Macaronesian Plants as Promising Biopesticides against the Crop Pest Ceratitis capitata
Abstract
1. Introduction
2. Results
2.1. The Extraction Yields
2.2. Ceratitis capitata Adults’ Mortality after Contact with Treated Artificial Fruits
2.3. Oviposition Deterrent Activity (OD)
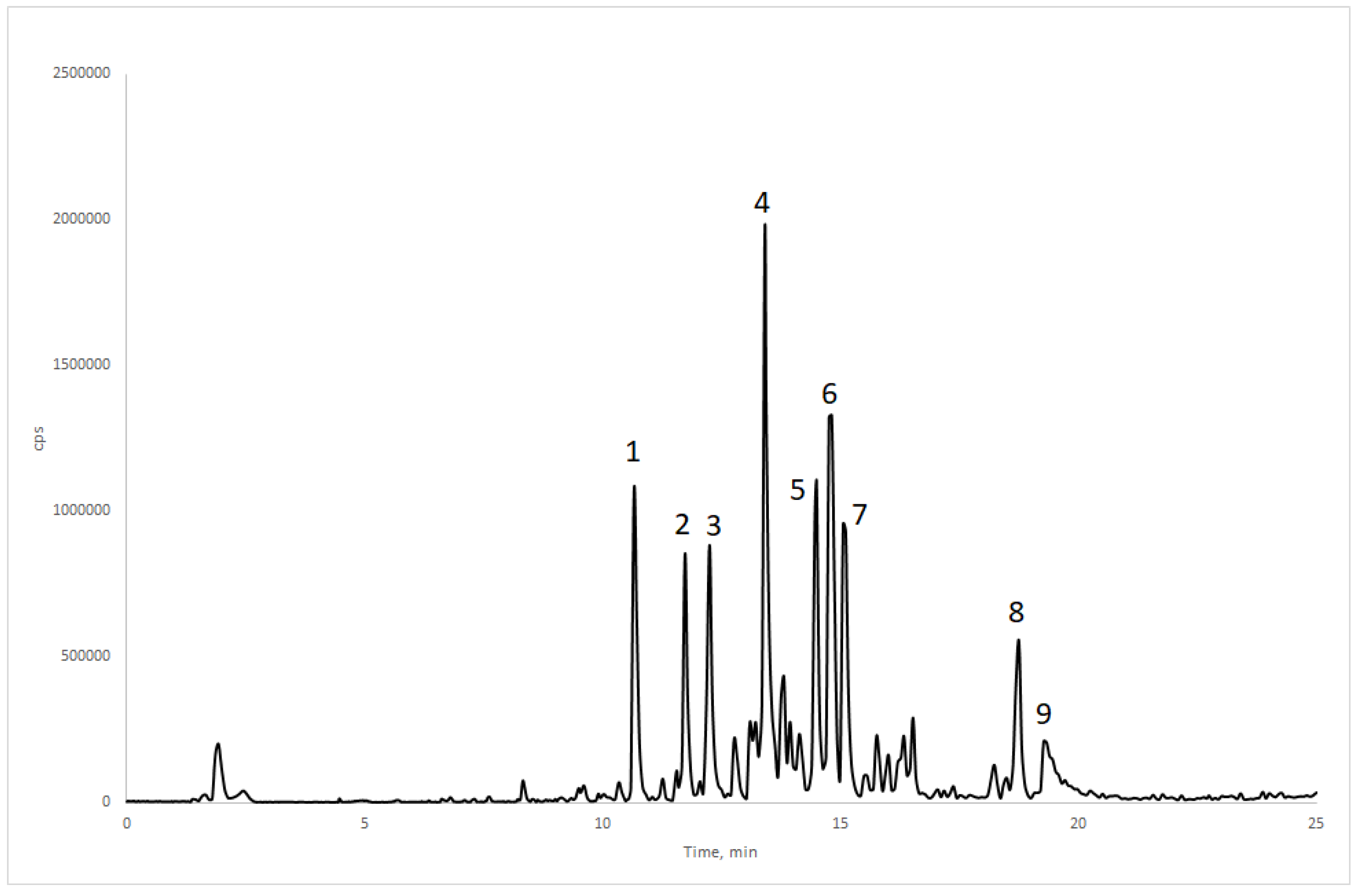
2.4. Phytochemical Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of Extracts and Fractions Thereof
4.3. Insecticidal Activity
4.3.1. Insect Rearing and Treatment
4.3.2. Oviposition and Adults’ Mortality Assay
- OD = oviposition deterrent activity;
- Cs = number of eggs laid on the control;
- Ts = number of eggs laid in the treated container.
4.3.3. Statistical Analysis
4.4. Phytochemical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sciarretta, A.; Tabilio, M.R.; Lampazzi, E.; Ceccaroli, C.; Colacci, M.; Trematerra, P. Analysis of the Mediterranean fruit fly [Ceratitis capitata (Wiedemann)] spatio-temporal distribution in relation to sex and female mating status for precision IPM. PLoS ONE 2018, 13, e0195097. [Google Scholar] [CrossRef]
- Ruiz-Arce, R.; Todd, T.N.; Deleon, R.; Barr, N.B.; Virgilio, M.; De Meyer, M.; McPheron, B.A. Worldwide phylogeography of Ceratitis capitata (Diptera: Tephritidae) using mitochondrial DNA. J. Econ. Entomol. 2020, 113, 1455–1470. [Google Scholar] [CrossRef]
- Deschepper, P.; Todd, T.N.; Virgilio, M.; De Meyer, M.; Barr, N.B.; Ruiz-Arce, R. Looking at the big picture: Worldwide population structure and range expansion of the cosmopolitan pest Ceratitis capitata (Diptera, Tephritidae). Biol. Invasions 2021, 23, 3529–3543. [Google Scholar] [CrossRef]
- Arias, M.B.; Hartle-Mougiou, K.; Taboada, S.; Vogler, A.P.; Riesgo, A.; Elfekih, S. Unveiling biogeographical patterns in the worldwide distributed Ceratitis capitata (medfly) using population genomics and microbiome composition. Mol. Ecol. 2022, 31, 4866–4883. [Google Scholar] [CrossRef] [PubMed]
- Ordax, M.; Piquer-Salcedo, J.E.; Santander, R.D.; Sabater-Muñoz, B.; Biosca, E.G.; López, M.M.; Marco-Noales, E. Medfly Ceratitis capitata as potential vector for fire blight pathogen Erwinia amylovora: Survival and transmission. PLoS ONE 2015, 10, e0127560. [Google Scholar] [CrossRef] [PubMed]
- Zaada, D.S.Y.; Ben-Yosef, M.; Yuval, B.; Jurkevitch, E. The host fruit amplifies mutualistic interaction between Ceratitis capitata larvae and associated bacteria. BMC Biotechnol. 2019, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Magaña, C.; Hernández-Crespo, P.; Brun-Barale, A.; Couso-Ferrer, F.; Bride, J.-M.; Castañera, P.; Feyereisen, R.; Ortego, F. Mechanisms of resistance to malathion in the medfly Ceratitis capitata. Insect Biochem. Mol. Biol. 2008, 38, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Badr, A.M. Organophosphate toxicity: Updates of malathion potential toxic effects in mammals and potential treatments. Environ. Sci. Pollut. Res. 2020, 27, 26036–26057. [Google Scholar] [CrossRef]
- Khursheed, A.; Rather, M.A.; Jain, V.; Wani, A.R.; Rasool, S.; Nazir, R.; Malik, N.A.; Majid, S.A. Plant based natural products as potential ecofriendly and safer biopesticides: A comprehensive overview of their advantages over conventional pesticides, limitations and regulatory aspects. Microb. Pathog. 2022, 173, 105854. [Google Scholar] [CrossRef]
- Grzywacz, D.; Stevenson, P.C.; Mushobozi, W.L.; Belmain, S.; Wilson, K. The use of indigenous ecological resources for pest control in Africa. Food Sec. 2014, 6, 71–86. [Google Scholar] [CrossRef]
- Stevenson, P.C.; Belmain, S.R. Pesticidal plants in African agriculture: Local uses and global perspectives. Outlooks Pest. Manag. 2016, 27, 226–230. [Google Scholar] [CrossRef]
- Mobolade, A.J.; Bunindro, N.; Sahoo, D.; Rajashekar, Y. Traditional methods of food grains preservation and storage in Nigeria and India. Ann. Agric. Sci. 2019, 64, 196–205. [Google Scholar] [CrossRef]
- Gonzalez-Coloma, A.; Reina, M.; Diaz, C.E.; Fraga, B.M.; Santana-Meridas, O. Natural product-based biopesticides for insect control. In Elsevier Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Waltham, MA, USA, 2013; pp. 1–57. [Google Scholar] [CrossRef]
- López, S.B.; López, M.L.; Aragón, L.M.; Tereschuk, M.L.; Slanis, A.C.; Feresin, G.E.; Zygadlo, J.A.; Tapia, A.A. Composition and anti-insect activity of essential oils from Tagetes L. species (Asteraceae, Helenieae) on Ceratitis capitata Wiedemann and Triatoma infestans Klug. J. Agric. Food Chem. 2011, 59, 5286–5292. [Google Scholar] [CrossRef]
- Furtado, R.; Baptista, J.; Lima, E.; Paiva, L.; Barroso, J.G.; Rosa, J.S.; Oliveira, L. Chemical composition and biological activities of Laurus essential oils from different Macaronesian Islands. Biochem. Syst. Ecol. 2014, 55, 333–341. [Google Scholar] [CrossRef]
- Ghalbane, I.; Alahyane, H.; Aboussaid, H.; Chouikh, N.-E.; Costa, J.; Romane, A.; El Messoussi, S. Chemical composition and insecticidal properties of Moroccan Lavandula dentata and Lavandula stoechas essential oils against Mediterranean fruit fly, Ceratitis capitata. Neotrop. Entomol. 2022, 51, 628–636. [Google Scholar] [CrossRef]
- Tavares, W.R.; Barreto, M.d.C.; Seca, A.M.L. Aqueous and ethanolic plant extracts as bio-insecticides—Establishing a bridge between raw scientific data and practical reality. Plants 2021, 10, 920. [Google Scholar] [CrossRef]
- Ghabbari, M.; Guarino, S.; Caleca, V.; Saiano, F.; Sinacori, M.; Baser, N.; Jemâa, J.M.-B.; Lo Verde, G. Behavior-modifying and insecticidal effects of plant extracts on adults of Ceratitis capitata (Wiedemann) (Diptera Tephritidae). J. Pest Sci. 2018, 91, 907–917. [Google Scholar] [CrossRef]
- Stupp, P.; Rakes, M.; Martins, L.N.; Piovesan, B.; Oliveira, D.C.; Miranda, J.A.C.; Ribeiro, L.P.; Nava, D.E.; Bernardi, D. Lethal and sublethal toxicities of acetogenin-based bioinsecticides on Ceratitis capitata and the parasitoid Diachasmimorpha longicaudata. Phytoparasitica 2020, 48, 477–489. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, A.; Kumar, B. Identification and characterization of phenolics and terpenoids from ethanolic extracts of Phyllanthus species by HPLC-ESI-QTOF-MS/MS. J. Pharm. Anal. 2017, 7, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Ablajan, K.; Abliz, Z.; Shang, X.-Y.; He, J.-M.; Zhang, R.-P.; Shi, J.-G. Structural characterization of flavonol 3,7-di-O-glycosides and determination of the glycosylation position by using negative ion electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2006, 41, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Cuyckens, F.; Claeys, M. Mass spectrometry in the structural analysis of flavonoids. J. Mass Spectrom. 2004, 39, 1–15. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, S.; Bajpai, V.; Reddy, T.J.; Rameshkumar, K.B.; Kumar, B. Structural characterization of flavonoid C- and O-glycosides in an extract of Adhatoda vasica leaves by liquid chromatography with quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2015, 29, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Roepke, J.; Bozzo, G.G. Biocatalytic synthesis of quercetin 3-O-glucoside-7-O-rhamnoside by metabolic engineering of Escherichia coli. Chembiochem. 2013, 14, 2418–2422. [Google Scholar] [CrossRef]
- Singh, A.P.; Chitme, H.; Sharma, R.K.; Kandpal, J.B.; Behera, A.; Abdel-Wahab, B.A.; Orabi, M.A.; Khateeb, M.M.; Habeeb, M.S.; Bakir, M.B. A comprehensive review on pharmacologically active phyto-constituents from Hedychium species. Molecules 2023, 28, 3278. [Google Scholar] [CrossRef] [PubMed]
- Sobral, F.; Calhelha, R.C.; Barros, L.; Dueñas, M.; Tomás, A.; Santos-Buelga, C.; Vilas-Boas, M.; Ferreira, I.C.F.R. Flavonoid composition and antitumor activity of bee bread collected in Northeast Portugal. Molecules 2017, 22, 248. [Google Scholar] [CrossRef] [PubMed]
- Guillem-Amat, A.; Sánchez, L.; López-Errasquín, E.; Ureña, E.; Hernández-Crespo, P.; Ortego, F. Field detection and predicted evolution of spinosad resistance in Ceratitis capitata. Pest Manag. Sci. 2020, 76, 3702–3710. [Google Scholar] [CrossRef]
- Williams, C.A.; Harborne, J.B. The leaf flavonoids of the Zingiberales. Biochem. Syst. Ecol. 1977, 5, 221–229. [Google Scholar] [CrossRef]
- Akhov, L.; Barl, B. Isolation of quercetin glycosides from leaves of sea buckthorn (Hippophae rhamnoides ssp. mongolica). Acta Hortic. 2003, 626, 389–395. [Google Scholar] [CrossRef]
- Guimarães, R.; Barros, L.; Dueñas, M.; Carvalho, A.M.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Characterization of phenolic compounds in wild fruits from Northeastern Portugal. Food Chem. 2013, 141, 3721–3730. [Google Scholar] [CrossRef]
- Jadhav, D.R.; Mallikarjuna, N.; Rathore, A.; Pokle, D. Effect of some flavonoids on survival and development of Helicoverpa armigera (Hübner) and Spodoptera litura (Fab) (Lepidoptera: Noctuidae). Asian J. Agric. Sci. 2012, 4, 298–307. [Google Scholar]
- Mitchell, M.J.; Keogh, D.P.; Crooks, J.R.; Smith, S.L. Effects of plant flavonoids and other allelochemicals on insect cytochrome P-450 dependent steroid hydroxylase activity. Insect Biochem. Mol. Biol. 1993, 23, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Shamkh, I.M.; Al-Majidi, M.; Shntaif, A.H.; Kai, P.T.D.; Nh-Pham, N.; Rahman, I.; Hamza, D.; Khan, M.S.; Elsharayidi, M.S.; Salah, E.T.; et al. Nontoxic and naturally occurring active compounds as potential inhibitors of biological targets in Liriomyza trifolii. Int. J. Mol. Sci. 2022, 23, 12791. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, W.; Wang, D.; Lei, Y.; Qi, G.; Chen, T.; Rizvi, S.A.H.; Sethuraman, V.; He, Y.; Lu, L. Evaluating the repellent effect of four botanicals against two Bactrocera species on mangoes. PeerJ 2020, 8, e8537. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, M.; Ghadamyari, M.; Sajedi, R.H.; Mahmoodi, N.O. Effects of 4-hexylresorcinol on the phenoloxidase from Hyphantria cunea (Lepidoptera: Arctiidae): In vivo and in vitro studies. Insect Sci. 2015, 22, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.M.; Samara, R.; Renaud, J.B.; Sumarah, M.W. Plant growth regulator-mediated anti-herbivore responses of cabbage (Brassica oleracea) against cabbage looper Trichoplusia ni Hübner (Lepidoptera: Noctuidae). Pestic. Biochem. Physiol. 2017, 141, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Huang, X.; Li, S.; Hao, K.; Chang, B.H.; Tu, X.; Pang, B.; Zhang, Z. Quercetin affects the growth and development of the grasshopper Oedaleus asiaticus (Orthoptera: Acrididae). J. Econ. Entomol. 2019, 112, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- De Geyter, E.; Swevers, L.; Caccia, S.; Geelen, D.; Smagghe, G. Saponins show high entomotoxicity by cell membrane permeation in Lepidoptera. Pest Manag. Sci. 2012, 68, 1199–1205. [Google Scholar] [CrossRef]
- Lin, M.; Yang, S.; Huang, J.; Zhou, L. Insecticidal triterpenes in Meliaceae: Plant species, molecules and activities: Part I (Aphanamixis-Chukrasia). Int. J. Mol. Sci. 2021, 22, 13262. [Google Scholar] [CrossRef]
- Lin, M.; Bi, X.; Zhou, L.; Huang, J. Insecticidal triterpenes in Meliaceae: Plant species, molecules and activities: Part II (Cipadessa, Melia). Int. J. Mol. Sci. 2022, 23, 5329. [Google Scholar] [CrossRef]
- Ma, Y.F.; Xiao, C. Push-pull effects of three plant secondary metabolites on oviposition of the potato tuber moth, Phthorimaea operculella. J. Insect Sci. 2013, 13, 128. [Google Scholar] [CrossRef]
- Albajes, R.; Santiago-Álvarez, C. Efectos de la densidad larvaria y de la alimentación en la proporción de sexos de Ceratitis capitata (Diptera: Tephritidae). Anales INIA Serie Agrícola 1980, 13, 175–182. [Google Scholar]
- Salles, L.A.B. Metodologia de criação de Anastrepha fraterculus (Wied., 1830) (Diptera: Ttephritidae) em dieta artificial em laboratório. ASEB 1992, 21, 479–486. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Hematpoor, A.; Liew, S.Y.; Azirun, M.S.; Awang, K. Insecticidal activity and the mechanism of action of three phenylpropanoids isolated from the roots of Piper sarmentosum Roxb. Sci. Rep. 2017, 7, 12576. [Google Scholar] [CrossRef]
| Plants | Ethanolic Extract Yield |
|---|---|
| Argyranthemum frutescens | 4.81 |
| Cistus symphytifolius | 14.13 |
| Hedychium gardnerianum | 6.89 |
| Laurus azorica | 13.21 |
| Maytenus canariensis | 14.77 |
| Salvia canariensis | 13.41 |
| Withania aristata | 12.68 |
| Plants (Extracts and Fractions) | Adults’ Mortality (% at 50 mg/mL) § | |||
|---|---|---|---|---|
| 24 h # | 48 h | 72 h | ||
| Argyranthemum frutescens EE | 2.82 ± 1.61 | 5.11 ± 4.17 a | 14.07 ± 7.87 abc | |
| Cistus symphytifolius EE | 4.93 ± 3.48 | 7.30 ± 4.89 a | 22.96 ± 5.42 abc | |
| Hedychium gardnerianum EE | −0.86 ± 0.00 | 9.82 ± 7.49 a | 21.15 ± 14.64 abc | |
| Laurus azorica EE | −0.86 ± 0.00 | −0.89 ± 1.85 a | 1.92 ± 4.74 ab | |
| Maytenus canariensis EE | 2.11 ± 1.18 | 2.92 ± 2.53 a | 14.07 ± 6.81 abc | |
| Salvia canariensis EE | 0.70 ± 1.41 | 7.30 ± 4.89 a | 27.41 ± 11.39 bc | |
| Withania aristata EE | 1.41 ± 1.09 | 7.30 ± 3.49 a | 20.00 ± 9.69 abc | |
| Hedychium gardnerianum EE | HF | 0.07 ± 0.89 | 11.46 ± 8.85 a | 32.69 ± 14.01 cdf |
| EAF | −0.86 ± 0.00 | 0.89 ± 2.54 a | −1.92 ± 5.26 a | |
| WF | 5.17 ± 4.03 | 31.25 ± 11.16 b | 57.69 ± 12.35 d | |
| Azadirachtin * | 6.34 ± 2.85 | 13.14 ± 6.01 a | 25.93 ± 7.24 bef | |
| Control | 0.00 ± 0.82 | 0.00 ± 2.24 a | 0.00 ± 4.95 ae | |
| F | 1.717 | 2.704 | 2.739 | |
| df | 11 | 11 | 11 | |
| p | 0.079 | 0.028 | 0.004 | |
| Samples | N * | LT50 1 | LT90 1 | Slope (±SEM) | Intercept (±SEM) | H 2 |
|---|---|---|---|---|---|---|
| Hexane fraction of H. gardnerianum EE | 116 | 95.29 (81.92–129.65) | 182.40 (132.87–366.01) | 4.55 ± 0.88 | −9.00 ± 1.58 | 0.46 |
| Water fraction of H. gardnerianum EE | 117 | 72.54 (64.62–85.71) | 167.5 (128.89–260.79) | 3.53 ± 0.48 | −6.60 ± 0.83 | 0.01 |
| Plants (Extracts and Fractions) | % OD (mean ± SEM) § | |
|---|---|---|
| Argyranthemum frutescens EE | 69.83 ± 6.25 c | |
| Cistus symphytifolius EE | 47.46 ± 6.51 bc | |
| Hedychium gardnerianum EE | 78.30 ± 7.06 c | |
| Laurus azorica EE | 55.11 ± 12.22 bc | |
| Maytenus canariensis EE | 7.46 ± 26.53 ab | |
| Salvia canariensis EE | 38.64 ± 13.04 bc | |
| Withania aristata EE | −44.41 ± 43.30 a | |
| Hedychium gardnerianum EE | HF | 78.70 ± 7.81 c |
| EAF | 35.51 ± 25.55 bc | |
| WF | 83.43 ± 6.22 c | |
| Azadirachtin | 35.25 ± 42.78 bc | |
| Control | 0.00 ± 21.21 ab | |
| F | 3.343 | |
| df | 11 | |
| p | 0.001 | |
| Peak Number | Retention Time (min) | [M-H]− (m/z) | MS/MS Ions (m/z) | Assigned Compound |
|---|---|---|---|---|
| 1 | 10.7 | 625 | 479; 317 | Myricetin-3-O-rhamnosyl-glucoside |
| 2 | 11.8 | 609 | 463; 447; 301 | Quercetin-3-O-glucoside-7-O-rhamnoside |
| 3 | 12.2 | 925 | 779; 633; 597 | Triterpene-3-O-di-rhamnoside |
| 4 | 13.4 | 625 | 317; 271; 179 | Myricetin-3-O-rutinoside |
| 5 | 14.5 | 609 | 301; 179 | Quercetin-3-O-rutinoside 1 |
| 6 | 14.9 | 625 | 463; 317 | Myricetin-3-O-glucosyl-rhamnoside |
| 7 | 15.1 | 477 | 301; 179 | Quercetin-3-O-glucuronide |
| 8 | 18.8 | 685 | 639; 621 | Triterpene derivative |
| 9 | 19.4 | 821 | 785; 767; 755; 737; 725 | Triterpene derivative |
| Plant Species 1 | Plant Part | Voucher Code |
|---|---|---|
| Argyranthemum frutescens (L.) Sch.Bip. | Leaves | TFC 54.141 2 |
| Cistus symphytifolius Lam. | Leaves | TFC 53.703 2 |
| Gymnosporia cassinoides (L’Hér.) Masf. (syn. Maytenus canariensis (Loes.) G.Kunkel & Sunding) | Leaves | TFC 53.244 2 |
| Hedychium gardnerianum Sheppard ex Ker Gawl. | Leaves and Stems | 3671 3 |
| Laurus azorica (Seub.) Franco | Leaves | 3670 3 |
| Salvia canariensis L. | Leaves | TFC 53.328 2 |
| Withania aristata (Aiton) Pauquy | Leaves | TFC 53.219 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares, W.R.; Jiménez, I.A.; Oliveira, L.; Kuhtinskaja, M.; Vaher, M.; Rosa, J.S.; Seca, A.M.L.; Bazzocchi, I.L.; Barreto, M.d.C. Macaronesian Plants as Promising Biopesticides against the Crop Pest Ceratitis capitata. Plants 2023, 12, 4122. https://doi.org/10.3390/plants12244122
Tavares WR, Jiménez IA, Oliveira L, Kuhtinskaja M, Vaher M, Rosa JS, Seca AML, Bazzocchi IL, Barreto MdC. Macaronesian Plants as Promising Biopesticides against the Crop Pest Ceratitis capitata. Plants. 2023; 12(24):4122. https://doi.org/10.3390/plants12244122
Chicago/Turabian StyleTavares, Wilson R., Ignacio A. Jiménez, Luísa Oliveira, Maria Kuhtinskaja, Merike Vaher, José S. Rosa, Ana M. L. Seca, Isabel L. Bazzocchi, and Maria do Carmo Barreto. 2023. "Macaronesian Plants as Promising Biopesticides against the Crop Pest Ceratitis capitata" Plants 12, no. 24: 4122. https://doi.org/10.3390/plants12244122
APA StyleTavares, W. R., Jiménez, I. A., Oliveira, L., Kuhtinskaja, M., Vaher, M., Rosa, J. S., Seca, A. M. L., Bazzocchi, I. L., & Barreto, M. d. C. (2023). Macaronesian Plants as Promising Biopesticides against the Crop Pest Ceratitis capitata. Plants, 12(24), 4122. https://doi.org/10.3390/plants12244122










