Harvesting Maturity Assessment of Newly Developed Citrus Hybrids (Citrus maxima Merr. × Citrus sinensis (L.) Osbeck) for Optimum Juice Quality
Abstract
:1. Introduction
2. Results
2.1. Changes in Physico-Chemical Fruit Quality Traits
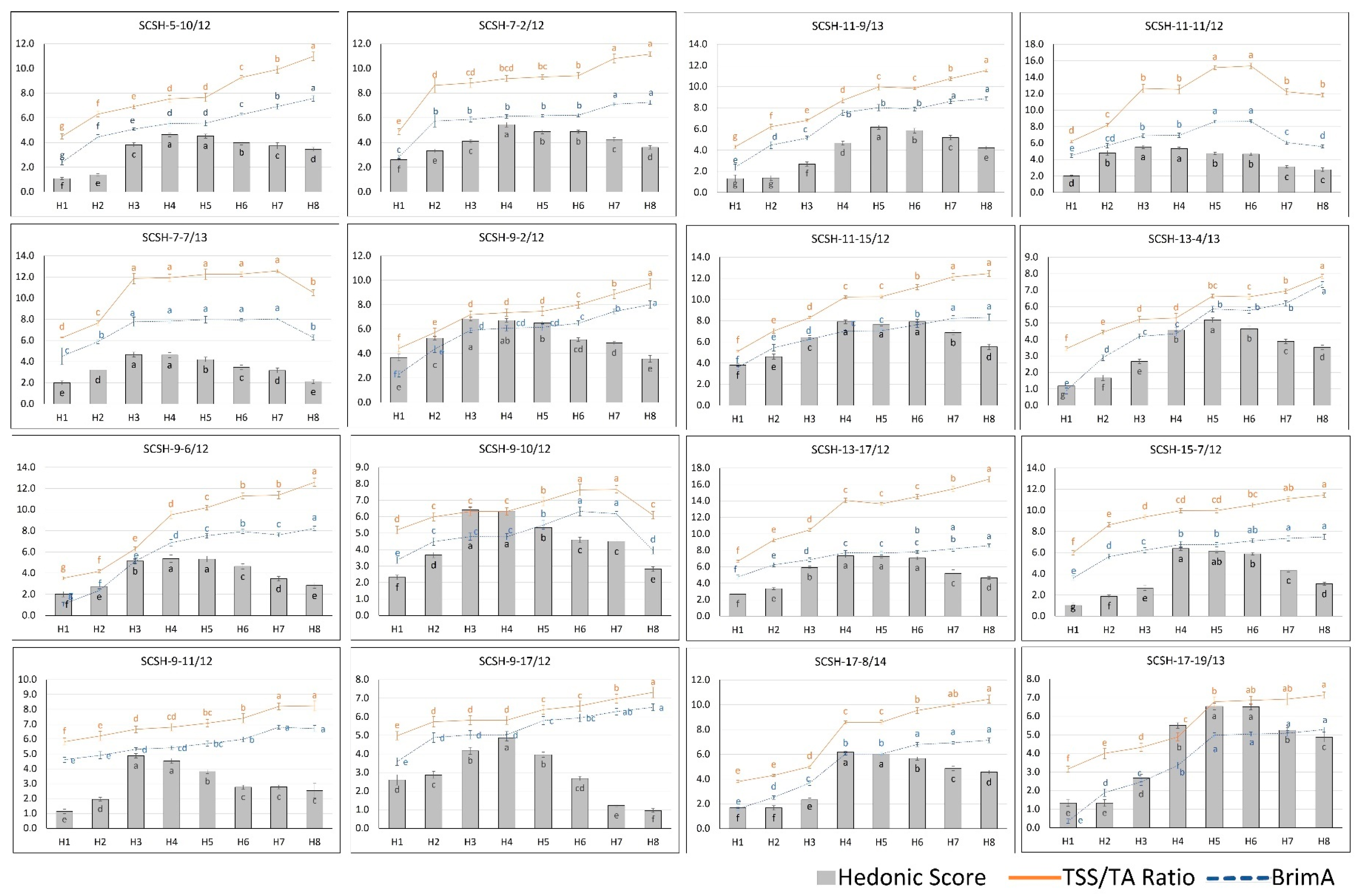
2.2. Consumer Preference Evaluation and Optimum Maturity Stage
2.3. Relations of Fruit Physico-Chemical Parameters and Hedonic Score
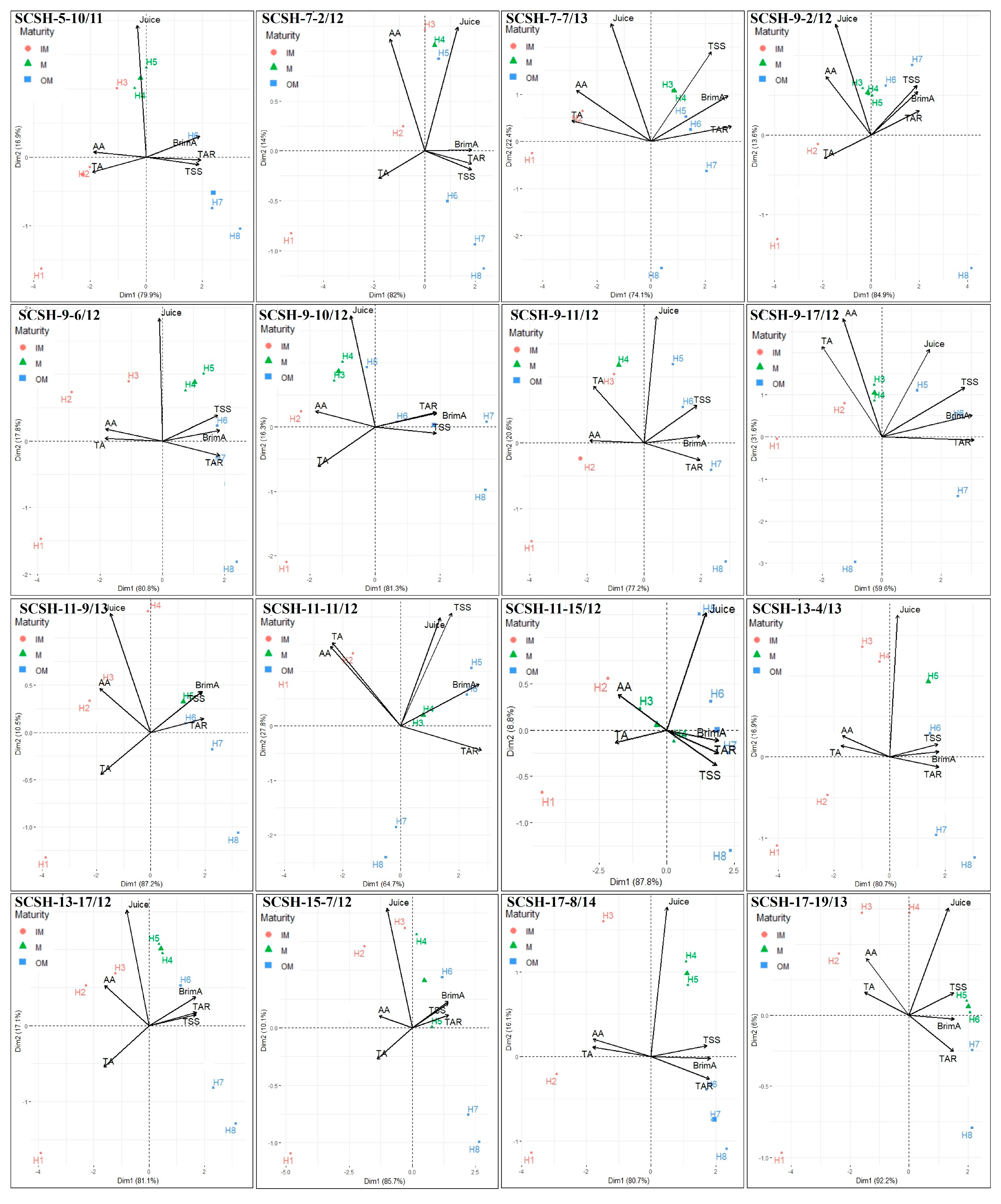
2.4. Discrimination of Harvest Maturity Stages Based on Principal Component Analysis (PCA)
3. Discussion
3.1. Harvesting Fruit Maturity
3.2. Correlation among the Fruit Physico-Chemical Attributes at Fruit Maturity
3.3. Discrimination of Harvest Maturity Stages Based on Principal Component Analysis (PCA)
4. Material and Methods
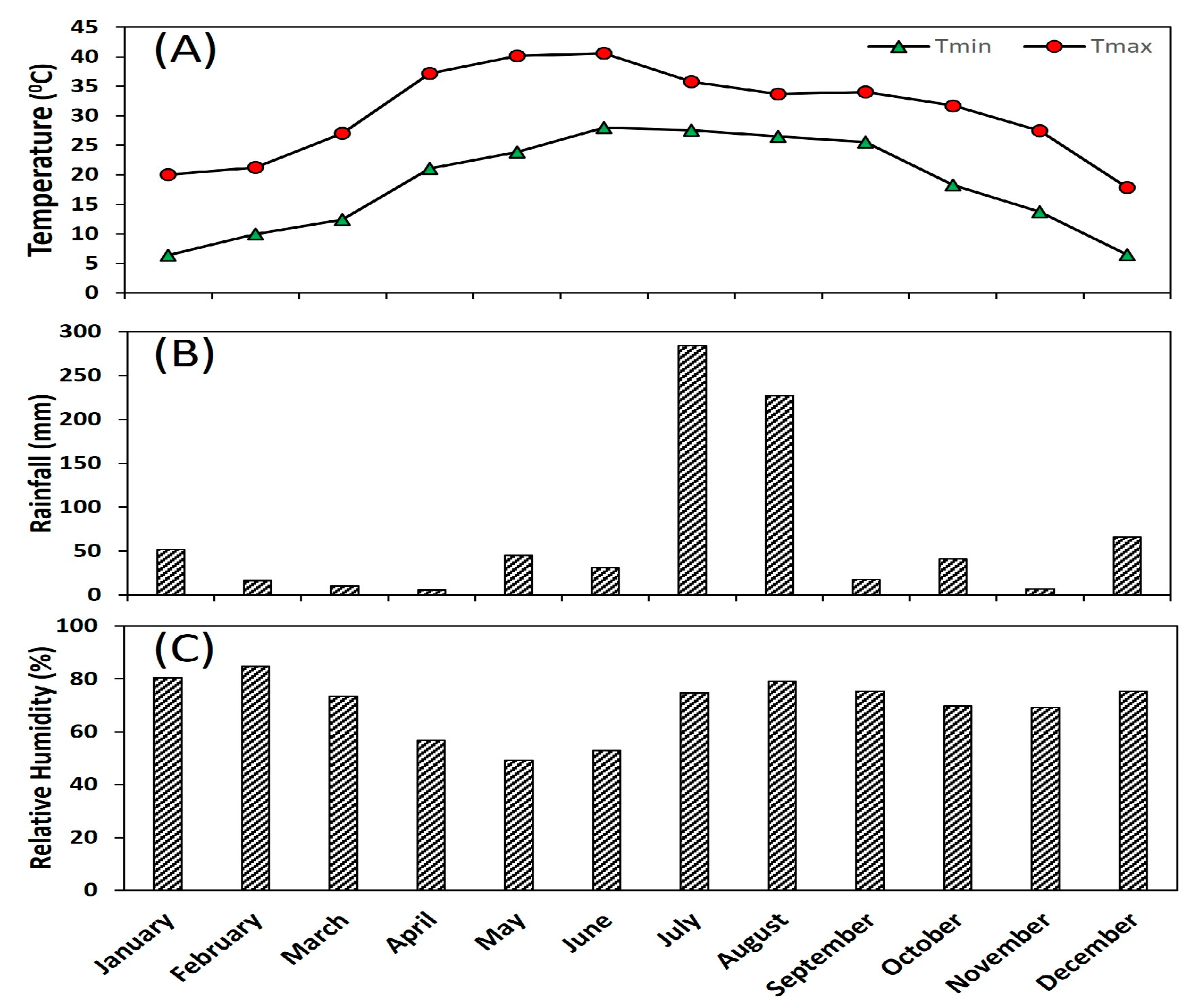
4.1. Study Site
4.2. Plant Materials
4.3. Estimation of Juice Content
4.4. Biochemical Attributes
4.5. Consumer Preference Evaluation and Optimum Maturity Stage
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). Citrus Fruit, Fresh and Processed: Statistical Bulletin 2020; FAO: Rome, Italy, 2020. [Google Scholar]
- Kowalska, M.; Konopska, J.; Feszterová, M.; Zbikowska, A.; Kowalska, B. Quality assessment of aatural juices and consumer preferences in the range of Citrus fruit juices. Appl. Sci. 2023, 13, 765. [Google Scholar] [CrossRef]
- Turner, T.; Burri, B.J. Potential nutritional benefits of current citrus consumption. Agriculture 2013, 3, 170–187. [Google Scholar] [CrossRef]
- Visvanathan, R.; Williamson, G. Effect of citrus fruit and juice consumption on risk of developing type 2 diabetes: Evidence on polyphenols from epidemiological and intervention studies. Trends Food Sci. Technol. 2021, 115, 133–146. [Google Scholar] [CrossRef]
- Lv, X.; Zhao, S.; Ning, Z.; Zeng, H.; Shu, Y.; Tao, O.; Xiao, C.; Lu, C.; Liu, Y. Citrus fruits as a treasure trove of active natural metabolites that potentially provide benefits for human health. Chem. Cent. J. 2015, 9, 1–14. [Google Scholar] [CrossRef]
- Raghavan, S.; Gurunathan, J. Citrus species—A golden treasure box of metabolites that is beneficial against disorders. J. Herb. Med. 2021, 28, 100438. [Google Scholar] [CrossRef]
- Sharma, B.D.; Hore, D.K.; Gupta, S.G. Genetic resources of Citrus of north-eastern India and their potential use. Genet. Resour. Crop Evol. 2004, 51, 411–418. [Google Scholar] [CrossRef]
- Arias, B.A.; Ramón-Laca, L. Pharmacological properties of citrus and their ancient and medieval uses in the Mediterranean region. J. Ethnopharmacol. 2005, 97, 89–95. [Google Scholar] [CrossRef]
- Abu-Goukh, A.B.A.; Almahi, A.A.M. Physico-chemical changes during growth and development of grapefruits (Citrus paradisi Macf.). I. Physical changes. Gezira J. Agric. Sci. 2017, 15, 13–25. [Google Scholar]
- Ministry of Agriculture & Farmers Welfare. Area and Production of Horticultural Crops for the Year 2021–22, New Delhi. Available online: https://agricoop.gov.in/en/StatHortEst (accessed on 30 July 2023).
- Wu, G.A.; Terol, J.; Ibanez, V.; López-García, A.; Pérez-Román, E.; Borredá, C.; Domingo, C.; Tadeo, F.R.; Carbonell-Caballero, J.; Alonso, R.; et al. Genomics of the origin and evolution of Citrus. Nature 2018, 554, 311–316. [Google Scholar] [CrossRef]
- Raveh, E.; Goldenberg, L.; Porat, R.; Carmi, N.; Gentile, A.; La Malfa, S. Conventional breeding of cultivated Citrus varieties. In The Citrus Genome: Compendium of Plant Genomes; Gentile, A., La Malfa, S., Deng, Z., Eds.; Springer: Cham, Switzerland, 2020; pp. 33–48. [Google Scholar]
- Salonia, F.; Ciacciulli, A.; Poles, L.; Pappalardo, H.D.; La Malfa, S.; Licciardello, C. New plant breeding techniques in citrus for the improvement of important agronomic traits. A Review. Front. Plant Sci. 2020, 11, 1234. [Google Scholar] [CrossRef]
- Anticona, M.; Fayos, M.C.; Esteve, M.J.; Frigola, A.; Blesa, J.; Lopez-Malo, D. Differentiation of juice of mandarin-like hybrids based on physicochemical characteristics, bioactive compounds, and antioxidant capacity. Eur. Food Res. Technol. 2022, 248, 2253–2262. [Google Scholar] [CrossRef]
- Kader, A.A.; Arpaia, M.L. Postharvest handling systems of sub-tropical fruits. In Postharvest Technology of Horticultural Crops; Kader, A.A., Ed.; University of California: Richmond, CA, USA, 2002; pp. 375–383. [Google Scholar]
- Ladaniya, M.S. Citrus Fruit: Biology, Technology and Evaluation; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Sadka, A.; Shlizerman, L.; Kamara, I.; Blumwald, E. Primary metabolism in citrus fruit as affected by its unique structure. Front. Plant Sci. 2019, 10, 1167. [Google Scholar] [CrossRef]
- Dubey, A.K.; Patel, R.K.; Singh, A.K. Standardization of fruit maturity indices in Khasi mandarin (Citrus reticulata Blanco.) under Meghalaya. Ann. Agric. Sci. 2003, 24, 559–562. [Google Scholar]
- Chen, Y.; Barzee, T.J.; Zhang, R.; Pan, Z.; Citrus. Integrated Processing Technologies for Food and Agricultural By-Products; Pan, Z., Zhang, R., Zicari, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 217–242. [Google Scholar]
- Lado, J.; Rodrigo, J.M.; Zacarias, L. Maturity indicators and citrus fruit quality. Stewart Postharvest Rev. 2014, 2, 1–6. [Google Scholar]
- Reuther, W. Climate and citrus behavoir. In The Citrus Industry: Production Technology; Reuther, W., Ed.; University of California: Oakland, CA, USA, 1973; Volume III. [Google Scholar]
- Ladaniya, M.S.; Mahalle, B. Fruit maturation and associated changes in ‘Mosambi’ orange (Citrus sinensis). Indian J. Agric. Sci. 2011, 81, 494–499. [Google Scholar]
- Jordan, R.; Seelye, R.; McGlone, A. A sensory-based alternative to brix/acid ratio. Food Technol. 2001, 55, 36–44. [Google Scholar]
- Obenland, D.; Collin, S.; Mackey, B.; Sievert, J.; Fjeld, K.; Arpaia, M.L. Determinants of flavor acceptability during the maturation of navel oranges. Postharvest Biol. Technol. 2009, 52, 156–163. [Google Scholar] [CrossRef]
- USDA. Oranges, grapefruit, tangerines, and tangelos grown in Florida: Change in the minimum maturity requirements for fresh grapefruit. Fed. Regist. 2002, 67, 71798–71803. [Google Scholar]
- Clark, C.J. Fast determination by Fourier-transform infrared spectroscopy of sugar–acid composition of citrus juices for determination of industry maturity standards. N. Z. J. Crop Hortic. Sci. 2016, 44, 69–82. [Google Scholar] [CrossRef]
- Obenland, D.; Campisi-Pinto, S.; Arpaia, M.L. Determinants of sensory acceptability in grapefruit. Sci. Hortic. 2018, 231, 151–157. [Google Scholar] [CrossRef]
- Zeb, A.; Qureshi, W.S.; Ghafoor, A.; Malik, A.; Imran, M.; Mirza, A.; Tiwana, M.I.; Alanazi, E. Towards sweetness classification of orange cultivars using short-wave NIR spectroscopy. Sci. Rep. 2023, 13, 325. [Google Scholar] [CrossRef]
- Bai, J.; Baldwin, E.A.; McCollum, G.; Plotto, A.; Manthey, J.A.; Widmer, W.W.; Luzio, G.; Cameron, R. Changes in volatile and non-volatile flavor chemicals of “Valencia” orange juice over the harvest seasons. Foods 2016, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhao, C.; Shi, H.; Liao, Y.; Xu, F.; Du, H.; Xiao, H.; Zheng, J. Nutrients and bioactives in citrus fruits: Different citrus varieties, fruit parts, and growth stages. Crit. Rev. Food Sci. Nutr. 2021, 63, 2018–2041. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.; Hussain, S.B.; Anjum, M.A.; Naseer, M.; Ahmad, R.; Ziogas, V. Effects of harvest time on the fruit quality of Kinnow and Feutrell’s early mandarins (Citrus reticulata Blanco). Agronomy 2023, 13, 802. [Google Scholar] [CrossRef]
- Marsh, K.; Gonzalez, P.; Echeverria, E. PPi formation by reversal of the tonoplast-bound H+-pyrophosphatase from ‘Valencia’ orange juice cells. J. Am. Soc. Hortic. Sci. 2000, 125, 420–424. [Google Scholar] [CrossRef]
- Albertini, M.V.; Carcouet, E.; Pailly, O.; Gambotti, C.; Luro, F.; Berti, L. Changes in organic acids and sugars during early stages of development of acidic and acidless citrus fruit. J. Agric. Food Chem. 2006, 54, 8335–8339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.Y.; Shan, Y.X.; Can, G.; Lian, H.E.; Zhang, L.Y.; Wei, L.; Liang, Y.; Zhong, B.L. Effect of harvest time on the chemical composition and antioxidant capacity of Gannan navel orange (Citrus sinensis L. Osbeck ‘Newhall’) juice. J. Integr. Agric. 2022, 21, 261–272. [Google Scholar] [CrossRef]
- Hiratsuka, S.; Nakayama, S.; Tamura, S.; Nada, K. Translocation and accumulation of fruit-fixed photosynthate in Satsuma mandarin. Plant Growth Regul. 2017, 81, 277–282. [Google Scholar] [CrossRef]
- Hussain, S.B.; Shi, C.Y.; Guo, L.X.; Kamran, H.M.; Sadka, A.; Liu, Y.Z. Recent advances in the regulation of citric acid metabolism in citrus fruit. Crit. Rev. Plant Sci. 2017, 36, 241–256. [Google Scholar] [CrossRef]
- Tadeo, F.R.; Terol, J.; Rodrigo, M.J.; Licciardello, C.; Sadka, A. Fruit growth and development. In The Genus Citrus; Talon, M., Caruso, M., Gmitter, F.G., Jr., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 245–269. [Google Scholar]
- Porat, R.; Daus, A.; Weiss, B.; Cohen, L.; Droby, S. Effects of combining hot water, sodium bicarbonate and biocontrol on postharvest decay of citrus fruit. J. Hortic. Sci. Biotechnol. 2002, 77, 441–445. [Google Scholar] [CrossRef]
- Swingle, W.T.; Robinson, T.R.; Savage, E.M. New Citrus Hybrids (No. 181); US Department of Agriculture: Washington, DC, USA, 1931. [Google Scholar]
- Soost, R.K.; Cameron, J.W. ‘Melogold’ a triploid pummelo–grapefruit hybrid. HortScience 1985, 20, 1134–1135. [Google Scholar] [CrossRef]
- Siebert, T.; Kahn, T. Tried and True or Something New? Selected Citrus Varieties for the Home Gardener; UCANR Publications: Oakland, CA, USA, 2011. [Google Scholar]
- Barry, G.H.; Caruso, M.; Gmitter, F.G., Jr. Commercial scion varieties. In The Genus Citrus; Talon, M., Caruso, M., Gmitter, F.G., Jr., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 83–104. [Google Scholar]
- Lubinska-Szczygeł, M.; Polkowska, Ż.; Dymerski, T.; Gorinstein, S. Comparison of the physical and sensory properties of hybrid citrus fruit Jaffa® Sweetie in relation to the parent fruits. Molecules 2020, 25, 2748. [Google Scholar] [CrossRef] [PubMed]
- Sadka, A.; Dahan, E.; Cohen, L.; Marsh, K.B. Aconitase activity and expression during the development of lemon fruit. Physiol. Plant. 2000, 108, 255–262. [Google Scholar] [CrossRef]
- Muhtaseb, J. Effect of harvesting date on fruit quality of grapefruit cv. ‘Red Blush’ under Jordan Valley conditions. Fruits 2007, 62, 107–113. [Google Scholar] [CrossRef]
- Caruso, P.; Russo, M.P.; Caruso, M.; Guardo, M.D.; Russo, G.; Fabroni, S.; Timpanaro, N.; Licciardello, C. A transcriptional analysis of the genes involved in the ascorbic acid pathways based on a comparison of the juice and leaves of navel and anthocyanin-rich sweet orange varieties. Plants 2021, 10, 1291. [Google Scholar] [CrossRef] [PubMed]
- Alós, E.; Rey, F.; Gil, J.V.; Rodrigo, M.J.; Zacarias, L. Ascorbic acid content and transcriptional profiling of genes involved in its metabolism during development of petals, leaves, and fruits of orange (Citrus sinensis cv. Valencia late). Plants 2021, 10, 2590. [Google Scholar] [CrossRef] [PubMed]
- Habibi, F.; Guillén, F.; Serrano, M.; Valero, D. Physicochemical changes, peel colour, and juice attributes of Blood orange cultivars stored at different temperatures. Horticulturae 2021, 7, 320. [Google Scholar] [CrossRef]
- Gao, X.T.; Li, H.Q.; Wang, Y.; Peng, W.T.; Chen, W.; Cai, X.D.; He, F.; Duan, C.Q.; Wang, J. Influence of the harvest date on berry compositions and wine profiles of Vitis vinifera L. cv. ‘Cabernet Sauvignon’ under a semiarid continental climate over two consecutive years. Food Chem. 2019, 292, 237–246. [Google Scholar] [CrossRef]
- Lavilla, T.; Recasens, I.; Lopez, M.L.; Puy, J. Multivariate analysis of maturity stages, including quality and aroma, in ‘Royal Glory’ peaches and ‘Big Top’ nectarines. J. Sci. Food Agric. 2002, 82, 1842–1849. [Google Scholar] [CrossRef]
- Satpathy, L.; Pradhan, N.; Dash, D.; Baral, P.P.; Parida, S.P. Quantitative determination of vitamin C concentration of common edible food sources by redox titration using iodine solution. Lett. Appl. NanoBioScience 2021, 10, 2361–2369. [Google Scholar]
- Heintz, C.M.; Kader, A.A. Procedures for the sensory evaluation of horticultural crops. HortScience 1983, 18, 18–22. [Google Scholar] [CrossRef]
- Kienzle, S.; Sruamsiri, P.; Carle, R.; Sirisakulwat, S.; Spreer, W.; Neidhart, S. Harvest maturity specification for mango fruit (Mangifera indica L. ‘Chok Anan’) in regard to long supply chains. Postharvest Biol. Technol. 2011, 61, 41–55. [Google Scholar] [CrossRef]


| Hybrid Genotypes | Total Soluble Solids (TSS) (%) | |||||||
| H1 | H2 | H3 | H4 | H5 | H6 | H7 | H8 | |
| SCSH-5-10/12 | 8.20 ± 0.5 e | 8.50 ± 0.53 d | 9.00 ± 0.42 c | 9.20 ± 0.54 c | 9.18 ± 0.53 c | 9.27 ± 0.49 c | 9.91 ± 0.55 b | 10.42 ± 0.57 a |
| SCSH-7-2/12 | 7.33 ± 0.4 c | 8.80 ± 0.31 b | 8.90 ± 0.41 b | 9.09 ± 0.4 b | 9.10 ± 0.32 b | 9.13 ± 0.38 b | 9.83 ± 0.37 a | 9.93 ± 0.46 a |
| SCSH-7-7/13 | 8.65 ± 0.42 c | 9.70 ± 0.44 b | 10.40 ± 0.75 ab | 10.46 ± 0.48 a | 10.56 ± 0.56 a | 10.52 ± 0.42 a | 10.56 ± 0.36 a | 8.76 ± 0.55 c |
| SCSH-9-2/12 | 7.20 ± 0.39 d | 9.2 ± 0.43 c | 10.13 ± 0.39 b | 10.27 ± 0.4 b | 10.29 ± 0.47 b | 10.38 ± 0.4 b | 11.25 ± 0.35 a | 11.57 ± 0.49 a |
| SCSH-9-6/12 | 7.18 ± 0.39 e | 8.4 ± 0.36 d | 9.8 ± 0.38 c | 10.07 ± 0.46 bc | 10.68 ± 0.46 a | 10.8 ± 0.37 a | 10.33 ± 0.41 b | 10.81 ± 0.4 a |
| SCSH-9-10/12 | 7.98 ± 0.33 d | 9 ± 0.36 c | 9.13 ± 0.4 c | 9.1 ± 0.34 c | 9.7 ± 0.45 b | 10.42 ± 0.4 a | 10.16 ± 0.39 a | 7.8 ± 0.42 d |
| SCSH-9-11/12 | 9.50 ± 0.43 d | 9.5 ± 0.46 d | 9.67 ± 0.32 cd | 9.71 ± 0.33 cd | 9.9 ± 0.44 bc | 10.06 ± 0.4 b | 10.72 ± 0.39 a | 10.54 ± 0.42 a |
| SCSH-9-17/12 | 9.00 ± 0.55 c | 10.25 ± 0.67 b | 10.4 ± 0.66 b | 10.4 ± 0.59 b | 10.95 ± 0.65 a | 10.92 ± 0.59 a | 11.02 ± 0.57 a | 11.05 ± 0.56 a |
| SCSH-11-9/13 | 7.80 ± 0.67 f | 8.8 ± 0.64 e | 9.2 ± 0.55 d | 11.5 ± 0.57 abc | 11.47 ± 0.6 bc | 11.33 ± 0.51 c | 11.93 ± 0.56 ab | 11.99 ± 0.53 a |
| SCSH-11-11/12 | 8.7 ± 0.44 c | 9 ± 0.46 bc | 9.07 ± 0.45 b | 9.13 ± 0.44 b | 10.72 ± 0.38 a | 10.72 ± 0.39 a | 8.03 ± 0.39 d | 7.48 ± 0.39 e |
| SCSH-11-15/12 | 8.67 ± 0.42 d | 9.5 ± 0.54 c | 9.76 ± 0.42 c | 9.93 ± 0.42 bc | 9.97 ± 0.46 bc | 10.41 ± 0.47 b | 10.92 ± 0.48 a | 10.96 ± 0.56 a |
| SCSH-13-4/13 | 7.21 ± 0.33 e | 8.9 ± 0.43 d | 9.86 ± 0.44 c | 10.03 ± 0.54 c | 10.67 ± 0.38 b | 10.6 ± 0.45 b | 10.93 ± 0.42 b | 11.89 ± 0.44 a |
| SCSH-13-17/12 | 8.67 ± 0.53 e | 9.23 ± 0.46 d | 9.6 ± 0.51 cd | 9.8 ± 0.49 bc | 9.83 ± 0.5 bc | 9.83 ± 0.41 bc | 10.16 ± 0.52 ab | 10.46 ± 0.48 a |
| SCSH-15-7/12 | 7.2 ± 0.35 e | 8.63 ± 0.41 d | 9.18 ± 0.41 c | 9.67 ± 0.45 b | 9.68 ± 0.41 b | 9.97 ± 0.34 ab | 10.08 ± 0.33 a | 10.16 ± 0.36 a |
| SCSH-17-8/14 | 7.6 ± 0.43 d | 8.33 ± 0.48 c | 9.2 ± 0.45 b | 9.27 ± 0.42 b | 9.27 ± 0.44 b | 9.91 ± 0.48 a | 9.9 ± 0.36 a | 10.01 ± 0.49 a |
| SCSH-17-19/13 | 6.95 ± 0.35 e | 7.6 ± 0.36 d | 8.04 ± 0.36 c | 8.71 ± 0.35 b | 8.93 ± 0.39 ab | 8.97 ± 0.42 ab | 8.97 ± 0.33 ab | 9.12 ± 0.3 a |
| Hybrid Genotypes | Titratable Acidity (TA) (%) | |||||||
| H1 | H2 | H3 | H4 | H5 | H6 | H7 | H8 | |
| SCSH-5-10/12 | 1.60 ± 0.02 a | 1.35 ± 0.02 b | 1.30 ± 0.01 b | 1.22 ± 0.02 c | 1.20 ± 0.02 c | 1.00 ± 0.02 d | 1.00 ± 0.01 d | 0.95 ± 0.01 e |
| SCSH-7-2/12 | 1.50 ± 0.03 a | 1.02 ± 0.02 b | 1.01 ± 0.01 bc | 0.99 ± 0.01 bcd | 0.98 ± 0.01 cd | 0.97 ± 0.01 d | 0.91 ± 0.02 e | 0.89 ± 0.02 e |
| SCSH-7-7/13 | 1.38 ± 0.02 a | 1.27 ± 0.03 b | 0.88 ± 0.01 c | 0.88 ± 0.02 c | 0.87 ± 0.02 cd | 0.86 ± 0.01 cd | 0.84 ± 0.01 d | 0.83 ± 0.01 d |
| SCSH-9-2/12 | 1.63 ± 0.03 a | 1.61 ± 0.02 a | 1.41 ± 0.01 b | 1.40 ± 0.02 b | 1.38 ± 0.02 b | 1.30 ± 0.01 c | 1.27 ± 0.01 c | 1.19 ± 0.02 d |
| SCSH-9-6/12 | 2.04 ± 0.02 a | 2.01 ± 0.03 a | 1.56 ± 0.03 b | 1.06 ± 0.01 c | 1.05 ± 0.01 c | 0.96 ± 0.01 d | 0.91 ± 0.01 de | 0.86 ± 0.01 e |
| SCSH-9-10/12 | 1.54 ± 0.03 a | 1.5 ± 0.02 ab | 1.45 ± 0.03 bc | 1.44 ± 0.02 c | 1.4 ± 0.02 cd | 1.37 ± 0.02 de | 1.33 ± 0.02 ef | 1.28 ± 0.01 f |
| SCSH-9-11/12 | 1.63 ± 0.02 a | 1.53 ± 0.02 b | 1.45 ± 0.01 c | 1.43 ± 0.02 cd | 1.4 ± 0.01 de | 1.36 ± 0.02 e | 1.31 ± 0.07 f | 1.28 ± 0.02 f |
| SCSH-9-17/12 | 1.81 ± 0.01 a | 1.79 ± 0.02 a | 1.79 ± 0.01 a | 1.79 ± 0.01 a | 1.72 ± 0.02 b | 1.66 ± 0.03 c | 1.58 ± 0.01 d | 1.51 ± 0.02 e |
| SCSH-11-9/13 | 1.80 ± 0.04 a | 1.40 ± 0.03 b | 1.35 ± 0.01 c | 1.32 ± 0.02 c | 1.15 ± 0.02 d | 1.15 ± 0.01 d | 1.11 ± 0.01 d | 1.04 ± 0.01 e |
| SCSH-11-11/12 | 1.40 ± 0.01 a | 1.10 ± 0.01 b | 0.72 ± 0.03 c | 0.73 ± 0.02 c | 0.71 ± 0.01 c | 0.70 ± 0.01 cd | 0.66 ± 0.01 de | 0.63 ± 0.01 e |
| SCSH-11-15/12 | 1.70 ± 0.02 a | 1.35 ± 0.02 b | 1.18 ± 0.02 c | 0.97 ± 0.01 d | 0.97 ± 0.01 d | 0.93 ± 0.01 de | 0.90 ± 0.01 ef | 0.88 ± 0.02 f |
| SCSH-13-4/13 | 2.10 ± 0.03 a | 2.00 ± 0.02 b | 1.89 ± 0.01 c | 1.89 ± 0.01 c | 1.61 ± 0.01 d | 1.61 ± 0.02 d | 1.58 ± 0.02 de | 1.52 ± 0.02 e |
| SCSH-13-17/12 | 1.30 ± 0.03 a | 1.00 ± 0.01 b | 0.92 ± 0.02 c | 0.70 ± 0.01 de | 0.72 ± 0.02 d | 0.68 ± 0.01 e | 0.66 ± 0.02 ef | 0.63 ± 0.02 f |
| SCSH-15-7/12 | 1.20 ± 0.021 a | 1.00 ± 0.012 b | 0.98 ± 0.01 bc | 0.97 ± 0.01 bc | 0.97 ± 0.01 bc | 0.95 ± 0.01 c | 0.91 ± 0.02 d | 0.89 ± 0.01 d |
| SCSH-17-8/14 | 2.00 ± 0.03 a | 1.94 ± 0.02 b | 1.84 ± 0.03 c | 1.08 ± 0.01 d | 1.08 ± 0.01 d | 1.04 ± 0.02 de | 0.99 ± 0.01 ef | 0.96 ± 0.01 f |
| SCSH-17-19/13 | 2.20 ± 0.01 a | 1.90 ± 0.03 b | 1.86 ± 0.02 b | 1.79 ± 0.02 c | 1.32 ± 0.02 d | 1.31 ± 0.02 d | 1.30 ± 0.02 d | 1.28 ± 0.01 d |
| Hybrid Genotypes | Juice (%) | |||||||
| H1 | H2 | H3 | H4 | H5 | H6 | H7 | H8 | |
| SCSH-5-10/12 | 36.23 ± 0.97 b | 36.93 ± 0.58 ab | 37.6 ± 0.85 a | 37.56 ± 0.67 a | 37.75 ± 0.81 a | 37.03 ± 1.08 ab | 36.45 ± 1.11 b | 36.25 ± 0.9 b |
| SCSH-7-2/12 | 21.62 ± 0.51 d | 23.33 ± 0.56 c | 25.5 ± 0.77 a | 25.47 ± 0.78 a | 25.5 ± 0.74 a | 24.65 ± 0.82 b | 24.08 ± 0.63 b | 24.05 ± 0.87 bc |
| SCSH-7-7/13 | 22.68 ± 0.83 ab | 22.91 ± 0.87 a | 22.9 ± 0.59 a | 22.79 ± 0.53 a | 21.77 ± 0.94 ab | 21.54 ± 1.35 b | 20.28 ± 0.72 c | 19.98 ± 0.64 c |
| SCSH-9-2/12 | 24.96 ± 0.87 a | 25.19 ± 0.5 a | 25.15 ± 0.65 a | 25.03 ± 0.54 a | 25 ± 0.58 a | 24.97 ± 0.62 a | 24.91 ± 0.73 a | 23.48 ± 0.72 b |
| SCSH-9-6/12 | 34.54 ± 1.03 bc | 35.53 ± 0.69 a | 35.51 ± 0.77 a | 35.51 ± 0.55 a | 35.6 ± 0.89 a | 35.23 ± 0.51 ab | 35.01 ± 0.55 abc | 34.28 ± 0.58 c |
| SCSH-9-10/12 | 39.14 ± 1.09 e | 40.56 ± 0.89 bcd | 41.83 ± 1.11 ab | 41.56 ± 0.77 abc | 42.08 ± 0.94 a | 41.04 ± 0.75 abcd | 40.32 ± 0.75 cde | 39.76 ± 1.01 de |
| SCSH-9-11/12 | 35.75 ± 0.88 b | 36.83 ± 0.87 a | 36.92 ± 0.77 a | 37.03 ± 0.6 a | 36.91 ± 0.71 a | 36.34 ± 0.64 ab | 36.31 ± 1.11 ab | 35.64 ± 0.66 b |
| SCSH-9-17/12 | 32.88 ± 0.78 b | 33.43 ± 1.02 ab | 34.24 ± 0.9 a | 34.33 ± 0.85 a | 34.41 ± 0.95 a | 34.16 ± 0.92 a | 33.75 ± 1.02 ab | 33.04 ± 0.7 b |
| SCSH-11-9/13 | 38.76 ± 0.69 ab | 39.45 ± 0.78 a | 39.42 ± 0.73 a | 39.45 ± 1.08 a | 38.41 ± 1 ab | 38.33 ± 1.19 b | 38.01 ± 0.85 bc | 37.21 ± 0.76 c |
| SCSH-11-11/12 | 37.33 ± 0.9 b | 38.87 ± 0.93 a | 38.85 ± 1.11 a | 38.83 ± 1.21 a | 38.86 ± 0.99 a | 38.2 ± 0.7 ab | 37.28 ± 0.8 b | 37.12 ± 1.06 b |
| SCSH-11-15/12 | 43.78 ± 0.91 d | 45.81 ± 0.66 c | 45.87 ± 1 bc | 45.86 ± 1.09 bc | 47.87 ± 1.12 a | 47.03 ± 1.25 ab | 46.73 ± 0.88 abc | 45.66 ± 0.78 c |
| SCSH-13-4/13 | 38.75 ± 1.21 c | 39.28 ± 0.61 bc | 40.82 ± 1.22 a | 40.8 ± 0.93 a | 40.72 ± 0.66 a | 40.23 ± 1.06 ab | 39.15 ± 0.91 bc | 39.01 ± 1.14 bc |
| SCSH-13-17/12 | 26.45 ± 0.81 b | 27.6 ± 0.82 a | 27.55 ± 0.84 a | 27.38 ± 0.92 a | 27.53 ± 0.88 a | 27.23 ± 0.66 a | 26.03 ± 0.87 bc | 25.45 ± 0.69 c |
| SCSH-15-7/12 | 24.33 ± 0.85 bc | 24.81 ± 0.78 ab | 24.85 ± 1.08 a | 24.45 ± 0.86 bc | 23.67 ± 0.81 c | 23.91 ± 1.29 bc | 22.43 ± 0.7 d | 22.07 ± 0.85 d |
| SCSH-17-8/14 | 26.78 ± 0.96 c | 27.33 ± 1.04 bc | 28.49 ± 1.02 a | 28.49 ± 0.87 a | 28.33 ± 1.02 ab | 27.56 ± 0.87 abc | 27.36 ± 1.14 bc | 27.21 ± 0.76 c |
| SCSH-17-19/13 | 44.32 ± 0.91 b | 46.57 ± 1.32 a | 47.11 ± 1.37 a | 47.95 ± 1.13 a | 48.24 ± 1.1 a | 48.21 ± 1.3 a | 48.01 ± 0.79 a | 47.23 ± 0.66 a |
| Hybrid Genotypes | Ascorbic Acid (mg/100 mL) | |||||||
| H1 | H2 | H3 | H4 | H5 | H6 | H7 | H8 | |
| SCSH-5-10/12 | 72.53 ± 1.57 a | 72.17 ± 1.98 ab | 68.45 ± 1.7 bc | 67.97 ± 3.61 c | 63.15 ± 1.84 d | 59.13 ± 2.79 e | 58.67 ± 1.6 e | 57.72 ± 3.1 e |
| SCSH-7-2/12 | 69.88 ± 3.14 a | 69.99 ± 2.74 a | 68.18 ± 1.22 a | 67.45 ± 0.93 a | 66.42 ± 1.34 a | 60.28 ± 1.45 b | 60.28 ± 2.49 b | 58.87 ± 1.08 b |
| SCSH-7-7/13 | 53.23 ± 2.08 a | 48.76 ± 1.81 b | 42.62 ± 1.65 c | 42.52 ± 1.83 c | 42.58 ± 1.48 c | 41.27 ± 2.11 cd | 39.45 ± 1.55 de | 37.82 ± 2.27 e |
| SCSH-9-2/12 | 76.28 ± 1.13 a | 73.26 ± 2.07 ab | 71.15 ± 1.97 b | 71.22 ± 2.04 b | 70.88 ± 2.85 b | 70.03 ± 1.98 bc | 67.34 ± 1.85 c | 55.43 ± 2.56 d |
| SCSH-9-6/12 | 73.42 ± 1.5 a | 70.06 ± 3.03 ab | 68.66 ± 2.45 b | 62.34 ± 2.2 c | 60.26 ± 1.94 cd | 59.43 ± 2.66 cde | 58.28 ± 1.38 de | 56.25 ± 1.8 e |
| SCSH-9-10/12 | 44.95 ± 1.55 a | 44.25 ± 1.66 ab | 44.59 ± 2.48 ab | 42.37 ± 2.22 ab | 41.99 ± 1.36 b | 38.27 ± 1.6 c | 23.25 ± 1.85 d | 23.22 ± 1.29 d |
| SCSH-9-11/12 | 54 ± 2.07 a | 53.76 ± 1.69 a | 53.18 ± 1.43 a | 53.07 ± 1.31 a | 52.55 ± 1.84 a | 52.19 ± 2 a | 46.25 ± 2.02 b | 47.28 ± 1.34 b |
| SCSH-9-17/12 | 48.22 ± 2.62 a | 48.02 ± 2.31 a | 45.43 ± 2.25 b | 44.22 ± 0.65 b | 40.21 ± 3.08 c | 40.33 ± 3.03 c | 38.23 ± 2.42 d | 38.23 ± 2.57 d |
| SCSH-11-9/13 | 72.87 ± 1.56 a | 69.27 ± 1.3 ab | 68.42 ± 3.15 bc | 65.25 ± 1.11 c | 60.93 ± 1.59 d | 58.23 ± 2.07 de | 52.1 ± 2.35 e | 46.94 ± 1.37 f |
| SCSH-11-11/12 | 52.57 ± 1.57 a | 46.23 ± 2.69 b | 38.21 ± 1.43 c | 37.86 ± 1.61 cd | 36.23 ± 1.58 cd | 36.3 ± 1.45 cd | 36.06 ± 2.24 cd | 35.45 ± 1.4 d |
| SCSH-11-15/12 | 54.38 ± 1.78 ab | 54.77 ± 3.1 a | 53.32 ± 1.3 abc | 52.11 ± 0.73 abcd | 50.58 ± 2.13 bcd | 50.42 ± 1.05 cd | 50.23 ± 0.77 cd | 49.03 ± 1.32 d |
| SCSH-13-4/13 | 59.83 ± 2.58 a | 57.33 ± 1.31 a | 57.51 ± 0.97 a | 50.66 ± 1.64 b | 48.78 ± 0.96 bc | 46.32 ± 0.4 c | 47.25 ± 1.24 bc | 42.22 ± 1.11 d |
| SCSH-13-17/12 | 50.77 ± 1.14 a | 51.23 ± 1.82 a | 49.62 ± 1.49 ab | 47.37 ± 1.79 b | 47.3 ± 1.53 b | 42.87 ± 2.64 c | 40.42 ± 1.62 c | 40.23 ± 2.1 c |
| SCSH-15-7/12 | 55.54 ± 1.67 a | 55.43 ± 1.25 a | 43.23 ± 1.12 c | 47.37 ± 1.33 b | 42.23 ± 1.42 cd | 42.35 ± 2.1 cd | 41.19 ± 1.74 cd | 40.4 ± 2.32 d |
| SCSH-17-8/14 | 51.25 ± 2.07 a | 52.52 ± 1.44 a | 48.42 ± 1.61 b | 38.92 ± 1.88 c | 37.43 ± 1.5 c | 37.39 ± 1.87 c | 37.33 ± 0.86 c | 32.25 ± 1.26 d |
| SCSH-17-19/13 | 60.23 ± 1.18 a | 62.56 ± 1.22 a | 60.21 ± 1.32 a | 44.52 ± 0.94 b | 37.86 ± 0.62 c | 36.43 ± 2.26 c | 33.22 ± 0.85 d | 33 ± 1.99 d |
| Hybrid Genotypes | R2 (Coefficient of Determination) | |
|---|---|---|
| Hedonic Score and TSS/TA Ratio | Hedonic Score and BrimA | |
| SCSH-5-10/12 | 0.33 | 0.46 |
| SCSH-7-2/12 | 0.27 | 0.31 |
| SCSH-7-7/13 | 0.39 | 0.57 |
| SCSH-9-2/12 | 0.01 | 0.02 |
| SCSH-9-6/12 | 0.14 | 0.32 |
| SCSH-9-10/12 | 0.18 | 0.25 |
| SCSH-9-11/12 | 0.02 | 0.02 |
| SCSH-9-17/12 | 0.26 | 0.41 |
| SCSH-11-9/13 | 0.72 | 0.78 |
| SCSH-11-11/12 | 0.27 | 0.45 |
| SCSH-11-15/12 | 0.46 | 0.48 |
| SCSH-13-4/13 | 0.52 | 0.58 |
| SCSH-13-17/12 | 0.38 | 0.42 |
| SCSH-15-7/12 | 0.38 | 0.46 |
| SCSH-17-8/14 | 0.76 | 0.78 |
| SCSH-17-19/13 | 0.73 | 0.78 |
| Sr. No. | Hybrid Genotypes | Parentage | Year of Planting |
|---|---|---|---|
| 1 | SCSH-5-10/12 | White Pummelo × Mosambi | 2012 |
| 2 | SCSH-7-2/12 | White Pummelo × Mosambi | 2012 |
| 3 | SCSH-7-7/13 | White Pummelo × Mosambi | 2013 |
| 4 | SCSH-9-2/12 | Red Pummelo × Mosambi | 2012 |
| 5 | SCSH-9-6/12 | White Pummelo × Mosambi | 2012 |
| 6 | SCSH-9-10/12 | White Pummelo × Mosambi | 2012 |
| 7 | SCSH-9-11/12 | White Pummelo × Mosambi | 2012 |
| 8 | SCSH-9-17/12 | White Pummelo × Mosambi | 2012 |
| 9 | SCSH-11-9/13 | White Pummelo × Mosambi | 2013 |
| 10 | SCSH-11-11/12 | White Pummelo × Mosambi | 2012 |
| 11 | SCSH-11-15/12 | White Pummelo × Mosambi | 2012 |
| 12 | SCSH-13-4/13 | White Pummelo × Mosambi | 2013 |
| 13 | SCSH-13-17/12 | White Pummelo × Mosambi | 2012 |
| 14 | SCSH-15-7/12 | White Pummelo × Mosambi | 2012 |
| 15 | SCSH-17-8/14 | White Pummelo × Mosambi | 2014 |
| 16 | SCSH-17-19/13 | White Pummelo × Mosambi | 2013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, N.; Sharma, R.M.; Dubey, A.K.; Awasthi, O.P.; Porat, R.; Saha, S.; Bharadwaj, C.; Sevanthi, A.M.; Kumar, A.; Sharma, N.; et al. Harvesting Maturity Assessment of Newly Developed Citrus Hybrids (Citrus maxima Merr. × Citrus sinensis (L.) Osbeck) for Optimum Juice Quality. Plants 2023, 12, 3978. https://doi.org/10.3390/plants12233978
Singh N, Sharma RM, Dubey AK, Awasthi OP, Porat R, Saha S, Bharadwaj C, Sevanthi AM, Kumar A, Sharma N, et al. Harvesting Maturity Assessment of Newly Developed Citrus Hybrids (Citrus maxima Merr. × Citrus sinensis (L.) Osbeck) for Optimum Juice Quality. Plants. 2023; 12(23):3978. https://doi.org/10.3390/plants12233978
Chicago/Turabian StyleSingh, Narendra, Radha Mohan Sharma, Anil Kumar Dubey, Om Prakash Awasthi, Ron Porat, Supradip Saha, Chellapilla Bharadwaj, Amitha Mithra Sevanthi, Amrender Kumar, Nimisha Sharma, and et al. 2023. "Harvesting Maturity Assessment of Newly Developed Citrus Hybrids (Citrus maxima Merr. × Citrus sinensis (L.) Osbeck) for Optimum Juice Quality" Plants 12, no. 23: 3978. https://doi.org/10.3390/plants12233978
APA StyleSingh, N., Sharma, R. M., Dubey, A. K., Awasthi, O. P., Porat, R., Saha, S., Bharadwaj, C., Sevanthi, A. M., Kumar, A., Sharma, N., & Carmi, N. (2023). Harvesting Maturity Assessment of Newly Developed Citrus Hybrids (Citrus maxima Merr. × Citrus sinensis (L.) Osbeck) for Optimum Juice Quality. Plants, 12(23), 3978. https://doi.org/10.3390/plants12233978









