Relict Plants Are Better Able to Adapt to Climate Change: Evidence from Desert Shrub Communities
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Species Distribution Survey
| Family | Species | Habitat and Distribution | Ecological Traits | |
|---|---|---|---|---|
| relict plants | Zygophyllaceae | Sarcozygium xanthoxylon | Deserts, steppe deserts and desertified grassland areas | Drought resistance |
| Nitrariaceae | Nitraria tangutorum | Desert and semidesert lake basin sands | Salt-tolerant, sand fixation | |
| Tamaricaceae | Reaumuria songarica | Deserts and desert steppe zones | Drought resistance, salt tolerance, sand collection | |
| Zygophyllaceae | Tetraena mongolica | Steppe desert, Yellow River terrace, steppe desert area | Drought tolerance | |
| Leguminosae | Ammopiptanthus mongolicus | Sandy and gravelly textures in desert areas | Super xerophytic structure, strong stress resistance | |
| typical desert plants | Rosaceae | Amygdalus mongolica | Gobi Desert Region in Central Asia | Drought tolerance, cold hardiness |
| Chenopodiaceae | Kalidium foliatum | Wet, fluffy saline soils of deserts, desert steppes and grasslands in Eurasia | Salt tolerance | |
| Compositae | Artemisia ordosica | Sandy areas of steppe, desert steppe to steppe deserts | Wind erosion resistance, sand burial resistance | |
| Chenopodiaceae | Halocnemum strobilaceum | Southern Europe, Western and Northern Asia and Northern Africa | Salt tolerance | |
| Convolvulaceae | Convolvulus tragacanthoides | Dry slopes in semidesert areas and mountain basins | Drought resistance |
2.2. Data Analysis
2.2.1. Species Distribution Model
- (1)
- Abiotic factors
- (2)
- Biological factors.
2.2.2. Climate Change Scenario
3. Results
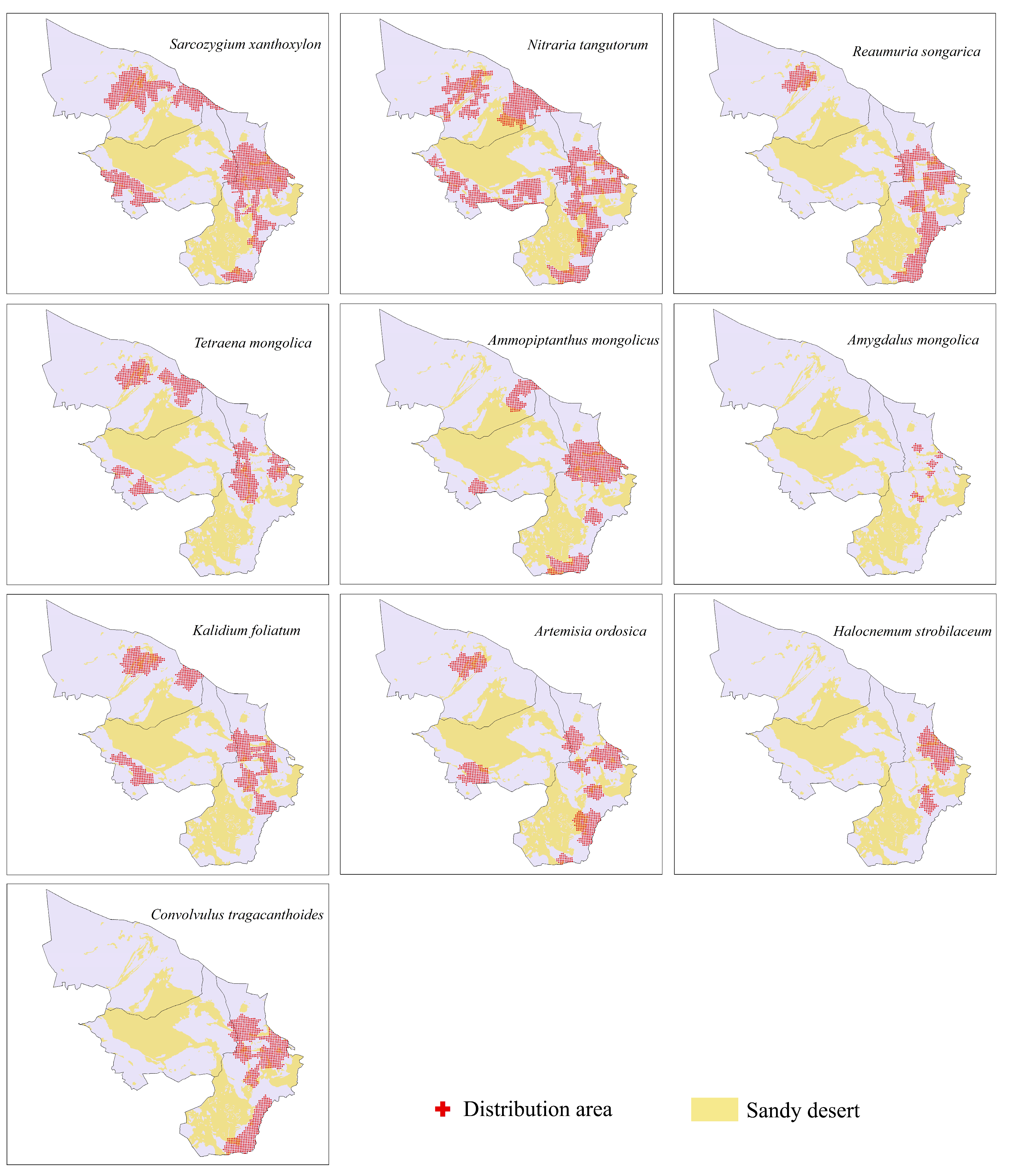
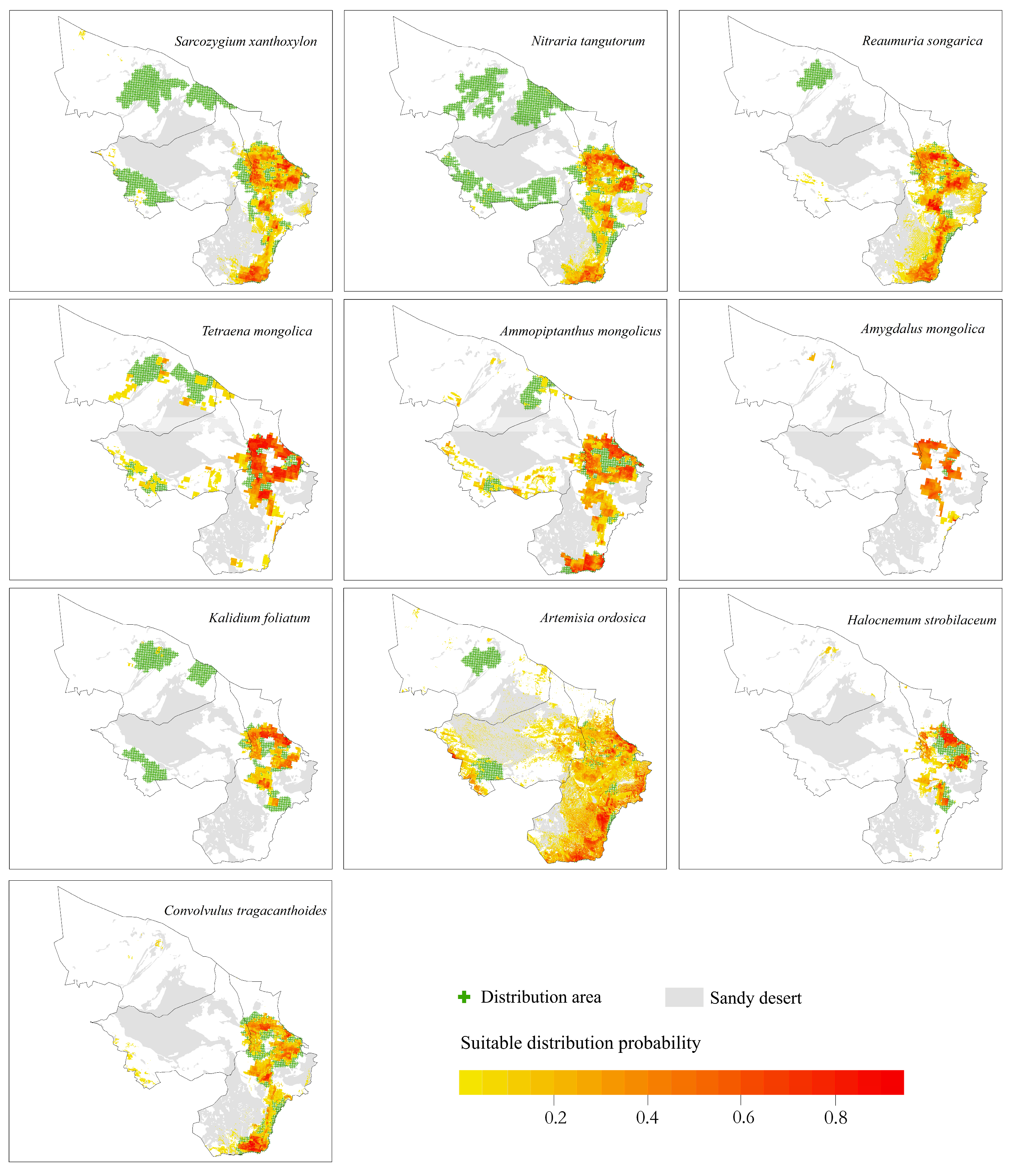
3.1. Shrub Species Distribution
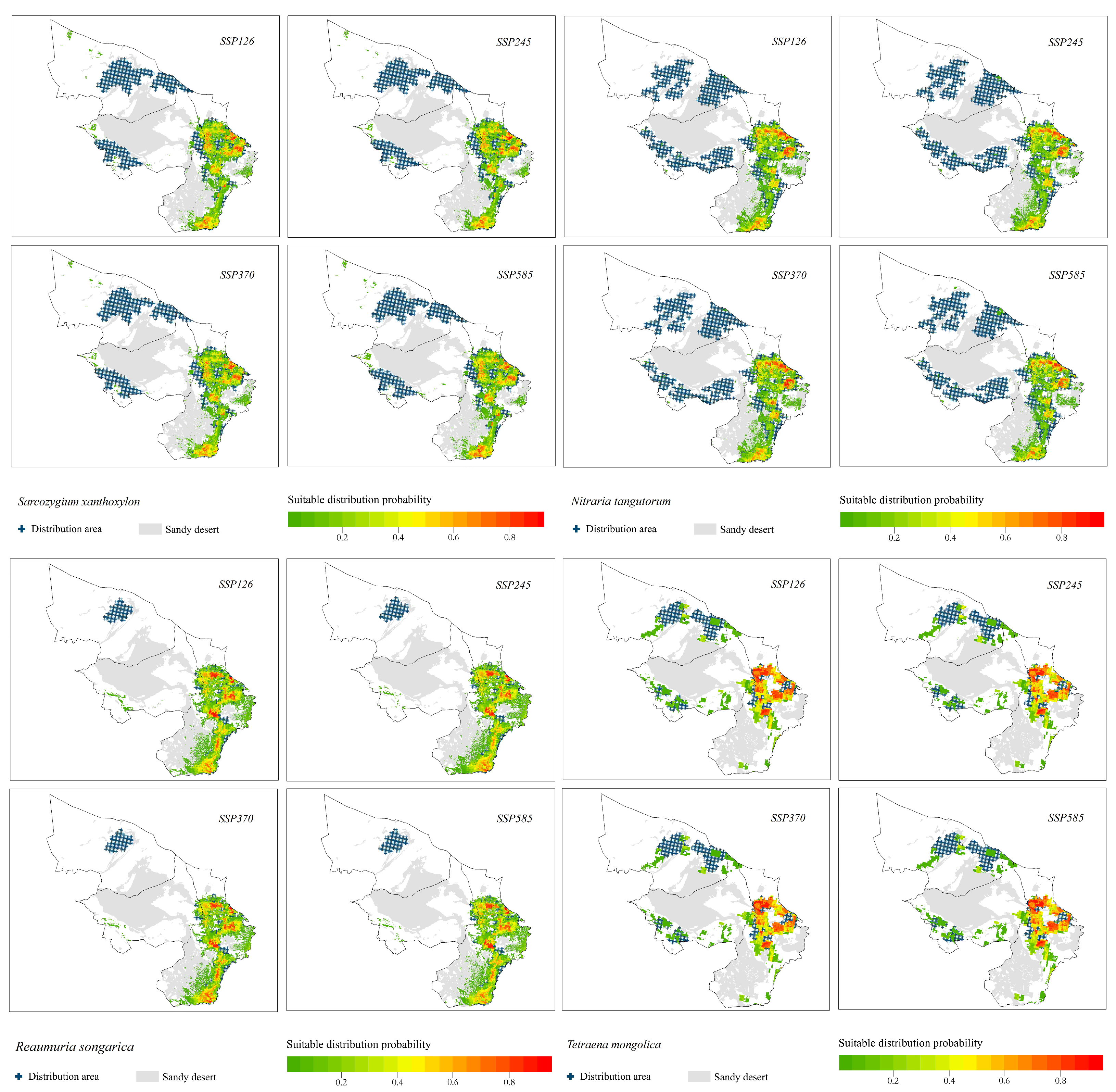
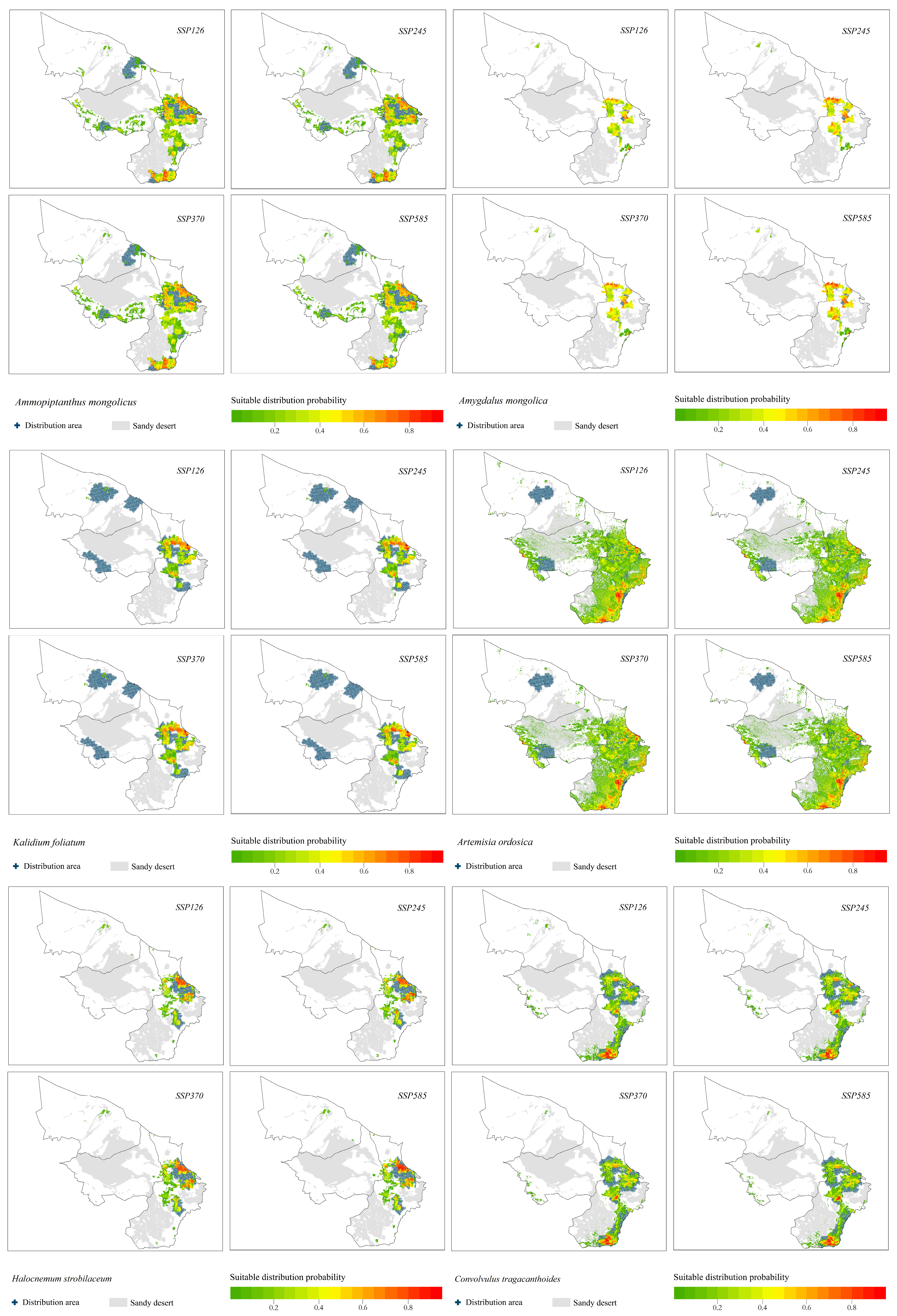
3.2. Prediction of Shrub Species Distribution under Different Climate Change Scenarios
- (1)
- The potential distribution areas of the relict plants N. tangutorum, S. xanthoxylon and R. songarica were mainly in the southeastern part of the study area, among which N. tangutorum had a higher probability of distribution in the eastern part of the study area. The overall suitable area of the S. xanthoxylon was biased toward the middle of the study area; the suitable distribution area of R. songarica in the southern part of the study area was large, and it was similar to the suitable area of S. xanthoxylon, which was related to the interaction between the species.
- (2)
- The endangered relict plant T. mongolica was mainly distributed in the east and north of the study area, and the distribution probability in the north was low.
- (3)
- The endangered relict plant A. mongolicus was mainly distributed in the central and eastern parts of the study area, and the distribution probability in the central part was low.
- (1)
- The typical desert plant A. mongolica was concentrated in the eastern part of the study area, and very few areas showed a high probability distribution.
- (2)
- H. strobilaceum and K. foliatum were distributed in the southeastern part of the study area, and the distribution area was scattered.
- (3)
- The suitable distribution area of A. ordosica was large, and it was often distributed in the eastern boundary of the study area.
- (4)
- The suitable distribution area of C. tragacanthoides was located in the southeast of the study area, and the distribution probability was high in the south.
- (1)
- The suitable distribution areas of the relict plants S. xanthoxylon, N. tangutorum and R. songarica under the four climate change scenarios were all concentrated in the southeastern part of the study area, which was consistent with the current distribution pattern of each species, but there was a trend toward a shift to the southern part of the study area.
- (2)
- The suitable distribution area of the relict plant T. mongolica was located in the north and east of the study area, and the suitable area in the north was facing the risk of contraction.
- (3)
- The suitable area of the relict plant A. mongolicus was located in the middle and east of the study area. The distribution probability in the middle was low, and the distribution patterns under the four climate scenarios were basically the same.
- (1)
- The suitable distribution area of A. mongolica, a typical desert plant, changed significantly under the four climate change scenarios. The suitable distribution areas under the SSP126, SSP370 and SSP585 climate change scenarios were located in the northern and eastern parts of the study area. The distribution area in the northern part of the study area disappeared under the SSP245 climate change scenario.
- (2)
- The suitable distribution areas of H. strobilaceum and K. foliatum were located in the southeastern part of the study area.
- (3)
- The probability of distribution of C. tragacanthoides in the eastern part of the study area was higher.
- (4)
- The suitable distribution area of A. ordosica was located in the middle and south of the study area.
3.3. Differences in Tolerance of Different Shrub Species to Climate Change
4. Discussion
4.1. Climate Change Is Not the Main Limiting Factor for the Distribution of Relict Plants
4.2. There Is a General Positive Correlation between Relict Shrub Species
4.3. Relict Shrub Species Have Developed Rich Ecological Strategies in Their Long Evolutionary History
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhat, I.A.; Fayaz, M.; Rafiq, S.; Guleria, K.; Qadir, J.; Wani, T.A.; Kaloo, Z.A. Predicting potential distribution and range dynamics of Aquilegia fragrans under climate change: Insights from ensemble species distribution modelling. Environ. Monit. Assess. 2023, 195, 623. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.F.; Wang, S.H.; Feng, J.W.; Ge, J.P.; Wang, T.M. Free-ranging livestock changes the acoustic properties of summer soundscapes in a Northeast Asian temperate forest. Biol. Conserv. 2023, 283, 110123. [Google Scholar] [CrossRef]
- Fausett, S.R.; Sandjak, A.; Billard, B.; Braendle, C. Higher-order epistasis shapes natural variation in germ stem cell niche activity. Nat. Commun. 2023, 14, 2824. [Google Scholar] [CrossRef] [PubMed]
- Varol, T.; Cetin, M.; Ozel, H.B.; Sevik, H.; Zeren Cetin, I. The Effects of Climate Change Scenarios on Carpinus betulus and Carpinus orientalis in Europe. Water Air Soil Pollut. 2022, 233, 45. [Google Scholar] [CrossRef]
- IPCC Sixth Assessment Report—Climate Change 2023 [EB/OL]. Available online: https://www.ipcc.ch/ (accessed on 26 May 2023).
- Urban, M.C. Climate change. Accelerating extinction risk from climate change. Science 2015, 348, 571–573. [Google Scholar] [CrossRef]
- Canturk, U.; Kulaç, S. The effects of climate change scenarios on Tilia ssp. in Turkey. Environ. Monit. Assess. 2021, 193, 771. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Meng, Y.H.; Xu, X.; Jiang, X.L.; Xu, G.B. Potential distribution modeling and analysis of Disanthus Maxim. Acta Ecol. Sin. 2019, 39, 2816–2825. [Google Scholar]
- Wu, J.G.; Lü, J.J.; Zhou, Q.F. Potential Effects of Climate Change on the Distribution of Six Desert Plants in China. Chin. Bull. Bot. 2010, 45, 723–738. [Google Scholar]
- Wan, J.Z.; Wang, C.J.; Han, S.J.; Yu, J.H. The Planning of Priority Protection Area for Taxus cuspidata under Climate Change. J. Shenyang Agric. Univ. 2014, 45, 28–32. [Google Scholar]
- Tao, C.; Li, X.X.; Wang, Q.C.; Cui, G.F. Relationships between Geographical Distribution of Endangered Pinus kwangtungensis and Climate in China. Plant Sci. J. 2012, 30, 577–583. [Google Scholar] [CrossRef]
- Shen, Y.F.; Tu, Z.H.; Zhang, Y.L.; Zhong, W.P.; Xia, H.; Hao, Z.Y.; Zhang, C.G.; Li, H.G. Predicting the impact of climate change on the distribution of two relict Liriodendron species by coupling the MaxEnt model and actual physiological indicators in relation to stress tolerance. J. Environ. Manag. 2022, 322, 116024. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.Y.; Lu, K.; Du, Z.Y.; Shi, J.G.; Chai, G.Q.; Zhang, Y.; Lei, K.Y.; Duan, Y.Z. Potential changes in the geographical distribution of the relict plant Polaninia mongolica Maxim. in China under climate change scenarios. Acta Ecol. Sin. 2022, 42, 4473–4484. [Google Scholar]
- Tan, X.; Zhang, L.; Zhang, A.P.; Wang, Y.; Huang, D.; Wu, X.G.; Sun, X.M.; Xiong, Q.L.; Pan, K.W. The suitable distribution area of Tsuga longibracteata revealed by a climate and spatial constraint model under future climate change scenarios. Acta Ecol. Sin. 2018, 38, 8934–8945. [Google Scholar]
- Ran, Q.; Wei, H.Y.; Zhao, Z.F.; Zhang, Q.Z.; Liu, J.; Gu, W. Impact of climate change on the potential distribution and habitat fragmentation of the relict plant Cathaya argyrophylla Chun et Kuang. Acta Ecol. Sin. 2019, 39, 2481–2493. [Google Scholar]
- Zhao, Z.F.; Wei, H.Y.; Guo, Y.L.; Luan, W.F.; Zhao, Z.B. Impact of climate change on the suitable habitat distribution of Gymnocarpos przewalskii, a relict plant. Desert China 2020, 40, 125–133. [Google Scholar]
- Zhai, X.Y.; Shen, Y.F.; Zhu, S.H.; Tu, Z.H.; Zhang, C.G.; Li, H.G. Potential Impacts of Climate Change in Future on the Geographical Distributions of Relic Liriodendron chinense. J. Trop. Subtrop. Bot. 2021, 29, 151–161. [Google Scholar]
- Shang, K.K.; Chen, B.; Da, L.J. Population structure and regeneration strategy of relict deciduous broadleaved trees on Mount Tianmu, Zhejiang Province, China. Chin. J. Appl. Ecol. 2018, 29, 361–368. [Google Scholar]
- Tang, C.Q.; Yang, Y.; Ohsawa, M.; Momohara, A.; Hara, M.; Cheng, S.; Fan, S. Population structure of relict Metasequoia glyptostroboides and its habitat fragmentation and degradation in south-central China. Biol. Conserv. 2011, 144, 279–289. [Google Scholar] [CrossRef]
- Tang, C.Q.; Yang, Y.; Ohsawa, M.; Yi, S.R.; Momohara, A.; Su, W.H.; Wang, H.C.; Zhang, Z.Y.; Peng, M.C.; Wu, Z.L. Evidence for the persistence of wild Ginkgo biloba (Ginkgoaceae) populations in the Dalou Mountains, southwestern China. Am. J. Bot. 2012, 99, 1408–1414. [Google Scholar] [CrossRef]
- Tang, C.Q.; Yang, Y.; Ohsawa, M.; Momohara, A.; Mu, J.; Robertson, K. Survival of a tertiary relict species, Liriodendron chinense (Magnoliaceae), in southern China, with special reference to village Fengshui forest. Am. J. Bot. 2013, 100, 2112–2119. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.Q.; Peng, M.C.; He, L.Y.; Ohsawa, M.; Wang, C.Y.; Xie, T.H.; Li, W.S.; Li, J.P.; Zhang, H.Y.; Li, Y.; et al. Population persistence of a Tertiary relict tree Tetracentron sinense on the Ailao Mountains, Yunnan, China. J. Plant Res. 2013, 126, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.Q.; Ohsawa, M. Tertiary relic deciduous forests on a humid subtropical mountain, Mt. Emei, Sichuan, China. Folia Geobot. 2002, 37, 93–106. [Google Scholar] [CrossRef]
- He, L.Y.; Tang, C.Q.; Wu, Z.L.; Wang, H.C.; Ohsawa, M.; Yan, K. Forest structure and regeneration of the Tertiary relict Taiwania cryptomerioides in the Gaoligong Mountains, Yunnan, southwestern China. Phytocoenologia 2015, 45, 135–156. [Google Scholar] [CrossRef]
- González, G.; Begoña, M. Life history and population size variability in a relict plant. Different routes towards long-term persistence. Divers. Distrib. 2008, 14, 106–113. [Google Scholar]
- Zhou, Z.Y.; Yan, S.Y.; Qin, Y.; Zou, L.N. The characters of shrubby diversity of Alxa arid desert region. Joumal Arid. Land Resour. Environ. 2009, 23, 146–150. [Google Scholar]
- Zhi, Y.B.; Li, J.M.; Wang, Z.L.; Han, X.; Zhang, H.L.; Su, Z.A.; Yu, Y.H.; Jia, X.T.; Bai, Z.Q. Pollen morphology of eight major sand plants and their historical significance in west ordos, Inner Mongolia, China. Acta Micropalaeontol. Sin. 2010, 27, 315–322. [Google Scholar]
- Zhu, Z.Y.; Ma, Y.Q.; Liu, Z.L.; Zhao, Y.Z. Endemic plants and floristic characteristics in Alashan-ordos biodiversity center. Arid. Zone Resour. Environ. 1999, 13, 1–16. [Google Scholar]
- Tiffney, B.H. The Eocene North Atlantic land bridge: Its importance in Tertiary and modern phytogeography of the Northern Hemisphere. J. Arnold Arbor. 1985, 66, 243–273. [Google Scholar] [CrossRef]
- Tiffney, B.H. Perspectives on the origin of the floristic similarity between eastern Asia and eastern North America. J. Arnold Arbor. 1985, 66, 73–94. [Google Scholar] [CrossRef]
- Wolfe, J.A. Tertiary climatic fluctuations and methods of analysis of Tertiary floras. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1971, 9, 27–57. [Google Scholar] [CrossRef]
- Tiffney, B.H.; Manchester, S.R. The use of geological and paleontological evidence in evaluating plant phylogeographic hypotheses in the Northern Hemisphere Tertiary. Int. J. Plant Sci. 2001, 162, S3–S17. [Google Scholar] [CrossRef]
- Milne, R.I.; Abbott, R.J. The origin and evolution of Tertiary relict floras. Adv. Bot. Res. 2002, 38, 281–314. [Google Scholar]
- Zhang, T.; Wang, W.; An, H.J.; Zhang, H.D.; Wu, J.L. Relevant Analysis on Changed Population Spot and Priority Protection Order of the Peculiar Endangered Plants in Eastern Alasham-western Erdos. J. Arid. Land Resour. Environ. 2005, 19, 179–184. [Google Scholar]
- Yang, Z.Y. Monitoring and Assessment of Ecological Environment in Alxa League under the Background of Climate Change. J. Agric. Catastrophology 2023, 13, 105–107. [Google Scholar]
- Chen, F.; Zhu, Y.; Li, J.; Shi, Q.; Jin, L.; Wünemann, B. Abrupt Holocene changes of the Asian monsoon at millennial- and centennial-scales: Evidence from lake sediment document in Minqin Basin, NW China. Chin. Sci. Bull. 2001, 46, 1942–1947. [Google Scholar] [CrossRef]
- Zhang, H.C.; Ma, Y.Z.; Li, J.J.; Wüennemann, B. A preliminary study on the Paleocene paleoclimatic change in the southern margin of Tengger Desert. Chin. Sci. Bull. 1998, 43, 1252–1258. [Google Scholar]
- Zhang, H.C.; Ma, Y.Z.; Wünnemann, B.; Pachur, H.J. A Holocene climatic record from arid northwestern China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2000, 162, 389–401. [Google Scholar] [CrossRef]
- You, L.; Shen, J.G.; Pei, H. Climate change in Inner Mongolia in the last 50 years and the trend in the next 10–20 years. Inn. Mong. Meteorol. 2002, 4, 14–18. [Google Scholar]
- Wang, Y.X.; Li, J.B.; Ma, W.; Yang, M.; Wang, Y. Preliminary analysis and mutation test of air temperature in Alashan League area. Inn. Mong. Sci. Econ. 2017, 06, 35–37. [Google Scholar]
- Wang, D.M.; Yang, Z.Y. Characterisation of changes in extreme cold weather events in Alashan League. Mod. Agric. 2017, 96–97. [Google Scholar]
- Alashan League Administration. About Alashan—Geography and Environment [EB/OL]. Available online: http://www.als.gov.cn/col/col3386/index.html#dlhj2022a (accessed on 20 November 2022).
- Alashan League Administration. Alashan Facts—Natural Resources [EB/OL]. Available online: http://www.als.gov.cn/col/col3386/index.html#mzzj2022b (accessed on 20 November 2022).
- Zhang, M.L.; Temirbayeva, K.; Sanderson, S.C.; Chen, X. Young dispersal of xerophil Nitraria lineages in intercontinental disjunctions of the Old World. Sci. Rep. 2015, 5, 13840. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Woutersen, A.; Jardine, P.E.; Silvestro, D.; Bogotá-Angel, R.G.; Zhang, H.-X.; Meijer, N.; Bouchal, J.; Barbolini, N.; Dupont-Nivet, G.; Koutsodendris, A.; et al. The evolutionary history of the Central Asian steppe-desert taxon Nitraria (Nitrariaceae) as revealed by integration of fossil pollen morphology and molecular data. Bot. J. Linn. Soc. 2023, 202, 195–214. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Tan, H.J.; Zhang, Z.S.; Jia, X.H.; Zhang, J.G.; Fan, H.W. Physiological Response and Adjustment Mechanism of Reaumuria soongorica and Salsola passerina to Extreme Environment. J. Desert Res. 2012, 32, 24–32. [Google Scholar]
- Guo, Z.T.; Ruddiman, W.F.; Hao, Q.Z.; Wu, H.B.; Qiao, Y.S.; Zhu, R.X.; Peng, S.Z.; Wei, J.J.; Yuan, B.Y.; Liu, T.S. Onset of Asian desertification by 22 Myr ago inferred from loess deposits in China. Nature 2002, 416, 159–163. [Google Scholar] [CrossRef]
- Dong, X.J.; Zhang, X.S. Some observations of the adaptations of sandy shrubs to the arid environment in the Mu Us Sandland: Leaf water relations and anatomic features. J. Arid. Environ. 2001, 48, 41–48. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, Z.; Wu, C. The research of the breeding characters of Zygophyllum xanthoxylum. Pratacult. Sci. 2006, 23, 38–41. [Google Scholar]
- Bellstedt, D.U.; Galley, C.; Pirie, M.D.; Linder, H.P. The migration of paleotropical arid flora: Zygophylloideae as an example. Syst. Bot. 2012, 37, 951–959. [Google Scholar] [CrossRef]
- Wu, S.D.; Lin, L.; Li, H.L.; Yu, S.X.; Zhang, L.J.; Wang, W. Evolution of Asian Interior Arid-Zone Biota: Evidence from the Diversification of Asian Zygophyllum (Zygophyllaceae). PLoS ONE 2015, 10, e0138697. [Google Scholar] [CrossRef]
- Shen, X.X.; Chai, M.W.; Xiang, J.; Li, R.L.; Qiu, G.Y. Survival strategies of Ammopiptanthus mongolicus and Zygophyllum xanthoxylon in saline and drought environments, northwest China. Acta Physiol. Plant 2015, 37, 213. [Google Scholar] [CrossRef]
- Zhengyi, W. China Vegetation; Science Press: Beijing, China, 1980. [Google Scholar]
- Liu, Y.H.; Wang, S.M.; Wang, H.S. A Study on the chromosomal geography of Ammopiptanthus Genus. Geogr. Res. 1996, 15, 40–47. [Google Scholar]
- Zhao, Y.Z. Study on Floristic Geographical Distribution of Amygdalus mongolica. Acta Sci. Nat. Univ. NeiMonggol (Nat. Sci.) 1995, 26, 713–715. [Google Scholar]
- Xinjiang Comprehensive Investigation Team of China Academy of Sciences, Institute of Botany, Chinese Academy of Sciences. Vegetation and Its Utilization in Xinjiang; Science Press: Beijing, China, 1978. [Google Scholar]
- Wang, H.S. Distribution of main halophyte communities in Xinjiang and their relationship with soil and groundwater. Plant Ecol. Geophys. Ser. 1964, 2, 57–69. [Google Scholar]
- Staples, G.W. Revision of Asiatic Poraneae (Convolvulaceae)—Cordisepalum, Dinetus, Duperreya, Porana, Poranopsis and Tridynamia. Blumea-Biodivers. Evol. Biogeogr. Plants 2006, 51, 403–491. [Google Scholar] [CrossRef]
- Staples, G.W.; Austin, D.F. Revision of neotropical Calucobolus and Porana (Convolvulaceae). Edinb. J. Bot. 2009, 66, 133–153. [Google Scholar] [CrossRef]
- Staples, G.W.; Noltie, H.J. Proposal to conserve the name Hewittia against Shuttereia Choisy (Convolvulaceae). Taxon 2007, 56, 262. [Google Scholar]
- Zhang, D.K.; Wang, J.H.; Ma, Q.L.; Liu, H.J. Summary of Artemisia ordosica studies. Pratacultural Sci. 2007, 24, 34–35. [Google Scholar]
- Inner Mongolia Flora Editorial Committee. Inner Mongolia Flora; Inner Mongolia People ‘s Publishing House: Hohhot, China, 1982. [Google Scholar]
- Department of Ecological Environment, Inner Mongolia Autonomous Region. Natural Ecological Protection and Management-Rare and Endangered Plant List of Inner Mongolia [EB/OL]. Available online: https://sthjt.nmg.gov.cn/stbh2021/zrstbhgl/202108/t20210829_1850525.html (accessed on 19 May 2023).
- National Environmental Protection Administration, Chinese Academy of Sciences. China Rare and Endangered Plant List; Science Publishing House: Beijing, China, 1987. [Google Scholar]
- Wang, S.; Xie, H. Red List of Chinese Species; Higher Education Press: Beijing, China, 1991. [Google Scholar]
- Liguo, F. China Plant Red Book; Science Publishing House: Beijing, China, 1991. [Google Scholar]
- Kong, L.S.; Ma, M.H. The bioecological characteristics of Halocnemum strobilaceum and its community on the border of oasis in Hutubi, Xinjiang. Acta Ecol. Sin. 1995, 15, 351–358. [Google Scholar]
- Ripley, B.D. The second-order analysis of stationary point processes. J. Appl. Probab. 1976, 13, 255–266. [Google Scholar] [CrossRef]
- Wang, G.; Xie, C.; Wei, L.; Gao, Z.; Yang, H.; Jim, C. Predicting Suitable Habitats for China’s Endangered Plant Handeliodendron bodinieri (H. Lév.) Rehder. Diversity 2023, 15, 1033. [Google Scholar] [CrossRef]
- Xie, S.; Si, H.; Sun, H.; Zhao, Q.; Li, X.; Wang, S.; Niu, J.; Wang, Z. Predicting the Potential Distribution of the Endangered Plant Eucommia ulmoides in China under the Background of Climate Change. Sustainability 2023, 15, 5349. [Google Scholar] [CrossRef]
- Dong, P.B.; Wang, L.Y.; Wang, L.J.; Jia, Y.; Li, Z.H.; Bai, G.; Zhao, R.M.; Liang, W.; Wang, H.Y.; Guo, F.X.; et al. Distributional Response of the Rare and Endangered Tree Species Abies chensiensis to Climate Change in East Asia. Biology 2022, 11, 1659. [Google Scholar] [CrossRef]
- Ferrier, S.; Guisan, A. Spatial modelling of biodiversity at the community level. J. Appl. Ecol. 2006, 43, 393–404. [Google Scholar] [CrossRef]
- Pollock, L.J.; Tingley, R.; Morris, W.K.; Golding, N.; O’Hara, R.B.; Parris, K.M.; Vesk, P.A.; McCarthy, M.A. Understanding co-occurrence by modelling species simultaneously with a Joint Species Distribution Model (JSDM). Methods Ecol. Evol. 2014, 5, 397–406. [Google Scholar] [CrossRef]
- McCullagh, P.; Nelder, J.A. Generalized Linear Models; Chapman and Hall: London, UK, 1989. [Google Scholar]
- Van Vuuren, D.P.; Stehfest, E.; Gernaat, D.E.; Doelman, J.C.; van den Berg, M.; Harmsen, M.; de Boer, H.S.; Bouwman, L.F.; Diaoglou, V.; Edelenbosch, O.Y. Energy, land-use and greenhouse gas emissions trajectories under a green growth paradigm. Glob. Environ. Chang. 2017, 42, 237–250. [Google Scholar] [CrossRef]
- Fan, M.E.; Wang, P.Y.; Chen, Y.; Liu, H.H.; Liu, Y.; Chen, Y.; Gang, C.C.; Ma, F.L. Spatial and Temporal Dynamics of Global Grassland and Net Primary Productivity under Different Future Climate Scenarios. Acta Agrestia Sin. 2023, 1–14. Available online: http://kns.cnki.net/kcms/detail/11.3362.S.20231101.1428.014.html (accessed on 1 December 2023).
- Doelman, J.C.; Stehfest, E.; Tabeau, A.; van Meijl, H.; Lassaletta, L.; Gernaat DE, H.J.; Hermans, K.; Harmsen, M.; Diaoglou, V.; Biemans, H.; et al. Exploring SSP land-use dynamics using the IMAGE model: Regional and gridded scenarios of land-use change and land-based climate change mitigation. Glob. Environ. Chang. 2018, 48, 119–135. [Google Scholar] [CrossRef]
- Popp, A.; Calvin, K.; Fujimori, S.; Havlik, P.; Humpenöder, F.; Stehfest, E.; Bodirsky, B.L.; Dietrich, J.P.; Doelmann, J.C.; Gusti, M.; et al. Land-use futures in the shared socio-economic pathways. Glob. Environ. Chang. 2017, 42, 331–345. [Google Scholar] [CrossRef]
- Hurtt, G.C.; Chini, L.; Sahajpal, R.; Frolking, S.; Bodirsky, B.L.; Calvin, K.; Doelman, J.C.; Fisk, J.; Fujimori, S.; Klein Goldewijk, K.; et al. Harmonization of global land use change and management for the period 850–2100 (LUH2) for CMIP6. Geosci. Model Dev. 2020, 13, 5425–5464. [Google Scholar] [CrossRef]
- Warton, D.I.; Blanchet, F.G.; O’Hara, R.B.; Ovaskainen, O.; Taskinen, S.; Walker, S.C.; Hui, F.K. So Many Variables: Joint Modeling in Community Ecology. Trends Ecol. Evol. 2015, 30, 766–779. [Google Scholar] [CrossRef]
- Zurell, D.; Pollock, L.J.; Thuiller, W. Do joint species distribution models reliably detect interspecific interactions from co-occurrence data in homogenous environments? Ecography 2018, 41, 1812–1819. [Google Scholar] [CrossRef]
- Chib, S.; Greenberg, E. Analysis of Multivariate Probit Models. Biometrika 1998, 85, 347–361. [Google Scholar] [CrossRef]
- Shi, Y.; Yan, X.; Zhao, P.; Yin, H.; Zhao, X.; Xiao, H.; Li, X.; Chen, G.; Ma, X.F. Transcriptomic analysis of a tertiary relict plant, extreme xerophyte Reaumuria soongorica to identify genes related to drought adaptation. PLoS ONE 2013, 8, e63993. [Google Scholar] [CrossRef]
- Cheng, J.; Kao, H.X.; Dong, S.B. Population genetic structure and gene flow of rare and endangered Tetraena mongolica Maxim. revealed by reduced representation sequencing. BMC Plant Biol. 2020, 20, 391. [Google Scholar] [CrossRef] [PubMed]
- Ivanov Leonid, A.; Migalina Svetlana, V.; Ronzhina Dina, A.; Tumurjav Shinekhuu Gundsambuu Tserenkhand Bazha Sergey, N.; Ivanova Larissa, A. Altitude-dependent variation in leaf structure and pigment content provides the performance of a relict shrub in mountains of Mongolia. Ann. Appl. Biol. 2022, 181, 321–331. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef]
- Inner Mongolia Ningxia Comprehensive Expedition Team, Chinese Academy of Sciences. Inner Mongolia Vegetation; Science Press: Beijing, China, 1985. [Google Scholar]
- Liguo, F. Chinese Plants; Science Press: Beijing, China, 1992. [Google Scholar]
- Xu, Q.; Liu, S.R.; Zang, R.G.; Guo, Q.S.; Hao, Y.G. The characteristics of reproductive ecology of endemic species Tetraena mongolica population in China. Sci. Silvae Sin. 2001, 37, 36–41. [Google Scholar]
- Gao, Q.; Yan, L.; Feng, Z.Q. The adjust diversity of the envronment dversity on the leaf structrue of 13 species of Zypophyllacdae. J. Inn. Mong. Agric. Univ. 2008, 29, 50–57. [Google Scholar]
- Meng, H.; Siqingaowa NaRen, H.; Gao, R.H. Adaptation diversity of Alashan desert shrub in inner mongolia. J. Inn. Mong. Agric. Univ. 2011, 32, 277–281. [Google Scholar]
- Zhi, Y.B.; Yang, C.; Wang, Z.S.; An, S.Q.; Wang, Z.L.; Li, H.L.; Su, Z.A.; Wang, Q. The endangered characteristics and mechanism of the endemic relict shrub Tetraena mongolica Maxim. Acta Ecol. Sin. 2008, 28, 0767–0776. [Google Scholar]
- Jiang, S.; Luo, M.X.; Gao, R.H.; Zhang, W.; Yang, Y.Z.; Li, Y.J.; Liao, P.C. Isolation-by-environment as a driver of genetic differentiation among populations of the only broad-leaved evergreen shrub Ammopiptanthus mongolicus in Asian temperate deserts. Sci Rep 2019, 9, 12008. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Z.; Luo, M.X.; Pang, L.D.; Gao, R.H.; Chang, J.T.; Liao, P.C. Parallel adaptation prompted core-periphery divergence of Ammopiptanthus mongolicus. Front. Plant Sci. 2022, 13, 956374. [Google Scholar] [CrossRef] [PubMed]




| Soil Property Factors | Topographic Factors | Biometeorological Factors | |
|---|---|---|---|
| Silt concentration | Slope | Average annual temperature | Average temperature of the warmest quarter |
| Silt content | Aspect of slope | Daily difference in mean temperature | Average temperature of the coldest quarter |
| Clay content | Altitude | Isothermality | Annual rainfall |
| Organic carbon content | Coefficient of seasonal variation of temperature | Precipitation in the wettest month | |
| PH | Maximum temperature in the hottest month | Precipitation in the driest month | |
| Carbonate or lime content | Minimum temperature in the coldest month | Seasonal variations in precipitation | |
| Sulfate content | Annual difference in temperature | Precipitation in the driest quarter | |
| Average temperature of the wettest quarter | Wettest quarter precipitation | ||
| Average temperature of the driest quarter | Warmest quarter precipitation | ||
| Coldest quarter precipitation | |||
| Species | Current Area (km2) | Area under SSP126 Scenario (km2) | Area under SSP245 Scenario (km2) | Area under SSP370 Scenario (km2) | Area under SSP585 Scenario (km2) |
|---|---|---|---|---|---|
| SX | 16,343 | 17,283 | 16,130 | 16,665 | 16,878 |
| NT | 14,760 | 14,816 | 14,947 | 15,217 | 14,366 |
| RS | 17,986 | 18,843 | 19,113 | 18,699 | 18,598 |
| AM | 21,554 | 21,276 | 20,064 | 22,410 | 21,145 |
| TM | 18,853 | 19,237 | 18,802 | 18,207 | 18,608 |
| Species | Current Area (km2) | Area under SSP126 Scenario (km2) | Area under SSP245 Scenario (km2) | Area under SSP370 Scenario (km2) | Area under SSP585 Scenario (km2) |
|---|---|---|---|---|---|
| CT | 12,311 | 11,480 | 12,038 | 11,552 | 10,161 |
| Amo | 12,857 | 11,555 | 12,423 | 13,025 | 12,470 |
| KF | 9649 | 10,023 | 9956 | 10,143 | 10,227 |
| HS | 7453 | 7054 | 7366 | 7454 | 7249 |
| AO | 30,014 | 30,748 | 30,686 | 32,172 | 29,650 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Zhang, B.; Zhang, M.; Jie, M.; Guo, S.; Wang, Y. Relict Plants Are Better Able to Adapt to Climate Change: Evidence from Desert Shrub Communities. Plants 2023, 12, 4065. https://doi.org/10.3390/plants12234065
Lu Y, Zhang B, Zhang M, Jie M, Guo S, Wang Y. Relict Plants Are Better Able to Adapt to Climate Change: Evidence from Desert Shrub Communities. Plants. 2023; 12(23):4065. https://doi.org/10.3390/plants12234065
Chicago/Turabian StyleLu, Ying, Boran Zhang, Min Zhang, Meiyu Jie, Siqi Guo, and Yange Wang. 2023. "Relict Plants Are Better Able to Adapt to Climate Change: Evidence from Desert Shrub Communities" Plants 12, no. 23: 4065. https://doi.org/10.3390/plants12234065
APA StyleLu, Y., Zhang, B., Zhang, M., Jie, M., Guo, S., & Wang, Y. (2023). Relict Plants Are Better Able to Adapt to Climate Change: Evidence from Desert Shrub Communities. Plants, 12(23), 4065. https://doi.org/10.3390/plants12234065





