Genome-Wide Analysis and Expression of Cyclic Nucleotide–Gated Ion Channel (CNGC) Family Genes under Cold Stress in Mango (Mangifera indica)
Abstract
1. Introduction
2. Results
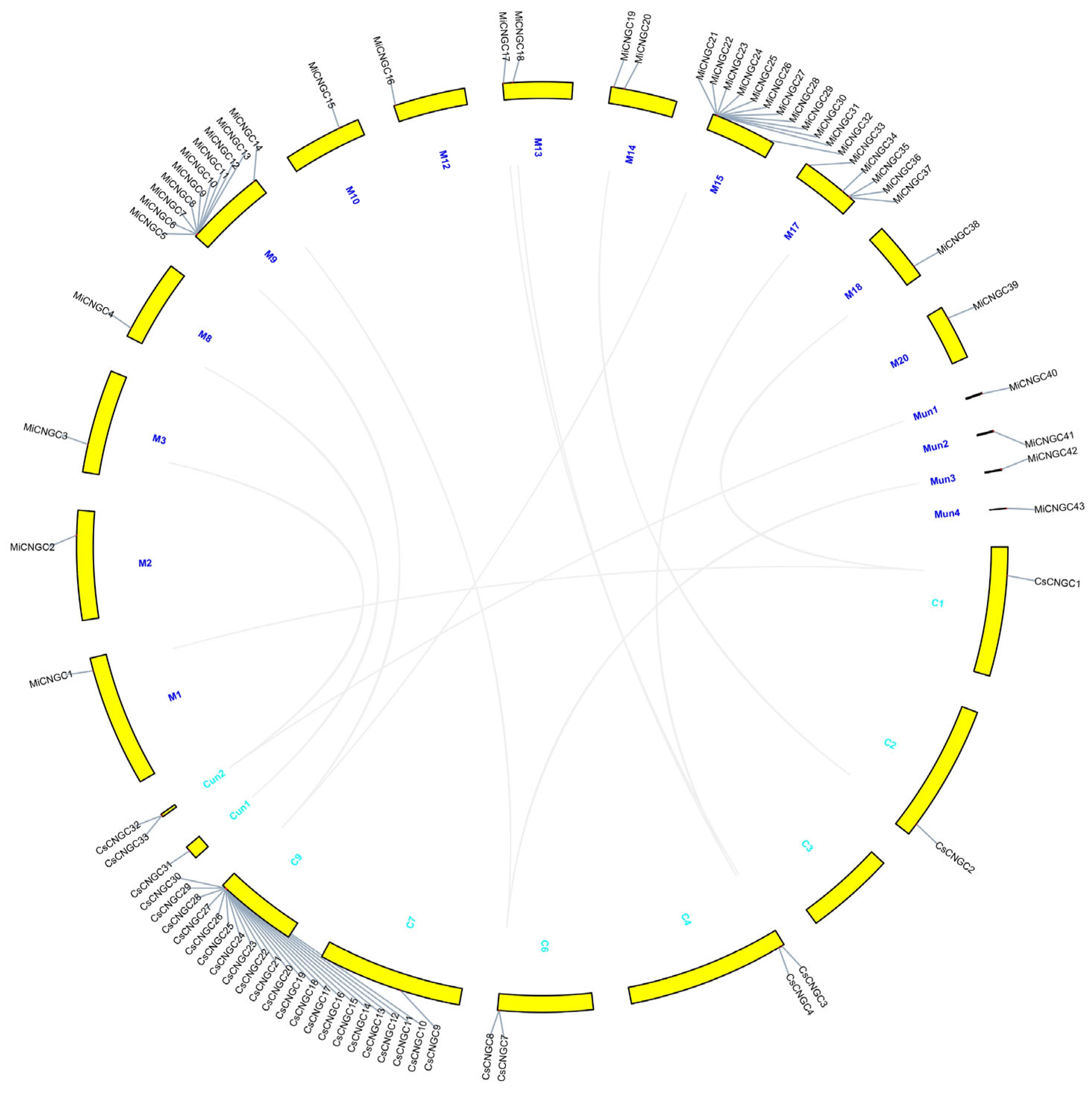
2.1. Characterisation of CNGC Family in Mango
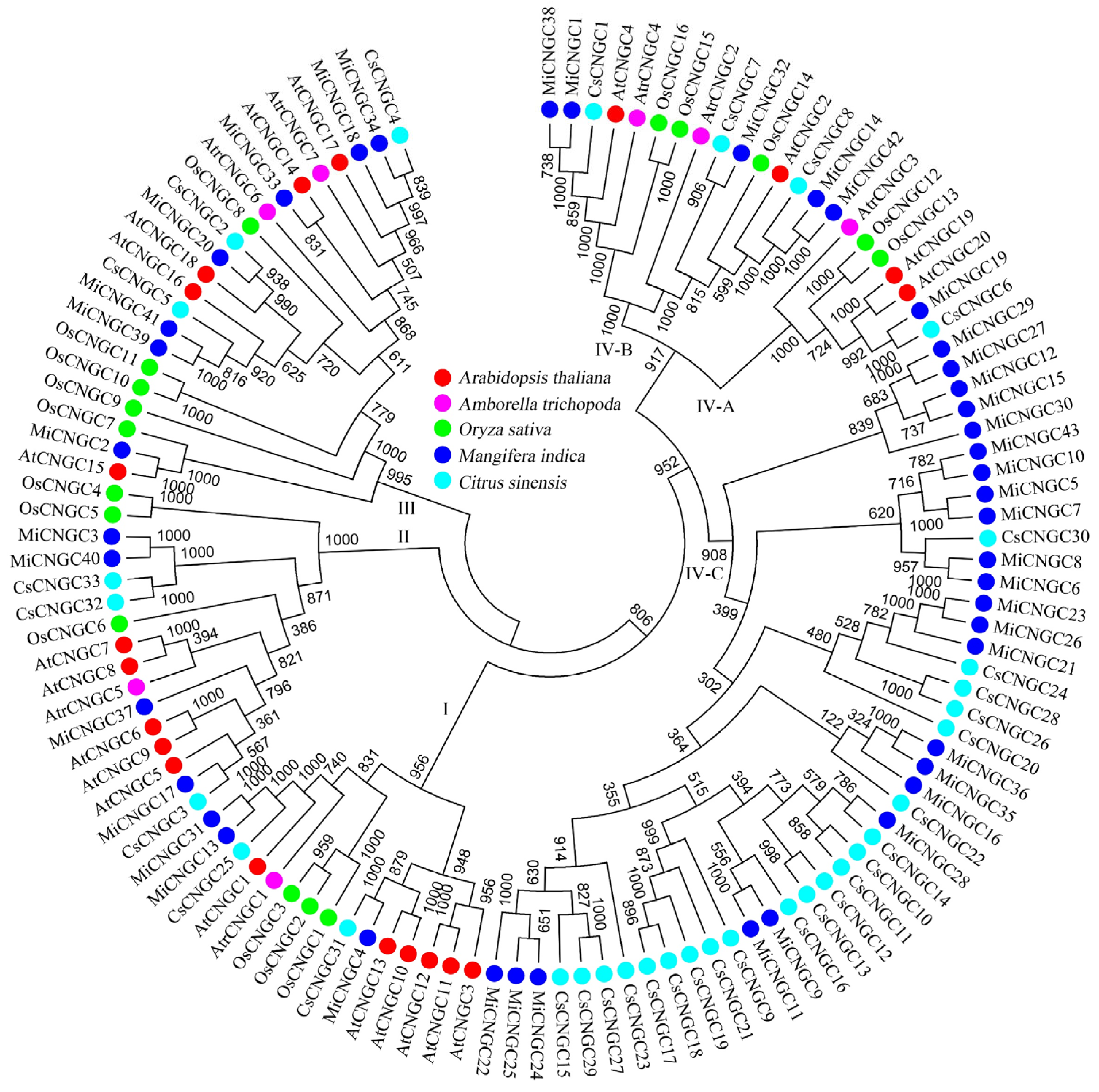
2.2. Phylogenetic Relationships of Mango CNGC Genes
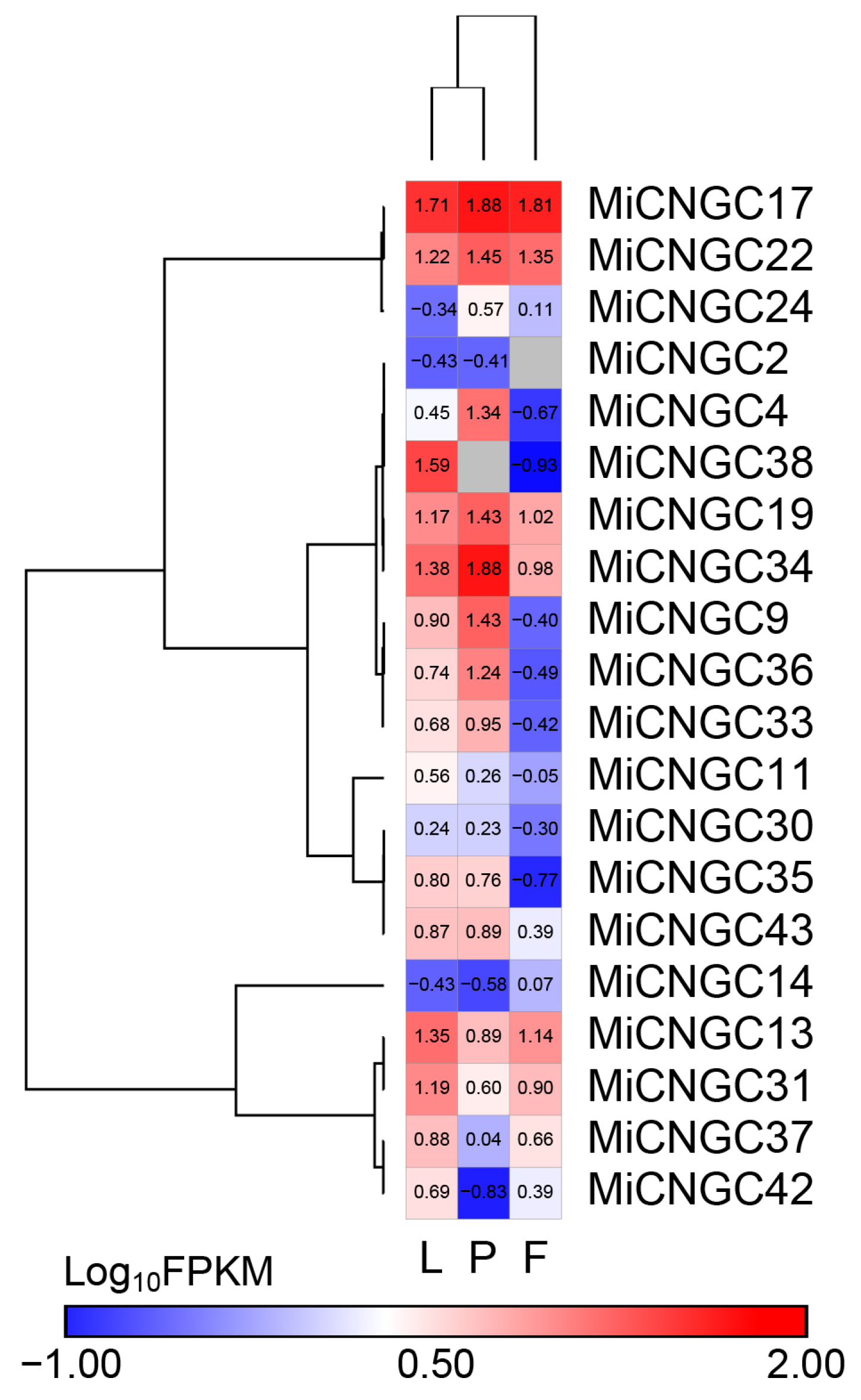
2.3. In Silico Expression of CNGC Genes in Mango Tissues
2.4. In Silico Expression of CNGC Genes under Cold Stress in Mango Fruit Peel
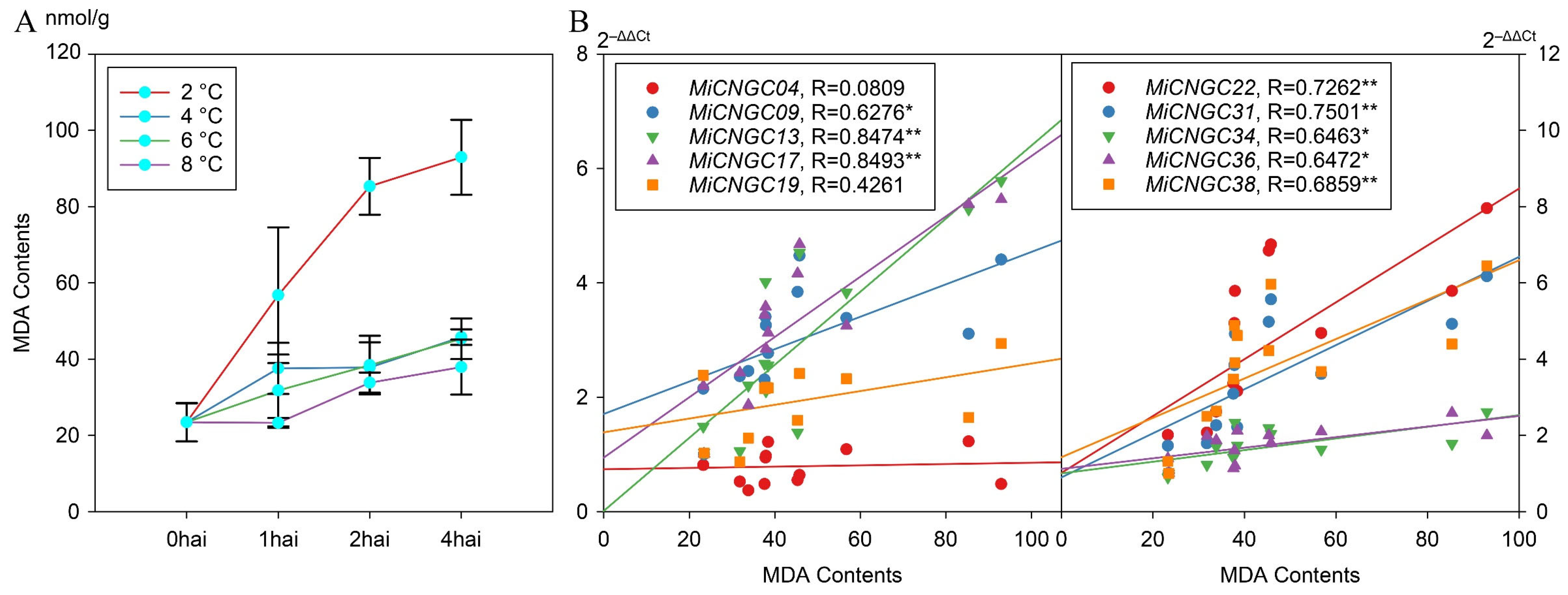
2.5. Expression Profiles of CNGC Genes under Cold Stress in Mango Leaf
2.6. Positively Correlated Mango CNGC Genes with MDA Contents
3. Discussion
3.1. Species-Specific Expansion of CNGC Family in Mango
3.2. Candidate CNGC Genes in Cold Stress Response in Mango
4. Materials and Methods
4.1. Bioinformatic Analysis of CNGC Genes
4.2. In Silico Gene Expression Analysis
4.3. Plant Materials and Treatment
4.4. Quantitative PCR and MDA Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaplan, B.; Sherman, T.; Fromm, H. Cyclic nucleotide-gated channels in plants. FEBS Lett. 2007, 581, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Baloch, A.A.; Raza, A.M.; Rana, S.S.A.; Ullah, S.; Khan, S.; Zaib-Un-Nisa; Zahid, H.; Malghani, G.K.; Kakar, K.U. BrCNGC gene family in field mustard: Genome-wide identification, characterization, comparative synteny, evolution and expression profiling. Sci. Rep. 2021, 11, 24203. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.K.; Sharma, M.; Pandey, G.K. Role of Cyclic Nucleotide Gated Channels in Stress Management in Plants. Curr. Genom. 2016, 17, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, Z.; Kakar, K.U.; Saand, M.A.; Shu, Q.Y. Cyclic nucleotide-gated ion channel gene family in rice, identification, characterization and experimental analysis of expression response to plant hormones, biotic and abiotic stresses. BMC Genom. 2014, 15, 853. [Google Scholar] [CrossRef] [PubMed]
- Saand, M.A.; Xu, Y.P.; Munyampundu, J.P.; Li, W.; Zhang, X.R.; Cai, X.Z. Phylogeny and evolution of plant cyclic nucleotide-gated ion channel (CNGC) gene family and functional analyses of tomato CNGCs. DNA Res. 2015, 22, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Zia, K.; Rao, M.J.; Sadaqat, M.; Azeem, F.; Fatima, K.; Tahir Ul Qamar, M.; Alshammari, A.; Alharbi, M. Pangenome-wide analysis of cyclic nucleotide-gated channel (CNGC) gene family in Citrus spp. Revealed their intraspecies diversity and potential roles in abiotic stress tolerance. Front. Genet. 2022, 13, 1034921. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Peng, S.; Cui, H.; Li, P.; Li, T.; Liu, L.; Zhang, H.; Tian, Z.; Shang, H.; Xu, R. Dynamic Expression, Differential Regulation and Functional Diversity of the CNGC Family Genes in Cotton. Int. J. Mol. Sci. 2022, 23, 2041. [Google Scholar] [CrossRef]
- Demidchik, V.; Shabala, S.; Isayenkov, S.; Cuin, T.A.; Pottosin, I. Calcium transport across plant membranes: Mechanisms and functions. New Phytol. 2018, 220, 49–69. [Google Scholar] [CrossRef]
- Gobert, A.; Park, G.; Amtmann, A.; Sanders, D.; Maathuis, F.J. Arabidopsis thaliana cyclic nucleotide gated channel 3 forms a non-selective ion transporter involved in germination and cation transport. J. Exp. Bot. 2006, 57, 791–800. [Google Scholar] [CrossRef]
- Gu, L.L.; Gao, Q.F.; Wang, Y.F. Cyclic nucleotide-gated channel 18 functions as an essential Ca2+ channel for pollen germination and pollen tube growth in Arabidopsis. Plant Signal. Behav. 2017, 12, e1197999. [Google Scholar] [CrossRef]
- Ma, W.; Smigel, A.; Walker, R.K.; Moeder, W.; Yoshioka, K.; Berkowitz, G.A. Leaf senescence signaling: The Ca2+-conducting Arabidopsis cyclic nucleotide gated channel2 acts through nitric oxide to repress senescence programming. Plant Physiol. 2010, 154, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.W.; DePew, C.L.; Miller, N.D.; Monshausen, G.B. The Cyclic Nucleotide-Gated Channel CNGC14 Regulates Root Gravitropism in Arabidopsis thaliana. Curr. Biol. 2015, 25, 3119–3125. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.Q.; Yang, Y.; Zhang, A.; Fei, C.F.; Gu, L.L.; Sun, S.J.; Xu, W.; Wang, L.; Liu, H.; Wang, Y.F. Three CNGC Family Members, CNGC5, CNGC6, and CNGC9, Are Required for Constitutive Growth of Arabidopsis Root Hairs as Ca2+-Permeable Channels. Plant Commun. 2019, 1, 100001. [Google Scholar] [CrossRef] [PubMed]
- Tunc-Ozdemir, M.; Rato, C.; Brown, E.; Rogers, S.; Mooneyham, A.; Frietsch, S.; Myers, C.T.; Poulsen, L.R.; Malhó, R.; Harper, J.F. Cyclic nucleotide gated channels 7 and 8 are essential for male reproductive fertility. PLoS ONE 2013, 8, e55277. [Google Scholar] [CrossRef]
- Tunc-Ozdemir, M.; Tang, C.; Ishka, M.R.; Brown, E.; Groves, N.R.; Myers, C.T.; Rato, C.; Poulsen, L.R.; McDowell, S.; Miller, G.; et al. A cyclic nucleotide-gated channel (CNGC16) in pollen is critical for stress tolerance in pollen reproductive development. Plant Physiol. 2013, 161, 1010–1020. [Google Scholar] [CrossRef]
- Chin, K.; DeFalco, T.A.; Moeder, W.; Yoshioka, K. The Arabidopsis cyclic nucleotide-gated ion channels AtCNGC2 and AtCNGC4 work in the same signaling pathway to regulate pathogen defense and floral transition. Plant Physiol. 2013, 163, 611–624. [Google Scholar] [CrossRef]
- Guo, K.M.; Babourina, O.; Christopher, D.A.; Borsics, T.; Rengel, Z. The cyclic nucleotide-gated channel, AtCNGC10, influences salt tolerance in Arabidopsis. Physiol. Plant. 2008, 134, 499–507. [Google Scholar] [CrossRef]
- Niu, W.T.; Han, X.W.; Wei, S.S.; Shang, Z.L.; Wang, J.; Yang, D.W.; Fan, X.; Gao, F.; Zheng, S.Z.; Bai, J.T.; et al. Arabidopsis cyclic nucleotide-gated channel 6 is negatively modulated by multiple calmodulin isoforms during heat shock. J. Exp. Bot. 2020, 71, 90–104. [Google Scholar] [CrossRef]
- Oranab, S.; Ghaffar, A.; Kiran, S.; Yameen, M.; Munir, B.; Zulfiqar, S.; Abbas, S.; Batool, F.; Farooq, M.U.; Ahmad, B.; et al. Molecular characterization and expression of cyclic nucleotide gated ion channels 19 and 20 in Arabidopsis thaliana for their potential role in salt stress. Saudi J. Biol. Sci. 2021, 28, 5800–5807. [Google Scholar] [CrossRef]
- Sunkar, R.; Kaplan, B.; Bouché, N.; Arazi, T.; Dolev, D.; Talke, I.N.; Maathuis, F.J.; Sanders, D.; Bouchez, D.; Fromm, H. Expression of a truncated tobacco NtCBP4 channel in transgenic plants and disruption of the homologous Arabidopsis CNGC1 gene confer Pb2+ tolerance. Plant J. 2000, 24, 533–542. [Google Scholar] [CrossRef]
- Yoshioka, K.; Moeder, W.; Kang, H.G.; Kachroo, P.; Masmoudi, K.; Berkowitz, G.; Klessig, D.F. The chimeric Arabidopsis CYCLIC NUCLEOTIDE-GATED ION CHANNEL11/12 activates multiple pathogen resistance responses. Plant Cell 2006, 18, 747–763. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.K. Calcium Channels, OST1 and Stomatal Defence: Current Status and Beyond. Cells 2023, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- Tharanathan, R.N.; Yashoda, H.M.; Prabha, T.N. Mango (Mangifera indica L.), “the king of fruits”—An overview. Food Rev. Int. 2006, 22, 29. [Google Scholar] [CrossRef]
- Wang, P.; Luo, Y.; Huang, J.; Gao, S.; Zhu, G.; Dang, Z.; Gai, J.; Yang, M.; Zhu, M.; Zhang, H.; et al. The genome evolution and domestication of tropical fruit mango. Genome Biol. 2020, 21, 60. [Google Scholar] [CrossRef]
- Sherman, A.; Rubinstein, M.; Eshed, R.; Benita, M.; Ish-Shalom, M.; Sharabi-Schwager, M.; Rozen, A.; Saada, D.; Cohen, Y.; Ophir, R. Mango (Mangifera indica L.) germplasm diversity based on single nucleotide polymorphisms derived from the transcriptome. BMC Plant Biol. 2015, 15, 277. [Google Scholar] [CrossRef]
- Sun, C.; Lin, W.; Huang, C.; Wu, L.; Chen, J.; Wang, J.; Lin, H. GIS-based Risk Zoning and Assessment of Mongo Cold and Freezing Injury in South China. Chin. J. Agrometeorol. 2022, 43, 563–575. [Google Scholar]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef]
- Sanches, A.G.; Da Silva, M.B.; Fernandes, T.F.S.; Pedrosa, V.M.D.; Wong, M.C.C.; Gratão, P.L.; Teixeira, G.H.A. Reducing chilling injury in ‘Palmer’ mangoes submitted to quarantine cold treatment. J. Sci. Food Agric. 2022, 102, 6112–6122. [Google Scholar] [CrossRef]
- Cui, Y.; Lu, S.; Li, Z.; Cheng, J.; Hu, P.; Zhu, T.; Wang, X.; Jin, M.; Wang, X.; Li, L.; et al. CYCLIC NUCLEOTIDE-GATED ION CHANNELs 14 and 16 Promote Tolerance to Heat and Chilling in Rice. Plant Physiol. 2020, 183, 1794–1808. [Google Scholar] [CrossRef]
- Amborella Genome Project. The Amborella genome and the evolution of flowering plants. Science 2013, 342, 1241089. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, L.L.; Ruan, X.; Chen, D.; Zhu, A.; Chen, C.; Bertrand, D.; Jiao, W.B.; Hao, B.H.; Lyon, M.P.; et al. The draft genome of sweet orange (Citrus sinensis). Nat. Genet. 2013, 45, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, W.; Wei, H.; He, Q.; Chen, J.; Zhang, B.; Zhu, S. Species-specific expansion and molecular evolution of the 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) gene family in plants. PLoS ONE 2014, 9, e94172. [Google Scholar] [CrossRef] [PubMed]
- Hunter, N. Meiotic Recombination: The Essence of Heredity. Cold Spring Harb. Perspect. Biol. 2015, 7, a016618. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liu, D.; Chong, K. Cold signaling in plants: Insights into mechanisms and regulation. J. Integr. Plant Biol. 2018, 60, 745–756. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools—An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- ProtParam Tool. Available online: web.expasy.org/protparam/ (accessed on 7 November 2022).
- Yu, C.S.; Chen, Y.C.; Lu, C.H.; Hwang, J.K. Prediction of protein subcellular localization. Proteins 2006, 64, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Krogh, A.; Larsson, B.; Von Heijne, G.; Sonnhammer, E.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- DNAMAN—Bioinformatics Solutions. Available online: www.lynnon.com (accessed on 7 November 2022).
- Sequence Read Archive. Available online: www.ncbi.nlm.nih.gov/sra (accessed on 7 November 2022).
- Sivankalyani, V.; Sela, N.; Feygenberg, O.; Zemach, H.; Maurer, D.; Alkan, N. Transcriptome Dynamics in Mango Fruit Peel Reveals Mechanisms of Chilling Stress. Front. Plant Sci. 2016, 7, 1579. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Sharma, A.; Rajput, R.; Sidhu, S.; Dhillon, H.; Verma, P.C.; Pandey, A.; Upadhyay, S.K. Molecular Characterization, Evolutionary Analysis, and Expression Profiling of BOR Genes in Important Cereals. Plants 2022, 11, 911. [Google Scholar] [CrossRef] [PubMed]
- Morpheus. Available online: software.broadinstitute.org/morpheus/ (accessed on 7 November 2022).
- Zolfaghari, F.; Khosravi, H.; Shahriyari, A.; Jabbari, M.; Abolhasani, A. Hierarchical cluster analysis to identify the homogeneous desertification management units. PLoS ONE 2019, 14, e0226355. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xiao, M.; Xi, J.; He, C.; Zheng, J.; Chen, H.; Gao, J.; Zhang, S.; Wu, W.; Liang, Y.; et al. De novo transcriptome assembly of Agave H11648 by Illumina sequencing and identification of cellulose synthase genes in Agave species. Genes 2019, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wu, J.B.; Zhan, R.L.; Ou, X.C. Transcription Profiling Analysis of Mango-Fusarium mangiferae Interaction. Front. Microbiol. 2016, 7, 1443. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, B.; Xi, J.; Zhang, Y.; He, C.; Zheng, J.; Gao, J.; Chen, H.; Zhang, S.; Wu, W.; et al. Transcriptome comparison reveals distinct selection patterns in domesticated and wild Agave species, the important CAM plants. Int. J. Genom. 2018, 2018, 5716518. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC(T) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sun, G.; Geng, S.; Zhang, H.; Jia, M.; Wang, Z.; Deng, Z.; Tao, S.; Liao, R.; Wang, F.; Kong, X.; et al. Matrilineal empowers wheat pollen with haploid induction potency by triggering postmitosis reactive oxygen species activity. New Phytol. 2022, 233, 2405–2414. [Google Scholar] [CrossRef]


| Gene ID | Accession Number | Chromosome Position | Coding Sequence (bp) | Predicted Protein (aa) | Molecular Weight (Da) | Theoretical pI | Subcellular Localization |
|---|---|---|---|---|---|---|---|
| MiCNGC1 | LOC123217317 | Chr1: 26617757–26622513 (+) | 2088 | 695 | 80,238.87 | 8.59 | Plasma Membrane (4.159) |
| MiCNGC2 | LOC123202006 | Chr2: 18877379–18881267 (−) | 2115 | 704 | 80,732.55 | 9.33 | Plasma Membrane (4.168) |
| MiCNGC3 | LOC123211819 | Chr3: 6181866–6187338 (+) | 2298 | 765 | 88,428.88 | 9.17 | Plasma Membrane (3.621) |
| MiCNGC4 | LOC123223438 | Chr8: 1942929–1947032 (+) | 2151 | 716 | 82,987.45 | 9.38 | Plasma Membrane (3.546) |
| MiCNGC5 | LOC123224898 | Chr9: 570085–574702 (−) | 1734 | 577 | 67,095.52 | 9.67 | Plasma Membrane (3.860) |
| MiCNGC6 | LOC123225620 | Chr9: 590058–593780 (−) | 1203 | 400 | 46,407.24 | 9.12 | Plasma Membrane (2.575) |
| MiCNGC7 | LOC123225300 | Chr9: 599306–603542 (+) | 1998 | 665 | 77,653.8 | 9.42 | Plasma Membrane (3.398) |
| MiCNGC8 | LOC123225621 | Chr9: 608291–611565 (+) | 1377 | 458 | 53,035.69 | 9.52 | Plasma Membrane (2.835) |
| MiCNGC9 | LOC123225476 | Chr9: 613450–633947 (−) | 1821 | 606 | 69,498.22 | 5.61 | Plasma Membrane (3.921) |
| MiCNGC10 | LOC123225622 | Chr9: 613655–614273 (+) | 516 | 171 | 19,510.66 | 4.88 | Nuclear (2.027) |
| MiCNGC11 | LOC123225623 | Chr9: 640657–644088 (−) | 1260 | 419 | 48,200.04 | 5.71 | Plasma Membrane (2.541) |
| MiCNGC12 | LOC123226204 | Chr9: 654424–660087 (−) | 1731 | 576 | 66,396.96 | 8.51 | Plasma Membrane (3.243) |
| MiCNGC13 | LOC123225899 | Chr9: 662666–667252 (−) | 2130 | 709 | 81,778.71 | 9.18 | Plasma Membrane (4.474) |
| MiCNGC14 | LOC123224819 | Chr9: 17603913–17607726 (−) | 2151 | 716 | 82,061.84 | 9.54 | Plasma Membrane (4.714) |
| MiCNGC15 | LOC123226709 | Chr10: 13110947–13113872 (+) | 1023 | 340 | 38,771.01 | 9.24 | Plasma Membrane (2.866) |
| MiCNGC16 | LOC123193626 | Chr12: 337616–340675 (−) | 1635 | 544 | 61,685.37 | 8.3 | Plasma Membrane (3.945) |
| MiCNGC17 | LOC123195239 | Chr13: 132576–141356 (+) | 2208 | 735 | 83,845.52 | 9.06 | Plasma Membrane (4.347) |
| MiCNGC18 | LOC123195166 | Chr13: 2244118–2249930 (+) | 2193 | 730 | 84,150.34 | 8.66 | Plasma Membrane (4.075) |
| MiCNGC19 | LOC123196387 | Chr14: 571224–582588 (−) | 2343 | 780 | 89,883.31 | 9.05 | Plasma Membrane (4.810) |
| MiCNGC20 | LOC123196103 | Chr14: 2913165–2916467 (+) | 2148 | 715 | 82,156.57 | 8.92 | Plasma Membrane (4.612) |
| MiCNGC21 | LOC123198417 | Chr15: 882187–884231 (+) | 729 | 242 | 28,545.32 | 9.19 | Nuclear (1.618) |
| MiCNGC22 | LOC123198080 | Chr15: 884570–888789 (+) | 1716 | 571 | 66,348.13 | 6.17 | Plasma Membrane (3.842) |
| MiCNGC23 | LOC123198017 | Chr15: 894806–897058 (+) | 1266 | 421 | 48,693.95 | 5.97 | Plasma Membrane (4.227) |
| MiCNGC24 | LOC123197882 | Chr15: 899688–903479 (+) | 1809 | 602 | 70,104.71 | 8.73 | Plasma Membrane (4.512) |
| MiCNGC25 | LOC123198019 | Chr15: 937711–939887 (−) | 744 | 247 | 28,653.79 | 8.06 | Nuclear (1.582) |
| MiCNGC26 | LOC123198301 | Chr15: 944971–947978 (−) | 1563 | 520 | 59,757.67 | 8.86 | Plasma Membrane (3.850) |
| MiCNGC27 | LOC123197889 | Chr15: 957259–959253 (−) | 708 | 235 | 27,110.7 | 9.57 | Extracellular (1.327) |
| MiCNGC28 | LOC123197890 | Chr15: 968337–969069 (−) | 399 | 132 | 14,907.27 | 8.37 | Extracellular (1.999) |
| MiCNGC29 | LOC123197893 | Chr15: 998581–1002107 (−) | 1524 | 507 | 58,308.01 | 8.81 | Plasma Membrane (4.361) |
| MiCNGC30 | LOC123197894 | Chr15: 1002388–1013277 (−) | 1926 | 641 | 74,391.25 | 8.83 | Plasma Membrane (4.326) |
| MiCNGC31 | LOC123198006 | Chr15: 1014910–1019679 (−) | 2130 | 709 | 81,816.51 | 9.13 | Plasma Membrane (4.548) |
| MiCNGC32 | LOC123197621 | Chr15: 13323057–13327587 (−) | 2028 | 675 | 77,023.97 | 9.41 | Plasma Membrane (4.667) |
| MiCNGC33 | LOC123199977 | Chr17: 659050–663360 (−) | 2163 | 720 | 83,381.02 | 8.99 | Plasma Membrane (4.360) |
| MiCNGC34 | LOC123200530 | Chr17: 10040631–10047558 (+) | 2178 | 725 | 83,488.05 | 9.09 | Plasma Membrane (4.053) |
| MiCNGC35 | LOC123201033 | Chr17: 11813705–11815638 (−) | 696 | 231 | 26,664.04 | 9.05 | Nuclear (1.519) |
| MiCNGC36 | LOC123200218 | Chr17: 11846751–11849774 (−) | 690 | 229 | 26,194.33 | 8.47 | Nuclear (1.544) |
| MiCNGC37 | LOC123200982 | Chr17: 12162919–12170581 (+) | 2136 | 711 | 81,639.43 | 9.03 | Plasma Membrane (4.349) |
| MiCNGC38 | LOC123201393 | Chr18: 10879862–10886175 (+) | 2061 | 686 | 79,312.97 | 9.09 | Plasma Membrane (4.272) |
| MiCNGC39 | LOC123204873 | Chr20: 2710625–2713311 (+) | 2052 | 683 | 79,142.16 | 9 | Plasma Membrane (4.212) |
| MiCNGC40 | LOC123206450 | NW_025401129.1: 250730–254202 (−) | 2229 | 742 | 85,358.83 | 9.37 | Plasma Membrane (3.988) |
| MiCNGC41 | LOC123206532 | NW_025401132.1: 147597–150404 (+) | 2052 | 683 | 79,185.18 | 9.05 | Plasma Membrane (4.213) |
| MiCNGC42 | LOC123206927 | NW_025401145.1: 36673–40482 (+) | 2151 | 716 | 82,061.84 | 9.54 | Plasma Membrane (4.714) |
| MiCNGC43 | LOC123208231 | NW_025401260.1: 224–2797 (−) | 1176 | 391 | 44,867.26 | 9.23 | Plasma Membrane (3.815) |
| Genes | Forward Primer | Reverse Primer | Product Size (bp) | Accession Number |
|---|---|---|---|---|
| MiCNGC4 | TTTACTGCTTCTGGTGGGGT | AGGGAGCAAACGATGAGACA | 236 | XM_044646600.1 |
| MiCNGC9 | TCAGCTTCCTCGTTGACCTT | CTTCCCGCTTCCAACATCAG | 226 | XM_044649431.1 |
| MiCNGC13 | TCGGGCTTCAGGATTCTTGT | CCCAGTCCACCTCTTCATGT | 196 | XM_044650230.1 |
| MiCNGC17 | CTTCAAACGAGCACCTTCCC | TCCTCGTGTTTCCAACCACT | 246 | XM_044608894.1 |
| MiCNGC19 | ACTTGGGAATGTCAGGAGCA | CCAACAACATGACCAGCCAA | 193 | XM_044610397.1 |
| MiCNGC22 | TCACATGGGCTCTAGATGGC | AAACAGAGTCATCCGGCAGA | 175 | XM_044612662.1 |
| MiCNGC31 | GACTTGGGCAGCTTGTTTCA | TGAATCCTTGCCTTCCGAGT | 216 | XM_044612575.1 |
| MiCNGC34 | CGCCGTTTTGACCAGTACAA | AGCATCTCGGTTACAGGGTC | 248 | XM_044615727.1 |
| MiCNGC36 | GCAAGACAGAGCAGTGGATG | TCCTCTAATGCCGATTCGCT | 232 | XM_044615370.1 |
| MiCNGC38 | ATTCTCCCTCTCCCTCAGGT | TGTGCCGAAAATGTAGCCAG | 183 | XM_044616876.1 |
| MiActin | CCACTGCTGAACGGGAAAT | GTGATGGCTGGAAGAGGAC | 192 | HQ585999.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Li, Y.; Yang, J.; Yang, X.; Chen, S.; Xie, Z.; Zhang, M.; Huang, Y.; Zhang, J.; Huang, X. Genome-Wide Analysis and Expression of Cyclic Nucleotide–Gated Ion Channel (CNGC) Family Genes under Cold Stress in Mango (Mangifera indica). Plants 2023, 12, 592. https://doi.org/10.3390/plants12030592
Zhang Y, Li Y, Yang J, Yang X, Chen S, Xie Z, Zhang M, Huang Y, Zhang J, Huang X. Genome-Wide Analysis and Expression of Cyclic Nucleotide–Gated Ion Channel (CNGC) Family Genes under Cold Stress in Mango (Mangifera indica). Plants. 2023; 12(3):592. https://doi.org/10.3390/plants12030592
Chicago/Turabian StyleZhang, Yajie, Yubo Li, Jing Yang, Xinli Yang, Shengbei Chen, Zhouli Xie, Mingjie Zhang, Yanlei Huang, Jinghong Zhang, and Xing Huang. 2023. "Genome-Wide Analysis and Expression of Cyclic Nucleotide–Gated Ion Channel (CNGC) Family Genes under Cold Stress in Mango (Mangifera indica)" Plants 12, no. 3: 592. https://doi.org/10.3390/plants12030592
APA StyleZhang, Y., Li, Y., Yang, J., Yang, X., Chen, S., Xie, Z., Zhang, M., Huang, Y., Zhang, J., & Huang, X. (2023). Genome-Wide Analysis and Expression of Cyclic Nucleotide–Gated Ion Channel (CNGC) Family Genes under Cold Stress in Mango (Mangifera indica). Plants, 12(3), 592. https://doi.org/10.3390/plants12030592







