Expression of Cry1Ab/2Aj Protein in Genetically Engineered Maize Plants and Its Transfer in the Arthropod Food Web
Abstract
1. Introduction
- The chosen species should face a high level of exposure to exogenous insecticidal proteins expressed by transgenic plants.
- The chosen species should be among those most likely to be sensitive to exogenous insecticidal proteins expressed by transgenic plants.
- The chosen species should represent providers of ecological or cultural services relevant for the cropping system in the region of anticipated use.
2. Material and Methods
2.1. Experimental Design
2.2. Sample Collection
2.3. ELISA Measurements
2.4. Statistical Analysis
3. Results
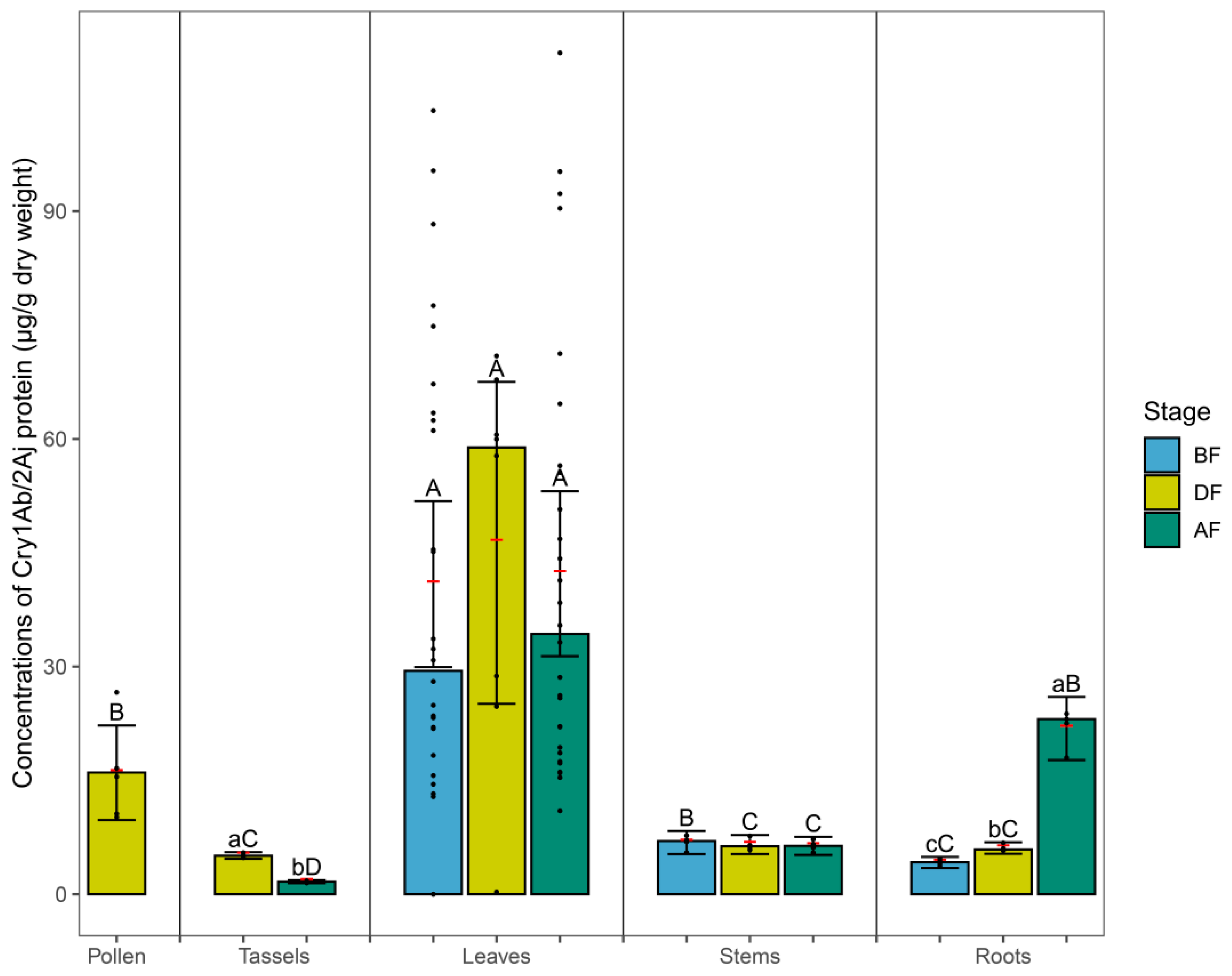
3.1. Cry Protein in Maize Tissues
3.2. Degradation of Bt Maize Leaves in Soil
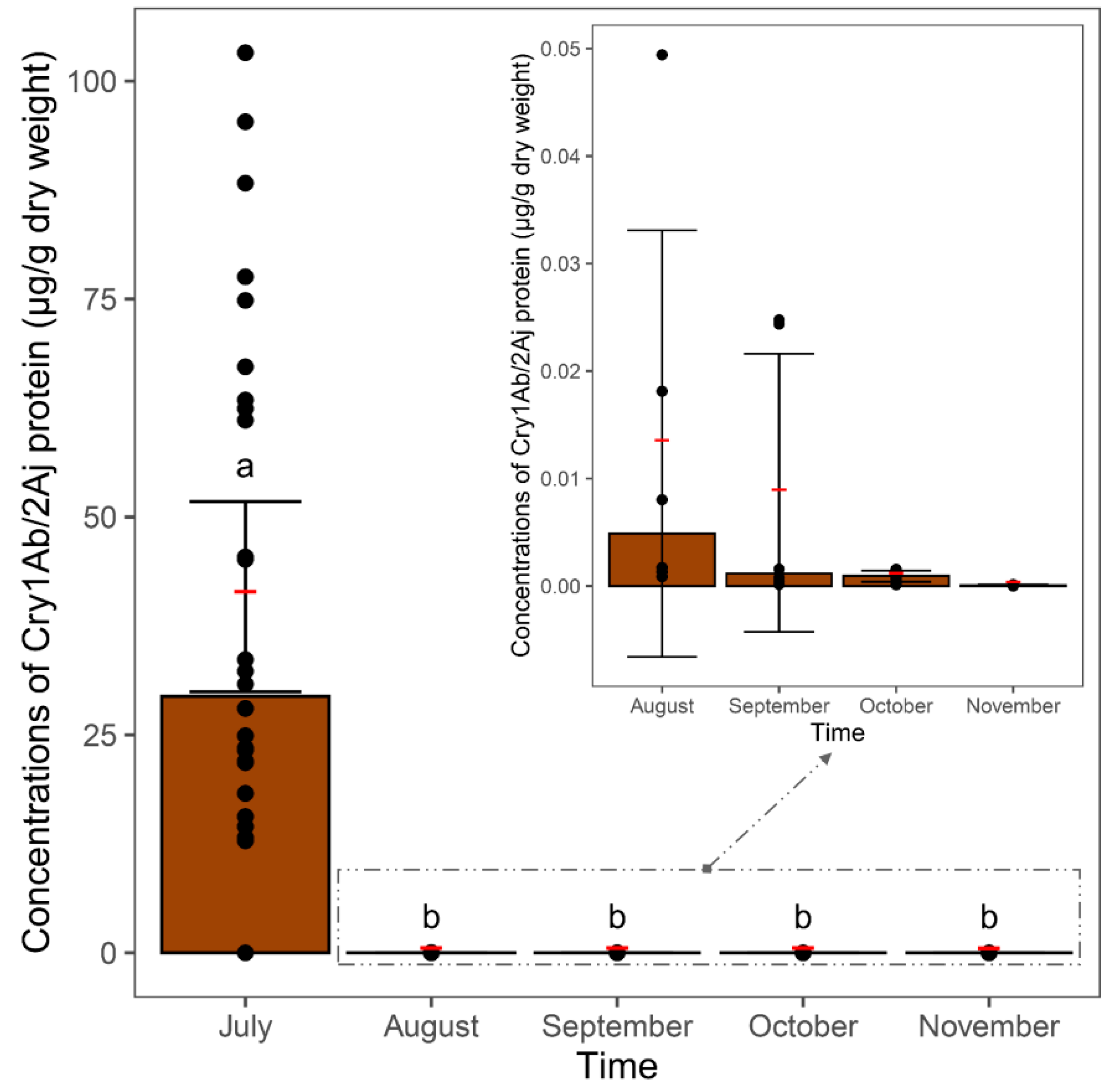
3.3. Cry Protein in Soil
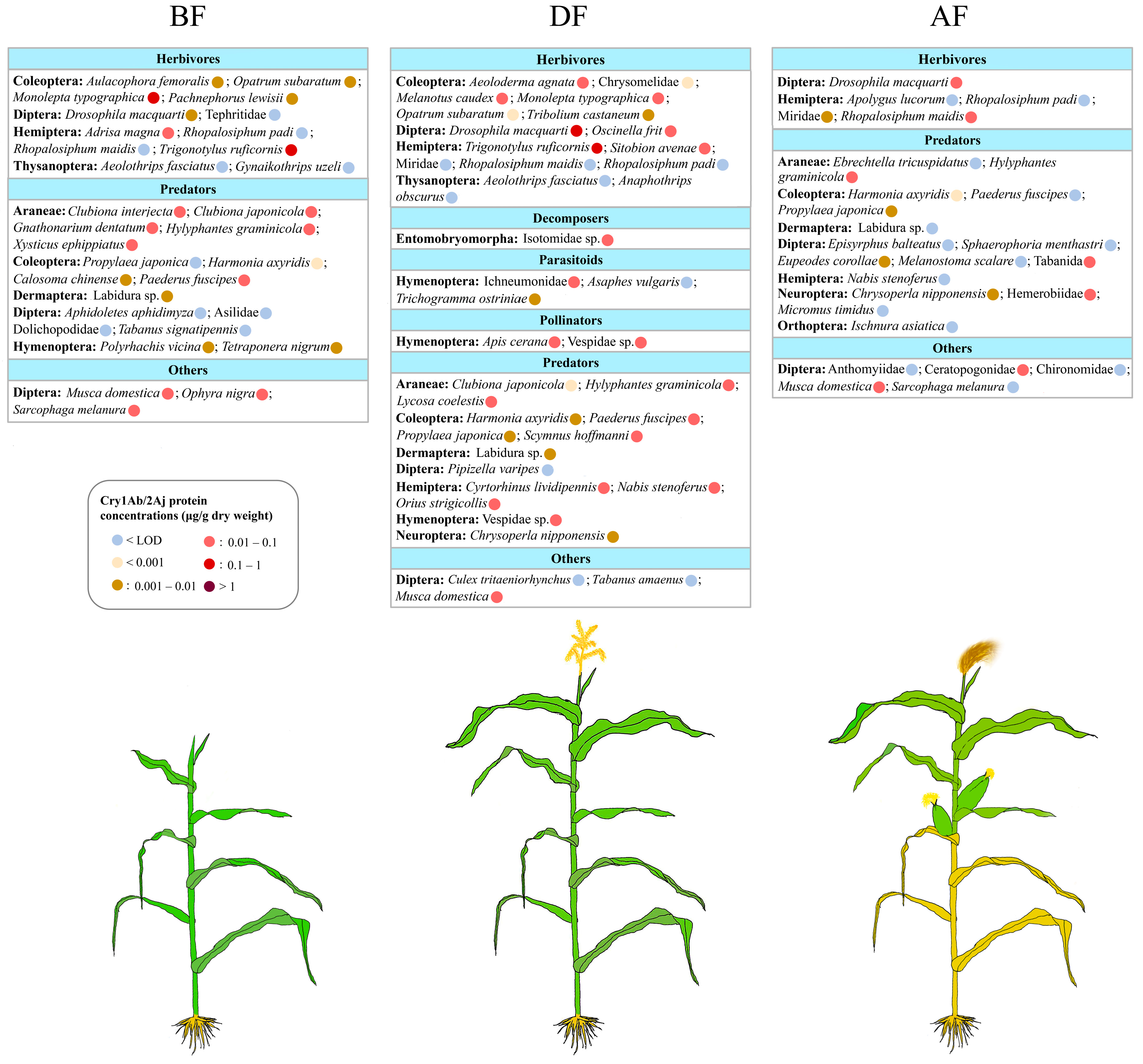
3.4. Exposure of Arthropods to Cry1Ab/2Aj Produced by Bt Maize
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Quemada, H. Lessons learned from the introduction of genetically engineered crops: Relevance to gene drive deployment in Africa. Transgenic Res. 2022, 31, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Brookes, G.; Barfoot, P. Environmental impacts of genetically modified (GM) Crop use 1996-2018: Impacts on pesticide use and carbon emissions. GM Crops Food 2020, 11, 215–241. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.; Carrière, Y. Surge in insect resistance to transgenic crops and prospects for sustainability. Nat. Biotechnol. 2017, 35, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Nap, J.P.; Metz, P.L.J.; Escaler, M.; Conner, A.J. The release of genetically modified crops into the environment: Part I. Overview of current status and regulations. Plant J. 2003, 33, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Conner, A.J.; Glare, T.R.; Nap, J.P. The release of genetically modified crops into the environment: Part II. Overview of ecological risk assessment. Plant J. 2003, 33, 19–46. [Google Scholar] [CrossRef] [PubMed]
- Romeis, J.; Bartsch, D.; Bigler, F.; Candolfi, M.P.; Gielkens, M.; Hartley, S.E.; Hellmich, R.L.; Huesing, J.E.; Jepson, P.C.; Layton, R.; et al. Assessment of risk of insect-resistant transgenic crops to nontarget arthropods. Nat. Biotechnol. 2008, 26, 203–208. [Google Scholar] [CrossRef] [PubMed]
- E 1850-04; Standard Guide for Selection of Resident Species as Test Organisms for Aquatic and Sediment Toxicity Tests. ASTM International: West Conshohocken, PA, USA, 2019.
- Candolfi, M.; Bigler, F.; Campbell, P.; Heimbach, U.; Schmuck, R.; Angeli, G.; Bakker, F.; Brown, K.; Carli, G.; Dinter, A.; et al. Principles for regulatory testing and interpretation of semi-field and field studies with non-target arthropods. J. Pest Sci. 2000, 73, 141–147. [Google Scholar] [CrossRef]
- Levin, S.A.; Harwell, M.A.; Kelly, J.R.; Kimball, K.D. Ecotoxicology: Problems and Approaches; Springer Advanced Text in Life Sciences; Springer: New York, NY, USA, 1989; pp. 3–7. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, R.; Sui, X.Y.; Chen, K.; Chen, Y.F. Ecological use of vertebrate surrogate species in ecosystem conservation. Glob. Ecol. Conserv. 2020, 24, e01344. [Google Scholar] [CrossRef]
- Caro, T.M.; O’Doherty, G. On the use of surrogate species in conservation biology. Conserv. Biol. 1999, 13, 805–814. [Google Scholar] [CrossRef]
- Duelli, P.; Obrist, M.K. Biodiversity indicators: The choice of values and measures. Agric. Ecosyst. Environ. 2003, 98, 87–98. [Google Scholar] [CrossRef]
- Bai, Y.Y.; Jiang, M.X.; Cheng, J.A. Temporal expression patterns of Cry1Ab insecticidal protein in Bt rice plants and its degradation in paddy soils. Acta. Ecol. Sin. 2005, 25, 1583–1590. [Google Scholar] [CrossRef]
- Knight, K.; Head, G.; Rogers, J. Season-long expression of Cry1Ac and Cry2Ab proteins in Bollgard II cotton in Australia. Crop Prot. 2013, 44, 50–58. [Google Scholar] [CrossRef]
- Cattaneo, M.G.; Yafuso, C.; Schmidt, C.; Huang, C.Y.; Rahman, M.; Olson, C.; Ellers-Kirk, C.; Orr, B.J.; Marsh, S.E.; Antilla, L.; et al. Farm-scale evaluation of the impacts of transgenic cotton on biodiversity, pesticide use, and yield. Proc. Natl. Acad. Sci. USA 2006, 103, 7571–7576. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, Y.; Xue, J.; Wang, Y.; Song, X.; Li, Y. Impact of Transgenic Cry1Ab/2Aj Maize on Abundance of Non-Target Arthropods in the Field. Plants 2022, 11, 2520. [Google Scholar] [CrossRef]
- Sun, H.W.; Xu, X.H.; Li, F.; Gao, R.; Yang, S.K.; Lu, X.B. Resistances of transgenic maize hybrid “Ruifeng 1-double resistance 12-5” with insect and herbicide resistance against three major lepidopteran pests. Shandong Agric. Sci. 2018, 50, 109–114. [Google Scholar] [CrossRef]
- Wang, H.Q.; Xiao, F.; Yang, L.; Miao, Q.M.; Zhang, X.D.; Zhang, X.J. Analysis of Verification Results by PCR Methods for Genetically Modified Double-resistant 12-5 Event-specific Maize. Biotechnol. Bull. 2020, 36, 48–55. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Liu, Q.S.; Song, X.Y.; Yang, X.W.; Han, L.Z.; Romeis, J.; Li, Y.H. Unintended changes in transgenic maize cause no nontarget effects. Plants People Planet 2022, 4, 392–402. [Google Scholar] [CrossRef]
- Scholte, E.J.; Dicke, M. Effects of insect-resistant transgenic crops on non-target arthropods: First step in pre-market risk assessment studies. In COGEM Report on Selecting Non-Target Organisms for ERA; COGEM: Bilthoven, The Netherlands, 2005; pp. 1–49. [Google Scholar]
- Harwood, J.D.; Wallin, W.G.; Obrycki, J.J. Uptake of Bt endotoxins by nontarget herbivores and higher order arthropod predators: Molecular evidence from a transgenic corn agroecosystem. Mol. Ecol. 2005, 14, 2815–2823. [Google Scholar] [CrossRef]
- Obrist, L.B.; Dutton, A.; Albajes, R.; Bigler, F. Exposure of arthropod predators to Cry1Ab toxin in Bt maize fields. Ecol. Entomol. 2006, 31, 143–154. [Google Scholar] [CrossRef]
- Álvarez-Alfageme, F.; Bigler, F.; Romeis, J. Laboratory toxicity studies demonstrate no adverse effects of Cry1Ab and Cry3Bb1 to larvae of Adalia bipunctata (Coleoptera: Coccinellidae): The importance of study design. Transgenic Res. 2011, 20, 467–479. [Google Scholar] [CrossRef]
- Fu, Q.; Lai, F.X.; Chen, Y.; Li, K.L. A review of the ecological safety of the insect-resistant transgenic rices to non-target organisms. Plant Physiol. J. 2013, 49, 655–663. [Google Scholar]
- Torres, J.B.; Ruberson, J.R.; Adang, M.J. Expression of Bacillus thuringiensis Cry1Ac protein in cotton plants, acquisition by pests and predators: A tritrophic analysis. Agric. For. Entomol. 2006, 8, 191–202. [Google Scholar] [CrossRef]
- EFSA GMO Panel (EFSA Panel on Genetically Modified Organisms); Mullins, E.; Bresson, J.L.; Dalmay, T.; Dewhurst, I.C.; Epstein, M.M.; Fribank, L.G.; Guerche, P.; Hejatko, J.; Moerno, F.J.; et al. Assessment of genetically modified soybean MON 87701 × MON 89788 for renewal authorisation under Regulation (EC) No 1829/2003 (application EFSA-GMO-RX-022). EFSA J. 2022, 20, e07684. [Google Scholar] [CrossRef]
- Dutton, A.; Klein, H.; Romeis, J.; Bigler, F. Uptake of Bt-toxin by herbivores feeding on transgenic maize and consequences for the predator Chrysoperla carnea. Ecol. Entomol. 2002, 27, 441–447. [Google Scholar] [CrossRef]
- Chang, X. Efficacy Evalution of Three Transgenic Bt Maize Events to Target Pests and the Risk Assessment to Non-Target Silk Worm and Predator Ladybird. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2013. [Google Scholar]
- Zhu, X. Efficacy Evalution of Transgenic Maize Event DR 12-5 to Target Pests and the Risk Assessment to Non-Target Animals. Master’s Thesis, Fuyang Normal University, Fuyang, China, 2019. [Google Scholar]
- Zhu, X.; Jiang, Y.Y.; Lai, Y.M.; Chen, X.Y.; Wang, X.F.; Xu, X.L.; Xu, J.F. Effects of transgenic maize (Zea mays) event DR 12-5 with cry1Ab/2Aj and g10evo-spsps genes on silkworm (Bombyx mori). J. Agric. Biotechnol. 2020, 28, 1269–1276. [Google Scholar]
- Hellmich, R.L.; Siegfried, B.D.; Sears, M.K.; Stanley-Horn, D.E.; Daniels, M.J.; Mattila, H.R.; Spencer, T.; Bidne, K.G.; Lewis, L.C. Monarch larvae sensitivity to Bacillus thuringiensis-purified proteins and pollen. Proc. Natl. Acad. Sci. USA 2001, 98, 11925–11930. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.K.; Schuppener, M.; Rauschen, S. Assessing the impact of Cry1Ab expressing corn pollen on larvae of Aglais urticae in a laboratory bioassay. GMOs Integr. Plant Prod. 2012, 73, 55–60. [Google Scholar]
- Wraight, C.L.; Zangerl, A.R.; Carroll, M.J.; Berenbaum, M.R. Absence of toxicity of Bacillus thuringiensis pollen to black swallowtails under field conditions. Proc. Natl. Acad. Sci. USA 2000, 97, 7700–7703. [Google Scholar] [CrossRef] [PubMed]
- Li, W.D.; Wu, K.M.; Wang, X.Q.; Wang, G.R.; Guo, Y.Y. Impact of pollen grains from Bt transgenic corn on the growth and development of Chinese tussah silkworm, Antheraea pernyi (Lepidoptera: Saturniidae). Environ. Entomol. 2005, 34, 922–928. [Google Scholar] [CrossRef]
- Romeis, J.; Hellmich, R.L.; Candolfi, M.P.; Carstens, K.; De Schrijver, A.; Gatehouse, A.M.; Waggoner, A.; Herman, R.A.; Huesing, J.E.; McLean, M.A.; et al. Recommendations for the design of laboratory studies on non-target arthropods for risk assessment of genetically engineered plants. Transgenic Res. 2011, 20, 1–22. [Google Scholar] [CrossRef]
- Virla, E.G.; Casuso, M.; Frias, E.A. A preliminary study on the effects of a transgenic corn event on the non-target pest Dalbulus maidis (Hemiptera: Cicadellidae). Crop Prot. 2010, 29, 635–638. [Google Scholar] [CrossRef]
- Kim, Y.H.; Hwang, C.E.; Kim, T.S.; Lee, J.H.; Lee, S.H. Assessment of potential impacts due to unintentionally released Bt maize plants on non-target aphid Rhopalosiphum padi (Hemiptera: Aphididae). J. Asia Pac. Entomol. 2012, 15, 443–446. [Google Scholar] [CrossRef]
- Erasmus, A.; Van den Berg, J. Effect of Bt-maize expressing Cry1Ab toxin on non-target Coleoptera and Lepidoptera pests of maize in South Africa. Afr. Entomol. 2014, 22, 167–179. [Google Scholar] [CrossRef]
- Romeis, J.; Raybould, A.; Bigler, F.; Candolfi, M.P.; Hellmich, R.L.; Huesing, J.E.; Shelton, A.M. Deriving criteria to select arthropod species for laboratory tests to assess the ecological risks from cultivating arthropod-resistant genetically engineered crops. Chemosphere 2013, 90, 901–909. [Google Scholar] [CrossRef]
- Shu, Y.; Du, Y.; Wang, J. Presence of Cry1Ab in the Bt maize–aphid (Rhopalosiphum maidis)–ladybeetle (Propylea japonica) system has no adverse effects on insect biological parameters. Entomol. Exp. Appl. 2019, 167, 553–560. [Google Scholar] [CrossRef]
- Bai, Y.Y.; Jiang, M.X.; Cheng, J.A. Effects of transgenic cry1Ab rice pollen on fitness of Propylea japonica (Thunberg). J. Pest Sci. 2005, 78, 123–128. [Google Scholar] [CrossRef]
- Liu, Y.M. Ecological Safety of Transgenic cry1Ab/2Aj and cry1Ac Maize Lines for Propylea japonica Thunberg and Chrysoperla sinica Tjedar. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2016. [Google Scholar]
- Cheng, Z.; Huang, J.; Liang, Y.; Cheng, S.; Xiong, H.; Chen, N.; Hu, S. Effect of transgenic Bt rice on the survival rate and predation of Paederus fuscipes Curtis adults. Chin. J. Appl. Entomol. 2014, 51, 1184–1189. [Google Scholar] [CrossRef]
- Liu, Y.M.; Li, Y.H.; Chen, X.P.; Song, X.Y.; Shen, P.; Peng, Y.F. No detrimental effect of Bt maize pollen containing Cry1Ab/2Aj or Cry1Ac on adult green lacewings Chrysoperla sinica Tjeder. J. Integr. Agric. 2019, 18, 893–899. [Google Scholar] [CrossRef]
- Yang, H.L.; Peng, Y.D.; Tian, J.X.; Wang, J.; Hu, J.L.; Song, Q.L.; Wang, Z. Review: Biosafety assessment of Bt rice and other Bt crops using spiders as example for non-target arthropods in China. Plant Cell Rep. 2017, 36, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Lu, Z.B.; Shen, Z.C.; Peng, Y.F.; Ye, G.Y. Bitrophic and Tritrophic Effects of Transgenic cry1Ab/cry2Aj Maize on the Beneficial, Nontarget Harmonia axyridis (Coleoptera: Coccinellidae). Environ. Entomol. 2017, 46, 1171–1176. [Google Scholar] [CrossRef]
- Dai, P.L.; Zhou, W.; Zhang, J.; Jiang, W.Y.; Wang, Q.; Cui, H.J.; Sun, J.; Wu, Y.; Zhou, T. The effects of Bt cry1ah toxin on worker honeybees (apis mellifera ligustica and apis cerana cerana). Apidologie 2012, 43, 384–391. [Google Scholar] [CrossRef]
- Li, J.; Guan, C.Y.; Li, X. Influence of Bt-transgenic insect-resistant rapeseed (B. napus L.) pollen on bees’ existance. Chin. J. Oil Crop Sci. 2003, 25, 78–79. [Google Scholar] [CrossRef]
- Duan, J.J.; Marvier, M.; Huesing, J.; Dively, G.; Huang, Z.Y. A meta-analysis of effects of Bt crops on honey bees (Hymenoptera: Apidae). PLoS ONE 2008, 3, e1415. [Google Scholar] [CrossRef] [PubMed]
- Hendriksma, H.P.; Küting, M.; Härtel, S.; Näther, A.; Dohrmann, A.B.; Steffan-Dewenter, I.; Tebbe, C.C. Effect of stacked insecticidal Cry proteins from maize pollen on nurse bees (Apis mellifera carnica) and their gut bacteria. PLoS ONE 2013, 8, e59589. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Ji, Y.; Lai, Y.M.; Zhu, X.; Chen, X.Y.; Xu, J.F.; Ma, L.J.; Xu, X.L. Potential effect of genetically modified maize DR 12-5 on honey bee (Apis mellifera). Acta Agric. Zhejiangensis 2019, 31, 1834–1840. [Google Scholar] [CrossRef]
- Malone, L.; Pham-Delègue, M.-H. Effects of transgene products on honey bees (Apis mellifera) and bumblebees (Bombus sp.). Apidologie 2001, 32, 287–304. [Google Scholar] [CrossRef][Green Version]
- Sanvido, O.; Widmer, F.; Winzeler, M.; Streit, B.; Szerencsits, E.; Bigler, F. Definition and feasibility of isolation distances for transgenic maize cultivation. Transgenic Res. 2008, 17, 317–335. [Google Scholar] [CrossRef]
- Mammadov, J.; Buyyarapu, R.; Guttikonda, S.K.; Parliament, K.; Abdurakhmonov, I.Y.; Kumpatla, S.P. Wild Relatives of Maize, Rice, Cotton, and Soybean: Treasure Troves for Tolerance to Biotic and Abiotic Stresses. Front. Plant Sci. 2018, 9, 886. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, Y.; Zhou, X.; Shen, P.; Peng, Y.F.; Li, Y.H. A laboratory assessment of the potential effect of Cry1Ab/Cry2Aj-containing Bt maize pollen on Folsomia candida by toxicological and biochemical analyses. Environ. Pollut. 2017, 222, 94–100. [Google Scholar] [CrossRef]
- Al-Deeb, M.A.; Wilde, G.E.; Blair, J.M.; Todd, T.C. Effect of Bt corn for corn rootworm control on nontarget soil microarthropods and nematodes. Environ. Entomol. 2003, 32, 859–865. [Google Scholar] [CrossRef]
- Sims, S.R.; Martin, J.W. Effect of Bacillus thuringiensis insecticidal proteins CryIA(b), CryIA(c), CryIIA, and CryIIIA on Folsomia candida and Xenylla grisea (Insecta: Collembola). Pedobiologia 1997, 41, 412–416. [Google Scholar]
- Bai, Y.Y.; Jiang, M.X.; Cheng, J.A. Impacts of transgenic cry1Ab rice on two collembolan species and predation of Microvelia horvathi (Hemiptera: Veliidae). Acta Entomol. Sin. 2005, 48, 42–47. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Meissle, M.; Xue, J.; Zhang, N.; Ma, S.; Guo, A.; Liu, B.; Peng, Y.; Song, X.; Yang, Y.; et al. Expression of Cry1Ab/2Aj Protein in Genetically Engineered Maize Plants and Its Transfer in the Arthropod Food Web. Plants 2023, 12, 4057. https://doi.org/10.3390/plants12234057
Chen Y, Meissle M, Xue J, Zhang N, Ma S, Guo A, Liu B, Peng Y, Song X, Yang Y, et al. Expression of Cry1Ab/2Aj Protein in Genetically Engineered Maize Plants and Its Transfer in the Arthropod Food Web. Plants. 2023; 12(23):4057. https://doi.org/10.3390/plants12234057
Chicago/Turabian StyleChen, Yi, Michael Meissle, Jiabao Xue, Nan Zhang, Shulin Ma, Anping Guo, Biao Liu, Yufa Peng, Xinyuan Song, Yan Yang, and et al. 2023. "Expression of Cry1Ab/2Aj Protein in Genetically Engineered Maize Plants and Its Transfer in the Arthropod Food Web" Plants 12, no. 23: 4057. https://doi.org/10.3390/plants12234057
APA StyleChen, Y., Meissle, M., Xue, J., Zhang, N., Ma, S., Guo, A., Liu, B., Peng, Y., Song, X., Yang, Y., & Li, Y. (2023). Expression of Cry1Ab/2Aj Protein in Genetically Engineered Maize Plants and Its Transfer in the Arthropod Food Web. Plants, 12(23), 4057. https://doi.org/10.3390/plants12234057







