Ozone and Temperature May Hinder Adaptive Capacity of Mediterranean Perennial Grasses to Future Global Change Scenarios
Abstract
1. Introduction
2. Results
2.1. Ozone Exposure and Temperature Increase
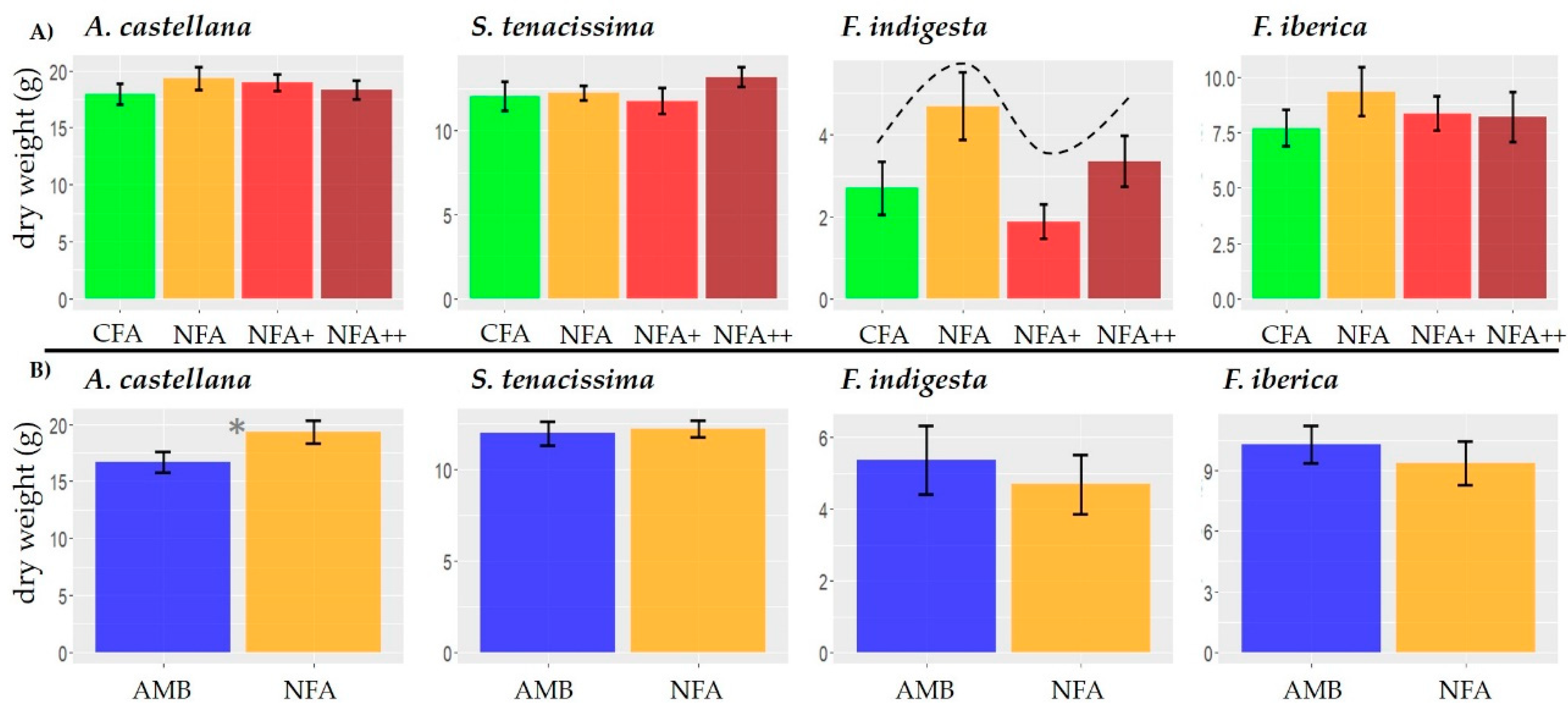
2.2. Vegetative Growth
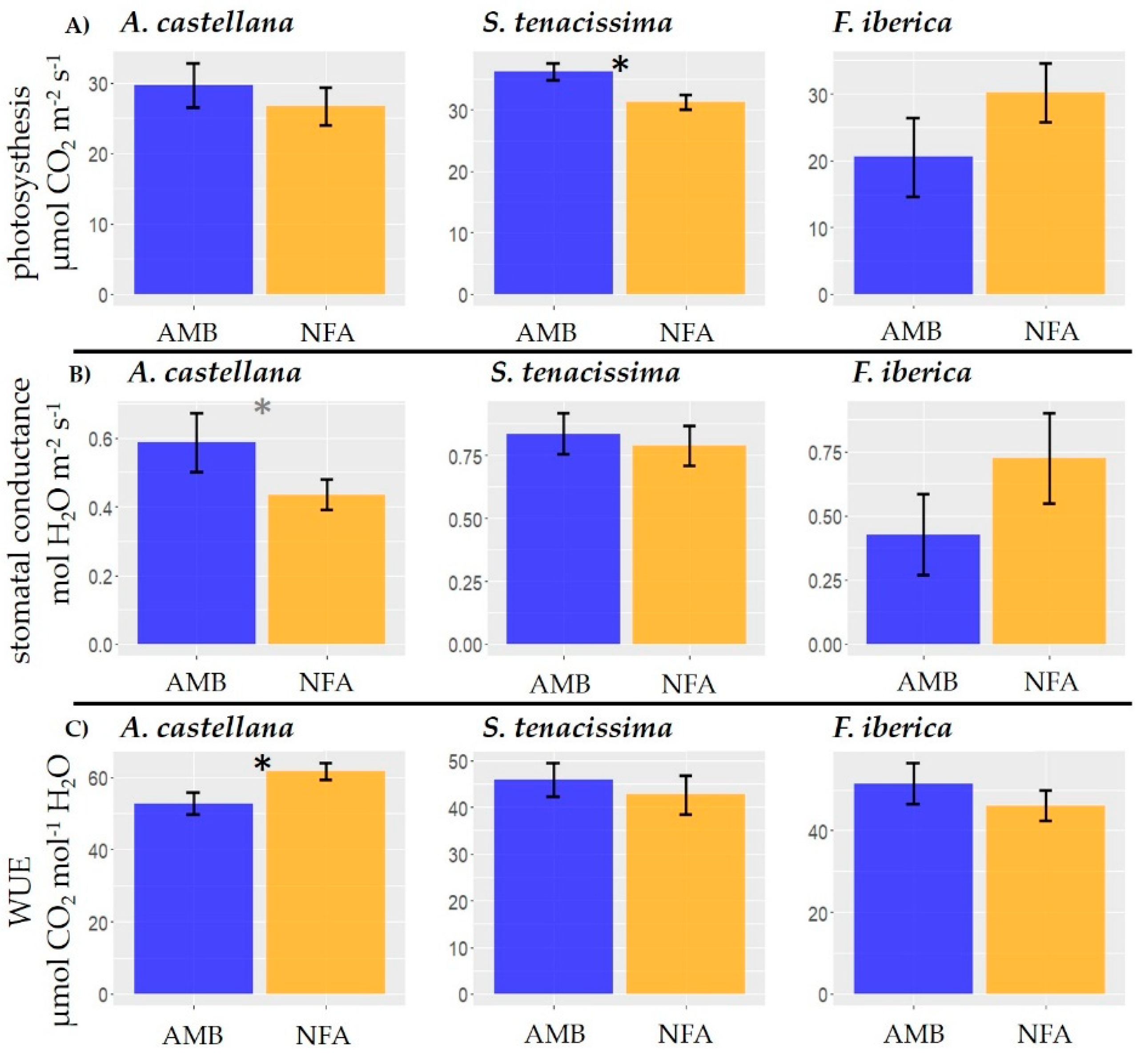
2.3. Gas Exchange
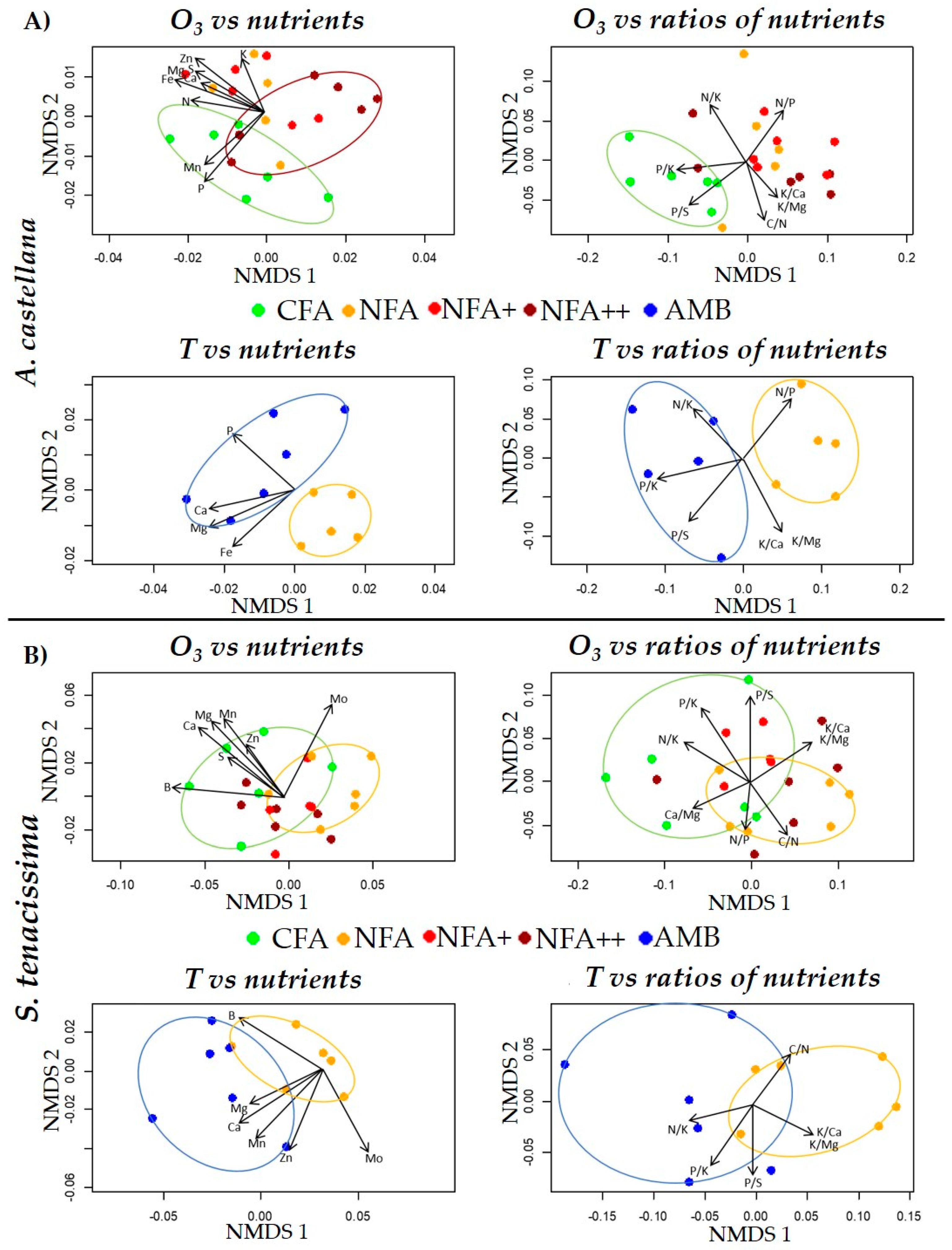
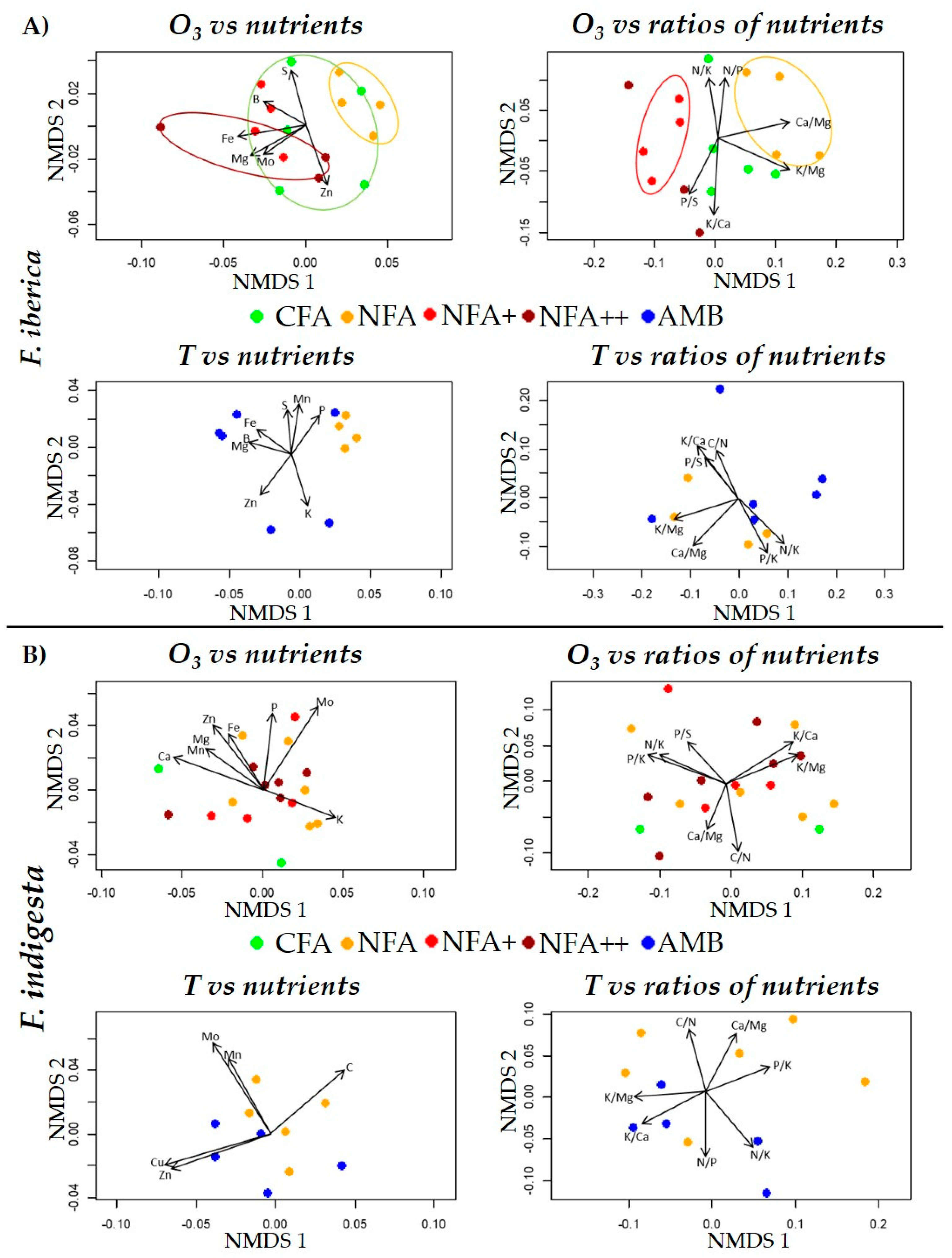
2.4. Nutrients and Nutrient Ratios
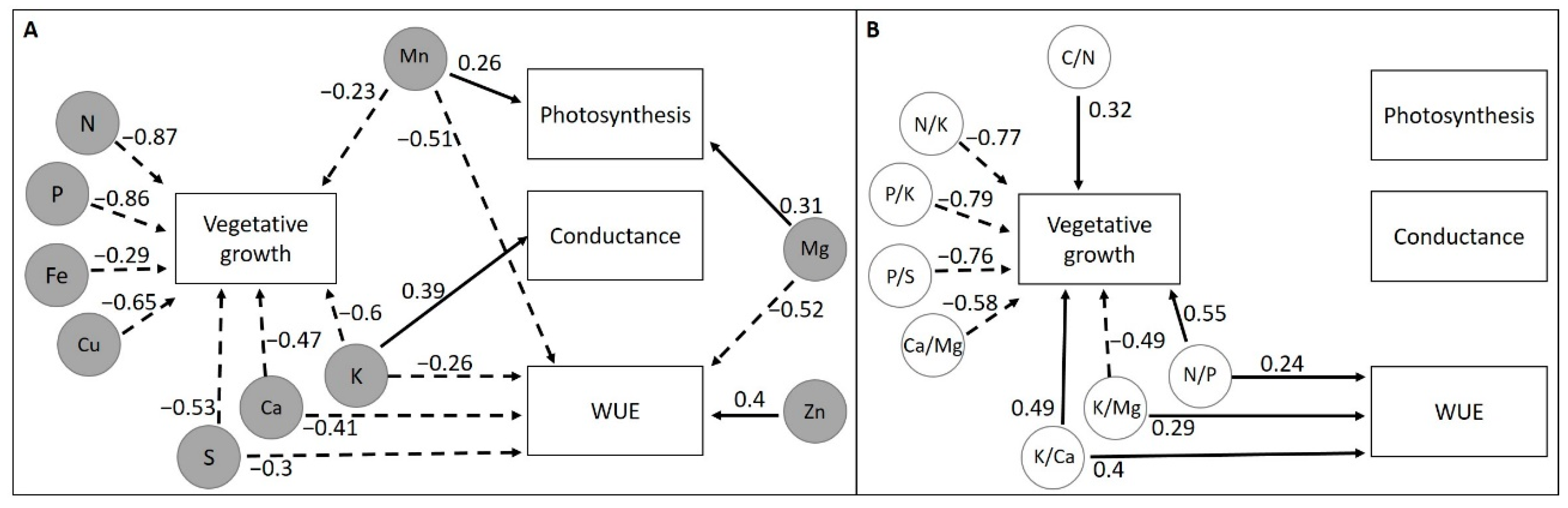
2.5. Correlations
3. Discussion
3.1. Effects on Plant Growth and Gas Exchange Parameters
3.2. Ozone and Temperature Effects on Foliage Nutrient Content
4. Material and Methods
4.1. OTC Experiment
4.2. Plant Material
4.3. Vegetative Growth
4.4. Gas Exchange
4.5. Macro- and Micronutrient Composition
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chappelka, A.H.; Samuelson, L.J. Ambient ozone effects on forest trees of the eastern United States: A review. New Phytol. 1998, 139, 91–108. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Yendrek, C.R.; Sitch, S.; Collins, W.J.; Emberson, L.D. The effects of tropospheric ozone on net primary productivity and implications for climate change. Annu. Rev. Plant Biol. 2012, 63, 637–661. [Google Scholar] [CrossRef]
- Leisner, C.P.; Ainsworth, E.A. Quantifying the effects of ozone on plant reproductive growth and development. Glob. Chang. Biol. 2012, 18, 606–616. [Google Scholar] [CrossRef]
- Mills, G.; Sharps, K.; Simpson, D.; Pleijel, H.; Frei, M.; Burkey, K.; Emberson, L.; Uddling, J.; Broberg, M.; Feng, Z.; et al. Closing the global ozone yield gap: Quantification and cobenefits for multistress tolerance. Glob. Chang. Biol. 2018, 24, 4869–4893. [Google Scholar] [CrossRef] [PubMed]
- Monks, P.S.; Archibald, A.T.; Colette, A.; Cooper, O.; Coyle, M.; Derwent, R.; Fowler, D.; Granier, C.; Law, K.S.; Mills, G.E.; et al. Tropospheric ozone and its precursors from the urban to the global scale from air quality to short-lived climate forcer. Atmos. Chem. Phys. 2015, 15, 8889–8973. [Google Scholar] [CrossRef]
- Querol, X.; Alastuey, A.; Gangoiti, G.; Perez, N.; Lee, H.K.; Eun, H.R.; Park, Y.; Mantilla, E.; Escudero, M.; Titos, G.; et al. Phenomenology of summer ozone episodes over the Madrid Metropolitan Area, central Spain. Atmos. Chem. Phys. Discuss. 2018, 18, 6511–6533. [Google Scholar] [CrossRef]
- Millán, M.; Salvador, R.; Mantilla, E.; Artíñano, B. Meteorology and photochemical air pollution in Southern Europe: Experimental results from EC research projects. Atmos. Environ. 1996, 30, 1909–1924. [Google Scholar] [CrossRef]
- Cristofanelli, P.; Bonasoni, P. Background ozone in the southern Europe and Mediterranean area: Influence of the transport processes. Environ. Pollut. 2009, 157, 1399–1406. [Google Scholar] [CrossRef]
- CLRTAP. Chapter III. Mapping Critical Levels for Vegetation; ICP Vegetation Programme Coordination Centre, Centre for Ecology and Hydrology: Bangor, UK, 2017; Volume 2017. [Google Scholar]
- MITERD Evaluación de la Calidad del Aire en España. Available online: https://www.miteco.gob.es/es/calidad-y-evaluacion-ambiental/temas/atmosfera-y-calidad-del-aire/Situación de la calidad del aire en España 2010_tcm30-182501.pdf (accessed on 1 January 2022).
- Díaz-de-Quijano, M.; Peñuelas, J.; Ribas, À. Increasing interannual and altitudinal ozone mixing ratios in the Catalan Pyrenees. Atmos. Environ. 2009, 43, 6049–6057. [Google Scholar] [CrossRef]
- Elvira, S.; González-Fernández, I.; Alonso, R.; Sanz, J.; Bermejo-Bermejo, V. Ozone levels in the Spanish Sierra de Guadarrama mountain range are above the thresholds for plant protection: Analysis at 2262, 1850, and 995 m a.s.l. Environ. Monit. Assess. 2016, 188, 1–20. [Google Scholar] [CrossRef]
- Bergmann, E.; Bender, J.; Weigel, H.-J. Assessment of the Impacts of Ozone on Biodiversity in Terrestrial Ecosystems: Literature Review and Analysis of Methods and Uncertainties in Current Risk Assessment Approaches. Part II: Literature Review of the Current State of Knowledge on the Impact of Ozone on Biodiversity in Terrestrial Ecosystems; Umweltbundesamt: Dessau-Roßlau, Germany, 2015; Volume 139. [Google Scholar] [CrossRef]
- Booker, F.; Muntifering, R.; Mcgrath, M.; Burkey, K.; Decoteau, D.; Fiscus, E.; Manning, W.; Krupa, S.; Chappelka, A.; Grantz, D. The ozone component of global change: Potential effects on agricultural and horticultural plant yield, product quality and interactions with invasive species. J. Integr. Plant Biol. 2009, 51, 337–351. [Google Scholar] [CrossRef]
- Grulke, N.E.; Heath, R.L. Ozone effects on plants in natural ecosystems. Plant Biol. 2020, 22, 12–37. [Google Scholar] [CrossRef]
- González-Fernández, I.; Elvira, S.; Calatayud, V.; Calvo, E.; Aparicio, P.; Sánchez, M.; Alonso, R.; Bermejo Bermejo, V. Ozone effects on the physiology and marketable biomass of leafy vegetables under Mediterranean conditions: Spinach (Spinacia oleracea L.) and Swiss chard (Beta vulgaris L. var. cycla). Agric. Ecosyst. Environ. 2016, 235, 215–228. [Google Scholar] [CrossRef]
- Wittig, V.E.; Ainsworth, E.A.; Naidu, S.L.; Karnosky, D.F.; Long, S.P. Quantifying the impact of current and future tropospheric ozone on tree biomass, growth, physiology and biochemistry: A quantitative meta-analysis. Glob. Chang. Biol. 2009, 15, 396–424. [Google Scholar] [CrossRef]
- Tiwari, S.; Agrawal, M.; Marshall, F.M. Seasonal variations in adaptational strategies of Beta vulgaris L. plants in response to ambient air pollution: Biomass allocation, yield and nutritional quality. Trop. Ecol. 2010, 51, 353–363. [Google Scholar]
- Fangmeier, A.; De Temmerman, L.; Black, C.; Persson, K.; Vorne, V. Effects of elevated CO2 and/or ozone on nutrient concentrations and nutrient uptake of potatoes. Eur. J. Agron. 2002, 17, 353–368. [Google Scholar] [CrossRef]
- Valkama, E.; Koricheva, J.; Oksanen, E. Effects of elevated O3, alone and in combination with elevated CO2, on tree leaf chemistry and insect herbivore performance: A meta-analysis. Glob. Chang. Biol. 2007, 13, 184–201. [Google Scholar] [CrossRef]
- Gimeno, B.S.; Bermejo, V.; Sanz, J.; De Torre, D.; Gil, J.M. Assessment of the effects of ozone exposure and plant competition on the reproductive ability of three therophytic clover species from Iberian pastures. Atmos. Environ. 2004, 38, 2295–2303. [Google Scholar] [CrossRef]
- Calvete-Sogo, H.; Elvira, S.; Sanz, J.; González-Fernández, I.; García-Gómez, H.; Sánchez-Martín, L.; Alonso, R.; Bermejo-Bermejo, V. Current ozone levels threaten gross primary production and yield of Mediterranean annual pastures and nitrogen modulates the response. Atmos. Environ. 2014, 95, 197–206. [Google Scholar] [CrossRef]
- Hayes, F.; Mills, G.; Jones, L.; Ashmore, M. Does a simulated upland grassland community respond to increasing background, peak or accumulated exposure of ozone? Atmos. Environ. 2010, 44, 4155–4164. [Google Scholar] [CrossRef]
- Fuhrer, J.; Val Martin, M.; Mills, G.; Heald, C.L.; Harmens, H.; Hayes, F.; Sharps, K.; Bender, J.; Ashmore, M.R. Current and future ozone risks to global terrestrial biodiversity and ecosystem processes. Ecol. Evol. 2016, 6, 8785–8799. [Google Scholar] [CrossRef]
- Agathokleous, E.; Feng, Z.; Oksanen, E.; Sicard, P.; Wang, Q.; Saitanis, C.J.; Araminiene, V.; Blande, J.D.; Hayes, F.; Calatayud, V.; et al. Ozone affects plant, insect, and soil microbial communities: A threat to terrestrial ecosystems and biodiversity. Sci. Adv. 2020, 6, eabc1176. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeler, R.A.; Mittermeler, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Girón, A.; Gavilán, R.G. Spatial patterns and interspecific relations analysis help to better understand species distribution patterns in a Mediterranean high mountain grassland. Plant Ecol. 2010, 210, 137–151. [Google Scholar] [CrossRef]
- López-angulo, J.; Pescador, D.S.; Sánchez, A.M.; Luzuriaga, A.L.; Cavieres, L.A.; Escudero, A. Science of the Total Environment Impacts of climate, soil and biotic interactions on the interplay of the different facets of alpine plant diversity. Sci. Total Environ. 2020, 698, 133960. [Google Scholar] [CrossRef]
- Stanisci, A.; Laura, M.; Pelino, G.; Chiarucci, A. Assessing the diversity pattern of cryophilous plant species in high elevation habitats. Plant Ecol. 2011, 595–600. [Google Scholar] [CrossRef]
- Le Houérou, H.N. Biogeography of the arid steppeland north of the Sahara. J. Arid Environ. 2001, 48, 103–128. [Google Scholar] [CrossRef]
- Gimeno, B.S.; Bermejo, V.; Sanz, J.; De La Torre, D.; Elvira, S. Growth response to ozone of annual species from Mediterranean pastures. Environ. Pollut. 2004, 132, 297–306. [Google Scholar] [CrossRef]
- Bermejo, V.; Gimeno, B.S.; Sanz, J.; De La Torre, D.; Gil, J.M. Assessment of the ozone sensitivity of 22 native plant species from Mediterranean annual pastures based on visible injury. Atmos. Environ. 2003, 37, 4667–4677. [Google Scholar] [CrossRef]
- Calvete-Sogo, H.; González-Fernández, I.; Sanz, J.; Elvira, S.; Alonso, R.; García-Gómez, H.; Ibáñez-Ruiz, M.A.; Bermejo-Bermejo, V. Heterogeneous responses to ozone and nitrogen alter the species composition of Mediterranean annual pastures. Oecologia 2016, 181, 1055–1067. [Google Scholar] [CrossRef]
- Sanz, J.; Bermejo, V.; Muntifering, R.; González-Fernández, I.; Gimeno, B.S.; Elvira, S.; Alonso, R. Plant phenology, growth and nutritive quality of Briza maxima: Responses induced by enhanced ozone atmospheric levels and nitrogen enrichment. Environ. Pollut. 2011, 159, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.; Calvete-Sogo, H.; González-Fernández, I.; Lin, J.; García-Gómez, H.; Muntifering, R.; Alonso, R.; Bermejo-Bermejo, V. Foliar senescence is the most sensitive response to ozone in Bromus hordeaceus and is modulated by nitrogen input. Grass Forage Sci. 2015, 70, 71–84. [Google Scholar] [CrossRef]
- Nussbaum, S.; Geissmann, M.; Fuhrer, J. Ozone exposure-response relationships for mixtures of perennial ryegrass and white clover depend on ozone exposure patterns. Atmos. Environ. 1995, 29, 989–995. [Google Scholar] [CrossRef]
- Van De Staaij, J.W.M.; Tonneijck, A.E.G.; Rozema, J. The effect of reciprocal treatments with ozone and ultraviolet-B radiation on photosynthesis and growth of perennial grass Elymus athericus. Environ. Pollut. 1997, 97, 281–286. [Google Scholar] [CrossRef]
- Bassin, S.; Volk, M.; Suter, M.; Buchmann, N.; Fuhrer, J. Nitrogen deposition but not ozone affects productivity and community composition of subalpine grassland after 3 yr of treatment. New Phytol. 2007, 175, 523–534. [Google Scholar] [CrossRef]
- REILING, K.; DAVISON, A.W. The response of native, herbaceous species to ozone: Growth and fluorescence screening. New Phytol. 1992, 120, 29–37. [Google Scholar] [CrossRef]
- Bassin, S.; Volk, M.; Fuhrer, J. Species Composition of Subalpine Grassland is Sensitive to Nitrogen Deposition, but Not to Ozone, After Seven Years of Treatment. Ecosystems 2013, 16, 1105–1117. [Google Scholar] [CrossRef]
- Bassin, S.; Werner, R.A.; Sörgel, K.; Volk, M.; Buchmann, N.; Fuhrer, J. Effects of combined ozone and nitrogen deposition on the in situ properties of eleven key plant species of a subalpine pasture. Oecologia 2009, 158, 747–756. [Google Scholar] [CrossRef]
- Hayes, F.; Mills, G.; Williams, P.; Harmens, H.; Büker, P. Impacts of summer ozone exposure on the growth and overwintering of UK upland vegetation. Atmos. Environ. 2006, 40, 4088–4097. [Google Scholar] [CrossRef]
- Ruiz-Labourdette, D.; Martínez, F.; Martín-López, B.; Montes, C.; Pineda, F.D. Equilibrium of vegetation and climate at the European rear edge. A reference for climate change planning in mountainous Mediterranean regions. Int. J. Biometeorol. 2011, 55, 285–301. [Google Scholar] [CrossRef]
- Luceño, M.; Vargas, P.; García, B. Guía de Campo del Sistema Central; Editorial Raices: Mexico City, Mexico, 2016; ISBN 9788486115852. [Google Scholar]
- Anthos Information System of the plants of Spain. Real Jardín Botánico, CSIC—Fundación Biodiversidad. Available online: http://www.anthos.es (accessed on 1 March 2022).
- Cortina, J.; Maestre, F.T.; Ramírez, D. Innovations in Semiarid Land Restoration. The case of Stipa tenacissima L. Steppes. In Land Restoration to Combat Desertification. Innovative Approaches, Quality Control and Project Evaluation; Fundación CEAM: Valencia, Spain, 2006; pp. 121–144. [Google Scholar]
- Soreng, R.J.; Peterson, P.M.; Romaschenko, K.; Davidse, G.; Zuloaga, F.O.; Judziewicz, E.J.; Filgueiras, T.S.; Davis, J.I.; Morrone, O. A worldwide phylogenetic classification of the Poaceae (Gramineae). J. Syst. Evol. 2015, 53, 117–137. [Google Scholar] [CrossRef]
- Prieto-Benítez, S.; Ruiz-Checa, R.; Bermejo-Bermejo, V.; Gonzalez-Fernandez, I. The Effects of Ozone on Visual Attraction Traits of Erodium paularense ( Geraniaceae ) Flowers: Modelled Perception by Insect Pollinators. Plants 2021, 10, 2750. [Google Scholar] [CrossRef]
- Giménez-Benavides, L.; Escudero, A.; García-Camacho, R.; García-Fernández, A.; Iriondo, J.M.; Lara-Romero, C.; Morente-López, J. How does climate change affect regeneration of Mediterranean high-mountain plants? An integration and synthesis of current knowledge. Plant Biol. 2018, 20, 50–62. [Google Scholar] [CrossRef]
- Nogués-Bravo, D.; Araújo, M.B.; Errea, M.P.; Martínez-Rica, J.P. Exposure of global mountain systems to climate warming during the 21st Century. Glob. Environ. Chang. 2007, 17, 420–428. [Google Scholar] [CrossRef]
- Vegas-Cañas, C.; Fidel González-Rouco, J.; Navarro-Montesinos, J.; García-Bustamante, E.; Lucio-Eceiza, E.E.; García-Pereira, F.; Rodríguez-Camino, E.; Chazarra-Bernabé, A.; álvarez-Arévalo, I. An assessment of observed and simulated temperature variability in Sierra de Guadarrama. Atmosphere 2020, 11, 985. [Google Scholar] [CrossRef]
- ECA&D European Climate Assessment & Dataset project. Available online: https://www.ecad.eu/ (accessed on 5 November 2022).
- Bender, J.; Muntifering, R.B.; Lin, J.C.; Weigel, H.J. Growth and nutritive quality of Poa pratensis as influenced by ozone and competition. Environ. Pollut. 2006, 142, 109–115. [Google Scholar] [CrossRef]
- Hayes, F.; Mills, G.; Ashmore, M. Effects of ozone on inter- and intra-species competition and photosynthesis in mesocosms of Lolium perenne and Trifolium repens. Environ. Pollut. 2009, 157, 208–214. [Google Scholar] [CrossRef] [PubMed]
- García-Romero, A.; Muñoz, J.; Andrés, N.; Palacios, D. Relationship between climate change and vegetation distribution in the Mediterranean mountains: Manzanares Head valley, Sierra De Guadarrama (Central Spain). Clim. Chang. 2010, 100, 645–666. [Google Scholar] [CrossRef]
- Rötter, R.; Van De Geijn, S.C. Climate change effects on plant growth, crop yield and livestock. Clim. Chang. 1999, 43, 651–681. [Google Scholar] [CrossRef]
- Dann, M.S.; Pell, E.J. Decline of Activity and Quantity of Ribulose Bisphosphate Carboxylase/Oxygenase and Net Photosynthesis in Ozone-Treated Potato Foliage. Plant Physiol. 1989, 91, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Lehnherr, B.; Grandjean, A.; Mächler, F.; Fuhrer, J. The Effect of Ozone in Ambient Air on Ribulosebisphosphate Carboxylase/Oxygenase Activity Decreases Photosynthesis and Grain Yield in Wheat. J. Plant Physiol. 1987, 130, 189–200. [Google Scholar] [CrossRef]
- Wittig, V.E.; Ainsworth, E.A.; Long, S.P. To what extent do current and projected increases in surface ozone affect photosynthesis and stomatal conductance of trees? A meta-analytic review of the last 3 decades of experiments. Plant, Cell Environ. 2007, 30, 1150–1162. [Google Scholar] [CrossRef]
- Davison, A.W.; Barnes, J.D. Effects of ozone on wild plants. New Phytol. 1998, 139, 135–151. [Google Scholar] [CrossRef]
- Volk, M.; Bungener, P.; Contat, F.; Montani, M.; Fuhrer, J. Grassland yield declined by a quarter in 5 years of free-air ozone fumigation. Glob. Chang. Biol. 2006, 12, 74–83. [Google Scholar] [CrossRef]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef]
- Sies, H. Biochemistry of oxidative stress. Angew. Chemie Int. Ed. Engl. 1986, 25, 1058–1071. [Google Scholar] [CrossRef]
- Alonso, R.; Elvira, S.; Castillo, F.J.; Gimeno, B.S. Interactive effects of ozone and drought stress on pigments and activities of antioxidative enzymes in Pinus halepensis. Plant, Cell Environ. 2001, 24, 905–916. [Google Scholar] [CrossRef]
- Pellegrini, E.; Hoshika, Y.; Dusart, N.; Cotrozzi, L.; Gérard, J.; Nali, C.; Vaultier, M.N.; Jolivet, Y.; Lorenzini, G.; Paoletti, E. Antioxidative responses of three oak species under ozone and water stress conditions. Sci. Total Environ. 2019, 647, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.P. Source-sink balance and carbon allocation below ground in plants exposed to ozone. New Phytol. 2003, 157, 213–228. [Google Scholar] [CrossRef]
- Agathokleous, E.; Belz, R.G.; Calatayud, V.; De Marco, A.; Hoshika, Y.; Kitao, M.; Saitanis, C.J.; Sicard, P.; Paoletti, E.; Calabrese, E.J. Predicting the effect of ozone on vegetation via linear non-threshold (LNT), threshold and hormetic dose-response models. Sci. Total Environ. 2019, 649, 61–74. [Google Scholar] [CrossRef]
- Amiro, B.D.; Gillespie, T.J.; Thurtell, G.W. Response Flux Density. Atmos. Environ. 1984, 18, 1207–1215. [Google Scholar] [CrossRef]
- Paoletti, E.; Grulke, N.E. Ozone exposure and stomatal sluggishness in different plant physiognomic classes. Environ. Pollut. 2010, 158, 2664–2671. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.; González-Fernández, I.; Elvira, S.; Muntifering, R.; Alonso, R.; Bermejo-Bermejo, V. Setting ozone critical levels for annual Mediterranean pasture species: Combined analysis of open-top chamber experiments. Sci. Total Environ. 2016, 571, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Wang, L.; Pleijel, H.; Zhu, J.; Kobayashi, K. Differential effects of ozone on photosynthesis of winter wheat among cultivars depend on antioxidative enzymes rather than stomatal conductance. Sci. Total Environ. 2016, 572, 404–411. [Google Scholar] [CrossRef]
- Calatayud, V.; Cerveró, J.; Sanz, M.J. Foliar, physiologial and growth responses of four maple species exposed to ozone. Water. Air. Soil Pollut. 2007, 185, 239–254. [Google Scholar] [CrossRef]
- Reich, P.B.; Lassoie, J.P. Effects of low level O3 exposure on leaf diffusive conductance and water-use efficiency in hybrid poplar. Plant. Cell Environ. 1984, 7, 661–668. [Google Scholar] [CrossRef]
- Moreno-Gutiérrez, C.; Dawson, T.E.; Nicolás, E.; Querejeta, J.I. Isotopes reveal contrasting water use strategies among coexisting plant species in a mediterranean ecosystem. New Phytol. 2012, 196, 489–496. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, Q. Growth and photosynthetic responses of two coniferous species to experimental warming and nitrogen fertilization. Can. J. For. Res. 2009, 39, 1–11. [Google Scholar] [CrossRef]
- Reich, P.B.; Sendall, K.M.; Stefanski, A.; Rich, R.L.; Hobbie, S.E.; Montgomery, R.A. Effects of climate warming on photosynthesis in boreal tree species depend on soil moisture. Nature 2018, 562, 263–267. [Google Scholar] [CrossRef]
- Loik, M.E.; Redar, S.P.; Harte, J. Photosynthetic responses to a climate-warming manipulation for contrasting meadow species in the rocky mountains, colorado, USA. Funct. Ecol. 2000, 14, 166–175. [Google Scholar] [CrossRef]
- Elvira, S.; Alonso, R.; Inclan, R.; Bermejo, V.; Castillo, F.J.; Gimeno, B.S. Ozone effects on Aleppo pine seedlings (Pinus halepensis Mill.) grown in open-top chambers. Water Air Soil Pollut. 1995, 85, 1387–1392. [Google Scholar] [CrossRef]
- Ribas, À.; Peñuelas, J.; Elvira, S.; Gimeno, B.S. Contrasting effects of ozone under different water supplies in two Mediterranean tree species. Atmos. Environ. 2005, 39, 685–693. [Google Scholar] [CrossRef]
- Pleijel, H.; Pihl Karlsson, G.; Sild, E.; Danielsson, H.; Skärby, L.; Selldén, G. Exposure of a grass-clover mixture to ozone in open-top chambers-Effects on yield, quality and botanical composition. Agric. Ecosyst. Environ. 1996, 59, 55–62. [Google Scholar] [CrossRef]
- Sanz, J.; González-Fernández, I.; Calvete-Sogo, H.; Lin, J.S.; Alonso, R.; Muntifering, R.; Bermejo, V. Ozone and nitrogen effects on yield and nutritive quality of the annual legume Trifolium cherleri. Atmos. Environ. 2014, 94, 765–772. [Google Scholar] [CrossRef]
- Frey, B.; Scheidegger, C.; Günthardt-Goerg, M.S.; Matyssek, R. The effects of ozone and nutrient supply on stomatal response in birch (Betula pendula) leaves as determined by digital image-analysis and X-ray microanalysis. New Phytol. 1996, 132, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Elvira, S.; Sanz, J.; Gonzalez-fernandez, I.; Bermejo-bermejo, V. Ozone Effects on the Quality of Swiss Chard. Peri-Urban Crops a Case Study †. 2021. Biol. Life Sci. Forum 2021, 11, 16. [Google Scholar]
- González-Fernández, I.; Bass, D.; Muntifering, R.; Mills, G.; Barnes, J. Impacts of ozone pollution on productivity and forage quality of grass/clover swards. Atmos. Environ. 2008, 42, 8755–8769. [Google Scholar] [CrossRef]
- Jonasson, S.; Michelsen, A.; Schmidt, I.K.; Nielsen, E.V. Responses in microbes and plants to changed temperature, nutrient, and light regimes in the arctic. Ecology 1999, 80, 1828–1843. [Google Scholar] [CrossRef]
- Martins, L.D.; Tomaz, M.A.; Lidon, F.C.; DaMatta, F.M.; Ramalho, J.C. Combined effects of elevated [CO2] and high temperature on leaf mineral balance in Coffea spp. plants. Clim. Chang. 2014, 126, 365–379. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J.; Prieto, P.; Estiarte, M. Changes in Ca, Fe, Mg, Mo, Na, and S content in a Mediterranean shrubland under warming and drought. J. Geophys. Res. Biogeosci. 2008, 113, 1–11. [Google Scholar] [CrossRef]
- Greenwood, D.J.; Lemaire, G.; Gosse, G.; Cruz, P.; Draycott, A.; Neeteson, J.J. Decline in percentage N of C3 and C4 crops with increasing plant mass. Ann. Bot. 1990, 66, 425–436. [Google Scholar] [CrossRef]
- Jarrell, W.M.; Beverly, R.B. The dilution effect in plant nutrition studies. Adv. Agron. 1981, 34, 197–224. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of Macronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 135–189. ISBN 9780123849052. [Google Scholar]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of Nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 191–248. ISBN 9780123849052. [Google Scholar]
- Larcher, W. Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups; Springer: Berlin/Heidelberg, Germany, 2003; ISBN 3-540-43516-6. [Google Scholar]
- Welch, R.M.; Shuman, L. Micronutrient Nutrition of Plants. In Critical Reviews in Plant Sciences; Taylor and Francis: Oxfordshire, UK, 2014; Volume 14, pp. 37–41. [Google Scholar]
- Fangmeier, A.; Grüters, U.; Högy, P.; Vermehren, B.; Jäger, H.J. Effects of elevated CO2, nitrogen supply and tropospheric ozone on spring wheat - II. Nutrients (N, P, K, S, Ca, Mg, Fe, Mn, Zn). Environ. Pollut. 1997, 96, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Heck, W.W.; Taylor, O.C.; Adams, R.; Bingham, G.; Miller, J.; Preston, E.; Weinstein, L. Assessment of Crop Loss from Ozone. J. Air Pollut. Control Assoc. 1982, 32, 353–361. [Google Scholar] [CrossRef]
- CLRTAP. Manual on Methodologies and Criteria for Modelling and Mapping Critical Loads & Levels and Air Pollution Effects, Risks and Trends; Umweltbundesamt: Berlin, Germany, 2004. [Google Scholar]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image Processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Schad, D.J.; Vasishth, S.; Hohenstein, S.; Kliegl, R. How to capitalize on a priori contrasts in linear (mixed) models: A tutorial. J. Mem. Lang. 2020, 110, 104038. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Team, R.C. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-155 2022. Available online: https://cran.r-project.org/web/packages/nlme/nlme.pdf (accessed on 10 June 2021).
- Core_Team_R. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMET-E: Plymouth, UK, 2006. [Google Scholar]
- Martinez Arbizu, P. pairwiseAdonis: Pairwise multilevel comparison using adonis. R Packag. version 0.4. 2020, 1. Available online: https://github.com/pmartinezarbizu/pairwiseAdonis (accessed on 28 November 2022).
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, H.H. Vegan: Community ecology package. R package version 2.5-7. Cran. R-Project. Org/I. 2020. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 10 June 2021).

| AOT40 (nL L−1 h) | O3 8h-Mean (nL L−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DaS | AMB | CFA | NFA | NFA+ | NFA++ | AMB | CFA | NFA | NFA+ | NFA++ | |
| S. tenacissima | 68 | 5780 | 189 | 4285 | 9340 | 16,677 | 43.4 | 21.1 | 40.7 | 50.8 | 63.3 |
| A. castellana | 63 | 4622 | 187 | 3399 | 8466 | 15,807 | 42.3 | 20.9 | 39.8 | 50.7 | 64.1 |
| F. iberica | 62 | 4456 | 187 | 3286 | 8349 | 15,692 | 42.2 | 20.8 | 39.7 | 50.8 | 64.4 |
| F. indigesta | 57 | 3964 | 187 | 2951 | 8072 | 15,321 | 41.9 | 20.9 | 39.8 | 51.6 | 66.2 |
| Mean | 63 | 4705 | 187 | 3480 | 8557 | 15,875 | 42.5 | 20.9 | 40.0 | 51.0 | 64.5 |
| Mean T (°C) | Max T (°C) | RH (%) | PAR (µmol m−2 s−1) | VDP (kPa) | |
|---|---|---|---|---|---|
| AMB | 20.4 | 37.2 | 49.4 | 1149 | 1.21 |
| OTCs | 25.7 | 43.2 | 47.4 | 991 | 1.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prieto-Benítez, S.; Ruiz-Checa, R.; González-Fernández, I.; Elvira, S.; Rucandio, I.; Alonso, R.; Bermejo-Bermejo, V. Ozone and Temperature May Hinder Adaptive Capacity of Mediterranean Perennial Grasses to Future Global Change Scenarios. Plants 2023, 12, 664. https://doi.org/10.3390/plants12030664
Prieto-Benítez S, Ruiz-Checa R, González-Fernández I, Elvira S, Rucandio I, Alonso R, Bermejo-Bermejo V. Ozone and Temperature May Hinder Adaptive Capacity of Mediterranean Perennial Grasses to Future Global Change Scenarios. Plants. 2023; 12(3):664. https://doi.org/10.3390/plants12030664
Chicago/Turabian StylePrieto-Benítez, Samuel, Raquel Ruiz-Checa, Ignacio González-Fernández, Susana Elvira, Isabel Rucandio, Rocío Alonso, and Victoria Bermejo-Bermejo. 2023. "Ozone and Temperature May Hinder Adaptive Capacity of Mediterranean Perennial Grasses to Future Global Change Scenarios" Plants 12, no. 3: 664. https://doi.org/10.3390/plants12030664
APA StylePrieto-Benítez, S., Ruiz-Checa, R., González-Fernández, I., Elvira, S., Rucandio, I., Alonso, R., & Bermejo-Bermejo, V. (2023). Ozone and Temperature May Hinder Adaptive Capacity of Mediterranean Perennial Grasses to Future Global Change Scenarios. Plants, 12(3), 664. https://doi.org/10.3390/plants12030664









