OsLEA1b Modulates Starch Biosynthesis at High Temperatures in Rice
Abstract
:1. Introduction
2. Results
2.1. OsLEA1b Encodes an Embryonic Protein Mainly Expressed in the Endosperm
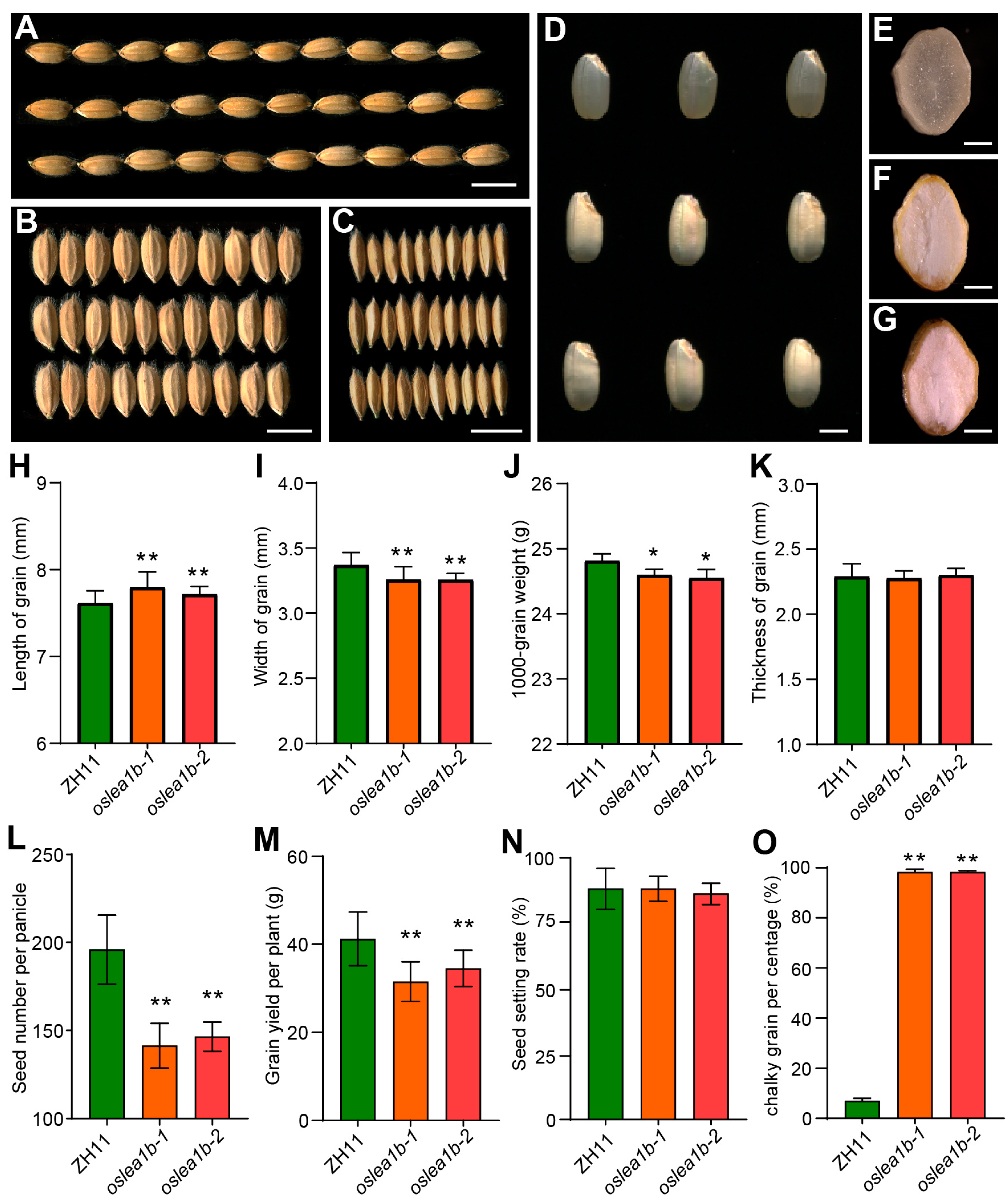
2.2. The oslea1b Mutants Exhibit Chalky Endosperm and Decreased Yield
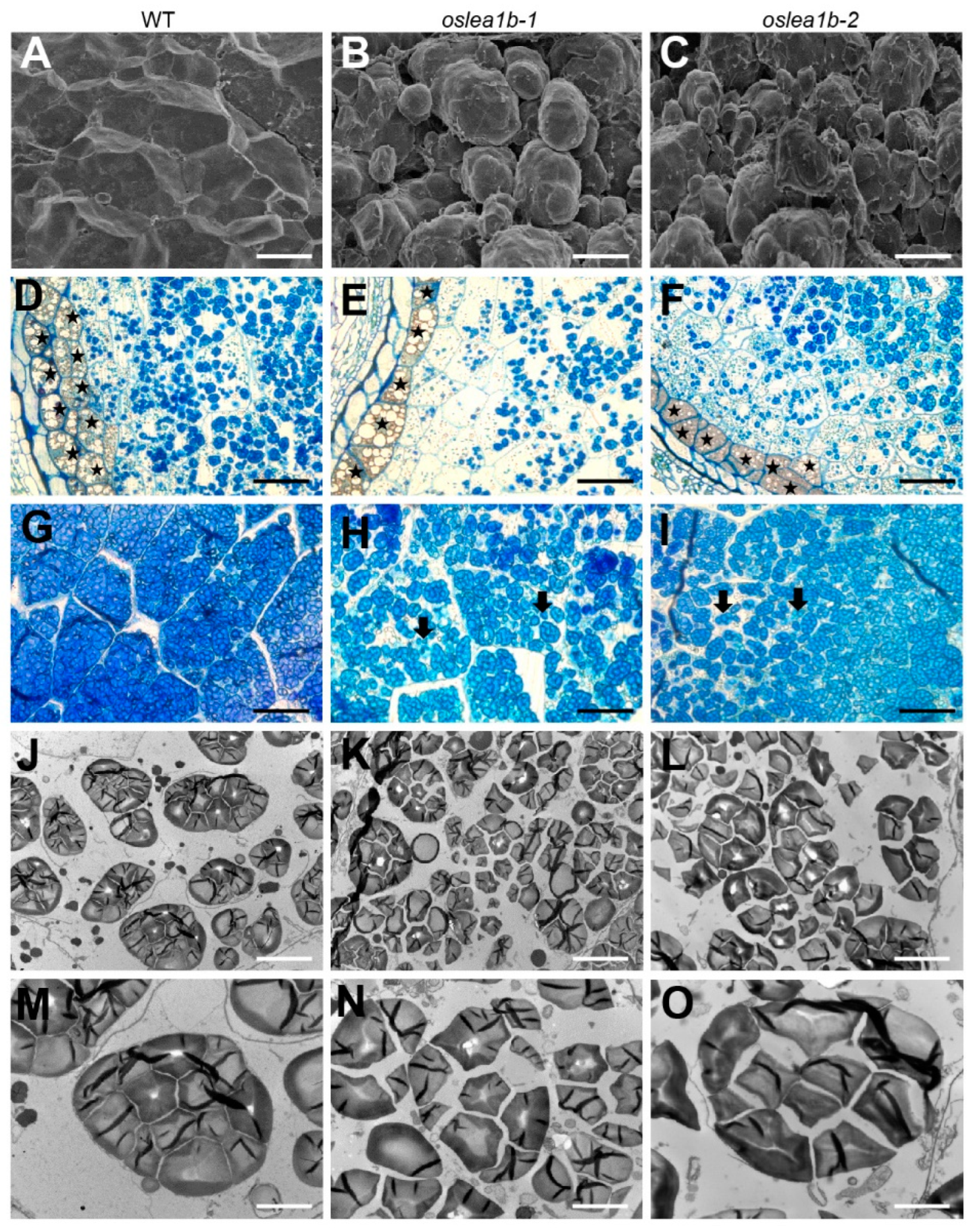
2.3. OsLEA1b Affects Starch Granule Development in the Endosperm
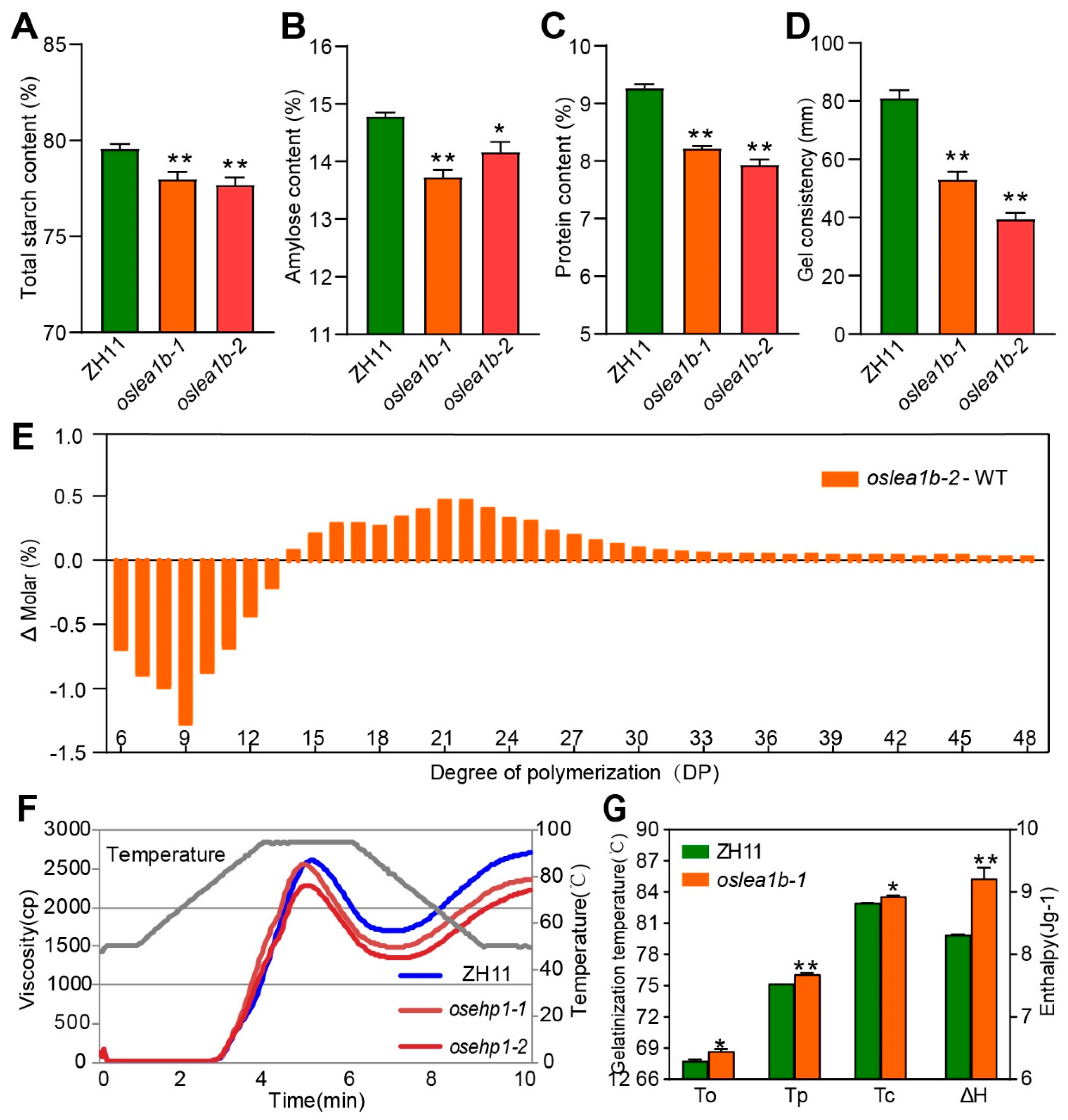
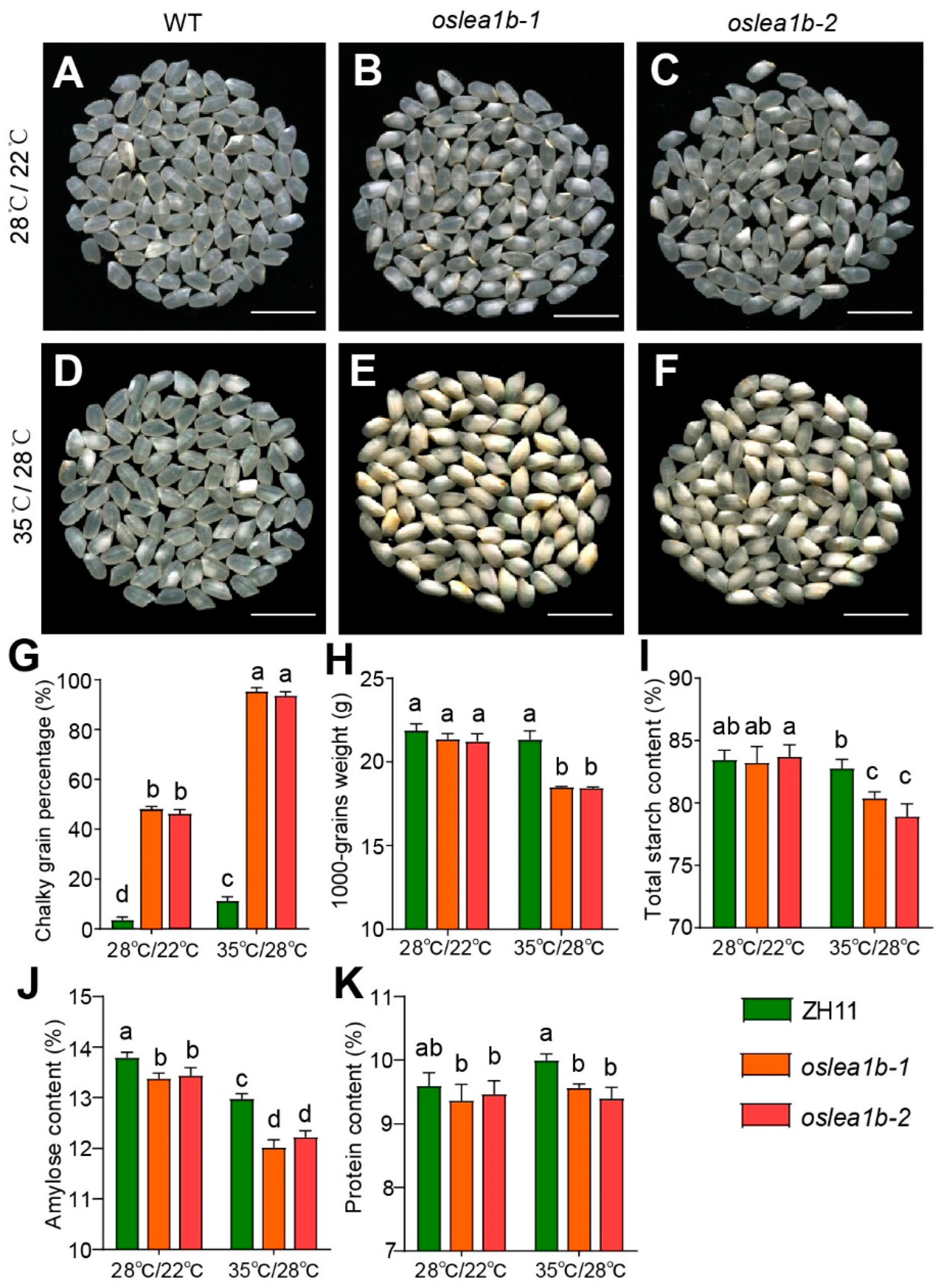
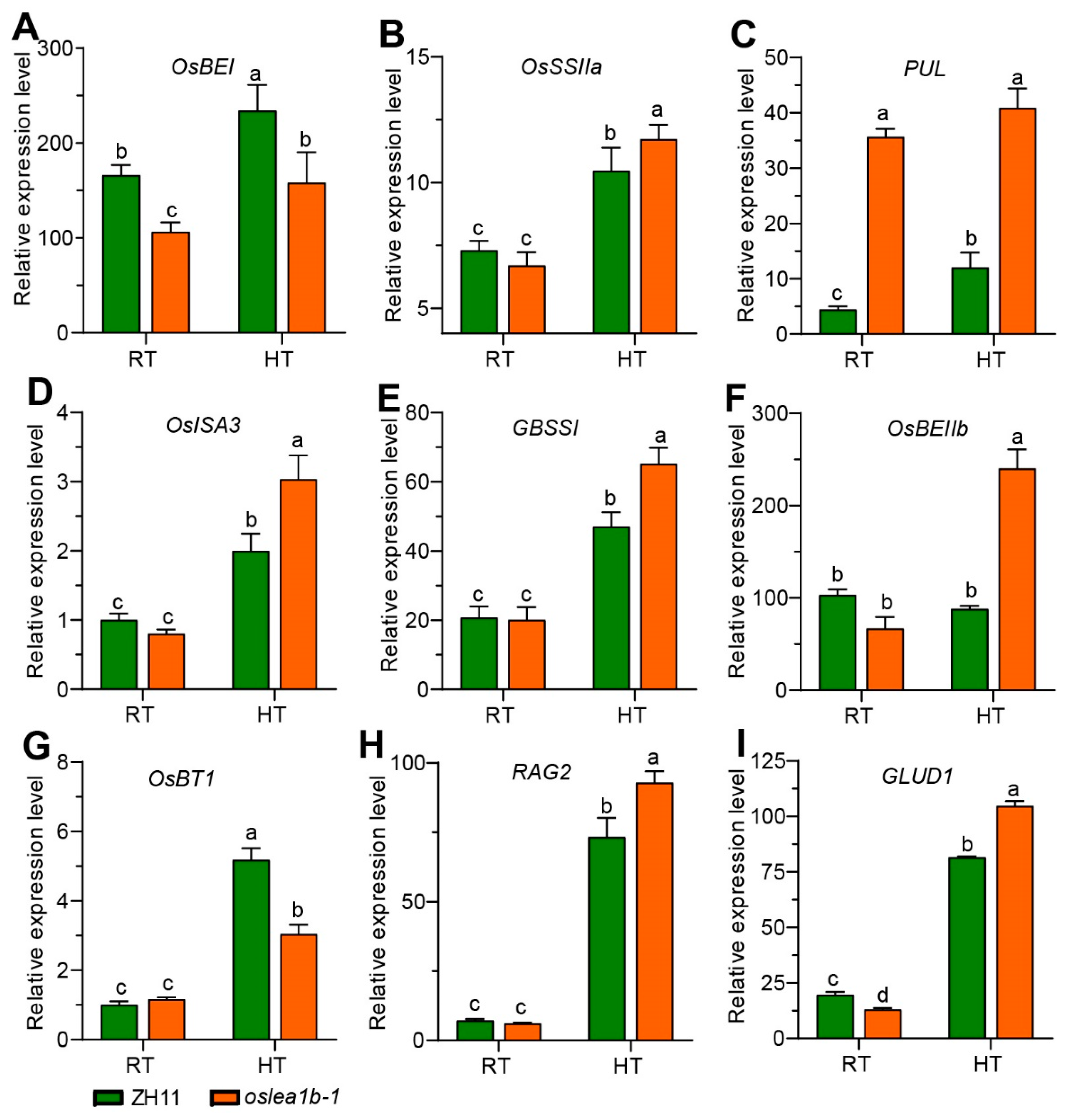
2.4. The oslea1b Mutants Showed Lower Grain Quality under High Temperature Conditions
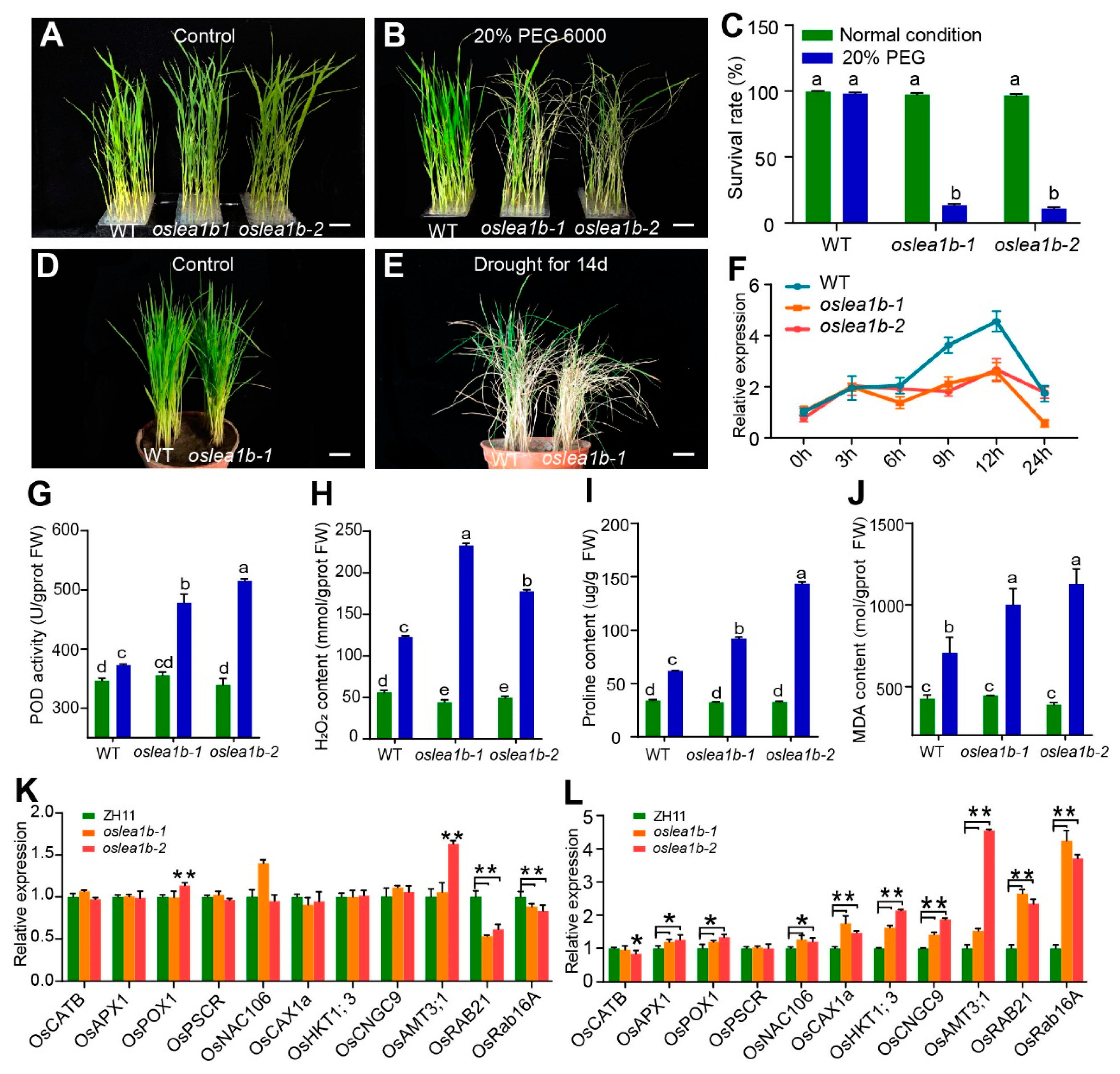
2.5. OsLEA1b Promotes Drought Tolerance
3. Discussion
3.1. The oslea1b Mutants Showed Greater Sensitivity to High Temperature
3.2. OsLEA1b Plays an Important Role in Rice Stress Responses
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Vector Construction and Plant Transformation
4.3. RNA Extraction, RT-qPCR Analysis, and GUS Staining
4.4. Subcellular Localization
4.5. Electron Microscopy
4.6. Analysis of Endosperm Starch Physicochemical Properties
4.7. Measurement of POD Activity and H2O2, Proline, and MDA Levels
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xiong, H.; Wang, W.; Sun, M. Endosperm development is an autonomously programmed process independent of embryogenesis. Plant Cell 2021, 33, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Matsukura, C.; Saitoh, T.; Hirose, T.; Ohsugi, R.; Perata, P.; Yamaguchi, J. Sugar uptake and transport in rice embryo. Expression of companion cell-specific sucrose transporter (OsSUT1) induced by sugar and light. Plant Physiol. 2000, 124, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Olsen, O. The Modular Control of Cereal Endosperm Development. Trends Plant Sci. 2020, 25, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Deschamps, P.; Haferkamp, I.; d’Hulst, C.; Neuhaus, H.E.; Ball, S.G. The relocation of starch metabolism to chloroplasts: When why and how. Trends Plant Sci. 2008, 13, 574–582. [Google Scholar] [CrossRef]
- Zhang, L.; Li, N.; Zhang, J.; Zhao, L.; Qiu, J.; Wei, C. The CBM48 domain-containing protein FLO6 regulates starch synthesis by interacting with SSIVb and GBSS in rice. Plant Mol. Biol. 2022, 108, 343–361. [Google Scholar] [CrossRef] [PubMed]
- Seung, D.; Soyk, S.; Coiro, M.; Maier, B.A.; Eicke, S.; Zeeman, S.C. PROTEIN TARGETING TO STARCH is required for localising GRANULE-BOUND STARCH SYNTHASE to starch granules and for normal amylose synthesis in Arabidopsis. PLoS Biol. 2015, 13, e1002080. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef]
- Pang, Y.; Hu, Y.; Bao, J. Comparative Phosphoproteomic Analysis Reveals the Response of Starch Metabolism to High-Temperature Stress in Rice Endosperm. Int. J. Mol. Sci. 2021, 22, 10546. [Google Scholar] [CrossRef]
- Tabassum, R.; Dosaka, T.; Ichida, H.; Morita, R.; Ding, Y.; Abe, T.; Katsube Tanaka, T. FLOURY ENDOSPERM11-2 encodes plastid HSP70-2 involved with the temperature-dependent chalkiness of rice (Oryza sativa L.) grains. Plant J. Cell Mol. Biol. 2020, 103, 604–616. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, H.; Feng, M.; Zhu, Y. Suppression of OsMADS7 in rice endosperm stabilizes amylose content under high temperature stress. Plant Biotechnol. J. 2018, 16, 18–26. [Google Scholar] [CrossRef]
- Hakata, M.; Kuroda, M.; Miyashita, T.; Yamaguchi, T.; Kojima, M.; Sakakibara, H.; Mitsui, T.; Yamakawa, H. Suppression of alpha-amylase genes improves quality of rice grain ripened under high temperature. Plant Biotechnol. J. 2012, 10, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Duan, L.; Dai, J.S.; Zhang, C.Q.; Li, J.; Gu, M.H.; Liu, Q.Q.; Zhu, Y. Major QTLs reduce the deleterious effects of high temperature on rice amylose content by increasing splicing efficiency of Wx pre-mRNA. Theor. Appl. Genet. 2014, 127, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Nie, D.M.; Ouyang, Y.D.; Wang, X.; Zhou, W.; Hu, C.G.; Yao, J. Genome-wide analysis of endosperm-specific genes in rice. Gene 2013, 530, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lai, Y.; Wu, X.; Wu, G.; Guo, C. Overexpression of OsEm1 encoding a group I LEA protein confers enhanced drought tolerance in rice. Biochem. Biophys. Res. Commun. 2016, 478, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Hundertmark, M.; Hincha, D.K. LEA (late embryogenesis abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genom. 2008, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; You, J.; Xie, K.; Xie, W.; Xiong, L. Systematic sequence analysis and identification of tissue-specific or stress-responsive genes of NAC transcription factor family in rice. Mol. Genet. Genom. 2008, 280, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Cai, W.; Park, S. OsLEA3-2, an abiotic stress induced gene of rice plays a key role in salt and drought tolerance. PLoS ONE 2012, 7, e45117. [Google Scholar] [CrossRef]
- Lin, C.; Li, C.; Lin, S.; Yang, F.; Huang, J.; Liu, Y.; Lur, H. Influence of High Temperature during Grain Filling on the Accumulation of Storage Proteins and Grain Quality in Rice (Oryza sativa L.). J. Agric. Food Chem. 2010, 58, 10545–10552. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, Y.; Yuan, J.; Xing, C. Grain quality evaluation of japonica rice effected by cultivars, environment, and their interactions based on appearance and processing characteristics. Food Sci. Nutr. 2021, 9, 2129–2138. [Google Scholar] [CrossRef]
- Misra, G.; Anacleto, R.; Badoni, S.; Butardo, V.; Molina, L.; Graner, A.; Demont, M.; Morell, M.K.; Sreenivasulu, N.; Rebetzke, G. Dissecting the genome-wide genetic variants of milling and appearance quality traits in rice. J. Exp. Bot. 2019, 70, 5115–5130. [Google Scholar] [CrossRef]
- Gao, B.; Hu, S.; Jing, L.; Wang, Y.; Zhu, J.; Wang, K.; Li, H.; Sun, X.; Wang, Y.; Yang, L. Impact of Elevated CO2 and Reducing the Source-Sink Ratio by Partial Defoliation on Rice Grain Quality–A 3-Year Free-Air CO2 Enrichment Study. Front. Plant Sci. 2021, 12, 788104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, L.; Zhu, Z.; Lu, H.; Zhou, X.; Qian, Y.; Li, Q.; Lu, Y.; Gu, M.; Liu, Q. Characterization of Grain Quality and Starch Fine Structure of Two Japonica Rice (Oryza sativa) Cultivars with Good Sensory Properties at Different Temperatures during the Filling Stage. J. Agric. Food Chem. 2016, 64, 4048–4057. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, X.; Li, Z.; Li, Y.; Chen, K.; Wu, L.; Fa, Y.; Xu, Z.; Xu, Q. Effects of Genetic Background and Environmental Conditions on Amylopectin Chain-Length Distribution in a Recombinant Inbred Line of an Inter-subspecies Rice Cross. J. Agric. Food Chem. 2020, 68, 7444–7452. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chu, C.; Yao, S. The impact of high-temperature stress on rice: Challenges and solutions. Crop J. 2021, 9, 963–976. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, S.; Jiang, L.; Li, W.; Tang, Y.; He, W.; Wang, M.; Xing, J.; Cui, Y.; Lin, Q.; et al. Transcription factor OsSGL is a regulator of starch synthesis and grain quality in rice. J. Exp. Bot. 2022, 73, 3417–3430. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Tan, H.; Zhang, C.; Li, Q.; Liu, Q. Starch biosynthesis in cereal endosperms: An updated review over the last decade. Plant Commun. 2021, 2, 100237. [Google Scholar] [CrossRef]
- Zhang, Z.; Tappiban, P.; Ying, Y.; Hu, Y.; Bao, J. Functional Interactions between Enzymes Involved in Amylose and Amylopectin Biosynthesis in Rice Based on Mathematical Models. Biomacromolecules 2022, 23, 1443–1452. [Google Scholar] [CrossRef]
- Crofts, N.; Iizuka, Y.; Abe, N.; Miura, S.; Kikuchi, K.; Matsushima, R.; Fujita, N. Rice Mutants Lacking Starch Synthase I or Branching Enzyme IIb Activity Altered Starch Biosynthetic Protein Complexes. Front. Plant Sci. 2018, 9, 1817. [Google Scholar] [CrossRef]
- Peng, Y.; Mao, B.; Zhang, C.; Shao, Y.; Wu, T.; Hu, L.; Hu, Y.; Tang, L.; Li, Y.; Tang, W.; et al. Influence of physicochemical properties and starch fine structure on the eating quality of hybrid rice with similar apparent amylose content. Food Chem. 2021, 353, 129461. [Google Scholar] [CrossRef]
- Magwanga, R.O.; Lu, P.; Kirungu, J.N.; Lu, H.; Wang, X.; Cai, X.; Zhou, Z.; Zhang, Z.; Salih, H.; Wang, K.; et al. Characterization of the late embryogenesis abundant (LEA) proteins family and their role in drought stress tolerance in upland cotton. BMC Genet. 2018, 19, 6. [Google Scholar] [CrossRef]
- Charfeddine, S.; Saïdi, M.N.; Charfeddine, M.; Gargouri-Bouzid, R. Genome-wide identification and expression profiling of the late embryogenesis abundant genes in potato with emphasis on dehydrins. Mol. Biol. Rep. 2015, 42, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Amara, I.; Odena, A.; Oliveira, E.; Moreno, A.; Masmoudi, K.; Pagès, M.; Goday, A. Insights into Maize LEA Proteins: From Proteomics to Functional Approaches. Plant Cell Physiol. 2012, 53, 312–329. [Google Scholar] [CrossRef] [PubMed]
- Kamarudin, Z.S.; Yusop, M.R.; Ismail, M.R.; Tengku, M.M.M.; Harun, A.R.; Yusuff, O.; Magaji, U.; Fatai, A. LEA Gene Expression Assessment in Advanced Mutant Rice Genotypes under Drought Stress. Int. J. Genom. 2019, 2019, 8406036. [Google Scholar] [CrossRef] [PubMed]
- Knox-Brown, P.; Rindfleisch, T.; Gunther, A.; Balow, K.; Bremer, A.; Walther, D.; Miettinen, M.S.; Hincha, D.K.; Thalhammer, A. Similar Yet Different-Structural and Functional Diversity among Arabidopsis thaliana LEA_4 Proteins. Int. J. Mol. Sci. 2020, 21, 2794. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xie, L.; Liang, X.; Zhang, S. CpLEA5, the Late Embryogenesis Abundant Protein Gene from Chimonanthus praecox, Possesses Low Temperature and Osmotic Resistances in Prokaryote and Eukaryotes. Int. J. Mol. Sci. 2015, 16, 26978–26990. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, Y.; Zhang, Y.; Wang, W.; Li, R. Soybean PM2 Protein (LEA3) Confers the Tolerance of Escherichia coli and Stabilization of Enzyme Activity Under Diverse Stresses. Curr. Microbiol. 2010, 60, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xu, H.; Zhang, L.; Zheng, Y. Fe Binding Properties of Two Soybean (Glycine max L.) LEA4 Proteins Associated with Antioxidant Activity. Plant Cell Physiol. 2011, 52, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Olvera-Carrillo, Y.; Campos, F.; Reyes, J.L.; Garciarrubio, A.; Covarrubias, A.A. Functional Analysis of the Group 4 Late Embryogenesis Abundant Proteins Reveals Their Relevance in the Adaptive Response during Water Deficit in Arabidopsis. Plant Physiol. 2010, 154, 373–390. [Google Scholar] [CrossRef]
- Aduse Poku, S.; Nkachukwu Chukwurah, P.; Aung, H.H.; Nakamura, I. Over-Expression of a Melon Y3SK2-Type LEA Gene Confers Drought and Salt Tolerance in Transgenic Tobacco Plants. Plants 2020, 9, 1749. [Google Scholar] [CrossRef]
- Imran, S.; Tsuchiya, Y.; Tran, S.; Katsuhara, M. Identification and Characterization of Rice OsHKT1;3 Variants. Plants 2021, 10, 2006. [Google Scholar] [CrossRef]
- Piao, W.; Kim, S.; Lee, B.; An, G.; Sakuraba, Y.; Paek, N. Rice transcription factor OsMYB102 delays leaf senescence by down-regulating abscisic acid accumulation and signaling. J. Exp. Bot. 2019, 70, 2699–2715. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, H.; Cho, Y.; Kim, S. Cold-Responsive Regulation of a Flower-Preferential Class III Peroxidase Gene, OsPOX1, in Rice (Oryza sativa L.). J. Plant Biol. Singmul Hakhoe Chi 2012, 55, 123–131. [Google Scholar] [CrossRef]
- Nagar, P.; Kumar, A.; Jain, M.; Kumari, S.; Mustafiz, A.; Prasad, M. Genome-wide analysis and transcript profiling of PSKR gene family members in Oryza sativa. PLoS ONE 2020, 15, e236349. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, M.; Roychoudhury, A.; Sengupta, D.N.; Datta, S.K.; Datta, K. Independent overexpression of OsRab16A and AtDREB1A exhibit enhanced drought tolerance in transgenic aromatic rice variety Pusa Sugandhi 2. J. Plant Biochem. Biot. 2020, 29, 503–517. [Google Scholar] [CrossRef]
- Huang, S.; Hu, L.; Zhang, S.; Zhang, M.; Jiang, W.; Wu, T.; Du, X. Rice OsWRKY50 Mediates ABA-Dependent Seed Germination and Seedling Growth, and ABA-Independent Salt Stress Tolerance. Int. J. Mol. Sci. 2021, 22, 8625. [Google Scholar] [CrossRef] [PubMed]
- Sakuraba, Y.; Piao, W.; Lim, J.; Han, S.; Kim, Y.; An, G.; Paek, N. Rice ONAC106 Inhibits Leaf Senescence and Increases Salt Tolerance and Tiller Angle. Plant Cell Physiol. 2015, 56, 2325–2339. [Google Scholar] [CrossRef]
- Sheng, C.; Yu, D.; Li, X.; Yu, H.; Zhang, Y.; Saqib Bilal, M.; Ma, H.; Zhang, X.; Baig, A.; Nie, P.; et al. OsAPX1 Positively Contributes to Rice Blast Resistance. Front. Plant Sci. 2022, 13, 843271. [Google Scholar] [CrossRef]
- Ye, N.; Zhu, G.; Liu, Y.; Li, Y.; Zhang, J. ABA controls H2O2 accumulation through the induction of OsCATB in rice leaves under water stress. Plant Cell Physiol. 2011, 52, 689–698. [Google Scholar] [CrossRef]
- Kamiya, T.; Akahori, T.; Ashikari, M.; Maeshima, M. Expression of the vacuolar Ca2+/H+ exchanger, OsCAX1a, in rice: Cell and age specificity of expression, and enhancement by Ca2+. Plant Cell Physiol. 2006, 47, 96–106. [Google Scholar] [CrossRef]
- Wang, J.; Ren, Y.; Liu, X.; Luo, S.; Zhang, X.; Liu, X.; Lin, Q.; Zhu, S.; Wan, H.; Yang, Y.; et al. Transcriptional activation and phosphorylation of OsCNGC9 confer enhanced chilling tolerance in rice. Mol. Plant 2021, 14, 315–329. [Google Scholar] [CrossRef]
- Ma, C.; Li, Q.; Song, Z.; Su, L.; Tao, W.; Zhou, B.; Wang, Q. Irrigation with Magnetized Water Alleviates the Harmful Effect of Saline–Alkaline Stress on Rice Seedlings. Int. J. Mol. Sci. 2022, 23, 10048. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Zhao, S.; Jiao, G.; Duan, Y.; Ma, L.; Dong, N.; Lu, F.; Zhu, M.; Shao, G.; Hu, S.; et al. OPAQUE3, encoding a transmembrane bZIP transcription factor, regulates endosperm storage protein and starch biosynthesis in rice. Plant Commun. 2022, 3, 100463. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tao, L.; Zeng, L.; Vega-Sanchez, M.E.; Umemura, K.; Wang, G.L. A highly efficient transient protoplast system for analyzing defence gene expression and protein-protein interactions in rice. Mol. Plant Pathol. 2006, 7, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Park, S.; Matsuoka, M.; An, G. White-core endosperm floury endosperm-4 in rice is generated by knockout mutations in the C-type pyruvate orthophosphate dikinase gene (OsPPDKB). Plant J. 2005, 42, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Wang, Y.; Liu, F.; Ren, Y.; Zhou, K.; Lv, J.; Zheng, M.; Zhao, S.; Zhang, L.; Wang, C.; et al. FLOURY ENDOSPERM6 encodes a CBM48 domain-containing protein involved in compound granule formation and starch synthesis in rice endosperm. Plant J. Cell Mol. Biol. 2014, 77, 917–930. [Google Scholar] [CrossRef]
- Li, S.; Wei, X.; Ren, Y.; Qiu, J.; Jiao, G.; Guo, X.; Tang, S.; Wan, J.; Hu, P. OsBT1 encodes an ADP-glucose transporter involved in starch synthesis and compound granule formation in rice endosperm. Sci. Rep. 2017, 7, 40124. [Google Scholar] [CrossRef]
- Tan, Y.F.; Li, J.X.; Yu, S.B.; Xing, Y.Z.; Xu, C.G.; Zhang, Q. Three important traits for cooking and eating quality of rice grains are controlled by a single locus in an elite rice hybrid, Shanyou 63. Theor. Appl. Genet. 1999, 99, 642–648. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Cao, R.; Ma, L.; Jiao, G.; Chen, P.; Dong, N.; Li, X.; Duan, Y.; Li, X.; Zhu, M.; et al. OsLEA1b Modulates Starch Biosynthesis at High Temperatures in Rice. Plants 2023, 12, 4070. https://doi.org/10.3390/plants12234070
Li G, Cao R, Ma L, Jiao G, Chen P, Dong N, Li X, Duan Y, Li X, Zhu M, et al. OsLEA1b Modulates Starch Biosynthesis at High Temperatures in Rice. Plants. 2023; 12(23):4070. https://doi.org/10.3390/plants12234070
Chicago/Turabian StyleLi, Gang, Ruijie Cao, Liuyang Ma, Guiai Jiao, Pengfei Chen, Nannan Dong, Xinwei Li, Yingqing Duan, Xiaoxue Li, Mingdong Zhu, and et al. 2023. "OsLEA1b Modulates Starch Biosynthesis at High Temperatures in Rice" Plants 12, no. 23: 4070. https://doi.org/10.3390/plants12234070
APA StyleLi, G., Cao, R., Ma, L., Jiao, G., Chen, P., Dong, N., Li, X., Duan, Y., Li, X., Zhu, M., Shao, G., Sheng, Z., Hu, S., Tang, S., Wei, X., Yu, Y., & Hu, P. (2023). OsLEA1b Modulates Starch Biosynthesis at High Temperatures in Rice. Plants, 12(23), 4070. https://doi.org/10.3390/plants12234070







