1. Introduction
Herbaceous peony (
Paeonia lactiflora Pall.) is documented as the oldest ornamental perennial herbaceous flower in the family Paeoniaceae. Due to its graceful appearance, rich color, broad application value, and various application forms,
P. lactiflora has fascinated people over the years, and it has also enjoyed the reputation of “Mayflower God” [
1,
2,
3]. Studies have also shown that
P. lactiflora roots have important medicinal values, with their therapeutical effects on cardiovascular and immune system diseases [
4,
5,
6].
Seed reproduction has long been the major mode of propagation of
P. lactiflora [
7]. The traditional reproduction method has a long cycle with a low reproduction coefficient, and seed germination is strongly dependent on the season. After sowing for 4–5 years, well-grown plants can normally flower, but they cannot maintain the intact characteristics of the female reproductive organs [
8,
9,
10]. Conventional growing methods are not conducive to breeding and cultivar improvement, thus tissue culturing has become an effective means for rapid/mass propagation and germplasm conservation in
P. lactiflora [
11]. Tissue culturing can also mitigate seed hypoplasia, germination difficulties and genetic instability in hybrid progeny of
P. lactiflora [
12]. Therefore, propagation using tissue culture technology provides a favorable technical approach for the large-scale regeneration and production in
P. lactiflora.
Plants can be regenerated in vitro through direct and indirect methods. The establishment of an indirect regeneration system is one of the key prerequisites for the development of
Agrobacterium-mediated genetic transformation and genome editing [
13]. The indirect shoot regeneration scheme based on callus, or indirect somatic organogenesis, can be divided into three basic stages—callus induction, callus proliferation, and callus regeneration into shoots followed by multiplication [
14].
In
P. lactiflora, different works have been published that explore explant selection, callus formation, proliferation and shoot regeneration [
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26]. Thus, various explants have been used for callus regeneration, including stem segments [
16,
17,
18], leaves [
9,
19], roots [
20], cotyledons [
21,
22] and various floral organs [
11,
23]. In the callus induction stage, the explants are transferred to the Murashige and Skoog (MS) [
27] medium [
22,
23,
24] supplemented with 2,4-Dichlorophenoxyacetic acid (2,4-D), 1-naphthaleneacetic acid (NAA), thidiazuron (TDZ), kinetin (KT) or 6-benzyleaminopurine (6-BA) [
22,
23,
24] to dedifferentiate the explants into callus. During shoot induction, the callus is transferred to the medium rich in plant growth regulators to form new adventitious shoots, and cytokinin plays an important role in shoot induction [
24]. Previous studies have shown that callus and a small amount of shoots can be successfully induced with TDZ [
24,
25], but the subsequent rooting of shoots has not been reported [
19,
24,
25,
26]. The induction of embryogenic callus and a subsequent somatic embryo in
P. lactiflora is hard to achieve, with only a few reports in the literature [
21,
23]. At the same time, there are still many problems in the process of callus formation, including low callus formation in explants, difficulty in inducing embryogenic callus, serious browning in the process of induction and proliferation, and difficulty in achieving redifferentiation, all of which seriously restricts the establishment of the regeneration system in
P. lactiflora [
11,
12,
19,
22,
23]. If the above problems can be solved, the propagation coefficient and regeneration frequency of
P. lactiflora will be substantially improved, and an efficient and stable regeneration system of
P. lactiflora can be built. Subsequently, the factory production of
P. lactiflora could be established, and the utilization and development of
P. lactiflora could be carried out in combination with various cultivation purposes to produce positive social and economic benefits, and to provide a reference for
P. lactiflora breeding, variety improvement, and genetic transformation [
28,
29,
30]. In this study, we improved the culture conditions and the plant growth regulators in the culture media for callus induction and regeneration in
P. lactiflora. Our results lay a foundation for subsequent genetic engineering research.
3. Discussion
The success of callus induction in
P. lactiflora is related to the explant type, culture conditions, basic media and plant growth regulators. There are great differences in the states of calluses formed from different explants [
31]. Studies have shown that cotyledons under suitable conditions can efficiently form high-quality calluses [
24,
32]. Coincidently, we discovered that using cotyledons as explants resulted in a higher rate of callus induction and a lower rate of browning. We observed that there was no significant difference in induction rate when callus were treated under dark or light conditions. However, it has been documented that
Rosa hybrida, lotus and
Datura innoxia have high callus induction rates only in dark environments [
33,
34,
35]. Our findings indicate that the explants not subjected to dark culturing were not completely dedifferentiated, and dark culturing for 20 days was more suitable for callus growth. This observation aligns with previous studies on
Rosa hybrida and
Clivia miniata, which also reported high callus quality under dark conditions [
33,
36]. We demonstrated that the MS medium significantly increased the induction speed of calluses, reduced the browning rate, and guaranteed a higher callus quality. This is similar to the results of studies on
Moso Bamboo,
Toona ciliata and
Ethiopian mustard [
32,
37,
38]. Furthermore, our experiment has revealed that the concentration of NAA influenced both the rate of callus induction and the time of callus emergence. This observation is consistent with the findings of Guo et al. (2021), who reported a positive effect of NAA on callus induction in
Abies koreana [
39]. However, it is worth noting that the previous study did not find a significant effect of NAA concentration on the formation time of black cumin callus [
40]. Many studies have found that PIC plays an important role in the induction of callus [
41,
42,
43,
44]. We consistently observed that the addition of 4 mg·L
−1 PIC into the medium induces more callus, with light yellow particles on the surface, which is similar to the results for peony [
45]. However, when the PIC concentration was higher, the browning rate of the callus was also increased. Therefore, we should be sure that the appropriate concentration of PIC is applied to reduce the browning rate of callus.
The callus proliferation of
P. lactiflora is primarily influenced by plant growth regulators. Our study has revealed that the combination of 1.0 mg·L
−1 NAA, 1.0 mg·L
−1 2,4-D and 0.5 mg·L
−1 TDZ increased the callus proliferation coefficient of
P. lactiflora to 3.13, and reduced the browning rate to 10.33%. Previous research has also demonstrated that the combination of NAA, 2,4-D and TDZ is beneficial to the callus proliferation of peony and
Dendrocalamus luodianense [
46,
47]. Interestingly, the combination of NAA and TDZ led to the highest callus proliferation rate of
Clivia miniata [
36]. In our experiment, the appropriate concentration of plant growth regulators significantly reduced the degree of browning during callus proliferation. Lower browning rates were observed when the concentration ratio of 2,4-D to NAA was 1:1 or 2:1. At the same time, we found that the frequent transferring of callus can also reduce callus browning.
The formation of adventitious shoot buds from callus of
P. lactiflora is a challenging process primarily influenced by plant growth regulators. Both cytokinin and auxin can promote the formation and proliferation of shoot buds [
48,
49,
50,
51,
52]. Our research demonstrated that TDZ combined with NAA successfully achieved the differentiation of adventitious shoot buds, and the buds grew robustly. These findings align with previous studies on the differentiation of adventitious buds in
Pogostemon cablin,
Cotoneaster wilsonii and
Allium hirtifolium [
53,
54,
55]. In the study of
Cotoneaster wilsonii, the effect of TDZ on adventitious bud differentiation was better than that of 6-BA [
54]. Our experiments have also confirmed this finding. When the concentration of TDZ was 0.5 mg·L
−1, the shoot buds differentiation rate of
P. lactiflora was the best. When the concentration of TDZ increased to 1.0 mg·L
−1, the differentiation of adventitious buds of
P. lactiflora was very difficult, although it was found that a small amount of callus of
P. lactiflora could differentiate into adventitious buds when 1.0 mg·L
−1 TDZ was added [
24,
25]. The main reason for the low differentiation rate of adventitious buds of
P. lactiflora is not only related to the combination and concentration of plant growth regulators, but also may be related to the number of callus subcultures. Multiple subculturing results in a reduction in meristematic activity, impeding the differentiation of calluses into organ primordia.
IBA, NAA and IAA are commonly used as plant growth regulators for rooting in plant tissue culture systems [
56,
57,
58]. Our results show that the addition of IAA or IBA to ½ MS media could significantly increase the rooting rate of
P. lactiflora, which is consistent with the findings of the study of Zhao et al. [
59]. Nevertheless, a prior investigation revealed that ½ MS media without plant growth regulators could also promote the root development of seedlings in
P. lactiflora [
9]. The rooting rate of peony was the highest in the combination of IBA and IAA, and was better than that in IAA or IBA alone [
60]. Interestingly, we observed the opposite results, and found that, compared with the combination of IAA and IBA, the rooting induction rate of
P. lactiflora with IBA alone was the highest. Overall, IBA treatment had the best rooting effect for
P. lactiflora, which is similar to the results derived for
Petunia hybrida and
Oryza sativa [
61,
62].
The establishment of a P. lactiflora regeneration system has long been challenging. Although we have made some progress on the regeneration of P. lactiflora, the rates of adventitious bud differentiation and rooting in P. lactiflora are still relatively low. Thus, the efficient establishment of a P. lactiflora regeneration system still has a long way to go, and further exploration is necessary to effectively achieve the regeneration of P. lactiflora via the callus pathway.
4. Materials and Methods
4.1. Plant Material and Culture Conditions
The intervarietal hybrid seeds “Fen Yunu” ♀ × “Fen Yulou” ♂ were used as materials, and were collected via pollination from the germplasm resources nursery in Shenyang Agricultural University, Liaoning province, China. The seeds that were collected 100 days after pollination in the current year were shade-dried, and stored in a refrigerator at 4 °C.
P. lactiflora hybrid seeds were soaked in water for 48 h, and we then peeled off the seed coats. Experiments of embryo germination were performed according to the protocol of Duan et al. [
22] with some modifications. Subsequently, seeds were treated with 75% ethanol for 30 s and sterilized with 0.1% (
w/
v) HgCl
2 for 5–6 min followed by washing 4 times with sterile distilled water. Mature zygotic embryos were cut from seeds and inoculated in MS medium supplemented with 0.5 mg·L
−1 GA
3 and 1.0 mg·L
−1 6-BA. After 20 days of culturing, the produced hypocotyls and cotyledons were used as explants for subsequent experiments. All culture media were added with 30 g/L sucrose and 6 g/L agar with pH of 5.8 and autoclaved at 121 °C for 20 min. Except for those subjected to special instructions, all cultures were maintained at 25 ± 1 °C, in a 16/8 h (light/dark) photoperiod with 2000–3000 lx light intensity. All components of the culture media used in this article were purchased from Beijing Solarbio Science & Technology Co., LTD (Beijing, China). All plant growth regulators (PGRs) were purchased from Shanghai Yien Chemical Technology Co., LTD (Shanghai, China).
4.2. Callus Induction by Explant Types and Auxins Combined with 6-BA
The zygotic embryos, cotyledons, and hypocotyls were cultured in MS medium supplemented with 1.0 mg·L−1 BA combined with 2.0 mg·L−1 PIC, 1.0 mg·L−1 BA combined with 2.0 mg·L−1 2,4-D or 1.0 mg·L−1 BA combined with 1.0 mg·L−1 NAA, respectively, to induce calluses under dark conditions. After 45 days, the callus induction rates of the three explants on different media were observed.
4.3. Callus Induction by Basal Medium and NAA Concentration
Experiments on callus induction were performed according to the protocol of Duan et al. [
22] with some modifications. The cotyledons were cultured in MS and ½ MS medium supplemented with 0.5 mg·L
−1 TDZ, 0.5 mg·L
−1 2,4-D and NAA (0.1, 0.3, 0.5, 1.0 mg·L
−1), respectively. After 30 days, the callus induction rate and browning rate were calculated, and the growth status of the callus in each medium was observed.
4.4. Callus Induction by Dark Culture Time
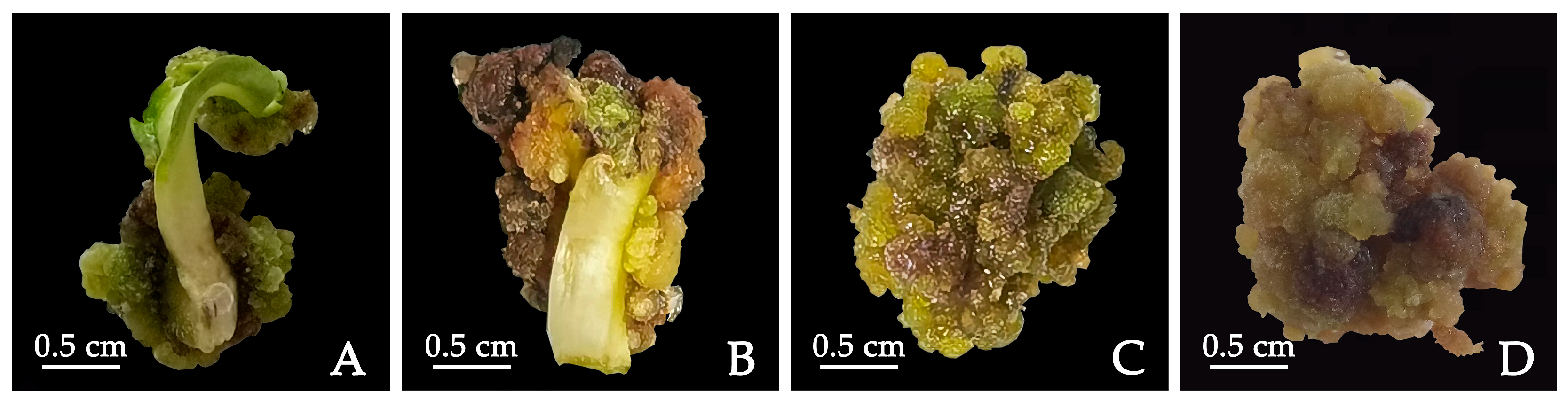
The cotyledons were transferred to MS medium supplemented with 0.5 mg·L−1 TDZ, 0.5 mg·L−1 2,4-D and 0.5 mg·L−1 NAA and cultured in the dark for 10, 20 and 30 days, respectively, while normal light culturing was used as the control treatment (16 h light/8 h dark photoperiod with 2000–3000 lx light intensity). After dark culturing, they were transferred to light culturing conditions. After 30 days, the callus induction of cotyledons under different dark culturing times was observed.
4.5. Callus Induction by PIC Concentration
Cotyledons were cultured in ½ MS medium supplemented with 1.0 mg·L−1 TDZ + 1.0 mg·L−1 2,4-D + 0.5 g·L−1 CH + 1.0 mg·L−1 PVP for 30 days to produce calluses, and then transferred to ½ MS medium supplemented with 1.0 mg·L−1 TDZ + 1.0 mg·L−1 NAA + 0.5 g·L−1 CH + 1.0 mg·L−1 PVP for callus proliferation. After 30 days, the obtained calluses were transferred to ½ MS (Ca2+) medium supplemented with 1.0 mg·L−1 TDZ + 0.5 g·L−1 CH + 1.0 mg·L−1 PVP + PIC (0, 2.0, 4.0 mg·L−1), and the calluses were induced under dark conditions. After 30 days, the induction of calluses was observed.
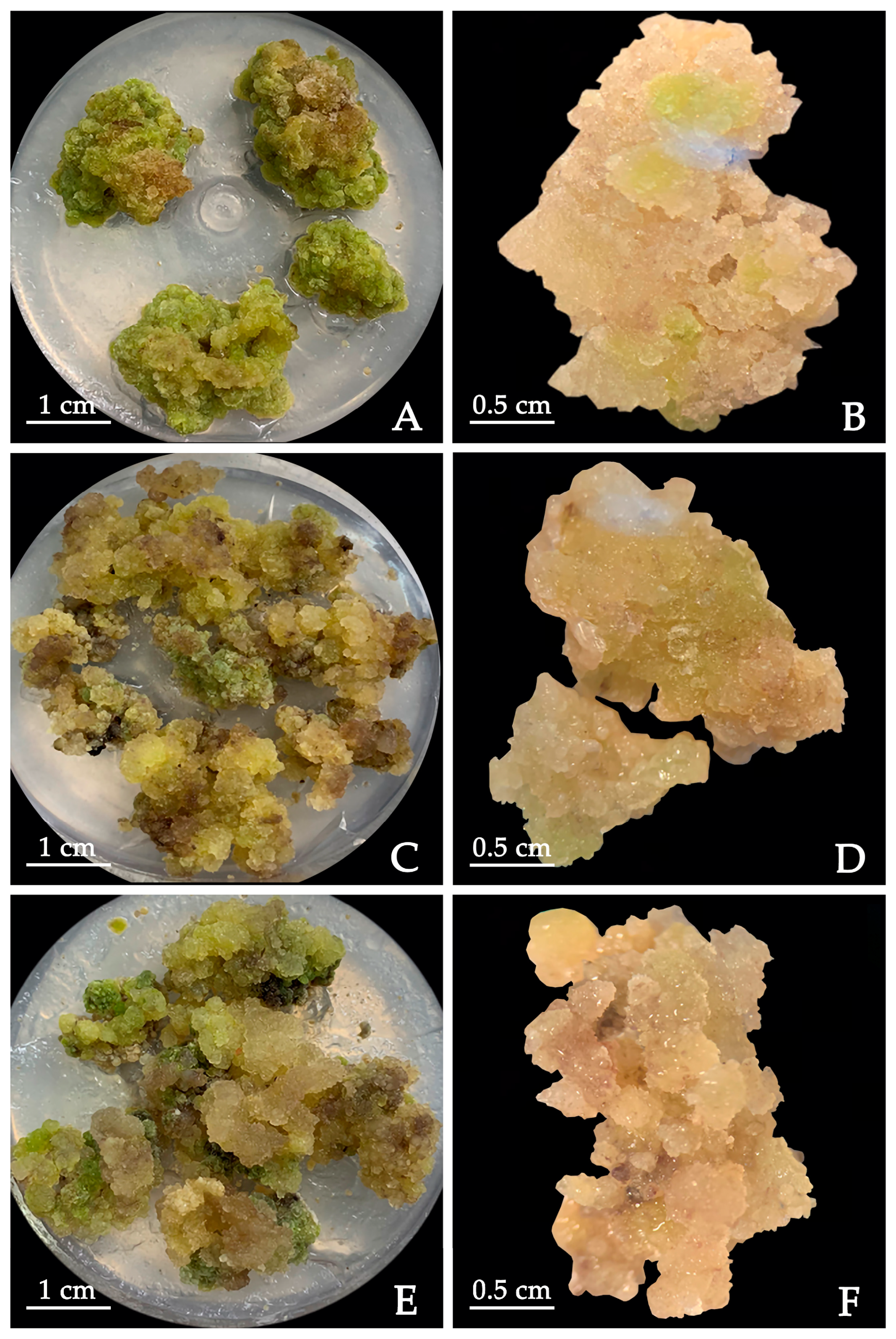
4.6. Callus Proliferation
The calluses induced in MS + 0.5 mg·L
−1 TDZ + 0.5 mg·L
−1 2,4-D + 0.5 mg·L
−1 NAA were selected as the material. Experiments on callus proliferation were performed according to the protocol of Duan et al. [
22] with some modifications. The most well-grown calluses were cut into 1 cm squares and transferred to the ½ MS medium supplemented with different concentrations of NAA (0.1, 0.5, 1.0 mg·L
−1), 2,4-D (0.1, 0.5, 1.0 mg·L
−1) and TDZ (0.5 mg·L
−1) for callus proliferation. Each treatment had 20 explants and 3 replicates. After 30 days, the callus proliferation coefficient and browning rate were measured.
4.7. Adventitious Bud Induction
The callus proliferated in ½ MS + 1.0 mg·L
−1 NAA + 1.0 mg·L
−1 2,4-D + 0.5 mg·L
−1 TDZ was used as the material. Experiments on adventitious bud induction were performed according to the protocol of Sun et al. [
24] with some modifications. Calluses were transferred to MS medium containing 0.2 mg·L
−1 NAA combined with TDZ (0.1, 0.3, 0.5, 1.0 mg·L
−1), 0.2 mg·L
−1 NAA combined with 6-BA (0.1, 0.3, 0.5, 1.0 mg·L
−1) or 0.2 mg·L
−1 NAA combined with KT (0.1, 0.3, 0.5, 1.0 mg·L
−1) for shoot bud differentiation. The medium was supplemented with 1.0 mg·L
−1 PVP to prevent browning. After 45 days, the shoot bud differentiation in each medium was observed.
4.8. Adventitious Bud Growth
Experiments on adventitious bud growth were performed according to the protocol of Liu et al. [
63] with some modifications. The adventitious buds were transferred into MS medium with 1.0 mg·L
−1 GA
3 and tested at different concentrations and ratios of NAA and BA (1:1, 0.5:1 and 1:0.5, in mg·L
−1). Each treatment had 20 explants and 3 replicates. After 30 days, the stem pumping rate, leaf expansion rate and shoot height of adventitious buds were recorded.
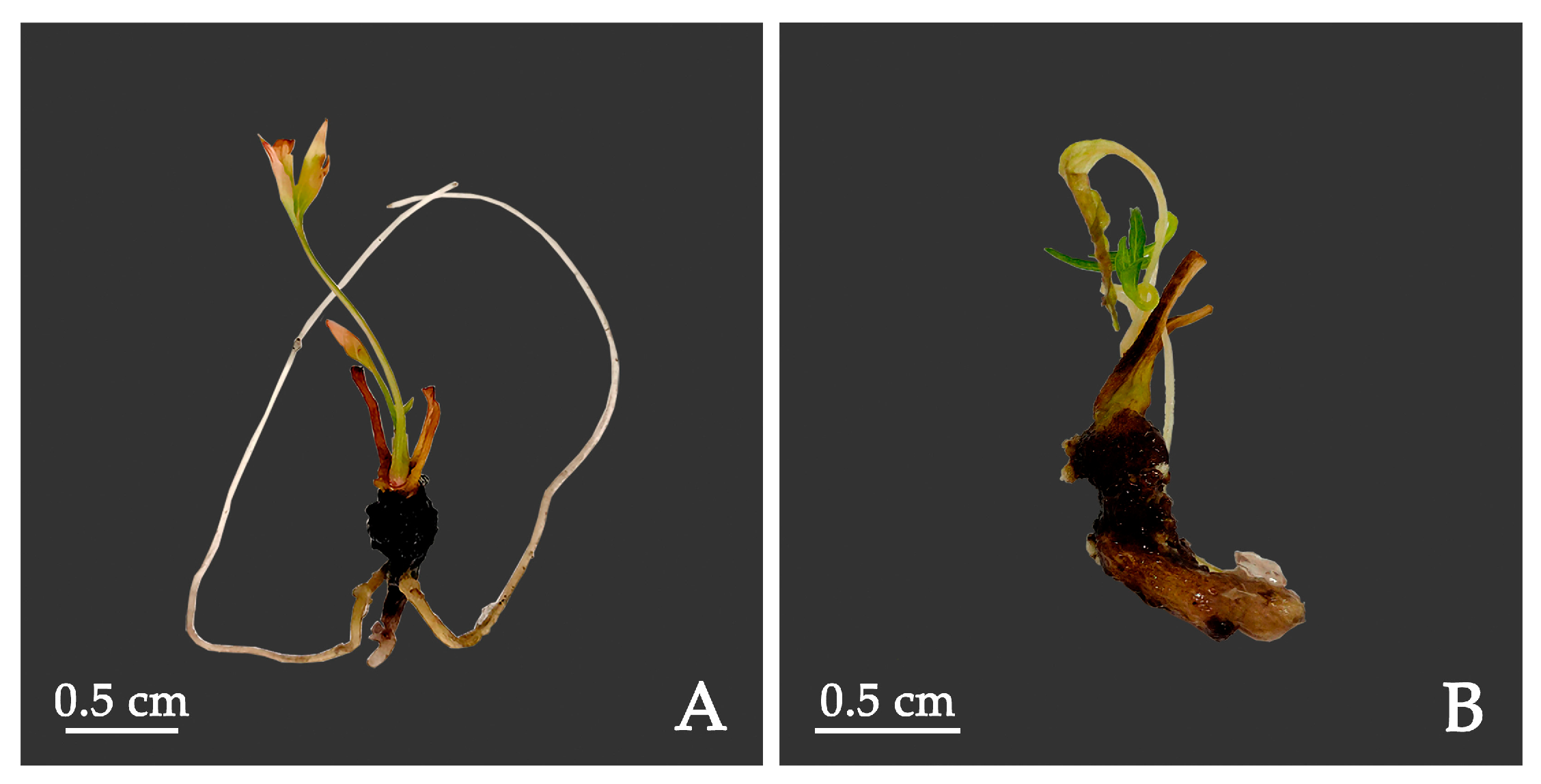
4.9. Root Induction
The shoots with 2–4 leaves and which were 1.5–2.5 cm in height were selected as materials. The shoots cultured for 30 d were transferred to ½ MS medium supplemented with 3.0 g·L−1 AC combined with different concentrations of IAA (0, 0.5, 1.0 mg·L−1) and IBA (0, 0.5, 1.0 mg·L−1) for root induction. The rooting was observed after 45 days.
4.10. Statistical Analysis
Except as noted, each treatment had 30 explants and 3 replicates. Statistical significance was calculated using SPSS software (Version 26.0) with the one-way analysis of variance (ANOVA) method, and significant difference was defined by Duncan’s multiple range test at p < 0.05. The significant data involved in this paper were calculated as follows: induction rate = (number of induced explants/total number of explants) × 100%; proliferation coefficient = fresh proliferation callus weight/inoculated callus weight; browning rate = (number of browning explants/total number of explants) × 100%; differentiation rate = (number of differentiated explants/total number of explants) × 100%; stem pumping rate: (number of stem pumping explants/total number of explants) × 100%; leaf expansion rate: (number of stems with expanded leaves/total number of stems) × 100%; rooting rate = (number of rooting explants/total number of explants) × 100%.