Abstract
The increasing use of nanofertilizers in modern agriculture and their impact on crop yield and pest management require further research. In this study, the effects of nano-Fe, -Zn, and -Cu (which are synthesized based on nanochelating technology), and urea (N) fertilizers on the antioxidant activities of wheat plants (cv. Chamran), and the wheat green aphid Schizaphis graminum (Rondani) are investigated. The authors observed the highest levels of phenolics in non-infested nano-Zn-treated plants (26% higher compared with control). The highest H2O2 levels are in the infested and non-infested nano-Zn-treated and infested nano-Fe-treated plants (in infested nano-Zn and nano-Fe treated plants, 18% and non-infested nano-Zn-treated plants, 28% higher compared with control). The highest peroxidase (POX) activity is observed in the infested and non-infested N-treated and non-infested water-treated plants (almost 14%, 37%, and 46% higher than control, respectively). The lowest activity is in the infested plants’ nano-Zn and -Fe treatments (almost 7 and 5 folds lower compared to the control, respectively). The highest and lowest catalase (CAT) activity are in the infested N-treated plants (almost 42% higher than control) and water-treated plants, respectively. The infested nano-Zn, -Fe, -Cu and Hoagland-treated plants showed the highest superoxide dismutase (SOD) activity. Regarding the antioxidant enzyme activities of S. graminum, the highest POX activity is in the nano-Cu treatment (more than two folds higher compared with control); the highest CAT and SOD activities are in the nano-Cu and -Zn treatments. It can be concluded that the application of nanofertilizers caused increasing effects on the wheat plant’s antioxidant system and its resistance to S. graminum.
1. Introduction
Wheat (Triticum aestivum L.) is one of the most important staple crops directly contributing to global food safety [1]. Numerous insect pests can damage this plant, leading to serious yield losses [2]. The wheat green aphid, Schizaphis graminum (Rondani), is one of the most devastating pests globally. The pest causes substantial economic damage by feeding the wheat plant and transmitting viral pathogens such as barley yellow dwarf virus (BYD) in most crops [3]. Its various biotypes can defeat resistant genes in wheat and sorghum and even detoxify pesticides [4]. Conventional pest control methods significantly contribute to applying insecticides to plants, resulting in significant risks to human and environmental security [5]. Thus, efficient alternative procedures, such as eco-friendly plant-derived agents [6,7], microencapsulation of pesticides, and nanotechnology, have been proposed to solve the issues mentioned above [2,8,9].
Recently, increasing demands for the application of nanotechnology in modern agriculture and environmental sciences have been witnessed, especially due to their exceptional and multipurpose characteristics [10,11]. Numerous studies have indicated the distinctive capability of nanomaterials concerning transportation through the cell wall and cellular membrane as functional barriers [12,13,14]. From this point of view, nanomaterials could be directly implicated in cellular biochemical pathways and reinforce them or act as carriers for other substances that may not be able to pass through the biological barriers alone [12,13].
Previous reports demonstrated the encouraging effects of nanomaterials on the efficiency of photosynthesis, the biosynthesis of secondary metabolites, the plant defense system, plant tolerance against environmental stresses, and the production of antioxidant compounds [15,16]. Since the absorption efficiency of nanomaterials is high, a smaller amount of the reagents would be needed. Accordingly, substituting traditional fertilizers with nanomaterials could decrease the probability of environmental pollution [17,18]. The localized application of high fertilizers such as ammonium salts, urea, nitrate, and phosphate compounds is detrimental. In any case, much of the fertilizers are unavailable to plants as they are lost as run-off leaching, which leads to pollution [19]. Thanks to the higher surface tension and slow release of nanofertilizers, plants could benefit more [20]. Moreover, nanofertilizers can either supply one or more nutrients to plants, resulting in enhanced growth and yield, or facilitate the better performance of conventional fertilizers without directly providing crops with nutrients [21]. Chelated fertilizers generally provide more benefits than other fertilizers, including high solubility, high stability, less environmental pollution, high efficiency in alkaline soils, high and fast mobility in plant roots and leaves, and rapid penetration into the plant [22].
Plants affect herbivores’ survival, nutrition, and reproduction through their biochemical, molecular, morphological, and physiological defense systems. Biotic and abiotic stresses cause opposing impacts on plant processes through changes in physiological and biochemical routes [23]. In this context, accumulating oxidative factors, mainly ROS (Reactive Oxygen Species) and free radicals, is nearly inevitable in all kinds of stresses.
The above-mentioned forms of reduced atmospheric oxygen with a high potential of reduction/oxidation (Redox) could induce lipid peroxidation, membrane damage, tissue necrosis, and other physiological disorders [24]. The increase in the activity of antioxidant enzymes in nanofertilizer-treated plants showed the importance of micronutrients in improving the growth and resistance of plants under stress conditions [25]. The most crucial antioxidant enzymes in both plants and animals are superoxide dismutase (SOD), peroxidase (POX), and catalase (CAT), which are activated during stress to eliminate ROS and H2O2 [26,27].
Plant phloem sap contains a ‘predigested’ food with a high concentration of sugars, providing an abundant source of carbon, nitrogen, and energy [28]. The growth and fecundity of herbivores, particularly sap-sucking insects, are generally influenced by plants’ nitrogen (N) content. Often, nitrogenous compounds are abundant in plant tissues, particularly in phloem sap [29].Therefore, aphids show a straight reaction to changes in the N level of host plants [30]. It has been demonstrated that applying nitrogen to wheat and oat plants can increase S. graminum population density [31]. Other nutritional materials have negative impacts on the reproduction and fertility of pests. For example, the fertility and fitness of the cabbage aphid (Brevicoryne brassicae L.) decreased by approximately 30% in plants treated with Cu and Pb [32]. Also, applying Zn led to detrimental effects on Spodopteralitura (F.) reproduction [33].
Advance chelate compounds technology (chelating and nanochelating) is a new method for producing compounds in different fields. In the last decade, studies proved the efficiency of nanofertilizers that were produced based on this technology. The scientific reports showed that using nano-chelated fertilizers can help plants to overcome against pests and stresses [34,35,36,37].
In the current research, we studied the effects of chelated nanofertilizers (Cu, Fe, and Zn) and N on the antioxidant system and biochemical properties of well-nourished wheat plants. We further attempted to elucidate the interaction of wheat-S. graminum under relatively high concentrations of nanofertilizer treatments by investigating the aphid antioxidant enzymes’ activity. Thus, the nature of the study was multidisciplinary. We developed new approaches to demonstrate how the physiological and biochemical properties of the host plant (wheat) could influence the enzymatic behavior of S. graminum. We also addressed the cascade of biochemical occurrences upon which the induced antioxidant enzymatic signal could be transported from the plant to this aphid. We aimed to investigate the effect of a relatively high concentration of mentioned nanofertilizers (Cu, Fe, and Zn) and N on metabolic processes. In addition, what is the effect of these substances on the antioxidant system of insects (part of the detoxification system), which can indicate the toxicity of these elements in insects, especially aphids?
2. Results
2.1. Total Phenolics Content
Data analysis revealed that the plants treated with different fertilizers had enhanced phenolics content compared with the control (F5,36 = 26.881, p value < 0.01). Similarly, infesting the plants with the aphid led to an increase in the phenolics content compared with the control (except nano-Fe and water) (F5,36 = 26.881, p value < 0.01). Also, the application of nano-Zn caused a significant increase in the production of phenolic compounds in the infested plants compared with the non-infested ones (F5,36 = 26.881, p value < 0.01). No significant effects were observed regarding the N, nano-Cu treatments (F5,36 = 26.881, p value < 0.01). The highest level of phenolic compounds was exhibited in the plants treated with nano-Zn in infested and non-infested plants (F5,36 = 26.881, p value < 0.01) (Figure 1).
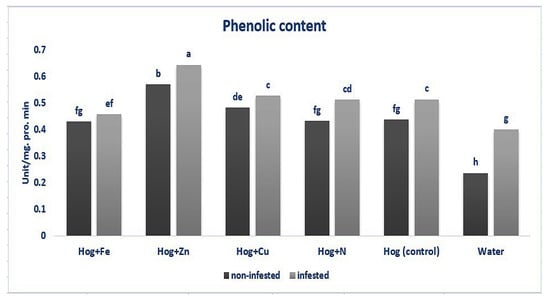
Figure 1.
Phenolic content in wheat plants treated with Fe, Zn, Cu, N, Hoagland (control), and water under Schizaphis graminum-infested and non-infested conditions. Different letters demonstrated significant differences at p = 0.05.
2.2. Wheat Lipid Peroxidation (MDA) Content
The MDA content was enhanced in aphid-infested and non-infested plants (F5,36 = 7.906, p value < 0.01). In non-infested plants, treated plants with fertilizers exhibited significantly higher MDA content than the control (except N- and water-treated plants) (F5,36 = 7.906, p value < 0.01). Furthermore, without considering the nutritional status, the aphid-infested plants showed higher levels of MDA than the non-infested ones (except nano-Zn) (F5,36 = 7.906, p value < 0.01). The highest MDA level was observed in the nano-Zn-treated plants in both infested and non-infested plants (F5,36 = 7.906, p value < 0.01) (Figure 2).
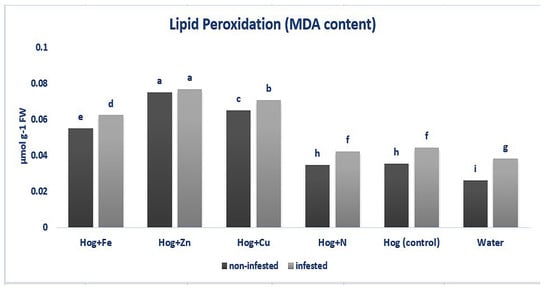
Figure 2.
Rate of malondialdehyde (MDA) in wheat plants treated with Fe, Zn, Cu, N, Hoagland (control), and water under Schizaphis graminum-infested and non-infested conditions. Different letters demonstrated significant differences at p = 0.05.
2.3. Wheat Proline Content
In non-infested plants, treating with nano-Cu and nano-Zn fertilizers caused a significant increase in the proline content compared with the control (F5,36 = 6.653, p value < 0.01). Also, plant infestation with aphids caused an enhancement in proline content compared with the non-infested ones (F5,36 = 6.653, p value < 0.01). Generally, aphid infestation has a higher impact on plant proline content than fertilization. However, significant differences were found in the aphid-infested and fertilizer-treated plants regarding proline amount (F5,36 = 6.653, p value < 0.01) (Figure 3).
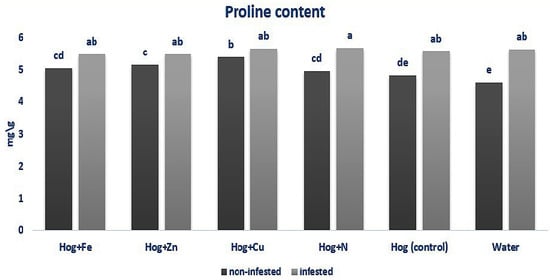
Figure 3.
Amount of proline content in wheat plants treated with Fe, Zn, Cu, N, Hoagland (control), and water under Schizaphis graminum-infested and non-infested conditions. Different letters demonstrated significant differences at p = 0.05.
2.4. Wheat Hydrogen Peroxide (H2O2)
As shown in Figure 4, fertilizer treatments caused significantly higher H2O2 levels than the control, except for water treatment (F5,36 = 3.316, p value < 0.05). Also, H2O2 was enhanced in the aphid-infested plants compared with the control (except in N and water) (F5,36 = 3.316, p value < 0.05). Considering the N and nano-Zn treatments, no significant difference was recorded between the infested and non-infested plants (F5,36 = 3.316, p value < 0.05). On the other hand, the aphid-infested plants in Hog, nano-Cu, nano-Fe, and water treatments demonstrated higher H2O2 content than the non-infested ones (F5,36 = 3.316, p value < 0.05). The highest H2O2 content was obtained in the aphid-infested plants treated with nano-Fe and nano-Zn and in the non-infested nano-Zn treatment (F5,36 = 3.316, p value < 0.05) (Figure 4).
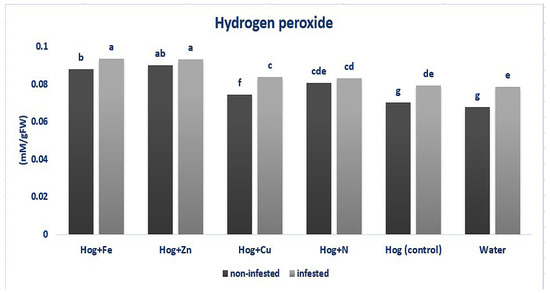
Figure 4.
Amount of hydrogen peroxide in wheat plants treated with Fe, Zn, Cu, N, Hoagland (control), and water under Schizaphis graminum-infested and non-infested conditions. Different letters demonstrated significant differences at p = 0.05.
2.5. Antioxidant Enzymes Assay in Wheat
The infestation of wheat plants with aphids led to a decrease in POX and an increase in CAT and SOD enzyme activities (Figure 5). A similar trend in the changes in POX activity was not observed in treated plants under both infested and non-infested conditions. In non-infested plants, except for the nano-Fe and nano-Zn treatments, the application of the treatments enhanced POX activity (F5,24 = 61.757, p value < 0.01) compared with the control. The lowest rate was detected in nano-Zn-subjected plants, and the highest amounts were ascertained in the water and N treatments.
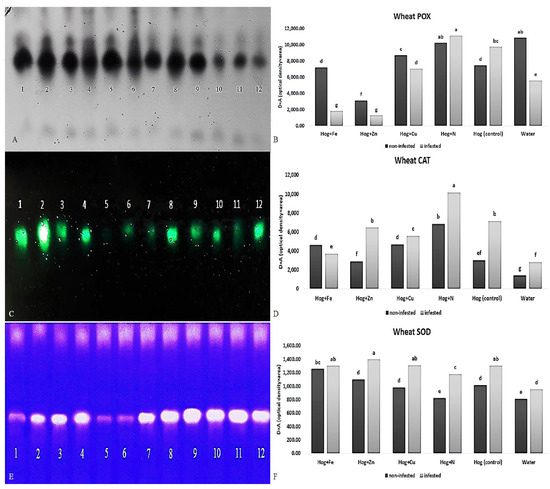
Figure 5.
Native-PAGE (polyacrylamide gel electrophoresis) analysis of peroxidase (POX) (A,B), catalase (CAT) (C,D), and superoxide dismutase (SOD) (E,F) activities in wheat plants treated with Fe, Zn, Cu, N, Hoagland (control), and water under Schizaphis graminum-infested and non-infested conditions. Note: 1, 3, 5, 7, 9, 11 identifying N, nano-Cu, water, Hoagland (control), nano-Fe, nano-Zn treatments under non-infested condition, respectively; 2, 4, 6, 8, 10, 12 identifying N, nano-Cu, water, Hoagland, nano-Fe, nano-Zn treatments under aphid-infested, respectively. Different letters demonstrated significant differences at p = 0.05.
Infestation with aphids increased the rate of wheat POX activity in some fertilizer treatments such as N (F5,24 = 61.757, p value < 0.01). However, the application of nano-Fe and nano-Zn decreased the POX activity compared with the control (F5,24 = 61.757, p value < 0.01). The highest activity of POX was observed in the N treatment, and the lowest was seen in nano-Fe and nano-Zn (F5,24 = 61.757, p value < 0.01) (Figure 5A,B). Aphid infestation caused a significant decrease in wheat POX activity in some treatments compared with non-infested ones (F5,24 = 61.757, p value < 0.01). The POX activity in the water, nano-Fe, and nano-Zn treatments was significantly decreased.
All fertilizer treatments enhanced CAT activity (F5,24 = 52.694, p value < 0.01) in the infested and non-infested plants compared with the control (except in water and nano-Zn). The levels of CAT were higher in the aphid-infested plants than the non-infested ones under the fertilizer treatments, except for the nano-Fe treatment (F5,24 = 52.694, p value < 0.01). Compared with the non-infested plants, aphid infestation caused a significant increase in wheat CAT activity (F5,24 = 52.694, p value < 0.01) (Figure 5C,D). In control and nano-Zn treatments, the CAT activity was significantly increased (F5,24 = 52.694, p value < 0.01). The application of N caused the highest CAT activity in the infested plants (F5,24 = 52.694, p value < 0.01) (Figure 5C,D).
Applying all fertilizers had significant effects on the SOD activity (F5,24 = 19.703, p value < 0.01) in the non-infested plants. Additionally, aphid feeding caused an increase in SOD activity in all fertilizer treatments compared with non-infested ones (F5,24 = 19.703, p value < 0.01). Water-treated plants showed a lower activity of the SOD than the control (F5,24 = 19.703, p value < 0.01) (Figure 5E,F).
2.6. Antioxidant Enzymes Assay in Aphid
Almost all fertilizer treatments significantly increased the aphid POX, CAT, and SOD activities compared with the control (Figure 6). Concerning the applied treatments, the highest and lowest aphid POX activities (F5,12 = 30.878, p value < 0.01) were recorded on nano-Cu and N treatments, respectively (Figure 6A,B). Aphid POX activity in nano-Cu treatment was twice higher than in control (F5,12 = 30.878, p value < 0.01). Moreover, the highest CAT (F5,12 = 26.347, p value < 0.01) (Figure 6C,D) and SOD (F5,12 = 9.851, p value < 0.01) (Figure 6E,F) activities were observed in the aphids reared on the plants treated with nano-Cu and nano-Zn. The lowest activity was obtained in the aphids reared on the N- and water-treated plants. Aphid CAT activity on nano-Cu and nano-Zn was approximately 1.5-fold higher than its activity on the control (F5,12 = 26.347, p value < 0.01) (Figure 6C,D). Also, the CAT activity was almost 2.5 times higher than the activity on the N treatment. Aphid SOD activity on nano-Cu and nano-Zn was 2.5 times higher than that of the water (F5,12 = 9.851, p value < 0.01) (Figure 6E,F).
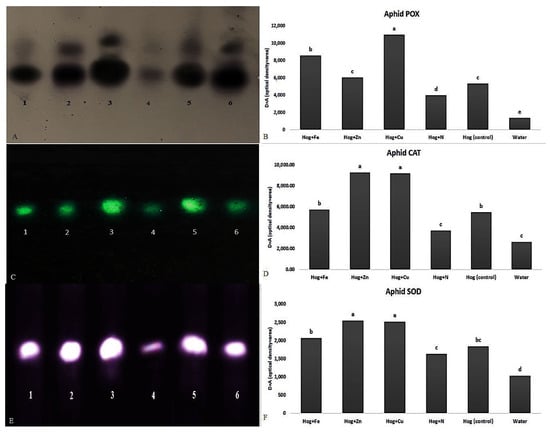
Figure 6.
Native-PAGE (polyacrylamide gel electrophoresis) analysis of peroxidase (POX) (A,B), catalase (CAT) (C,D), and superoxide dismutase (SOD) (E,F) activities in Schizaphis graminum reared on wheat plants treated with Fe, Zn, Cu, N, Hoagland (control), and water. Note: 1, 2, 3, 4, 5, and 6 identifying the enzymatic activities of S. graminum reared on wheat plants treated with N, Hoagland (control), nano-Cu, water, nano-Zn, and nano-Fe, respectively. Different letters demonstrated significant differences at p = 0.05.
As shown in Figure 7, the non-infested plants treated with all fertilizers demonstrated different correlations among the measured factors. The proline content showed a 70 and 79% correlation with phenolics and MDA, respectively. Phenolic contents had 80, 72, and 70% associations with MDA, H2O2, and POX, respectively. MDA displayed 64, 76, and 51% relationships with H2O2, POX, and SOD, respectively. Also, H2O2 demonstrated 63 and 51% correlations with POX and SOD, respectively.
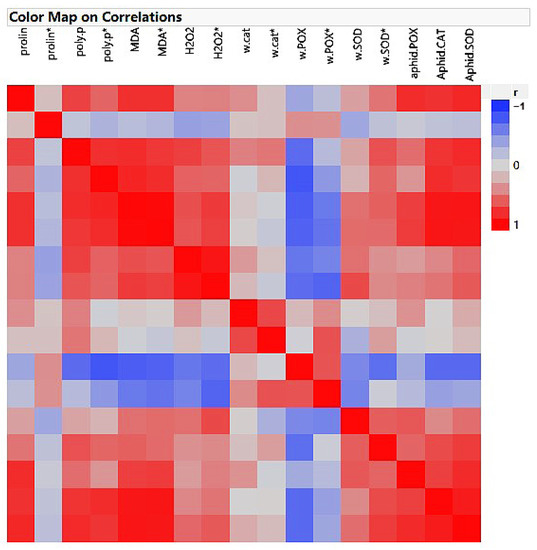
Figure 7.
Heatmap of correlation in the non-infested, Schizaphis graminum-infested, and treated plants. Parameters marked with * were infested by the aphid.
Concerning the heatmap data in the infested and treated plants, phenolic compounds displayed 80, 57, 80, 55, 81, and 76% correlations with the MDA, H2O2, POX, SOD, aphid CAT, and SOD, respectively. MDA showed 71, 64, 53, 73, 92, and 90% correlations with H2O2, POX, SOD, aphid POX, CAT, and SOD, respectively. H2O2 had 73, 60, and 50% associations with wheat POX, SOD, and aphid CAT, respectively (Figure 7).
3. Discussion
According to the symptoms of the nanofertilizer-treated plants (Fe, Zn, and Cu) (Figure 8), the relatively high concentration of the elements probably caused abiotic stress compared to the control, which severely affected the plant antioxidant system and the secondary metabolites. In addition, water-treated plants experienced extreme stress due to nutrient deficiency. Furthermore, aphid activity on the treated plants altered the levels of secondary metabolites and enzymes that can indicate biotic and abiotic stress.

Figure 8.
The appearance of plants treated with different nutrients (nano-Fe, nano-Zn, nano-Cu, N, Hoagland, and water).
Adding micronutrients (i.e., Zn, Cu, and Fe) can induce oxidative stress, but plants have developed various defensive mechanisms, such as enzymatic (including CAT, POX, SOD, ascorbate peroxidase, and glutathione reductase) and non-enzymatic (including ascorbate, glutathione, flavonoids, phenolic compounds, tocopherol, and carotenoids) systems, that allow the scavenging of free radicals [38,39]. Due to the novelty of this field, a few studies have been conducted on this topic.
Our results demonstrated that when plants were fertilized with nano-Zn, nano-Cu, and nano-Fe, the amount of phenolics increased. Consequently, it seems that when plants are infested with aphids, phenolic compounds are available to eliminate radicals produced by aphid feeding. In other words, when biotic and abiotic stresses combined, phenolics increased to eliminate ROS. Phenolics are water-soluble antioxidants with the potential to protect plant cells, besides other critical roles [23]. They could also improve the efficiency of mineral absorption and accelerate the mobilization of elements in plants [40].
Furthermore, some studies have indicated that phenolic compounds substantially protect plants from oxidative stress under metal stresses [41,42,43]. So, it can be concluded that nanofertilizers, in a small amount, may derive plants by increasing phenolics and consequently increasing the absorption of elements. Additionally, increased phenolics may enhance plant resistance to pest attacks and high concentrations of elements. However, the increase in phenolics is not always beneficial to plants. For instance, accumulations of phenolics in plant tissues cause a reduction in plant growth and diminished carbohydrate and N content in phloem sap. However, this could be a restriction factor for aphid feeding on the plants, which is confirmed by the current study. The ZnO NPs application with NaCl in rapeseed (Brassica napus L.) caused the enhancement of phenolics in the seeds [44].
According to research done on Imperta cylidrica, the amount of phenolics in the shoots increased after 21 days of copper exposure [45]. Several studies have demonstrated that Fe deficiency increases the amount of phenolics in plants and their secretion from the roots because phenolics in the rhizosphere cause the chelation of insoluble Fe in the rhizosphere and transfer it to the plant. It can be concluded that the low amount of phenolics in nano-Fe treated plants is due to the sufficient availability of Fe to plant [46]. Chrzanowski & Leszczyński [47] stated that infestation of winter triticale seedlings with Sitobion avenae F. induced phenolics and subsequently induced resistance. Kariyat et al. [48] reported that the flavonoids (as derivatives of phenolic compounds) in wild sorghum caused significant mortality and reduced population growth in Rhopalosiphum maidis Fitch. In general, due to the high level of phenolics in the nano-Zn treatment, this element seems to induce more resistance to biotic and abiotic stress factors.
Aphids, as an external enemy, cause damage to the cell wall and increase the MDA content of the plant [49]. Also, the fact that lipid peroxidation is high in the aphid-infested wheat plants fertilized with different nutrient solutions would deduce that the vigorous plants have a favorable inner condition for encountering external enemies. MDA is the most important indicator for lipid peroxidation of cell membranes [49], affecting other cellular components by damaging proteins, nucleic acids, and polysaccharides [50]. Previous studies verified that the levels of H2O2 and MDA increased in plant cells due to ROS accumulation, the main factor in polyunsaturated lipid oxidation [51,52]. Our results agree with the notion that the levels of MDA often correlate with the extent of aphid activity. In other words, a high level of Zn in plant tissues could influence the production of ROS and MDA levels [53]. Zhang et al. [54] reported that plants fertilized with urea demonstrated a decrease in the MDA content of their leaves. It seems that N prevents the destruction of cell walls in plants or is one of the reasons that makes plants suitable for aphid feeding. Similarly, the current study observed the highest amount of MDA in the aphid-infested plants treated with nano-Zn due to Zn role in antioxidant enzyme activities and MDA production. It can be concluded that the more complete the plant nutrition, the lower the MDA level, and the less cell damage.
Proline is the only proteinogenic secondary amino acid excluding amino groups produced by plants to regulate the osmotic situation during stress conditions. This protects the structure of macromolecules and membranes during severe dehydration [55]. Based on our results, using the nanofertilizers and aphid infestation caused an increase in proline content, which refers to proline’s antioxidant activity and proline role in plant stresses. Proline contents correlate indirectly with water content, stomatal conductivity, photosynthesis, and antioxidant enzyme activity. They have been used as indices of plant stress [56,57]. Proline lowers cytoplasmic pH, maintains the proper ratio of NADP+/NADPH in metabolism, increases various enzymes activities [58], and, importantly, acts as a source of energy, carbon, and nitrogen for tissue recovery under stress conditions [59]. The aphid-infested plants with enhanced proline content may contain lower water content to decrease aphid feeding and help plants cope with stress. Proline enhancement in the infested plants would increase resistance to stress conditions through better photosynthesis. Also, proline enhancement in the infested and fertilizer-treated plants demonstrated higher antioxidant enzyme activities, which agrees with the above-mentioned studies. During the application of micronutrients (including Fe), proline enhancement has been previously confirmed in rice plants [60,61], probably due to a decrease in the activity of the electron transport system [62,63], which resulted in the accumulation of NADH and H+. The higher the metal ion concentration, the higher the protein content in Spirulina and Anabaena [64,65]. This is probably caused by increased proline with metal ion chelating ability.
H2O2 in low concentrations is detoxified by the plant antioxidant system and acts as a signal transduction molecule in plant defense systems against stresses. At higher levels, H2O2 destroys the cell wall and also has a high tendency to DNA, proteins, carbohydrates, and lipids [66,67]. Our results showed that fertilization and aphid infestation increased the amount of H2O2, perhaps because of cell wall destruction and fertilizer effects. One of the side effects of the fertilizers utilized in the current study is their participation in photosynthesis, which enhances H2O2 levels. As essential parts of proteins and enzymes, Fe, Cu, and Zn are positively involved in photosynthesis, which may indirectly increase the levels of H2O2 due to the running of photorespiration [68]. The mechanism by which H2O2 is produced is well understood. It is produced in peroxisomes, and RuBisCO is the key enzyme in this process [69,70]. The fulfilment of photosynthesis in healthy plants is obligatorily tied to oxygenation as a form of H2O2 [71]. Superoxide anion and H2O2 act as wound signaling factors generated in the damaged tissues under biotic stress conditions [72], confirming our results.
Nonetheless, the balance between the generation and removal of H2O2 would determine if the molecule would act as a signaling factor or a destructive element [73,74]. In this regard, low levels of H2O2 would participate in wound stress signaling. However, high reagent levels could turn on the antioxidant system in the plant.
The significant correlation obtained in the current study for the levels of H2O2, the application of the nanofertilizers, and the aphid activity on the plants agreed with the above-mentioned subjects. Fe could improve the photosynthetic efficiency of plants [75]. Moreover, it is an essential part of proteins and enzymes, resulting in an important function in plants’ physiological and biological processes [68]. Consequently, attributable to the encouraging effects of Zn and Fe on photosynthesis, respiration, cellular metabolism, and antioxidant enzymes, the increase in the level of H2O2 in nano-Zn and nano-Fe treatments might be explained.
H2O2 and ROS are detoxified by the plant antioxidant system and several enzymes, including SOD, POX, and CAT, to control ROS levels and protect cells under suboptimal conditions [26]. In addition, wounding due to aphid activity could activate antioxidant enzymes to eliminate ROS from the injured cells and lessen their effects [72]. Therefore, augmenting the antioxidant enzymatic activity and ascending the levels of antioxidants could be assessed as a primary symptom of stress in plants, which causes an obvious diminish in their growth and induces resistance to the stress factors. In addition, the existence of Zn is correlated with the activity of antioxidant enzymes, which consequently increases the levels of ROS and MDA [51,76]. Some reports have indicated that Zn could act as a cofactor for antioxidant enzymes at low levels. However, higher concentrations of Zn lead to toxic effects by which the activity of the related enzymes could be increased, in line with the current findings to some extent. Cu, a redox-active transition metal in Fenton chemistry, catalyzes hydroxyl radical production. Likewise, Zn, a redox-inactive metal, disables the cellular antioxidant pool, disrupting the metabolic balance and then enhancing a load of ROS [77,78].
Similarly, upper Cu or Fe amounts enhanced ROS generation via the Fenton reaction [79,80]. Furthermore, several studies have shown evidence of stress in plants exposed to ZnO NPs-treated soils [21,81,82]. According to our findings, fertilizing with nano-Zn, nano-Fe, and nano-Cu could benefit the plant, leading to the relative activity of the antioxidant system and making the plant potentially resistant to biotic and abiotic stresses.
Our study indicated that the water-treated wheat plants are demonstrating high activity of POX, which probably refers to the accumulation of H2O2 through photorespiration and the attempt of the plant to remove ROS by exploiting the POX enzyme. The decrease in the POX activity in the infested plants (with any treatments) may be due to diminishing photosynthesis and reducing photorespiration, which may lead to a decrease in the H2O2 levels. In this situation, the production of H2O2 would be low.
Concerning the water-treated wheat plants, the high activity of POX probably indicated nutritional stress in the plant. The decrease in the POX activity of the infested plants receiving any treatments may be due to the plant’s inability to maintain its defensive state and survive. Generally, higher plants induce POX in response to the absorption of microelements (e.g., Cu, Zn, Cd, and Pb) [83]. The lowest POX activity in the infested nano-Zn and nano-Fe treatments probably refers to their higher amounts of H2O2 and even MDA leading to problems in their decomposition by the enzyme. CAT likely played an essential role in neutralizing H2O2 in these treatments.
The CAT is the main antioxidant enzyme inhibiting H2O2, and its activity is enhanced in plants under stress conditions [84]. The increase in CAT activity is considered an indicator of higher ROS production. However, in some cases, a decrease in the activity of antioxidant enzymes has been reported under stress conditions [85]. In the current study, N increased CAT activity in the aphid-infested wheat plants, which may be related to its role in enhancing aphid feeding and the polemical role of N, in which it increased CAT activity in some plants while decreasing it in others. The role of N in the oxidative state of the plant is very controversial; for instance, in some cases, high N concentrations enhance the antioxidant defense in plants [54,86] and decrease lipid peroxidation (MDA) [54]. In plants such as beet [86], maize [54], and poplar [87], N content increases CAT activity. Rengel & Graham [88] showed that Zn fertilizer reduced the CAT activity of wheat plants. Zn and Fe are critical parts of many fundamental enzymes, such as CAT and SOD, and also take part in the synthesis of chlorophyll and indole-3-acetic acid (IAA) [89,90].
As our findings demonstrated, the aphid-infested plants treated with nano-Zn and nano-Cu revealed higher levels of SOD activity. This might be related to the cupro-zinc property of SOD. It could occur due to the plant’s ability to maintain the homeostatic state of Zn under oxidative stress conditions, as confirmed by several subsequent reports in line with the current outcomes. Studies have stated that SOD, the first line of defense to scavenge ROS [91], is a cupro-zinc protein. Thus it becomes essential to maintain Zn homeostasis in plant cells to protect them from oxidative stress [92]. SOD and CAT are the most important antioxidant enzymes that are functionally related because SOD converts superoxide free radical (O2) to H2O2, which is removed by CAT [93]. The infested wheat plants receiving different nano-nutrients demonstrated increased SOD and CAT activities, with the highest activities in nano-Zn, nano-Fe, and nano-Cu, introducing the enzymes as the first line of defense against oxidative stress [94]. Enhanced activity of SOD was reported in algal chloroplasts under Cu application [95,96]. In addition, SOD activity was enhanced in Scenedesmus bijugatus exposed to different Cu concentrations [97]. Since Zn is one of the metal cofactors of the antioxidative enzyme superoxide dismutase (SOD), its application in higher dosages could initiate oxidative stress [98].
Insects need low micronutrients, but higher concentrations of these elements are toxic and cause stress [91,92,93]. High concentrations of elements cause destructive effects on different processes in animals, including survival, growth, reproduction, metabolism, and the innate immune system [99,100]. Several studies confirmed the importance of ROS-dependent immunity in the immune system of invertebrates for survival [101]. The effects of nanomaterials on the immune defense mechanisms of insects have been poorly investigated. Furthermore, using antioxidant systems in response to ROS is one of the primary techniques for analyzing the toxicity of these compounds. Several studies confirmed this [102,103,104]. As an illustration, utilizing CuO NPs on Blapus sulcata demonstrated destructive effects on the DNA. Also, it disrupted the antioxidant system, including increased SOD activity, on the other hand decreased CAT and acetyl cholinesterase (AChE) activities [103]. In invertebrates, hemolymph enzymes are essential in humoral defense [105]. Therefore, innate immunity and antioxidant enzymes have been used as indicators for stress tolerance in invertebrates and to determine the level of environmental pollution [106]. It has been reported that the influence of nanoparticles on antioxidant enzyme activity varies based on numerous aspects, such as size, concentration, and the treated organism [107]. The activity of antioxidant enzymes may extremely change during the onset of high concentrations of elemental exposure but return to normal levels after a few days [108]. It seems that in the presence of non-toxic amounts of micro-fertilizers, the antioxidant system of insects continued its activity. The increase in the activity of antioxidant enzymes in aphids under different nanofertilizer treatments might confirm the transfer of these substances into the food chain. The insect midgut is the main site of food absorption, where the metals are mainly concentrated. The main site for micronutrient accumulation in insects is fat bodies involved in several homeostatic functions, such as regulating the synthesis and storage of nutrients or providing several metabolic pathways [109,110].
In this study, the highest activities of antioxidant enzymes in aphids were frequently observed in nano-Cu and nano-Zn treatments. The following studies support the findings of current research: Abd El-Wahab and Anwar [111] indicated that Cu and Zn nanoparticles increased SOD enzyme activity in Spodoptera littoralis Boisduval larvae. Investigations conducted on Drosophila sp. [112] and Glyphodes pyloalis Walker [113] also established the effects of metal nanoparticles on the antioxidant activity of these insects. In S. littoralis, ZnO NPs increased the activities of SOD and CAT [107]. Gomes et al. [114] similarly showed that the SOD and CAT activities increased after one week of exposure to CuO NPs in the digestive gland of Monochamus galloprovincialis Olivier. According to our results, the nano-Zn treatment causes an increase in enzyme activity, which confirms the critical role of Zn in enzyme function. A conclusion that may be drawn is that, in addition to the positive role of Zn in insects’ metabolism, the utilization of nano-Zn acts as an abiotic stress agent for insects and may be beneficial to pest management. In insects, Zn plays a vital role in the synthesis of lipids, proteins, carbohydrates and the duration of larval and pupal stages [115]. Also, Zn inhibits ROS production by competing with transition metals, especially Fe [116].
Insects could minimize ROS impacts through the sequestration of Fe in non-reactive forms due to the effect of free Fe (or Cu) in the accumulations of superoxide and H2O2. Although low-molecular-weight cellular components may chelate Fe ions, these Fe chelates can still participate in Fenton-type reactions. Based on our findings, considering the low levels of antioxidant enzymes in nano-Fe treatment, it may be that nano-Fe was not toxic enough to the aphids because specific proteins are responsible for transporting and storing Fe in its non-reactive form in the most efficient way [117] in which “transferrin” is the major one in insects as well as vertebrates [118]. Besides, “ferritin” facilitates Fe transport and storage in insects. Ferritin preserves Fe in a non-toxic form at relatively high concentrations so that it can be used for the biosynthesis of Fe-containing proteins [118]. The application of FeSO4 on Schistocerca gregaria generated oxidative stress leading to macromolecule damage and higher antioxidant enzyme activities (e.g., SOD, CAT), presenting a somewhat negative effect of Fe-containing fertilizers on insect physiology [119].
Regarding function, the xylem and phloem are tightly associated in plants. In short, stem bundles and minor veins without the cambium of cereal, xylem and phloem interact more closely and directly [120,121]. Various studies have demonstrated that high concentrations of elements and micronutrients are transported through the phloem [122,123]. Zn has high mobility in the phloem [122], but Fe is less mobile than Zn [124]. Despite the absence of toxins in the phloem, aphids feed on phloem sap. Because of the high concentration of micronutrients in the phloem, they transfer to the insect body and cause toxicity; hence, this may be the reason for the antioxidant system activation in these insects. As the toxicity of nanoparticles has not yet been clearly studied and is, of course, not entirely understood, it is clear that they cannot be employed carelessly. It is obvious that micronutrients affect crop nutrition positively and induce plant resistance to biotic and abiotic stresses. The application of nanotechnology in agriculture, particularly manufacturing nanofertilizers or nanopesticides, has also improved their performance and efficiency. Considering that few studies have been done on environmental pollution and transmission of elements to higher food chains and that there is little understanding of the quantity and mechanism of accumulation of nanomaterials, more studies are needed. Also, a comparison should be made between conventional fertilizers and nanofertilizers, as well as their transmission speed and volume.
4. Materials and Methods
The study was conducted at the research greenhouse and laboratory of Experimental Plant Physiology, Faculty of Agriculture, University of Tabriz (Tabriz, Iran).
4.1. Plant Material
The plants were obtained by sowing wheat seeds (cv. Chamran) in medium-sized (3 L) pots filled with perlite (10 plants per pot). The pots were then transferred to the greenhouse and kept at 22 ± 1 °C, 60 ± 5% RH and 16:8 (L:D) h photoperiod. These seeds were irrigated with tap water every other day until germination. Afterwards, germinated plants were irrigated with half-strength Hoagland’s solution [125] for the initial stages of germination for up to seven days. Then full-strength Hoagland’s solution was used (for two weeks). The pots were washed every five days to remove excess ions. For treatment application, the plant was treated with nanofertilizers every other day. Plant samples were taken for biochemical assays one week after the application of the treatments (Figure 8).
4.2. Nanomaterials Tested
The exploited nanomaterials, including nano-zinc (Zn), nano-copper (Cu), and nano-iron (Fe), were obtained from Khazra Company (Tehran, Iran) (Figure S1 and Table S1), and added to the nutrient solution. In addition, N was added in the form of urea (Merck Co., Darmstadt, Germany) into the nutrient solution. Based on the manufacturing company’s instructions, we used the average high and low values of the recommended concentrations for each pot (Table 1). Water-treated plants (as a treatment of nutrient deficiency stress to compare nutrient excess treatments) received only tap water. Hoagland-treated plants are considered as a control for this experiment.

Table 1.
Fertilizer amounts recommended by the manufacturer per hectare and amounts used per pot in this experiment.
4.3. Insect Culture
The initial population of S. graminum was obtained from the previously existing colony in the Department of Plant Protection, University of Mohaghegh Ardabili, Iran, and reared on cultivated wheat plants (with three leaves). Infested plants were gradually replaced by healthy ones to ensure the availability of adequate aphids for the experiment. For the biochemical studies and investigating plant-aphid interactions, after one week of plant treatment, the leaves were infested with aphids, and feeding adult aphids on the leaves was enclosed using the plastic straw (0.5 × 10 cm) to avoid the aphids escaping from the target plants (Figure 9). All the assays were done with the same leaves and samples. The leaf samples were powdered using liquid nitrogen and then kept in a −80 °C freezer. Finally, all the assessments were done using these samples. All newborn nymphs were removed from the plants, and only primary mother entities were permitted to feed. Almost 72 h after feeding on treated wheat and control plants, 100 selected adult aphids were transferred into 1.5 mL microtubes and homogenized by a homogenizer. Then, the resulting mixture was centrifuged (15,000 rpm at 4 °C for 15 min), and obtained supernatant was used for the enzymatic assay by smearing Whatman® papers and gelling. All measurements were performed in aphid-infested and non-infested conditions.
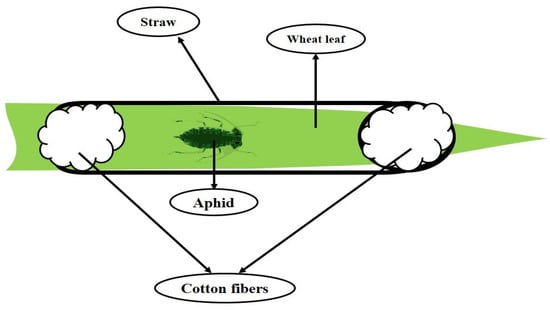
Figure 9.
General scheme of limiting aphids on a wheat leaf.
4.4. Total Phenolics Content
The total phenolics were measured (in four replicates) using the Folin-Ciocalteau reagent. Fresh, expanded leaves (0.1 g) were homogenized in 5 mL of 95% ethanol and centrifuged (10,000 rpm for 10 min). The digested mixture was kept in the dark, and then 1 mL supernatant, 1 mL of 95% ethanol, and 3 mL distilled water were mixed. Subsequently, Folin-Ciocalteau (1 mL) reagent, together with sodium carbonate 20% (5 mL) and deionized water (10 mL), was added to the prepared solution (100 mL). This mixture was completely stirred, and the absorbance was read at 750 nm using a spectrophotometer (UV-1800 Shimadzu, Kyoto, Japan). The phenolics content was expressed as mg per g of fresh leaves [126].
4.5. Lipid Peroxidation Content
Malondialdehyde (MDA) content, as an index of general lipid peroxidation, was measured (in four replicates) based on the colorimetric method [127,128]. Fresh, expanded leaf samples (0.1 g) were homogenized in 3 mL of trichloroacetic acid (TCA) 1% (w/v) at 4 °C. After centrifuging (15,000 rpm for 20 min), the same volume of TCA 20% was added to the test tubes at 96 °C for 30 min. Prepared extracts were then retained at 0 °C for 5 min, centrifuged (10,000 rpm, 5 min), and their absorbance was measured at 532 and 600 nm using a spectrophotometer. The MDA content was calculated based on the standardization procedure [128].
4.6. Proline Assay
The proline content (in four replicates) was assayed using standard phytochemical procedures with minor modifications [23]. For this purpose, samples from fully-expanded leaves (0.5 g) were homogenized in pre-cooled aqueous sulfosalicylic acid 3%. The provided solution was centrifuged (6000 rpm at 4 °C for 7 min), and supernatant (2 mL) was mixed with ninhydrin acid (2 mL) and glacial acetic acid (2 mL) and then incubated at 100 °C for 1 h. The reaction mixture was extracted with toluene under vigorous shaking for 20 s. The absorbance of the samples was determined at 520 nm by exploiting the spectrophotometer. The proline values were measured using the standard curve [129].
4.7. Hydrogen Peroxide Content
Fresh expanded leaf tissues (0.5 g) was extracted with 2 mL of 0.1% (w/v) TCA in an ice bath and subsequently centrifuged (12,000 rpm at 4 °C for 15 min). Afterwards, 0.5 mL potassium phosphate buffer (pH 7) and 1 mL potassium iodide (KI) were added to the 0.5 mL supernatant. The absorbance of the prepared solution was finally determined at 390 nm using a spectrophotometer, and the hydrogen peroxide content was measured based on the standard curve [130]. This measurement was done in four replications.
4.8. Native Polyacrylamide Gel Electrophoresis (Native PAGE)
The activity of antioxidative enzymes, including superoxide dismutase (SOD), peroxidase (POX), and catalase (CAT), was determined on a native PAGE. For this purpose, an extract of fresh leaf tissues was obtained using an extraction buffer (Tris-HCl, pH 7.5) based on the procedure described by Naderi, et al. [131]. Extracted samples were centrifuged (10,000 rpm at 4 °C for 10 min), at which point small pieces of filter paper (3 × 5 mm, Whatman® 3) were immediately submerged. After absorbing the extract with papers, they were put in a 7.5% horizontal slab of the polyacrylamide gels (0.6 × 15 × 12 cm), which were prepared using Poulik buffer [132] and TBE (Tris-Borate-EDTA) electrode buffer (pH 8.8). Electrophoresis of the provided gels was carried out (4 °C, 3 h, 30 mA, 180 V), followed by staining of SOD and CAT [132], as well as POX [133]. Subsequently, the gels were fixed, and images were immediately acquired.
A similar method was fulfilled for the aphid samples. After the emergence of adult aphids on different treatments, 100 adults were randomly collected in 1.5 mL microtubes containing 0.5 mL distilled water. Then, the obtained samples were homogenized using a homogenizer and centrifuged (15,000 rpm at 4 °C for 15 min). The provided supernatant was used for the aphid enzymatic assay.
4.9. Statistical Analysis
The gel images were saved in TIFF format and analyzed using ImageJ software (ImageJ 1.52). The figures were drawn using Excel 2013, and the numerical data were imported into the statistical software SPSS ver. 20. The plant factors experiments were conducted on a factorial design based on a completely randomized design (CRD) at two levels (aphid-infested and non-infested). Analysis of variances was performed using two-way ANOVA and the means comparing was done using the Duncan Multiple Range Test (DMRT) at p = 0.05. The measurements of aphid antioxidant activity were carried out based on CRD, with DMRT to compare the means (at p = 0.05).
5. Conclusions
Considering the results of this study, nano-Zn application caused the highest antioxidant levels in wheat plants. In addition, the application of nano-Cu, nano-Fe, and nitrogen (N) activated antioxidant systems. All led to the hypothesis that the proportionate and combined use of nanofertilizers could enhance plant resistance to the pest; moreover, the toxicity impact of nano-Zn, nano-Fe, and nano-Cu on the aphid, identified by the activation of the insect’s antioxidant system, might demonstrate a reflection of the unfavorable influence of these elements on the aphid. More definitive results require more studies, particularly on the nanofertilizers effects on the life table, fertility, and length of the insect’s development, plus the environmental effects of the nanofertilizers application. However, it can be recommended that further research be carried out on the effects of relatively high element concentrations on wheat yield and the effects on aphid life table parameters, given the increasing effects that were found on the activity of antioxidant enzymes in aphids.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12142602/s1, Table S1: Size of nano-chelated fertilizers used in the experiment; Figure S1: The transmission electron microscope (TEM) images of nano-chelated fertilizers (A) Fe nano-chelated fertilizer (B) Cu nano-chelated fertilizer (C) Zn nano-chelated fertilizer.
Author Contributions
Conceptualization, B.N., J.E. and H.R.-D.; methodology, M.C. and B.N.; formal analysis, M.C. and J.E.; investigation, M.C. and B.N.; writing—original draft preparation, M.C. and B.N.; writing—review and editing, M.C., B.N., A.E. and F.P.; supervision, B.N., J.E. and H.R.-D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data that support the findings of this study are available upon reasonable request.
Acknowledgments
The paper was revised by Mohammadreza Dadpour and Sima Panahi Rad (Department of Horticulture, University of Tabriz, Iran), and their helpful recommendations made a significant improvement. These two honorable scientists are sincerely appreciated. The cytogenetic laboratory, which the authors used to carry out the project’s research, was kindly provided by Mostafa Valizadeh, a retired professor, and Siamak Alavi-Kia (Department of Plant Breeding, University of Tabriz, Iran). We would like to thank Milad Ghasemi (a former Ph.D. student, Department of Plant Breeding, University of Tabriz, Iran), who helped us with statistical analysis. The authors appreciate the assistance of Fatemeh Shariat and Amir Kohnmoei, technicians in the cytogenetic and molecular breeding laboratories (Plant Breeding Department, University of Tabriz, Iran), who assisted us in carrying out this study. Furthermore, we profoundly thank the university of Tabriz and University of Mohaghegh Ardabili for providing the necessary facilities to conduct this research. For supplying the important details on nanofertilizers, we especially thank the reputable Khazra Company and Negar Serajzade.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shewry, P.R. Wheat. J. Exp. Bot. 2009, 60, 1537–1553. [Google Scholar] [CrossRef] [PubMed]
- Lopes, T.; Hatt, S.; Xu, Q.; Chen, J.; Liu, Y.; Francis, F. Wheat (Triticum Aestivum L.)-Based Intercropping Systems for Biological Pest Control. Pest Manag. Sci. 2016, 72, 2193–2202. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.M. Intraspecific Variability of Luteovirus Transmission within Aphid Vector Populations. In Luteoviridae; Smith, H.G., Barker, H., Eds.; CABI Publisher: Wallingford, UK, 1999. [Google Scholar]
- Gray, S.M.; Smith, D.M.; Barbierri, L.; Burd, J. Virus Transmission Phenotype Is Correlated with Host Adaptation among Genetically Diverse Populations of the Aphid Schizaphis Graminum. Phytopathology 2002, 92, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, P.R.R.; Oliveira, M.D.; Barros, E.M.; Michaud, J.P.; Torres, J.B. Differential Impacts of Six Insecticides on a Mealybug and Its Coccinellid Predator. Ecotoxicol. Environ. Saf. 2018, 147, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Ebadollahi, A.; Ziaee, M.; Palla, F. Essential Oils Extracted from Different Species of the Lamiaceae Plant Family as Prospective Bioagents against Several Detrimental Pests. Molecules 2020, 25, 1556. [Google Scholar] [CrossRef]
- Russo, R.; Palla, F. Plant Essential Oils as Biocides in Sustainable Strategies for the Conservation of Cultural Heritage. Sustainability 2023, 15, 8522. [Google Scholar] [CrossRef]
- Biondo, F.; Baldassarre, F.; Vergaro, V.; Ciccarella, G. Controlled Biocide Release from Smart Delivery Systems: Materials Engineering to Tune Release Rate, Biointeractions, and Responsiveness. In Nanotechnology-Based Sustainable Alternatives for the Management of Plant Diseases; Balestra, G.M., Fortunati, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 31–147. ISBN 9780128225882. [Google Scholar]
- Gad, H.A.; Al-Anany, M.S.; Atta, A.A.M.; Abdelgaleil, S.A.M. Efficacy of Low-Dose Combinations of Diatomaceous Earth, Spinosad and Trichoderma Harzianum for the Control of Callosobruchus Maculatus and Callosobruchus Chinensis on Stored Cowpea Seeds. J. Stored Prod. Res. 2021, 91, 101778. [Google Scholar] [CrossRef]
- Hermes, P.H.; Gabriela, M.P.; Ileana, V.R.; Fusaro, C.; Fernando, L.V.; Mariana, M.A.; Padilla-Rodríguez, C.; Fabián, F.L. Carbon Nanotubes as Plant Growth Regulators: Prospects. In Nanotechnology in the Life Sciences; Springer Science and Business Media B.V.: Berlin/Heidelberg, Germany, 2020; pp. 77–115. [Google Scholar]
- Suriya Prabha, A.; Angelin Thangakani, J.; Renuga Devi, N.; Dorothy, R.; Nguyen, T.A.; Senthil Kumaran, S.; Rajendran, S. Nanotechnology and Sustainable Agriculture. In Nanosensors for Smart Agriculture; Denizli, A., Nguyen, T.A., Rajendran, S., Yasin, G., Nadda, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 25–39. ISBN 9780128245545. [Google Scholar]
- Serag, M.F.; Kaji, N.; Gaillard, C.; Okamoto, Y.; Terasaka, K.; Jabasini, M.; Tokeshi, M.; Mizukami, H.; Bianco, A.; Baba, Y. Trafficking and Subcellular Localization of Multiwalled Carbon Nanotubes in Plant Cells. ACS Nano 2011, 5, 493–499. [Google Scholar] [CrossRef]
- Su, Y.; Ashworth, V.; Kim, C.; Adeleye, A.S.; Rolshausen, P.; Roper, C.; White, J.; Jassby, D. Delivery, Uptake, Fate, and Transport of Engineered Nanoparticles in Plants: A Critical Review and Data Analysis. Environ. Sci. Nano 2019, 6, 2311–2331. [Google Scholar] [CrossRef]
- Nair, R.; Varghese, S.H.; Nair, B.G.; Maekawa, T.; Yoshida, Y.; Kumar, D.S. Nanoparticulate Material Delivery to Plants. Plant Sci. 2010, 179, 154–163. [Google Scholar] [CrossRef]
- Shweta; Sood, S.; Sharma, A.; Chadha, S.; Guleria, V. Nanotechnology: A Cutting-Edge Technology in Vegetable Production. J. Hortic. Sci. Biotechnol. 2021. [Google Scholar] [CrossRef]
- Vera-Reyes, I.; Vázquez-Núñez, E.; Lira-Saldivar, R.H.; Méndez-Argüello, B. Effects of Nanoparticles on Germination, Growth, and Plant Crop Development. In Agricultural Nanobiotechnology; López-Valdez, F., Fernández-Luqueño, F., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 77–110. [Google Scholar]
- Salama, D.M.; Abd El-Aziz, M.E.; Rizk, F.A.; Abd Elwahed, M.S.A. Applications of Nanotechnology on Vegetable Crops. Chemosphere 2021, 266. [Google Scholar] [CrossRef]
- Scott, N.R.; Chen, H.; Cui, H. Nanotechnology Applications and Implications of Agrochemicals toward Sustainable Agriculture and Food Systems. J. Agric. Food Chem. 2018, 66, 6451–6456. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.A.; Tran, N.H.; Milev, A.S.; Kannangara, G.S.K.; Volk, H.; Lu, G.Q.M. Nanomaterials in Soils. Geoderma 2008, 146, 291–302. [Google Scholar] [CrossRef]
- Villagarcia, H.; Dervishi, E.; De Silva, K.; Biris, A.S.; Khodakovskaya, M.V. Surface Chemistry of Carbon Nanotubes Impacts the Growth and Expression of Water Channel Protein in Tomato Plants. Small 2012, 8, 2328–2334. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Potentials of Engineered Nanoparticles as Fertilizers for Increasing Agronomic Productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef]
- Liu, G.; Hanlon, E.; Li, Y. Understanding and Applying Chelated Fertilizers Effectively Based on Soil PH: HS1208/HS1208, 11/2012. EDIS 2012, 2012. [Google Scholar] [CrossRef]
- Gohari, G.; Panahirad, S.; Sepehri, N.; Akbari, A.; Zahedi, S.M.; Jafari, H.; Dadpour, M.R.; Fotopoulos, V. Enhanced Tolerance to Salinity Stress in Grapevine Plants through Application of Carbon Quantum Dots Functionalized by Proline. Environ. Sci. Pollut. Res. 2021, 28, 42877–42890. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Ali, B.; Rani, I.; Hayat, S.; Ahmad, A. Effect of 4-Cl-Indole-3-Acetic Acid on the Seed Germination of Cicer Arietinum Exposed to Cadmium. Acta Bot. Croat. 2007, 66, 57–65. [Google Scholar]
- Apel, K.; Hirt, H. Reactive Oxygen Species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Wang, Y.; Oberley, L.W.; Murhammer, D.W. Evidence of Oxidative Stress Following the Viral Infection of Two Lepidopteran Insect Cell Lines. Free Radic. Biol. Med. 2001, 31, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.A.; Schulz, A. Macromolecular Trafficking in the Phloem. Trends Plant Sci. 1999, 4, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Mattson, W.J., Jr. Herbivory in Relation to Plant Nitrogen Content. Annu. Rev. Ecol. Syst. 1980, 11, 119–161. [Google Scholar] [CrossRef]
- Van Emden, H.F. Studies on the Relations of Insect and Host Plant: III. A Comparison of the Reproduction of Brevicoryne Brassicae and Myzus Persicae:(Hemiptera: Aphididae) on Brussels Sprout Plants Supplied with Different Rates of Nitrogen and Potassium. Entomol. Exp. Appl. 1966, 9, 444–460. [Google Scholar] [CrossRef]
- Daniels, N.E. Greenbug Populations and Their Damage to Winter Wheat as Affected by Fertilizer Applications. J. Econ. Entomol. 1957, 50, 793–794. [Google Scholar] [CrossRef]
- Gorur, G. Effects of Host Plant Contaminated with Heavy Metals on the Life History Traits of Aphids (Brevicoryne Brassicae L.). Polish J. Ecol. 2007, 55, 113. [Google Scholar]
- Shu, Y.; Zhou, J.; Tang, W.; Lu, K.; Zhou, Q.; Zhang, G. Molecular Characterization and Expression Pattern of Spodoptera Litura (Lepidoptera: Noctuidae) Vitellogenin, and Its Response to Lead Stress. J. Insect Physiol. 2009, 55, 608–616. [Google Scholar] [CrossRef]
- Fakharzadeh, S.; Hafizi, M.; Baghaei, M.A.; Etesami, M.; Khayamzadeh, M.; Kalanaky, S.; Akbari, M.E.; Nazaran, M.H. Using Nanochelating Technology for Biofortification and Yield Increase in Rice. Sci. Rep. 2020, 10, 4351. [Google Scholar] [CrossRef]
- Astaneh, N.; Bazrafshan, F.; Zare, M.; Amiri, B.; Bahrani, A. Nano-Fertilizer Prevents Environmental Pollution and Improves Physiological Traits of Wheat Grown under Drought Stress Conditions. Sci. Agropecu. 2021, 12, 41–47. [Google Scholar] [CrossRef]
- Ahmadian, K.; Jalilian, J.; Pirzad, A. Nano-Fertilizers Improved Drought Tolerance in Wheat under Deficit Irrigation. Agric. Water Manag. 2021, 244, 106544. [Google Scholar] [CrossRef]
- Rahemi, M.; Gharechahi, S.R.; Sedaghat, S. The Application of Nano-Iron Chelate and Iron Chelate to Soil and as Foliar Application: Treatments against Chlorosis and Fruit Quality in Quince. Int. J. Fruit Sci. 2020, 20, 300–313. [Google Scholar] [CrossRef]
- Schutzendubel, A.; Polle, A. Plant Responses to Abiotic Stresses: Heavy Metal-induced Oxidative Stress and Protection by Mycorrhization. J. Exp. Bot. 2002, 53, 1351–1365. [Google Scholar] [CrossRef] [PubMed]
- Gratão, P.L.; Polle, A.; Lea, P.J.; Azevedo, R.A. Making the Life of Heavy Metal-Stressed Plants a Little Easier. Funct. Plant Biol. 2005, 32, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, G.; Jayasinghearachchi, H.S. Mycelial Colonization by Bradyrhizobia and Azorhizobia. J. Biosci. 2003, 28, 243. [Google Scholar] [CrossRef]
- Kaur, R.; Yadav, P.; Sharma, A.; Thukral, A.K.; Kumar, V.; Kohli, S.K.; Bhardwaj, R. Castasterone and Citric Acid Treatment Restores Photosynthetic Attributes in Brassica Juncea L. under Cd (II) Toxicity. Ecotoxicol. Environ. Saf. 2017, 145, 466–475. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Sharma, A.; Gautam, V.; Arora, S.; Bhardwaj, R.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Interaction of 24-Epibrassinolide and Salicylic Acid Regulates Pigment Contents, Antioxidative Defense Responses, and Gene Expression in Brassica Juncea L. Seedlings under Pb Stress. Environ. Sci. Pollut. Res. 2018, 25, 15159–15173. [Google Scholar] [CrossRef]
- Handa, N.; Kohli, S.K.; Sharma, A.; Thukral, A.K.; Bhardwaj, R.; Abd_Allah, E.F.; Alqarawi, A.A.; Ahmad, P. Selenium Modulates Dynamics of Antioxidative Defence Expression, Photosynthetic Attributes and Secondary Metabolites to Mitigate Chromium Toxicity in Brassica Juncea L. Plants. Environ. Exp. Bot. 2019, 161, 180–192. [Google Scholar] [CrossRef]
- Hezaveh, T.A.; Pourakbar, L.; Rahmani, F.; Alipour, H. Effects of ZnO NPs on Phenolic Compounds of Rapeseed Seeds under Salinity Stress. J. Plant Process Funct. 2020, 8, 11–18. [Google Scholar]
- Vidal, C.; Ruiz, A.; Ortiz, J.; Larama, G.; Perez, R.; Santander, C.; Ferreira, P.A.A.; Cornejo, P. Antioxidant responses of phenolic compounds and immobilization of copper in Imperata cylindrica, a plant with potential use for bioremediation of Cu contaminated environments. Plants 2020, 9, 1397. [Google Scholar] [CrossRef]
- Chong, W.J.; Guang, Y.Y.; Yun, F.H.; Tang, C.; Wu, P.; Shao, J.Z. Iron Deficiency-Induced Secretion of Phenolics Facilitates the Reutilization of Root Apoplastic Iron in Red Clover. Plant Physiol. 2007, 144, 278–285. [Google Scholar] [CrossRef]
- Chrzanowski, G.; Leszczyński, B. Induced Accumulation of Phenolic Acids in Winter Triticale (Triticosecale Wittm.) under Insects Feeding. Herba Pol. 2008, 54. [Google Scholar]
- Kariyat, R.R.; Gaffoor, I.; Sattar, S.; Dixon, C.W.; Frock, N.; Moen, J.; De Moraes, C.M.; Mescher, M.C.; Thompson, G.A.; Chopra, S. Sorghum 3-Deoxyanthocyanidin Flavonoids Confer Resistance against Corn Leaf Aphid. J. Chem. Ecol. 2019, 45, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Benamar, A.; Corbineau, F.; Côme, D. Changes in Malondialdehyde Content and in Superoxide Dismutase, Catalase and Glutathione Reductase Activities in Sunflower Seeds as Related to Deterioration during Accelerated Aging. Physiol. Plant. 1996, 97, 104–110. [Google Scholar] [CrossRef]
- Jiang, M.Y.; Jing, J.H.; Wang, S.T. Generation of Hydroxyl Radical in Plants and Its Relation to the Initiation of Lipid Peroxidation. Plant Physiol. Commun. 1993, 29, 300–305. [Google Scholar]
- García-Gómez, C.; Obrador, A.; González, D.; Babín, M.; Fernández, M.D. Comparative Effect of ZnO NPs, ZnO Bulk and ZnSO4 in the Antioxidant Defences of Two Plant Species Growing in Two Agricultural Soils under Greenhouse Conditions. Sci. Total Environ. 2017, 589, 11–24. [Google Scholar] [CrossRef]
- Perveen, S.; Saeed, M.; Parveen, A.; Javed, M.T.; Zafar, S.; Iqbal, N. Modulation of Growth and Key Physiobiochemical Attributes after Foliar Application of Zinc Sulphate (ZnSO4) on Wheat (Triticum Aestivum L.) under Cadmium (Cd) Stress. Physiol. Mol. Biol. Plants 2020, 26, 1787–1797. [Google Scholar] [CrossRef]
- Gaschler, M.M.; Stockwell, B.R. Lipid Peroxidation in Cell Death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Zhang, H.; Liang, Z. Nitrogen Rates and Water Stress Effects on Production, Lipid Peroxidation and Antioxidative Enzyme Activities in Two Maize (Zea Mays L.) Genotypes. J. Agron. Crop Sci. 2007, 193, 387–397. [Google Scholar] [CrossRef]
- Farhoudi, R. Effect of Salt Stress on Physiological and Morphological Parameters of Rapeseed Cultivars. Adv. Environ. Biol. 2011, 5, 2501–2508. [Google Scholar]
- Maccaferri, M.; Sanguineti, M.C.; Demontis, A.; El-Ahmed, A.; Garcia del Moral, L.; Maalouf, F.; Nachit, M.; Nserallah, N.; Ouabbou, H.; Rhouma, S. Association Mapping in Durum Wheat Grown across a Broad Range of Water Regimes. J. Exp. Bot. 2011, 62, 409–438. [Google Scholar] [CrossRef]
- Ashraf, M.; Parveen, N. Photosynthetic Parameters at the Vegetative Stage and during Grain Development of Two Hexaploid Wheat Cultivars Differing in Salt Tolerance. Biol. Plant. 2002, 45, 401–407. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A Multifunctional Amino Acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Slama, I.; Ghnaya, T.; Hessini, K.; Messedi, D.; Savouré, A.; Abdelly, C. Comparative Study of the Effects of Mannitol and PEG Osmotic Stress on Growth and Solute Accumulation in Sesuvium Portulacastrum. Environ. Exp. Bot. 2007, 61, 10–17. [Google Scholar] [CrossRef]
- Saradhi, P.P. Proline Accumulation under Heavy Metal Stress. J. Plant Physiol. 1991, 138, 554–558. [Google Scholar]
- Bassi, R.; Sharma, S.S. Proline Accumulation in Wheat Seedlings Exposed to Zinc and Copper. Phytochemistry 1993, 33, 1339–1342. [Google Scholar] [CrossRef]
- Sawhney, V.; Sheoran, I.S.; Singh, R. Nitrogen Fixation, Photosynthesis and Enzymes of Ammonia Assimilation and Ureide Biogenesis in Nodules of Mungbean (Vigna Radiata) Grown in Presence of Cadmium. Indian J. Exp. Biol. 1990, 28, 883–886. [Google Scholar]
- Saradhi, P.P.; Mohanty, P. Proline in Relation to Free Radical Production in Seedlings of Brassica Juncea Raised under Sodium Chloride Stress. In Proceedings of the Plant Nutrition—From Genetic Engineering to Field Practice: Proceedings of the Twelfth International Plant Nutrition Colloquium, Perth, Australia, 21–26 September 1993; Springer: Berlin/Heidelberg, Germany, 1993; pp. 731–734. [Google Scholar]
- Jetley, U.K.; Choudhary, M.; Fatma, T. Evaluation of Bio-Chemical Productivity in Cyanobacterium Spirulina Platensis-S5 under Heavy Metal Stress. Asian J. Chem. 2004, 16, 1524–1528. [Google Scholar]
- Kumar, S.; Jetley, U.K.; Fatma, T. Tolerance of Spirulina Platensis-S5 and Anabaena Sp to Endosulfan an Organochlorine Pesticide. Ann. Plant Physiol. 2004, 18, 103–107. [Google Scholar]
- Jajic, I.; Sarna, T.; Strzalka, K. Senescence, Stress, and Reactive Oxygen Species. Plants 2015, 4, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Sofo, A.; Scopa, A.; Roychoudhury, A.; Gill, S.S.; Iqbal, M.; Lukatkin, A.S.; Pereira, E.; Duarte, A.C.; Ahmad, I. Lipids and Proteins—Major Targets of Oxidative Modifications in Abiotic Stressed Plants. Environ. Sci. Pollut. Res. 2015, 22, 4099–4121. [Google Scholar] [CrossRef]
- Kim, J.; Rees, D.C. Structural Models for the Metal Centers in the Nitrogenase Molybdenum-Iron Protein. Science 1992, 257, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Weisany, W.; Sohrabi, Y.; Heidari, G.; Siosemardeh, A.; Ghassemi-Golezani, K. Changes in Antioxidant Enzymes Activity and Plant Performance by Salinity Stress and Zinc Application in Soybean (Glycine Max L.). Plant Omics 2012, 5, 60–67. [Google Scholar]
- Dat, J.; Vandenabeele, S.; Vranova, E.; Van Montagu, M.; Inzé*, D.; Van Breusegem, F. Dual Action of the Active Oxygen Species during Plant Stress Responses. Cell. Mol. Life Sci. C 2000, 57, 779–795. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Tansley Review No. 112 Oxygen Processing in Photosynthesis: Regulation and Signalling. New Phytol. 2000, 146, 359–388. [Google Scholar] [CrossRef]
- León, J.; Rojo, E.; Sánchez-Serrano, J.J. Wound Signalling in Plants. J. Exp. Bot. 2001, 52, 1–9. [Google Scholar] [CrossRef]
- Demiral, T.; Türkan, I. Comparative Lipid Peroxidation, Antioxidant Defense Systems and Proline Content in Roots of Two Rice Cultivars Differing in Salt Tolerance. Environ. Exp. Bot. 2005, 53, 247–257. [Google Scholar] [CrossRef]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative Modifications to Cellular Components in Plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef]
- Briat, J.-F.; Curie, C.; Gaymard, F. Iron Utilization and Metabolism in Plants. Curr. Opin. Plant Biol. 2007, 10, 276–282. [Google Scholar] [CrossRef]
- Mosavat, N.; Golkar, P.; Yousefifard, M.; Javed, R. Modulation of Callus Growth and Secondary Metabolites in Different Thymus Species and Zataria Multiflora Micropropagated under ZnO Nanoparticles Stress. Biotechnol. Appl. Biochem. 2019, 66, 316–322. [Google Scholar] [CrossRef]
- Stohs, S.J.; Bagchi, D. Oxidative Mechanisms in the Toxicity of Metal Ions. Free Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Briat, J.-F. Metal Ion-Activated Oxidative Stress and Its Control. Oxidative Stress Plants 2002, 171–189. [Google Scholar]
- Chaoui, A.; Mazhoudi, S.; Habib Ghorbal, M.; Ferjani, E. Cadmium and Zinc Induction of Lipid Peroxidation and Effects on Antioxidant Enzyme Activities in Bean (Phaseolus vulgaris L.). Plant Sci. 1997, 127, 139–147. [Google Scholar] [CrossRef]
- Luna, C.M.; González, C.A.; Trippi, V.S. Oxidative Damage Caused by an Excess of Copper in Oat Leaves. Plant Cell Physiol. 1994, 35, 11–15. [Google Scholar]
- Mukherjee, A.; Peralta-Videa, J.R.; Bandyopadhyay, S.; Rico, C.M.; Zhao, L.; Gardea-Torresdey, J.L. Physiological Effects of Nanoparticulate ZnO in Green Peas (Pisum Sativum L.) Cultivated in Soil. Metallomics 2014, 6, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, S.; Lee, I. Alteration of Phytotoxicity and Oxidant Stress Potential by Metal Oxide Nanoparticles in Cucumis Sativus. Water Air Soil Pollut. 2012, 223, 2799–2806. [Google Scholar] [CrossRef]
- Van Assche, F.; Clijsters, H. Effects of Metals on Enzyme Activity in Plants. Plant. Cell Environ. 1990, 13, 195–206. [Google Scholar] [CrossRef]
- Harinasut, P.; Poonsopa, D.; Roengmongkol, K.; Charoensataporn, R. Salinity Effects on Antioxidant Enzymes in Mulberry Cultivar. Sci. Asia 2003, 29, 109–113. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Mukherjee, A.K. Salt Stress-Induced Cytosolute Accumulation, Antioxidant Response and Membrane Deterioration in Three Rice Cultivars during Early Germination. Seed Sci. Technol. 2002, 30, 279–287. [Google Scholar]
- Štajner, D.; Kevrešan, S.; Gašić, O.; Mimica-Dukić, N.; Zongli, H. Nitrogen and Azotobacter Chroococcum Enhance Oxidative Stress Tolerance in Sugar Beet. Biol. Plant. 1997, 39, 441–445. [Google Scholar] [CrossRef]
- Zhang, F.; Wan, X.; Zheng, Y.; Sun, L.; Chen, Q.; Zhu, X.; Guo, Y.; Liu, M. Effects of Nitrogen on the Activity of Antioxidant Enzymes and Gene Expression in Leaves of Populus Plants Subjected to Cadmium Stress. J. Plant Interact. 2014, 9, 599–609. [Google Scholar] [CrossRef]
- Rengel, Z.; Graham, R.D. Wheat Genotypes Differ in Zn Efficiency When Grown in Chelate-Buffered Nutrient Solution: I. Growth. Plant Soil 1995, 176, 307–316. [Google Scholar] [CrossRef]
- Jeong, J.; Connolly, E.L. Iron Uptake Mechanisms in Plants: Functions of the FRO Family of Ferric Reductases. Plant Sci. 2009, 176, 709–714. [Google Scholar] [CrossRef]
- Li, M.; Hu, C.; Zhu, Q.; Chen, L.; Kong, Z.; Liu, Z. Copper and Zinc Induction of Lipid Peroxidation and Effects on Antioxidant Enzyme Activities in the Microalga Pavlova Viridis (Prymnesiophyceae). Chemosphere 2006, 62, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of Superoxide Dismutases (SODs) in Controlling Oxidative Stress in Plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Asmar, F.; Singh, T.; Nielsen, N.E. Barley Genotypes Differ in Activity of Soluble Extracellular Phosphatase and Depletion of Organic Phosphorus in the Rhizosphere Soil. Plant Soil 1995, 172, 117–122. [Google Scholar] [CrossRef]
- Li, Z.-H.; Li, P.; Dzyuba, B.; Randak, T. Influence of Environmental Related Concentrations of Heavy Metals on Motility Parameters and Antioxidant Responses in Sturgeon Sperm. Chem. Biol. Interact. 2010, 188, 473–477. [Google Scholar] [CrossRef]
- Pandey, S.; Parvez, S.; Sayeed, I.; Haque, R.; Bin-Hafeez, B.; Raisuddin, S. Biomarkers of Oxidative Stress: A Comparative Study of River Yamuna Fish Wallago Attu (Bl. & Schn.). Sci. Total Environ. 2003, 309, 105–115. [Google Scholar]
- Okamoto, O.K.; Pinto, E.; Latorre, L.R.; Bechara, E.J.H.; Colepicolo, P. Antioxidant Modulation in Response to Metal-Induced Oxidative Stress in Algal Chloroplasts. Arch. Environ. Contam. Toxicol. 2001, 40, 18–24. [Google Scholar]
- Rijstenbil, J.W.; Derksen, J.W.M.; Gerringa, L.J.A.; Poortvliet, T.C.W.; Sandee, A.; Van den Berg, M.; Van Drie, J.; Wijnholds, J.A. Oxidative Stress Induced by Copper: Defense and Damage in the Marine Planktonic Diatom Ditylum Brightwellii, Grown in Continuous Cultures with High and Low Zinc Levels. Mar. Biol. 1994, 119, 583–590. [Google Scholar] [CrossRef]
- Nagalakshmi, N.; Prasad, M.N.V. Responses of Glutathione Cycle Enzymes and Glutathione Metabolism to Copper Stress in Scenedesmus Bijugatus. Plant Sci. 2001, 160, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, A.; Vangronsveld, J.; Clijsters, H. The Redox Status of Plant Cells (AsA and GSH) Is Sensitive to Zinc Imposed Oxidative Stress in Roots and Primary Leaves of Phaseolus Vulgaris. Plant Physiol. Biochem. 2001, 39, 657–664. [Google Scholar] [CrossRef]
- Galloway, T.S.; Depledge, M.H. Immunotoxicity in Invertebrates: Measurement and Ecotoxicological Relevance. Ecotoxicology 2001, 10, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Dubovskiy, I.M.; Grizanova, E.V.; Ershova, N.S.; Rantala, M.J.; Glupov, V.V. The Effects of Dietary Nickel on the Detoxification Enzymes, Innate Immunity and Resistance to the Fungus Beauveria Bassiana in the Larvae of the Greater Wax Moth Galleria Mellonella. Chemosphere 2011, 85, 92–96. [Google Scholar] [CrossRef]
- Ha, E.-M.; Oh, C.-T.; Bae, Y.S.; Lee, W.-J. A Direct Role for Dual Oxidase in Drosophila Gut Immunity. Science 2005, 310, 847–850. [Google Scholar] [CrossRef]
- Alaraby, M.; Hernández, A.; Marcos, R. New Insights in the Acute Toxic/Genotoxic Effects of CuO Nanoparticles in the in Vivo Drosophila Model. Nanotoxicology 2016, 10, 749–760. [Google Scholar] [CrossRef]
- El-Samad, L.M.; El-Gendy, A.H.; Abdel-Moneim, A.M.; El-Ashram, S.; Augustyniak, M. CuO NPs-Induced Damage to Testes and Deregulation of the Antioxidant System in Wild Terrestrial Organism Blaps Sulcata (Coleoptera: Tenebrionidae). Environ. Nanotechnol. Monit. Manag. 2022, 18, 100751. [Google Scholar] [CrossRef]
- Sezer Tuncsoy, B.; Tuncsoy, M.; Gomes, T.; Sousa, V.; Teixeira, M.R.; Bebianno, M.J.; Ozalp, P. Effects of Copper Oxide Nanoparticles on Tissue Accumulation and Antioxidant Enzymes of Galleria Mellonella L. Bull. Environ. Contam. Toxicol. 2019, 102, 341–346. [Google Scholar] [CrossRef]
- Lemaitre, B.; Hoffmann, J. The Host Defense of Drosophila Melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef]
- Rodrıguez, J.; Le Moullac, G. State of the Art of Immunological Tools and Health Control of Penaeid Shrimp. Aquaculture 2000, 191, 109–119. [Google Scholar] [CrossRef]
- Ibrahim, A.M.A.; Ali, A.M. Silver and Zinc Oxide Nanoparticles Induce Developmental and Physiological Changes in the Larval and Pupal Stages of Spodoptera Littoralis (Lepidoptera: Noctuidae). J. Asia. Pac. Entomol. 2018, 21, 1373–1378. [Google Scholar] [CrossRef]
- Hansen, B.H.; Rømma, S.; Garmo, Ø.A.; Olsvik, P.A.; Andersen, R.A. Antioxidative Stress Proteins and Their Gene Expression in Brown Trout (Salmo Trutta) from Three Rivers with Different Heavy Metal Levels. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 143, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Kafel, A.; Bednarska, K.; Augustyniak, M.; Witas, I.; Szuliñska, E. Activity of Glutathione S-Transferase in Spodoptera Exigua Larvae Exposed to Cadmium and Zinc in Two Subsequent Generations. Environ. Int. 2003, 28, 683–686. [Google Scholar] [CrossRef]
- Xia, Q.; Sun, H.; Hu, X.; Shu, Y.; Gu, D.; Zhang, G. Apoptosis of Spodoptera Litura Larval Hemocytes Induced by Heavy Metal Zinc. Chinese Sci. Bull. 2005, 50, 2856–2860. [Google Scholar] [CrossRef]
- Abd El-Wahab, R.A.; Anwar, E.M. The Effect of Direct and Indirect Use of Nanoparticles on Cotton Leaf Worm, Spodoptera Littoralis. Int. J. Chem. Biol. Sci. 2014, 1, 17–24. [Google Scholar]
- Ahmed, A.; Ghallab, E.H.; Shehata, M.; Zekri, A.-R.N.; Ahmed, O.S. Impact of Nano-Conjugate on Drosophila for Early Diagnosis of Alzheimer’s Disease. Nanotechnology 2020, 31, 365102. [Google Scholar] [CrossRef]
- Memarizadeh, N.; Ghadamyari, M.; Adeli, M.; Talebi, K. Biochemical Biomarkers of Glyphodes Pyloalis Walker (Lepidoptera: Pyralidae) in Exposure to TiO2 Nanoparticles. Invertebr. Surviv. J. 2014, 11, 47–53. [Google Scholar]
- Gomes, T.; Pereira, C.G.; Cardoso, C.; Pinheiro, J.P.; Cancio, I.; Bebianno, M.J. Accumulation and Toxicity of Copper Oxide Nanoparticles in the Digestive Gland of Mytilus Galloprovincialis. Aquat. Toxicol. 2012, 118, 72–79. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Kaliwal, B.B. The Biochemical Effects of Potassium Chloride on the Silkworm, (Bombyx Mori L.). Insect Sci. 2005, 12, 95–100. [Google Scholar] [CrossRef]
- Bray, T.M.; Bettger, W.J. The Physiological Role of Zinc as an Antioxidant. Free Radic. Biol. Med. 1990, 8, 281–291. [Google Scholar] [CrossRef]
- Felton, G.W.; Summers, C.B. Antioxidant Systems in Insects. Arch. Insect Biochem. Physiol. 1995, 29, 187–197. [Google Scholar] [CrossRef]
- Locke, M.; Nichol, H. Iron Economy in Insects: Transport, Metabolism, and Storage. Annu. Rev. Entomol. 1992, 37, 195–215. [Google Scholar] [CrossRef]
- Renault, D.; Dorrah, M.A.; Mohamed, A.A.; Abdelfattah, E.A.; Renault, D. Assessment of Oxidative Stress and Activities of Antioxidant Enzymes Depicts the Negative Systemic Effect of Iron-Containing Fertilizers and Plant Phenolic Compounds in the Desert Locust. Environ. Sci. Pollut. Res. 2016. [Google Scholar] [CrossRef]
- Huang, Y.; Han, Y.; Wei, L.; Wang, J. Comparative studies of tracheary element structure of some gymnosperms with angiosperms. Am. J. Plant Sci. 2017, 8, 959–984. [Google Scholar] [CrossRef]
- Van Bel, A.J.E. Xylem-Phloem Exchange via the Rays: The Undervalued Route of Transport. J. Exp. Bot. 1990, 41, 631–644. [Google Scholar] [CrossRef]
- Page, V.; Weisskopf, L.; Feller, U. Heavy Metals in White Lupin: Uptake, Root-to-shoot Transfer and Redistribution within the Plant. New Phytol. 2006, 171, 329–341. [Google Scholar] [CrossRef]
- Riesen, O.; Feller, U. Redistribution of Nickel, Cobalt, Manganese, Zinc, and Cadmium via the Phloem in Young and Maturing Wheat. J. Plant Nutr. 2005, 28, 421–430. [Google Scholar] [CrossRef]
- Page, V.; Feller, U. Heavy Metals in Crop Plants: Transport and Redistribution Processes on the Whole Plant Level. Agronomy 2015, 5, 447–463. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 1–32. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- López-Hidalgo, C.; Meijón, M.; Lamelas, L.; Valledor, L. The Rainbow Protocol: A Sequential Method for Quantifying Pigments, Sugars, Free Amino Acids, Phenolics, Flavonoids and MDA from a Small Amount of Sample. Plant. Cell Environ. 2021, 44, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the Thiobarbituric Acid-Reactive-Substances Assay for Estimating Lipid Peroxidation in Plant Tissues Containing Anthocyanin and Other Interfering Compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.A.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative Stress and Some Antioxidant Systems in Acid Rain-Treated Bean Plants: Protective Role of Exogenous Polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Naderi, R.; Valizadeh, M.; Toorchi, M.; Shakiba, M.R. Antioxidant Enzyme Changes in Response to Osmotic Stress in Wheat (Triticum Aestivum L.) Seedling. Acta Biol. Szeged. 2014, 58, 95–101. [Google Scholar]
- Soltis, D.E. Isozymes in Plant Biology; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 9400918402. [Google Scholar]
- Olson, P.D.; Varner, J.E. Hydrogen Peroxide and Lignification. Plant J. 1993, 4, 887–892. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).