Abstract
Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) is an invasive pest native to the American continent. The present study focused on bio-intensive tactics like intercropping, using natural enemies, botanical insecticides and biopesticides for managing S. frugiperda for the organic production of maize in Indian conditions. A total of eight different parasitoids attacking the different stages of S. frugiperda viz., eggs and larvae were found in the study area. The total parasitism rate due to all the parasitoids ranged from 28.37 to 42.44%. The egg-larval parasitoid, Chelonus formosanus Sonan (Hymenoptera: Braconidae) was the dominant parasitoid (12.55%), followed by Chelonus nr. blackburni (Hymenoptera: Braconidae) (10.98%) and Coccygydium sp. (4.85%). About 36.58 percent of the egg masses collected was parasitized by egg parasitoids, among which Telenomus remus (Nixon) (Hymenoptera: Scelionidae) was the dominant parasitoid. The botanicals insecticides such as citronella and annona extract were most effective, resulting in 100% mortality of FAW larvae (168 h after treatment). The essential oil of garlic (100%) was found highly effective in inhibiting egg hatching, followed by geraniol (90.76%). The maize intercropped with lady’s finger (okra) recorded significantly the lowest pest infestation and recorded higher grain yield (6.17 q/ha) than other intercropping systems and control (5.10 q/ha). The overall bioefficacy of commercial biopesticides against the larvae of S. frugiperda was in the following order azadirachtin > Metarhizium anisopliae (Metch.) Sorokin (Hypocreales: Clavicipitaceae) > Beauveria bassiana (Balsamo) Vuillemin (Hypocreales: Clavicipitaceae) at 168 h after treatment.
Keywords:
biocontrol; Chelonus formosanus; botanicals; fall armyworm; intercropping; Telenomus remus 1. Introduction
Fall Armyworm (FAW), Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae), is a highly polyphagous invasive pest native to the Americas, that has expanded its distribution from western to eastern hemisphere [1,2]. It was first discovered in Africa in 2016 and is now found in over 70 countries throughout Asia and Oceania [1,3,4]. Since its introduction, FAW has emerged as a serious threat to cereal crop productivity, particularly maize and sorghum, two of the major staple food crops of Asia and Africa’s smallholder farmers, threatening regional food security [5,6]. To contain the FAW spread, many African and Asian countries have recommended, distributed, and applied synthetic pesticides [7,8]. For instance, in 2017, Zimbabwe distributed nearly 102,000 L of pesticide worth $1.97 million USD to farmers [9]. Despite government subsidies, the use of synthetic insecticide as the sole control measure is unsustainable due to its high cost, increased pesticide resistance, pest resurgence, and risk to human health and the environment.
The use of cover crops/hedge rows/intercrops/flower strip crops in the main crop field to conserve beneficial insect fauna is known as habitat manipulation [10,11]. Conservation biological control is a method of customizing crop habitat to support and sustain the population of native parasitoids and predators for biological control of pests [12,13]. Intercrops not only help to reduce pest infestations but also improve soil fertility and serve as a refuge for parasitoids and predators [14,15]. Despite the availability of successful modules on the use of habitat manipulation for managing other maize borer pests such as stem borers [16], extensive research on agro-ecological approach for the management of Fall Armyworm under Indian conditions is lacking. Furthermore, using vegetable crops as intercrops to reduce the incidence is a novel concept as it also generates additional income for farmers [17].
The Fall Armyworm in India is attacked by various natural enemies [18], including insect parasitoids and predators, and entomopathogens such as fungi, bacteria, viruses, and nematodes. Efficient natural enemies of FAW could be identified and integrated into an Integrated Pest Management (IPM) system to achieve economic growth and environmental safety and sustainability paving the way for organic production [19]. Keerthi et al. [15] identified Eocanthecona furcellata (Wolff) (Hemiptera: Pentatomidae), as an efficient predator of FAW from central India and also, larval parasitoids like Cotesia ruficrus (Haliday) (Hymenoptera: Braconidae), Campoletis chlorideae Uchida (Hymenoptera: Ichneumonidae), and Aleiodes sp. from field [3]. Chelonus formosanus was identified as the most dominant egg-larval parasitoid of FAW in northern India by Sagar et al. [20]. Udayakumar et al. [21] and Navik et al. [22] also documented the distribution of egg parasitoids such as Trichogramma chilonis Ishii (Hymenoptera: Trichogrammatidae) and Telenomus remus (Nixon) (Hymenoptera: Scelionidae) in southern India. Sivakumar et al. [23] reported the natural occurrence of entomopathogens on FAW like Bacillus thuringiensis Berliner (Bacillales: Bacillaceae), Nomuraea rileyi (Farl.) Samson (Hypocreales: Clavicipitaceae), SpfrNPV (Lefavirales: Baculoviridae). Hence, we hypothesize that, identifying relatively dominant and efficient natural enemies for their use under field conditions might leads to development of bio-intensive management option for suppressing FAW in the India.
Effective control of the borer complex of maize in the America has mainly relied on the genetically modified maize hybrids expressing Bt insecticidal proteins for over a decade [24]. However, the management of FAW exclusively depends on the synthetic pesticides in Africa and Asia [8,25]. Because of high cost involved in pesticide control and risk to human health and the environment, the use of synthetic pesticides as the sole control measure is unsustainable in long run [26]. Botanicals are one of the suitable alternatives to synthetic pesticides and have a great potential to use under field conditions. Among botanicals, essential oils are complex secondary metabolite mixtures that evolved in plant defense mechanisms against insects and that, when extracted and applied exogenously, can confer insecticidal, repellent, or antifeedant activities [27]. Sombra et al. [28] evaluated the efficacy of three essential oils on the different stages of FAW, among which Lippia origanoides Kunth, registered an average of 97.8% ovicidal activity and 81.3% pupicidal activity. Negrini et al. [29] also documented 100% mortality of second instar larvae of FAW due to the application of Corymbia citriodora (Hook.) K.D. Hill & L.A.S. Johnson (Myrtales: Myrtaceae) and Lippia microphylla Cham. (Lamiales: Verbenaceae). Being natural in origin, a research on the phytosanitary use of botanicals derived from various aromatic plants has increased [30]. Owing to its revamped distribution range, it would be prudent to document the native biocontrol agents associated with the invasive pest and evaluate ecofriendly pest management options against the pest to develop an Integrated Pest Management (IPM) module in India.
2. Results
2.1. Field Parasitism by Egg and Larval Parasitoids of FAW
During the study period, a total of 287 egg masses and 2741 larvae were collected from maize fields during the main cropping season. Table 1 shows the total number of FAW larvae collected during each cropping season month, as well as the average parasitization rates. Six parasitoids emerged from field collected larvae, including two egg-larval parasitoids viz., Chelonus formosanus and Chelonus nr. blackburni (Hymenoptera: Braconidae) and four larval parasitoids Coccygidium sp. (Hymenoptera: Braconidae), Temelucha sp. (Hymenoptera: Ichneumonidae), Cotesia ruficrus (Haliday) (Hymenoptera: Braconidae) and Campoletis chlorideae Uchida (Hymenoptera: Ichneumonidae). The combined parasitism rate due to all the parasitoids ranged from 28.37 to 42.44, and the highest parasitism rate (42.44) was recorded during July, 2021.

Table 1.
Relative abundance and parasitism rate of native parasitoids on S. frugiperda in New Delhi, India.
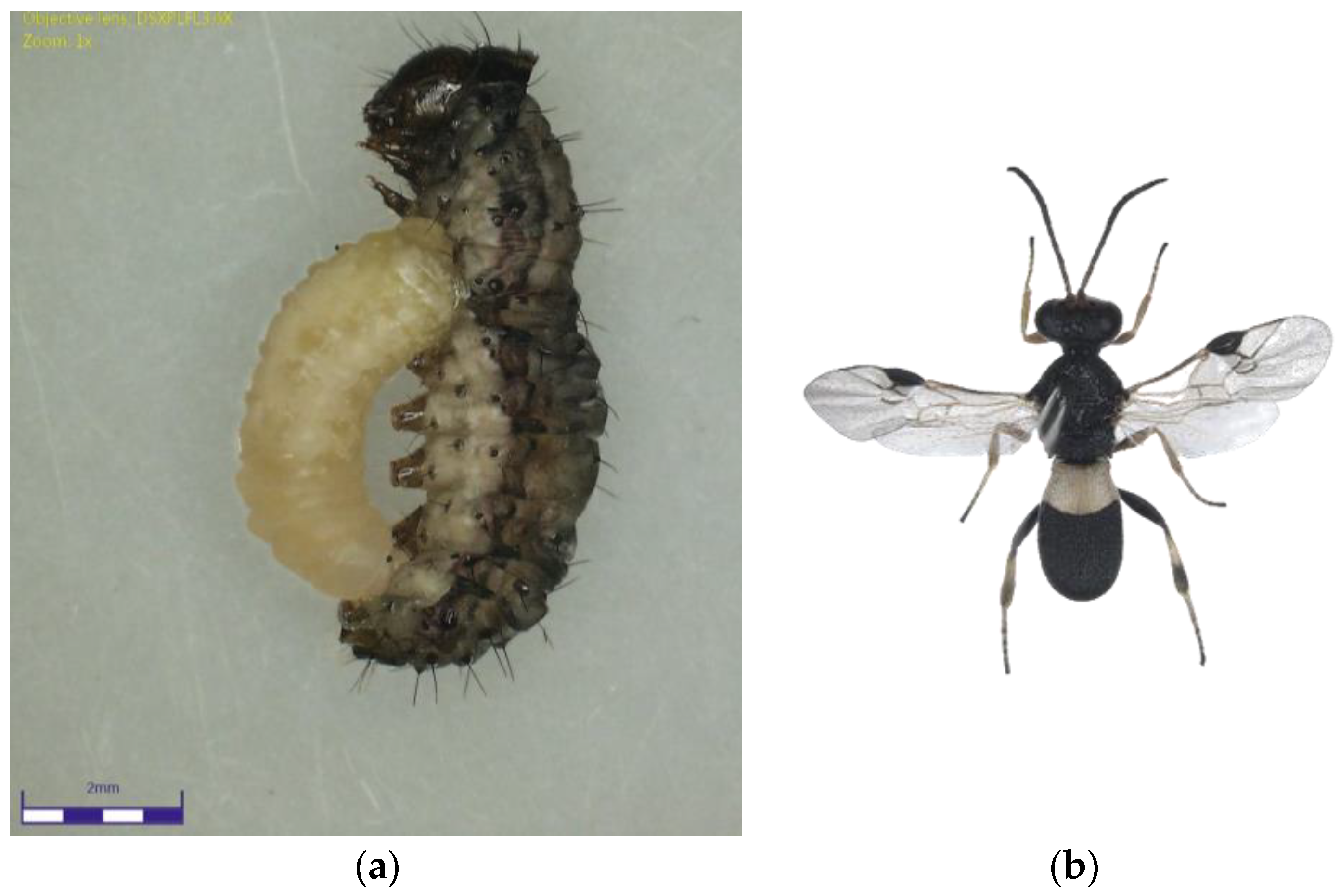
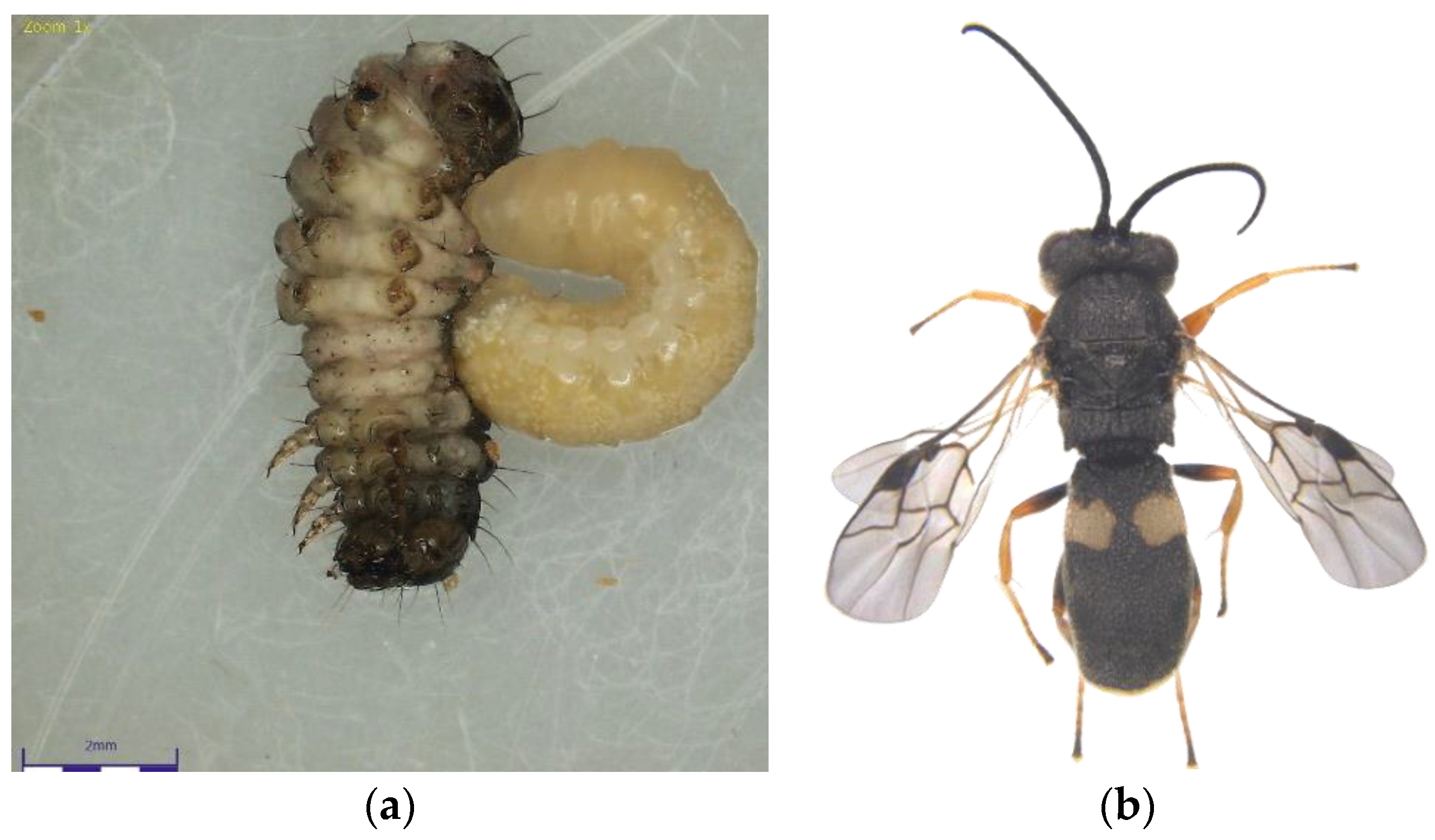
The Chelonus nr. blackburni was the most abundant and contributed the most to parasitism (18.57%) during July, 2021 (Figure 1a,b). During August and September, the most abundant parasitoid was C. formosanus (32.56 and 45.57%), which contributed significantly to the FAW’s natural mortality, respectively (Figure 2a,b). Even though Coccygidium sp. was active throughout the cropping season, it was most abundant (34.33%) in October 2021 and contributed significantly to parasitism. Temelucha sp., C. ruficrus, and C. chlorideae, on the other hand, were the least abundant parasitoids recovered from field-collected larvae throughout the study period. C. formosanus (12.55%) was the most active parasitoid and contributed to the highest for the total parasitism, followed by Chelonus nr. blackburni (10.98%). Surprisingly, nearly 2% of the field-collected larvae died due to entomopathogens infection. Furthermore, dead parasitoids accounted for 5.73 percent of total parasitism.
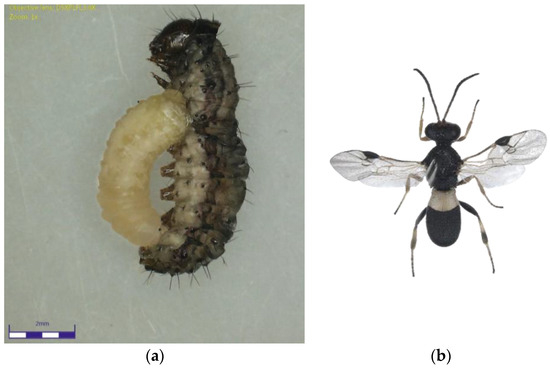
Figure 1.
(a) Chelonus nr. blackburni larva emerging from the Fall Armyworm larvae; (b) Chelonus nr. blackburni adult.
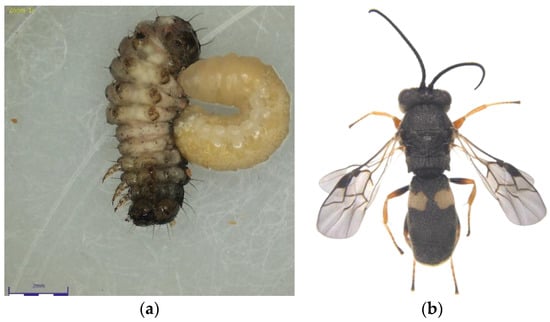
Figure 2.
(a) Chelonus formosanus larva emerging from the Fall Armyworm larvae; (b) C. formosanus adult.
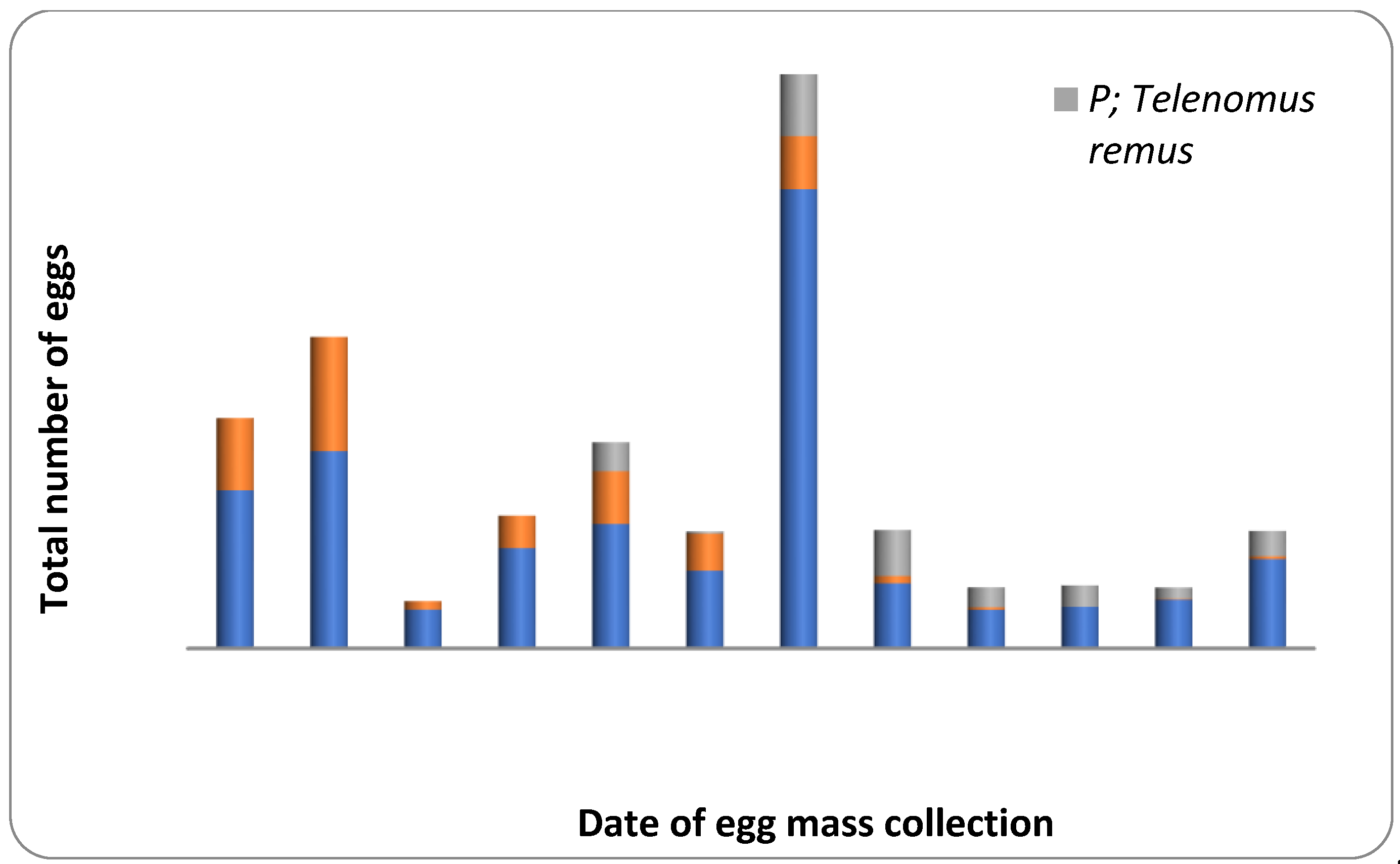
About 36.58 percent of the egg masses collected in the field was parasitized by egg parasitoids like T. chilonis and T. remus (Table 2; Figure 3). Natural parasitism caused by T. chilonis was 56.25 percent in July, 2021 and 24.05 percent in August, 2021. In comparison, the activity of T. remus was not recorded during July and August, 2021. However, the percentage natural parasitism due to T. remus was 23.48 during September, 2021. However, the percent of egg masses parasitized due to both the parasitoid was 7.41 and 6.96 during August and September, 2021, respectively. The activity of T. chilonis was observed during the third week of July 2021, and the incidence fluctuated throughout the crop growth period (Figure 3). In contrast, the incidence of T. remus started during the second week of August and remained active until the end of the crop growth period (Figure 3).

Table 2.
Percentage of Fall Armyworm egg mass parasitized by Trichogramma chilonis and Telenomus remus.
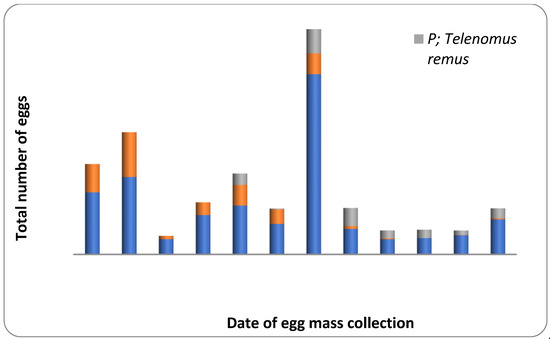
Figure 3.
Parasitism level on Fall Armyworm eggs collected from maize agro-ecosystem during July–September, 2021. The orange color: Trichogramma chilonis; The grey color: Telenomus remus; The blue color: Unparasitized.
2.2. Effect of Different Botanicals on Developmental Stages of FAW
Significant differences were found among the botanicals with respect to its ovicidal (F4,14 = 26.183; p value < 0.0001) (Table 3), larvicidal, and pupicidal (Table 4) activity. The toxicity of botanicals at a uniform concentration was tested against FAW eggs, and the percent inhibition of egg hatching was recorded (Table 3). Eggs masses treated with double distilled water (control) hatched in 99 percent of the cases. All of the botanicals used in the study showed ovicidal activity for FAW eggs, but the percentage inhibition of hatching ranged from 4.69 to 100%. Essential oil of garlic was more toxic than other botanicals because, it completely inhibited hatching (100%) on FAW egg masses followed by geraniol (90.76 ± 8.88). Annona methanoic extract also recorded significant ovicidal action (60.63 ± 13.11). However, the geranium, picro, and Karanjin were least effective in inhibiting the hatching with 4.69 ± 5.15, 7.31 ± 3.34, and 8.35 ± 10.59 percentage, respectively.

Table 3.
Ovicidal action of different botanicals on eggs of Fall Armyworm.

Table 4.
Percent mortality of larvae and pupae of Fall Armyworm after exposure to different botanicals.
Botanicals were toxic to S. frugiperda larvae at a single concentration (1%), and the maximum mortality was observed after 72 h. Geranium oil resulted in the highest mean larval mortality after 24 h of treatment (40%). Citronella oil treatment resulted in 60 and 90 percent mortality of treated larvae after 48 and 72 h, respectively. After 72 h of treatment, annona, geranium oil, geraniol, clove compound, sesame oil, chilli, garlic are equally effective (40%) against the S. frugiperda. Citronella oil and annona were most effective at achieving 100 percent mortality of exposed insect after 168 h, followed by citronellol and geranium oil (90%). However, annona methane extract and Annona acetone extract were found ineffective against larvae of S. frugiperda.
The essential oil of chilli caused significant larval mortality (40% at 168 h), but it led to phytotoxicity in exposed maize leaves. All of the survived larvae from each treatment were observed until pupation and adult emergence. All the survived larvae treated with citronellal and sesame oil went to pupation. The percentage of larvae that develop into pupae after being exposed to geraniol was the lowest (16.7). Aside from the toxicological effect, the botanicals of annona, citronellol, and clove had antifeedant activity on the treated larvae. Furthermore, adults emerged from sesame oil, and karanjin-treated larvae showed wing deformation. The adults emerged from the pupae treated with annona acetone extract (40%), and karanjin (30%) showed wing deformation.
2.3. Effect of Intercropping on the Incidence of FAW
The plants with excreta, damage severity scale, and numbers of live larvae per plant were recorded at 5, 6, 7, 8, and 9 weeks after sowing (WAS) and presented in Table 5. The percent plant damage of maize differed significantly between treatments at six (F2,6 = 3.830; p < 0.0023), seven (F2,6 = 7.307; p < 0.002), eight (F2,6 = 11.872; p < 0.0001), and nine weeks (F2,6 = 10.711; p < 0.0001). During 5 WAS, the percent incidence was non-significant in the intercropping systems. The percent plant incidence was highest in monocrop (33.02, 48.86, 61.99, and 67.10 percent) and lowest in maize + lady’s finger (11.39, 12.2, 12.74 and 13.81 percent), during 6, 7, 8, and 9 WAS, respectively (Table 6).

Table 5.
Mean number of larvae, damage scale and mean number of plants with excreta in intercropped and sole crop of maize.

Table 6.
Effect of vegetable intercropping on maize infestation by Fall Armyworm at different weeks after sowing (WAS).
There was a significant difference in the number of live larvae between the intercropped and monocropped maize plots at 7 (F2,6 = 3.612; p < 0.0028), 8 (F2,6 = 10.231; p < 0.0001) and 9 WAS (F2,6 = 11.063; p < 0.0001). The least number of live larvae was recorded in maize + Lady’s finger intercropping in all the observed weeks. Monocrop of maize recorded the highest number of live larvae/plant viz., 1.10, 1.01, and 1.28/plant during 7, 8, and 9 WAS, respectively. The severity of damage was non-significant among the different intercropping systems during 5 (F2,6 = 4.069; p < 0.019), and 6 WAS (F2,6 = 1.131; p < 0.401). Whereas, during 7 and 8WAS, the severity of damage was highest in control plots (5.95 ± 0.47 and 5.86 ± 0.39, respectively) but remained non-significant among the different intercropping systems.
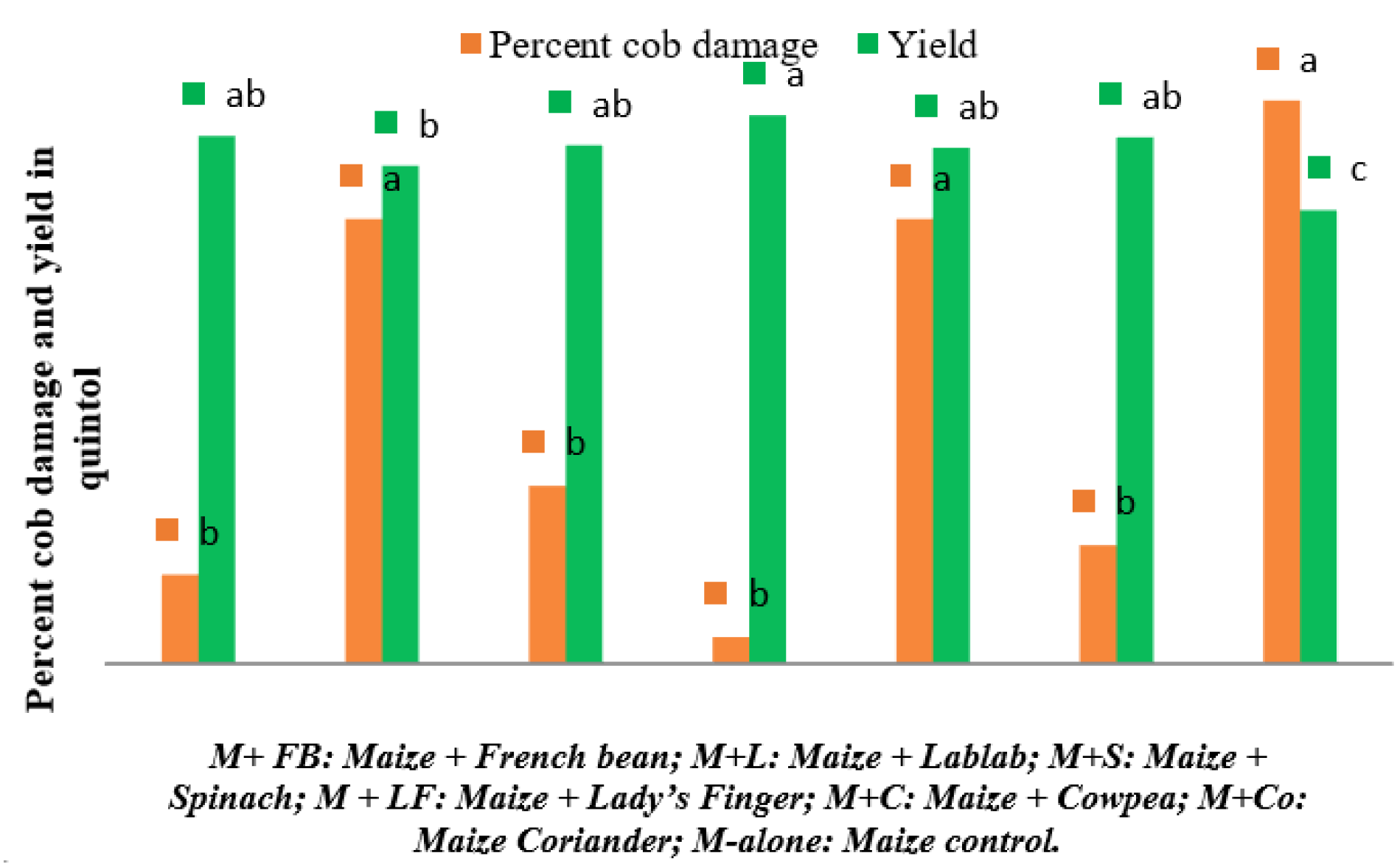
Similarly, the highest number of plants carrying excreta was observed in control plots during 8 (29.00 ± 9.85) and 9 WAS (34.00 ± 16), however it remains non-significant among different intercropping systems during 9 WAS. Figure 4 depicts the percentage cob damage and yield under the intercropping systems. The cob damage was significantly superior in control plots (6.33) and lablab intercropped field (5.00), and the lowest cob damage was observed in the lady’s finger intercropping system (0.30). The maize + lady’s finger recorded highest grain yield (6.17 quintal/ha), whereas, the control plot recorded the lowest grain yield (5.13 quintal/ha).
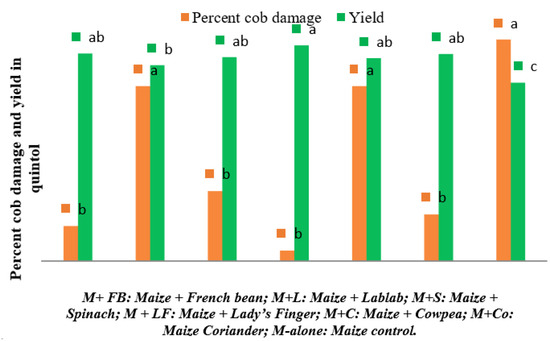
Figure 4.
Percent cob damage by S. frugiperda and maize grain yield under different intercropping systems in New Delhi, India. [Bars of same colour marked with different letters differ significantly; p < 0.05, Duncan’s multiple range test].
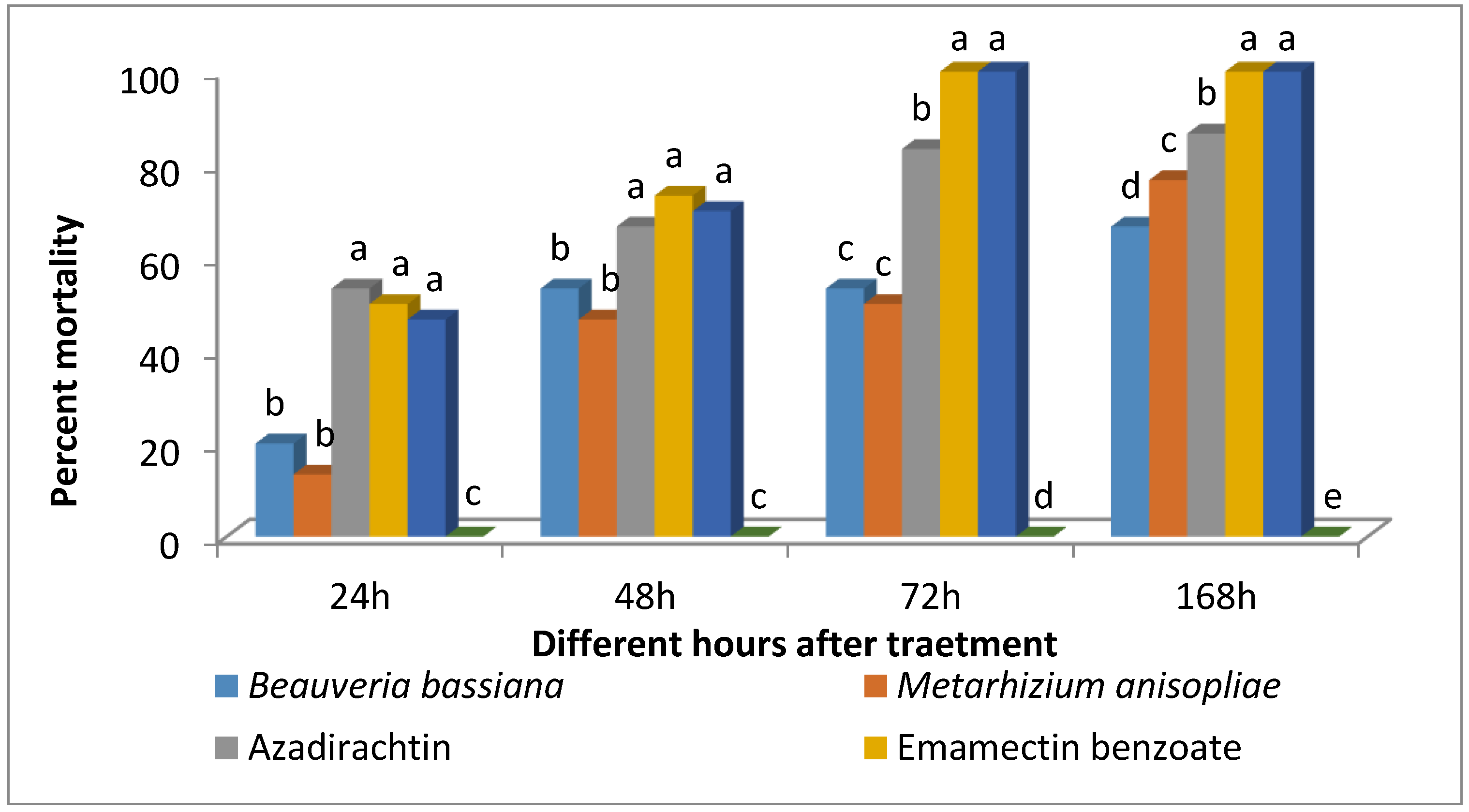
2.4. Efficacy of Biopesticides against Larvae of FAW
The analysis of variance (ANOVA) revealed that the efficacy of biopesticides against S. frugiperda varied significantly after 24 h (F2,9 = 29.98; p < 0.0001), 48 h (F2,9 = 57.1; p < 0.0001), 72 h (F2,9 = 99.05; p < 0.0001), and 168 h (F2,9 = 252.6; p < 0.0001) of exposure to treatments (Figure 5). At 24 h after treatment, the mortality caused by Azadirachtin, Emamectin benzoate, and Chlorantraniliprole was equally significant. In contrast, mortality caused by B. bassiana and M. anisopliae was less effective, with no difference observed between them. A similar trend in mortality was observed at 48 h after treatment. All the larvae on Emamectin benzoate and Chlorantraniliprole treated leaves died at 72 h after treatment, while, Azadirachtin treated larvae showed 83.33% mortality. The overall efficacy of biopesticides formulation was in the following order azadirachtin > M. anisopliae > B. bassiana at 168 h after treatment.
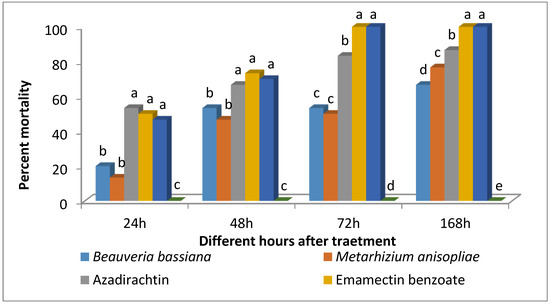
Figure 5.
Efficacy of different biopesticides and insecticides against 3rd instar larvae of S. frugiperda. Bars marked with different letters differ significantly; p < 0.05, Duncan’s multiple range test.
3. Discussion
The FAW is a polyphagous exotic and invasive pest which causes significant crop damage and threatening food and nutritional security [5]. The sole reliance on chemical control not only eliminated the natural defender population, but also, led to a slew of environmental issues such as pesticide residues, resistance to pesticides, pest resurgence, and effects on non-target organisms [31]. The current study focuses on potential biological and maize agroecosystem manipulation (intercropping different plants) interventions for S. frugiperda management in India.
Eight different parasitoid species attacking the eggs and larvae of S. frugiperda were found in northern India. In similar surveys carried out in central and North India, Keerthi et al. [3] and Sagar et al. [20] documented the emergence of several parasitoids from central and northern India, respectively. Furthermore, Sharanabasappa et al. [18] reported five parasitoids from South India. Our results showed that larval parasitism levels ranged from 28.37 to 42.44% during the different months of larval collection. At the same time, the percent egg parasitism varied from 30.44 to 56.25. Similarly, Navik et al. [22] reported the parasitism of FAW eggs between 15.81–23.87 and 5.44–8.78 percent due to T. chilonis and T. remus, respectively.
Among the parasitoids reported in the present study, C. formosanus was the most dominant parasitoids (12.55) collected during the study period, followed by Chelonus nr. blackburni (10.98) and Coccygydium sp. (4.85). The members of Chelonus sp. are arrhenotokous, solitary, egg-larval parasitoids of several important lepidopterous pests distributed throughout the world. C. nr blackburni and C. formosanus are the widely distributed parasitoids of lepidopteran pests in the Neotropical and Oriental region [32]. However, Sagar et al. [20] reported Chelonus nr. blackburni was the most abundant parasitoid in northern India; higher sampling size carrying out in the present study might have contributed to the variations. Further, Coccygidium sp.(Hymenoptera: Braconidae) was one of the most dominating parasitoids identified in the study. The parasitoid was less active in the early cropping season, but it replaced other parasitoids and remained the most abundant in the later cropping season. Coccygidium sp. is known to parasitize the larvae of many noctuids, including Spodoptera spp. [33]. Many researchers have reported that, different species of Coccygydium sp. parasitized the larvae of S. frugiperda [33,34]. Average parasitism across India was higher than the parasitism rate reported in other studies of Fall Armyworm in corn (0.76%, Sagar et al. [20]; 0.001%, Sharanabasappa et al. [18]). However, the parasitism rate in the present study was lesser than what reported in the African countries (3.5–19.3, Agboyi et al. [35]; 9.2%, Otim et al. [36]). The variation in the parasitism might be due to the variable distribution of different species of Coccygidium sp. and also the different general equilibrium levels of FAW.
Two major egg parasitoids (T. chilonis and T. remus) have emerged from the field-collected egg masses. T. chilonis was the most dominant parasitoid reported in the early crop period, later replaced by T. remus. The Trichogramma sp. and T. remus are the most important parasitoid used in the biological control of S. frugiperda in Latin American countries such as Venezuela, Colombia, Brazil and Mexico [37]. The earlier workers in India also reported the field parasitization by the aforementioned parasitoids [21,22]. Competition is a dynamic, ongoing process. As a result, an interspecific competition that results in displacement has far-reaching evolutionary repercussions for the interacting species. Two species can survive for an extended period, but eventually, one will evolve better competitive powers, displacing the other [38,39,40]. The displacement might be due to variation in the temporal distribution of parasitoids and also the effect of environmental factors [41].
The plant botanicals are among the promising alternatives to synthetic pesticide as it offered added advantages like repellent, and antifeedant and that’s why, emphasized as an important tool in Integrated Pest Management (IPM) [28]. In the present work, we evaluated fourteen different botanicals against eggs, larvae, and pupae of S. frugiperda. The obtained results using different botanicals substantiate the literature and provide additional information on the biocidal potential of extracts and botanicals [28,42].
The botanicals like citronella oil and annona extracts were most effective, resulting in 100% mortality of S. frugiperda larvae. When botanicals having insecticidal property interact with insect integument, they may influence digestive and neurological enzymes [43]. The essential oil of the garlic was highly effective in inhibiting egg hatching (100%), followed by geraniol (90.76%). Allyl disulfide is an important component of the garlic oil and it is known for exhibiting ovicidal and larvicidal activity and the same might have contributed to ovicidal action against the eggs of S. frugiperda [44]. Similarly, geraniol also known to exhibit ovicidal action against the housefly eggs, which reduced the 99% of hatching [45]. Among the screened botanicals, none of the compounds are known to exhibit significant pupicidal activity against S. frugiperda. However, significant adult wing deformation was noticed in the pupae treated with annona acetone extract. The insecticidal activity of acetone extract of Annona squamosa L. (Magnoliales: Annonaceae) was also reported by several workers [46,47]. The differences in the insecticidal activity of different botanicals are primarily attributable to their composition and modes of action, which resulted in morphological, physiological, and behavioral changes in S. frugiperda at various growth stages [48,49].
In the present study, maize intercropped with lady’s finger recorded significantly the lowest percent plant damage during all the observed weeks. At the same time, the percent damage in maize intercropped with French bean, lablab, spinach, cowpea, and coriander was on par with each other. The present study was the first of its kind in evaluating the effect of vegetable intercropping on the incidence of FAW in India. However, the results of French bean and cowpea intercropping systems are in accordance with the findings of Hailu et al. [50] and Udayakumar et al. [21]. Because of the presence of natural enemies, maize intercropped with beans recorded a lower incidence [21]. The number of plants with excreta was significantly lower in the lady’s finger intercropped plot and highest in the control plot. The suppression of herbivore populations and reduction of damage has already been demonstrated in several intercropping systems; Saminathan et al. [51] documented the lowest pest activity and higher activity of natural defender population in cotton intercropped with lady’s finger. It is well known that insect pest populations are fewer in diverse ecosystems or intercrops [52,53]. The current study supports the previously reported findings that intercropped maize with leguminous and other crops resulted in much decreased FAW infestation compared to mono-cropped maize [50].
The infestation of FAW on maize cob was highest in the lablab and cowpea intercrops, and it is on par with the control plots. The percent cob damage was lowest in French bean, spinach, lady’s finger, and coriander intercropping system and on par with each other. Similarly, the highest grain yield was obtained in maize + lady’s finger intercropping system followed by French bean, spinach, cowpea, and coriander intercropping system and was on par with each other. Similar results were reported by Tanyi et al. [54], who stated the intercropping of maize with different beans reduced the incidence of S. frugiperda. Pest management through habitat manipulation via intercropping system was established in many cropping systems. It was based on the hypothesis that confusing olfactory and visual cues received from the companion beans plants probably served as the push component that repelled FAW larvae away from the maize plants. In the case of the lady’s finger plot, the green leaf volatiles emitted by it might be repelling the FAW adults and larvae to move away from maize [54]. The low maize yield recorded in control plots was consistent with the FAW severity and maize infestation. In contrast, the highest grain yield was recorded in the maize + lady’s finger intercropping system. The improved maize yield in the plots intercropped with beans could be due to a combination of low FAW severity and maize infestation [54]. The disparity observed is probably due to the crop morphology, ecosystem, and management practices [53].
In laboratory bioassays, moderate to high larval mortality was achieved with M. anisopliae, B. bassiana, Azadirachtin, Emamectin benzoate, and Chlorantraniliprole. Bio-pesticides are vital in IPM and are the best alternative to chemical control [31]. Azadirachtin was the most effective against the larvae among the biopesticides, followed by M. anisopliae and B. bassiana. The results follow the earlier findings of Dhobi et al. [55]. Synthetic insecticides are important management options in FAW control, as is common with other insect pest species. In India, chemical control of FAW in maize is achieved by applying Cyantraniliprole, Emamectin benzoate, and Thiamethoxam, among other synthetic insecticides [8]. Emamectin benzoate and Chlorantraniliprole are highly effective against the S. frugiperda resulting in 100% mortality after 72 h after exposure. Deshmukh et al. [56] also evaluated the field efficacy of insecticides and reported chlorantraniliprole was the most effective, followed by emamectin benzoate.
4. Materials and Methods
4.1. Insect Rearing
The rearing was established from larvae collected in experimental maize fields of ICAR- Indian Agricultural Research Institute, Pusa, New Delhi, and maintained using an artificial diet proposed by Gujar et al. [57] with few modifications (Chickpea flour was reduced to 90 g instead of 108 g, and streptomycin sulphate was increased to 0.48 g instead of 0.2 g). The field collected larvae were reared for two generations under laboratory conditions, and the successive developmental stages of FAW were used for laboratory evaluations.
4.2. Field Collection of FAW (Egg Masses and Larvae) and Laboratory Rearing
Egg masses and larvae of FAW were collected from different maize fields of ICAR- Indian Agricultural Research Institute (IARI), Pusa, New Delhi (28.08° N, 77.12° E) during July–October 2021. Plants with visible FAW attack were selected in each field and checked for the presence of FAW egg masses and larvae. Larvae were pulled from the whorl and placed individually in small Petri dishes (2 cm height × 5.8 cm diameter) containing an artificial diet until parasitoid emergence. To avoid damage to any eggs in the batch, the FAW egg masses were collected from infested maize plants along with a piece of fresh leaves. The individual egg mass was placed in Petri dishes. The collected egg masses and larvae were maintained in the insect rearing laboratory under climatized room (27 ± 1 °C, 65 ± 5% RH and 14 h: 10 h L: D photophase), in ICAR-IARI, New Delhi. The parasitoids that emerged from the collected larvae were regularly preserved in 70% alcohol. At every week, an attempt was made to collect at least 250 larvae and 25 egg masses; however, larger or smaller numbers of larvae and egg masses were collected during some weeks.
4.3. Evaluation of Botanicals against FAW
Bioassays were carried out to evaluate the activity of 14 different botanicals on the egg (n = 5 egg masses per treatment), larvae (n = 10), and pupae (n = 10) of FAW in a Completely Randomized Design (CRD) under laboratory conditions. Each treatment of botanicals on different developmental stages of FAW was replicated thrice. The negative control consisted of distilled water, and 5.0% (v/v) of neutral detergent (Tween 80) used to correct the results obtained with botanicals.
4.3.1. Preparation of Plant Extracts
The mature and dried seeds of custard apple, Annona squamosa L. and sesame, Sesamum indicum L. (Lamiales: Pedaliaceae) were purchased from the local market, cleaned and powdered. The powdered seeds of A. squamosa were extracted sequentially with hexane, acetone and methanol to obtained different extracts while sesame oil was obtained from the powdered sesame seeds with hexane using Soxhlet apparatus. The solvents from the different extracts were removed using rotary evaporator to obtain the dried extracts.
4.3.2. Chemicals and Solvent
Geranium oil and citronella oil (Cymbopogon spp.: Poales: Poaceae) were purchase from M/s Shiv Sales Corporation, New Delhi, citronellol and citronellal (Cymbopogon spp) from M/s SRL Pvt. Ltd., India, while tween-80 from M/s Ranchem Pvt Ltd., India. Garlic oil (Allium sativum L. Plantae: Amaryllidaceae) and Capsicum oleoresin (Capsicum spp. Solanales: Solanaceae) were obtained from M/S Bio-India Biologicals, Corporation, Hyderabad. Geraniol, oleanolic acid and karanjin were isolated and identified from palmarosa oil (Cymbopogon martini (Roxb.): Poales: Poaceae), clove buds (Syzygium aromatic (L.) Merr. & L.M.: Myrtales: Myrtaceae) and karanj seed (Millettia pinnata (L.) Panigrahi, Fabales: Fabaceae).
4.3.3. Preparation of Test Solution
Stock solution (1.0%) of the oils, pure compounds and extracts were prepared with Tween-80 (3.0%) in distilled water using lab stirrer by stirring the content for 1 h. A uniform concentration of 1% was used for all the botanicals.
4.3.4. Bioassay for Ovicidal Activity
The eggs of same age groups (n = 5) were collected from the laboratory culture, and each egg mass was sprayed with different botanicals (1%) using hand held atomizer. Only a desired quantity (2 mLfor each egg mass) of botanicals was used for uniform application and sprayed on the FAW egg masses. After spraying, the egg mass was individually transferred into Petri dishes. The deterioration or hatching of S. frugiperda eggs was observed after 7 days of treatment by counting the dead larvae and unhatched eggs in each Petri plate. The hatchability percent was worked out as per the following formula as given by Sangha et al. [58].
4.3.5. Larvicidal Activity Bioassay by Leaf Dipping Method
Under this, 1% botanicals solution was taken in the Petri dish (9 cm). The leaf disc of maize (5 cm diameter) was dipped in the solution for a minute and later shade dried before exposing larvae for feeding. Accumulated mortalities were assessed at 24, 48, 72, and 168 h after application of treatments including control. Observed mortality was also corrected for the control using the formulas proposed by Abbott [59]. After 72 h, the surviving larvae in each treatment were transferred to new Petri dishes containing an artificial diet. Observations were made on the remaining larvae to document the number of pupae formed (larval viability) and the number of adults formed (pupal viability). During the observations, the presence of feeding marks on the leaves and fecal pellets in the Petri dishes was noted for the antifeedant activity. In addition, the phytotoxicity effect of botanicals on the maize leaves was observed by recording the change of leaf colour, leaf drying, and early loss of turgidity by comparing it with the control.
4.3.6. Bioassay for Pupicidal Activity
Pupicidal activity (Baskar et al. [60]) was determined using pupae (48 h old) of S. frugiperda. Pupae (batch of 10) were placed on filter paper and sprayed with 2 mLof different botanicals solutions and control using an atomizer. After 30 min, the pupae were transferred to Petri dishes (9 cm diameter) lined with filter paper. The observations on percent adult emergence and the wings deformations among the emerged adults were recorded.
4.4. Effect of Intercropping on the Incidence and Damage by FAW
The experiment was conducted in the winter season of 2020 in Randomized Block Design (RBD) with a plot size of 5 m × 5 m. The treatments were seven and replicated thrice. The different treatments were maize + coriander (Coriander sativum L.; Figure 6), maize + lablab (Lablab purpureus (L.) Sweet), maize + French bean (Phaseolus vulgaris L.), maize + vegetable cowpea (Vigna unguiculata (L.) Walp.), maize + lady’s finger (Abelmoschus esculentus L.; Figure 7), maize + spinach (Spinacia oleracea L.) and maize as solo crop. The maize crop was planted with a 75 × 20 cm inter-row and intra-row spacing.

Figure 6.
Maize + coriander, Coriander sativum intercropping system.

Figure 7.
Maize + Lady’s finger, Abelmoschus esculentus intercropping system.
Intercrops were sown in between the rows of maize in the intercropped plot. The total plants in each plot were observed for the damage signs during each week. The percent plant damage was calculated by dividing the total number of plants in the plot by the number of damaged plants. The leaf damage caused by S. frugiperda was assessed visually and scored on a 0–9 scale [61]. In addition, total numbers of plants having excreta were also recorded during each observed week. In the later growth stage, the total numbers of cobs damaged in each plot were noted. The yield of cobs in each plot was recorded, and the yield/acre was calculated by extrapolation.
4.5. Evaluation of Selected Bio-Pesticides against FAW
The current study was conducted in the biocontrol laboratory, Division of Entomology, ICAR-IARI, Pusa, New Delhi, in Kharif, 2021. Treatments comprised of three biopesticides viz., Beauveria bassiana (Balsamo) Vuillemin1.15% WP (1×108 CFU/g at 5g/liter) (Hypocreales: Clavicipitaceae), Metarhizium anisopliae (Mechnikov) Sorokin 1% WP (Balsamo) Vuillemin (Hypocreales: Clavicipitaceae) (1×108 CFU/g at 5g/liter) and Azadirachtin E.C. 1% w/w (10,000 ppm) at 1mL/liter. In addition, two insecticides viz., Emamectin benzoate 5SG and Chlorantraniliprole 18.5SC were used as a positive control and water as a negative control. The leaf dip (5 cm diameter) bioassay method was followed to evaluate the toxicity against 3rd instar larvae of S. frugiperda. The fresh leaves of maize were made into leaf discs then dipped in the different insecticides and shade dried; later, were offered to the 12 h starved larvae of S. frugiperda (n = 10). The post treatment observations on surviving larvae were recorded at 24, 48, 72, and 168 h after treatment. The observations recorded were analyzed statistically using Abbott’s formula [59] to determine the corrected mortality and the relative efficacy of different biopesticides used in the study was analyzed.
4.6. Data Analysis
Analysis of variance-one way [62] was used to compare the effect of intercrops on the percent pest incidence, the number of live larvae per damaged plants, plants showing excreta, where significant difference was detected treatment means were separated using Duncan’s multiple range test (0.5%). The percent pest incidence values are arc signed, while live larvae per plant and plant with excreta are square transformed.
Larval mortality due to unknown factors, microbial infections, adult emergence and larval parasitisation rates were calculated for all observed months. The formula [63], was used to calculate the parasitism rate of parasitoids, where Pi is the number of parasitized individuals of species i and Pt the total number of larvae collected. Similarly, the formula [64], was used to calculate the relative abundance of a particular parasitoid (RA), where Ni is the number of individuals of each parasitoid species and Nt is the total number of parasitoids emerged from field collected larvae. The relative contribution of each parasitoid species to total parasitism (RP) was calculated by using ; Where, PS = total number of FAW larvae parasitized by each parasitoid; LS = total number of FAW larvae collected.
5. Conclusions
The incidence of Fall Armyworm ranged from 33.02 to 67.10% and the total parasitism rate due to all parasitoids ranged from 28.37 to 42.44%, indicating the potential for biological control through conservation of natural enemies. In the study area, eight different parasitoid species attacked the eggs and larvae of S. frugiperda. Among these parasitoids, C. formosanus and C. blackburni were the most important egg larval parasitoids, and T. remus and T. chilonis were the most important egg parasitoids. Under laboratory conditions, essential oil of citronella and annona extract were effective against S. frugiperda larvae, while essential oil of garlic showed strong ovicidal action against S. frugiperda. However, field efficacy must be validated. The maize + lady’s finger intercropping system had the lowest infestation and the highest grain yield. Among the commercial biopesticides evaluated, azadirachtin 10,000 ppm was effective against larvae of S. frugiperda, followed by Metarhizium anisopliae.
Author Contributions
Conceptualization, Methodology, Software and Validation, M.C.K. and S.S.S.; Investigation, M.C.K.; Formal analysis, M.C.K., S.S.S., S.D. and A.G.; Resources, V.S.R., R.C., H.O.E., A.M. and N.A.S.; Data curation, Writing original draft preparation, M.C.K., K.T.S. and H.S.M.; Writing review and editing, M.C.K., R.C., H.O.E., K.T.S. and H.S.M.; Supervision, S.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
Researchers Supporting Project (RSP2023R118), King Saud University for publication of this manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data included in the main text.
Acknowledgments
Authors are thankful to ICAR-IARI, New Delhi, ICAR-IGFRI, Jhansi and Firoz Hossain, ICAR-IARI, New Delhi for providing facilities. The authors would like to thank Researchers Supporting Project (RSP2023R118), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First report of outbreaks of the Fall Armyworm Spodoptera frugiperda (JE Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef]
- Ganiger, P.C.; Yeshwanth, H.M.; Muralimohan, K.; Vinay, N.; Kumar, A.R.V.; Chandrashekara, K. Occurrence of the new invasive pest, fall armyworm, Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae), in the maize fields of Karnataka, India. Curr. Sci. 2018, 115, 621–623. [Google Scholar] [CrossRef]
- Keerthi, M.C.; Mahesha, H.S.; Manjunatha, N.; Gupta, A.; Saini, R.P.; Shivakumara, K.T.; Bhargavi, H.A.; Gupta, G.; Kulkarni, N.S. Biology and oviposition preference of Fall Armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) on fodder crops and its natural enemies from Central India. Int. J. Pest Manag. 2021, 1–10. [Google Scholar] [CrossRef]
- Qi, G.J.; Ma, J.; Wan, J.; Ren, Y.L.; McKirdy, S.; Hu, G.; Zhang, Z.F. Source regions of the first immigration of Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) invading Australia. Insects 2021, 12, 1104. [Google Scholar] [CrossRef] [PubMed]
- Devi, S. Fall Armyworm threatens food security in southern Africa. Lancet 2018, 391, 727. [Google Scholar] [CrossRef]
- Koffi, D.; Agboka, K.; Adenka, D.K.; Osae, M.; Tounou, A.K.; AnaniAdjevi, M.K.; Fening, K.O.; Meagher, R.L., Jr. Maize infestation offal Armyworm (Lepidoptera:Noctuidae) within agro-ecological zones of Togo and Ghana in West Africa 3 year after its invasion. Environ. Entomol. 2020, 49, 645–650. [Google Scholar] [CrossRef]
- Rwomushana, I.; Bateman, M.; Beale, T.; Beseh, P.; Cameron, K.; Chiluba, M.; Clottey, V.; Davis, T.; Day, R.; Early, R.; et al. Fall Armyworm: Impacts and implication for Africa; Evidence Note Update; CAB International: Wallingford, UK, 2018. [Google Scholar]
- DPPQS. 2019. Available online: http://ppqs.gov.in/sites/default/files/faw_do.pdf (accessed on 2 March 2022).
- Timilsena, B.P.; Niassy, S.; Kimathi, E.; Abdel-Rahman, E.M.; Seidl-Adams, I.; Wamalwa, M.; Tonnang, H.E.; Ekesi, S.; Hughes, D.P.; Rajotte, E.G.; et al. Potential distribution of Fall Armyworm in Africa and beyond, considering climate change and irrigation patterns. Sci. Rep. 2022, 12, 539. [Google Scholar] [CrossRef]
- Gurr, G.M.; Wratten, S.D.; Barbosa, P. Success in conservation biological control of arthropods. In Biological Control: Measures of Success; Springer: Dordrecht, The Netherlands; Kluwer Academic: Dordrecht, The Netherlands, 2000; pp. 105–132. [Google Scholar]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef]
- Pollard, K.A.; Holland, J.M. Arthropods within the woody element of hedge rows and their distribution pattern. Agric. For. Entomol. 2006, 8, 203–211. [Google Scholar] [CrossRef]
- Bone, N.J.; Thomson, L.J.; Ridland, P.M.; Cole, P.; Hoffmann, A.A. Cover crops in Victorian apple orchards: Effects on production, natural enemies and pests across a season. Crop Prot. 2009, 28, 675–683. [Google Scholar] [CrossRef]
- Ratnadass, A.; Fernandes, P.; Avelino, J.; Habib, R. Plant species diversity for sustainable management of crop pests and diseases in agroecosystems: A review. Agron. Sustain. Dev. 2012, 32, 273–303. [Google Scholar] [CrossRef]
- Keerthi, M.C.; Sravika, A.; Mahesha, H.S.; Gupta, A.; Bhargavi, H.A.; Ahmed, S. Performance of the native predatory bug, Eocanthecona furcellata (Wolff) (Hemiptera: Pentatomidae), on the Fall Armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae), and its limitation under field condition. Egypt J. Biol. Pest Control. 2020, 30, 1–4. [Google Scholar] [CrossRef]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The use of push-pull strategies in integrated pest management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef] [PubMed]
- Huss, C.P.; Holmes, K.D.; Blubaugh, C.K. Benefits and risks of intercropping for crop resilience and pest management. J. Econ. Entomol. 2022, 115, 1350–1362. [Google Scholar] [CrossRef]
- Sharanabasappa, S.; Kalleshwaraswamy, C.M.; Poorani, J.; Maruthi, M.S.; Pavithra, H.B.; Diraviam, J. Natural enemies of Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae),a recent invasive pest on maize in South India. Fla. Entomol. 2019, 102, 619–623. [Google Scholar]
- Myrick, S.; Norton, G.W.; Selvaraj, K.N.; Natarajan, K.; Muniappan, R. Economic impact of classical biological control of papaya mealybug in India. Crop Prot. 2014, 56, 82–86. [Google Scholar] [CrossRef]
- Sagar, D.; Sachin, S.S.; Keerthi, M.C.; Poorani, J.; Gupta, A.; Rahul, K.C. Native parasitoid complex of the invasive Fall Armyworm, Spodoptera frugiperda (J.E. Smith) from Northern India. Int. J. Trop. Insect Sci. 2021, 42, 2773–2778. [Google Scholar] [CrossRef]
- Udayakumar, A.; Shivalingaswamy, T.M.; Bakthavatsalam, N. Legume–based intercropping for the management of Fall Armyworm, Spodoptera frugiperda L. in maize. J. Plant Dis. Prot. 2021, 128, 775–779. [Google Scholar] [CrossRef]
- Navik, O.; Shylesha, A.N.; Patil, J.; Venkatesan, T.; Lalitha, Y.; Ashika, T.R. Damage, distribution and natural enemies of invasive Fall Armyworm, Spodoptera frugiperda (JE smith) under rainfed maize in Karnataka, India. Crop Prot. 2021, 143, 105536. [Google Scholar] [CrossRef]
- Sivakumar, G.; Mohan, M.; Kannan, M.; Elango, K.; RamKumar, P.; Venkatesan, T.; Rangeshwaran, R.; Mahesh, S.Y.; Dhanyakumar, O. Natural occurrence of entomopathogens on the invasive Fall Armyworm, Spodoptera frugiperda (J.E. Smith) in South India. Curr. Sci. 2020, 120, 619. [Google Scholar]
- Tiwari, S.; Youngman, R.R. Transgenic Bt corn hybrids and pest management in the USA. In Alternative Farming Systems, Biotechnology, Drought Stress and Ecological Fertilization; Springer: Dordrecht, The Netherlands, 2011; pp. 15–37. [Google Scholar]
- Tambo, J.A.; Day, R.K.; Lamontagne-Godwin, J.; Silvestri, S.; Beseh, P.K.; Oppong-Mensah, B.; Phiri, N.A.; Matimelo, M. Tackling fall armyworm (Spodoptera frugiperda) outbreak in Africa: An analysis of farmers’ control actions. Int. J. Pest Manag. 2020, 66, 298–310. [Google Scholar] [CrossRef]
- Matova, P.M.; Kamutando, C.N.; Magorokosho, C.; Kutywayo, D.; Gutsa, F.; Labuschagne, M. Fall-armyworm invasion, control practices and resistance breeding in Sub-Saharan Africa. Crop Sci. 2020, 60, 2951–2970. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plantvolatiles: Recent advances and future perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Sombra, K.E.; deAguiar, C.V.; deOliveira, S.J.; Barbosa, M.G.; Zocolo, G.J.; Pastori, P.L. Potential pesticide of three botanicals against Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae). Chil. J. Agric. Res. 2020, 80, 617–628. [Google Scholar] [CrossRef]
- Negrini, M.; Fidelis, E.G.; Schurt, D.A.; Silva, F.D.S.; Pereira, R.S.; Bizzo, H.R. Insecticidal activity of botanicals in controlling Fall Armyworm, Spodoptera frugiperda. Arq. Inst. Biológico 2019, 86, 1–9. [Google Scholar]
- Mendoza-García, E.E.; Ortega-Arenas, L.D.; Serrato-Cruz, M.Á.; Villanueva-Jiménez, J.A.; López-Arroyo, J.I.; Pérez-Pacheco, R. Chemical composition, toxicity, and repellence of plant botanicals against Diaphorina citri (Hemiptera: Liviidae). Chil. J. Agric. Res. 2019, 79, 636–647. [Google Scholar] [CrossRef]
- Rani, A.T.; Kammar, V.; Keerthi, M.C.; Rani, V.; Majumder, S.; Pandey, K.K.; Singh, J. Biopesticides: An Alternative to Synthetic Insecticides. In Microbial Technology for Sustainable Environment; Springer: Singapore, 2021; pp. 439–466. [Google Scholar] [CrossRef]
- Yu, D.S.; Van Achterberg, C.; Horstmann, K. World Ichneumonoidea 2015: Taxonomy, Biology, Morphology and Distribution; CD/DVD; Taxapad: Nepean, ON, Canada, 2016. [Google Scholar]
- Achterberg, V.C. Order Hymenoptera, family Braconidae. The subfamily Agathidinae from the United Arab Emirates, with a review of the fauna of the Arabian Peninsula. Arthropod Fauna United Arab Emir. 2011, 4, 286–352. [Google Scholar]
- Sisay, B.; Simiyu, J.; Malusi, P.; Likhayo, P.; Mendesil, E.; Elibariki, N.; Wakgari, M.; Ayalew, G.; Tefera, T. First report of the Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), natural enemies from Africa. J. Appl. Entomol. 2018, 142, 800–804. [Google Scholar] [CrossRef]
- Agboyi, L.K.; Goergen, G.; Beseh, P.; Mensah, S.A.; Clottey, V.A.; Glikpo, R.; Buddie, A.; Cafà, G.; Offord, L.; Day, R.; et al. Parasitoid complex of Fall Armyworm, Spodoptera frugiperda in Ghana and Benin. Insects 2020, 11, 68. [Google Scholar] [CrossRef]
- Otim, M.H.; AdumoAropet, S.; Opio, M.; Kanyesigye, D.; NakeletOpolot, H.; TekTay, W. Parasitoid distribution and parasitism of the Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) in different maize producing regions of Uganda. Insects 2021, 12, 121. [Google Scholar] [CrossRef]
- Van Lenteren, J.C.; Bueno, V.H. Augmentative biological control of arthropods in Latin America. BioControl 2003, 48, 123–139. [Google Scholar] [CrossRef]
- Gause, G.F. The Struggle for Existence; Williams & Wilkins: Baltimore, MD, USA, 1934; 163p. [Google Scholar]
- Mitchell, P.; Arthur, W. Extinction due to evolution of a competitor. Evolution 1991, 45, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Reitz, S.R.; Trumble, J.T. Competitive displacement among insects and arachnids. Annu. Rev. Entomol. 2002, 47, 435–465. [Google Scholar] [CrossRef]
- Ribeiro, L.P.; Dequech, S.T.; Camera, C.; Sturza, V.S.; Poncio, S.; Vendramim, J.D. Vertical and temporal distribution of Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) egg masses, parasitized and non-parasitized, on maize plants. Maydica 2014, 59, 315–320. [Google Scholar]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Botanicals in insect control: Low risk products in a high-state world. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. A renaissance for botanical insecticides? Pest Manag. Sci. 2015, 71, 1587–1590. [Google Scholar] [CrossRef]
- Muturi, E.J.; Ramirez, J.L.; Zilkowski, B.; Flor-Weiler, L.B.; Rooney, A.P. Ovicidal and larvicidal effects of garlic and asafoetidabotanicals against West Nile virus vectors. J. Insect Sci. 2018, 18, 43. [Google Scholar] [CrossRef]
- Rice, P.J.; Coats, J.R. Insecticidal properties of several monoterpenoids to the house fly (Diptera: Muscidae), red flour beetle (Coleoptera: Tenebrionidae), and Southern corn root worm (Coleoptera: Chrysomelidae). J. Econ. Entomol. 1994, 87, 1172–1179. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Murasaki, C.; Shimada, H.; Nishioka, S.; Kakinuma, K.; Singh, S.; Singh, M.; Gupta, Y.K.; Sahai, M. Annonaceous acetogenins from the seeds of Annona squamosa. Non-adjacent bis-tetrahydrofuran icacetogenins. Chem. Pharm. Bull. 1994, 42, 1175–1184. [Google Scholar] [CrossRef]
- Khalequzzaman, M.; Sultana, S. Insecticidal activity of Annona squamosa L. seed extracts against the red flour beetle, Tribolium castaneum (Herbst). J. Biosci. 2006, 14, 107–112. [Google Scholar] [CrossRef]
- El-Wakeil, N.E. Botanical pesticides and their mode of action. Gesunde Pflanz. 2013, 65, 125–149. [Google Scholar] [CrossRef]
- Campos, E.V.; Proença, P.L.; Oliveira, J.L.; Bakshi, M.; Abhilash, P.C.; Fraceto, L.F. Use of botanical insecticides for sustainable agriculture: Future perspectives. Ecol. Indic. 2018, 105, 1–13. [Google Scholar] [CrossRef]
- Hailu, G.; Niassy, S.; Khan, Z.R.; Ochatum, N.; Subramanian, S. Maize–legume intercropping and push–pull for management of Fall Armyworm, stemborers, and striga in Uganda. Agron. J. 2018, 110, 2513–2522. [Google Scholar] [CrossRef]
- Saminathan, V.R.; Murali Baskaran, R.K.; Mahadevan, N.R. Studies on the influence of intercropping on the conservation of Chrysoperla carnea (Stephens) for management of insect pests of cotton, Abstract. In Biotechnological Application for Integrated Pest Management; Ignacimuthu, S., Sen, A., Janarthanan, S., Eds.; Oxford & IBH Publishing Co., Pvt. Ltd.: New Delhi, India, 2002; pp. 107–110. [Google Scholar]
- Risch, S.J. A comparison by sweep sampling of the insect fauna from corn and sweet potato monoculture and dicultures inCosta Rica. Oecologia 1979, 42, 195–211. [Google Scholar] [CrossRef]
- Degri, M.M.; Mailafiya, D.M.; Mshelia, J.S. Effect of intercropping pattern on stem borer infestation in pearlmillet (Pennisetum glaucum L.) grown in the Nigerian Sudan Savannah. Adv. Entomol. 2014, 2, 81–86. [Google Scholar] [CrossRef][Green Version]
- Tanyi, C.B.; Nkongho, R.N.; Okolle, J.N.; Tening, A.S.; Ngosong, C. Effect of intercropping beans with maize and botanical extract on Fall Armyworm (Spodoptera frugiperda) infestation. Int. J. Agron. 2020, 26, 1–7. [Google Scholar] [CrossRef]
- Dhobi, C.B.; Zala, M.B.; Verma, H.S.; Sisodiya, D.B.; Thumar, R.K.; Patel, M.B.; Patel, J.K.; Borad, P.K. Evaluation of Bio-pesticides against Fall Armyworm, Spodoptera frugiperda (JE Smith) in Maize. Int. J. Curr. Microbiol. App. Sci. 2020, 9, 1150–1160. [Google Scholar] [CrossRef]
- Deshmukh, S.; Pavithra, H.B.; Kalleshwaraswamy, C.M.; Shivanna, B.K.; Maruthi, M.S.; Mota-Sanchez, D. Field efficacy of insecticides for management of invasive Fall Armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) on maize in India. Fla. Entomol. 2020, 103, 221–227. [Google Scholar] [CrossRef]
- Gujar, G.T.; Mittal, A.; Kumari, A.; Kalia, V. Host crop influence on the susceptibility of the American bollworm, Helicoverpa armigera, to Bacillus thuringiensis ssp. kurstaki HD-73. Entomol. Exp. Appl. 2004, 113, 165–172. [Google Scholar] [CrossRef]
- Sangha, J.S.; Astatkie, T.; Cutler, G.C. Ovicidal, larvicidal, and behavioral effects of some plant botanicals on diamond backmoth (Lepidoptera: Plutellidae). Can. Entomol. 2017, 149, 639–648. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Baskar, K.; Kingsley, S.; Vendan, S.E.; Paulraj, M.G.; Duraipandiyan, V.; Ignacimuthu, S. Antifeedant, larvicidal and pupicidal activities of Atalantia monophylla (L) Correa against Helicoverpa armigera Hubner (Lepidoptera: Noctuidae). Chemosphere 2009, 75, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.M.; Williams, W.P. Visual Rating Scales for Screening Whorl-Stage Corn for Resistance to Fall Armyworm; Technical Bulletin186; Mississippi Agricultural and Forestry Research Experiment Station, Mississippi State University: Starkville, MS, USA, 1992. [Google Scholar]
- WASP-Web Agri Stat Package 2.0. Available online: https://ccari.icar.gov.in/wasp2.0/index.php (accessed on 20 April 2022).
- Legaspi, C.J.; French, J.V.; Garza Zuniga, A.; Legaspi, B.C. Population dynamics of the citrus leaf miner, Phyllocnistic citrella (Lep: Gracillariidae), and its natural enemies in Texas and México. Biol. Control. 2001, 21, 84–90. [Google Scholar] [CrossRef]
- Molina-Ochoa, J.; Hamm, J.J.; Lezama-Gutiérrez, R.; Lopez-Edwards, M.; Gonzalez-Ramirez, M.; Pescador-Rubio, A. A survey of Fall Armyworm (Lepidoptera: Noctuidae) parasitoids in the Mexican states of Michoacán, Colima, Jalisco, and Tamaulipas. Fla. Entomol. 2001, 84, 31–36. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).