosphate hydrolases superfamily protein |
| TubNum_C | solcap_snp_c2_15676 | 5 | 18718517 | G | T | −25.38 | 0.0438 | 0.0003 | 0.0222 | RNA-binding CRS1/YhbY (CRM) domain-containing protein |
| TubNum_C | solcap_snp_c2_37217 | 11 | 1818959 | A | G | −32.80 | 0.0006 | 0.0486 | 0.05 | - |
| TubNum_C | ST4.03ch11_2070850 | 11 | 2070850 | A | T | 42.25 | 0.0153 | 0.0355 | 0.0416 | Di-glucose binding protein with Kinesin motor domain |
| NDVI50_D | solcap_snp_c1_6462 | 2 | 2450782 | G | T | 0.03 | 0.0744 | 2.53 × 10−6 | 0.0027 | Plant protein with unknown function |
| SPAD70_D | PotVar0039950 | 6 | 53985614 | C | T | −3.65 | 0.0712 | 4.25 × 10−6 | 0.0083 | Radical SAM superfamily protein |
| NDVI70_D | solcap_snp_c2_43735 | 4 | 64055406 | A | G | −0.07 | 0.0095 | 0.0432 | 0.0444 | GroES-like zinc-binding dehydrogenase family protein |
| NDVI70_D | PotVar0113919 | 4 | 64089292 | A | G | −0.07 | 0.0049 | 0.0461 | 0.0472 | Ascorbate peroxidase |
| SC70_D | solcap_snp_c2_45637 | 1 | 12022163 | A | G | −182.03 | 0.0729 | 3.26 × 10−6 | 0.0055 | Hypothetical protein |
| Yield_D | solcap_snp_c2_26653 | 8 | 54286889 | G | T | −0.75 | 0.071 | 4.37 × 10−6 | 0.0111 | Osmotin |
| TubNum_D | PotVar0064470 | 11 | 787325 | G | T | −10.25 | 0.0614 | 2.03 × 10−5 | 0.0166 | Alternative oxidase family protein |
| TubNum_D | solcap_snp_c2_55085 | 10 | 20334943 | A | G | 27.36 | 0.0586 | 3.23 × 10−5 | 0.0194 | Transketolase |
| RS_D | solcap_snp_c1_3746 | 2 | 7050595 | C | T | 0.14 | 0.034 | 0.0016 | 0.025 | Cofactor assembly of complex C |
| RS_D | solcap_snp_c2_25284 | 4 | 65872176 | A | G | 0.10 | 0.0041 | 0.0277 | 0.0388 | Sucrose transporter |
| RS_D | solcap_snp_c2_55785 | 4 | 65970953 | G | T | 0.11 | 0.0952 | 0.01 | 0.0277 | Leucine-rich receptor-like protein kinase family protein |
| RS_D | solcap_snp_c2_55783 | 4 | 65971150 | A | G | 0.11 | 0.0952 | 0.01 | 0.0305 | Leucine-rich receptor-like protein kinase family protein |
| RS_D | solcap_snp_c2_55775 | 4 | 65972399 | C | T | 0.11 | 0.0952 | 0.01 | 0.0333 | Leucine-rich receptor-like protein kinase family protein |
| Year 2019 | |||||
| 14–31 May | June | July | August | 1–17 September | |
| Max. temperature (°C) | 18.1 | 25.4 | 7.1 | 27.5 | 22.2 |
| Min. temperature (°C) | 5.7 | 10.0 | 3.1 | 13.0 | 10.0 |
| Humidity (%) | 79.9 | 70.3 | 2.3 | 73.5 | 75.1 |
| Precipitation (L/m2) | 33.9 | 17 | 2.1 | 24.7 | 30.5 |
| Year 2020 | |||||
| 26–31 May | June | July | August | 1–28 September | |
| Max. temperature (°C) | 26.8 | 22.2 | 6.4 | 27.7 | 24.6 |
| Min. temperature (°C) | 9.2 | 0.7 | 2.4 | 13.0 | 11.0 |
| Humidity (%) | 68.3 | 7.2 | 3.2 | 72.3 | 71 |
| Precipitation (L/m2) | 0 | 5.8 | 8.8 | 31.4 | 33.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez-Morezuelas, A.; Barandalla, L.; Ritter, E.; Ruiz de Galarreta, J.I. Genome-Wide Association Study of Agronomic and Physiological Traits Related to Drought Tolerance in Potato. Plants 2023, 12, 734. https://doi.org/10.3390/plants12040734
Alvarez-Morezuelas A, Barandalla L, Ritter E, Ruiz de Galarreta JI. Genome-Wide Association Study of Agronomic and Physiological Traits Related to Drought Tolerance in Potato. Plants. 2023; 12(4):734. https://doi.org/10.3390/plants12040734
Chicago/Turabian StyleAlvarez-Morezuelas, Alba, Leire Barandalla, Enrique Ritter, and Jose Ignacio Ruiz de Galarreta. 2023. "Genome-Wide Association Study of Agronomic and Physiological Traits Related to Drought Tolerance in Potato" Plants 12, no. 4: 734. https://doi.org/10.3390/plants12040734
APA StyleAlvarez-Morezuelas, A., Barandalla, L., Ritter, E., & Ruiz de Galarreta, J. I. (2023). Genome-Wide Association Study of Agronomic and Physiological Traits Related to Drought Tolerance in Potato. Plants, 12(4), 734. https://doi.org/10.3390/plants12040734






