The Fungus Beauveria bassiana Alters Amounts of Sterols, Fatty Acids, and Hydroxycinnamic Acids in Potato Solanum tuberosum
Abstract
1. Introduction
2. Results
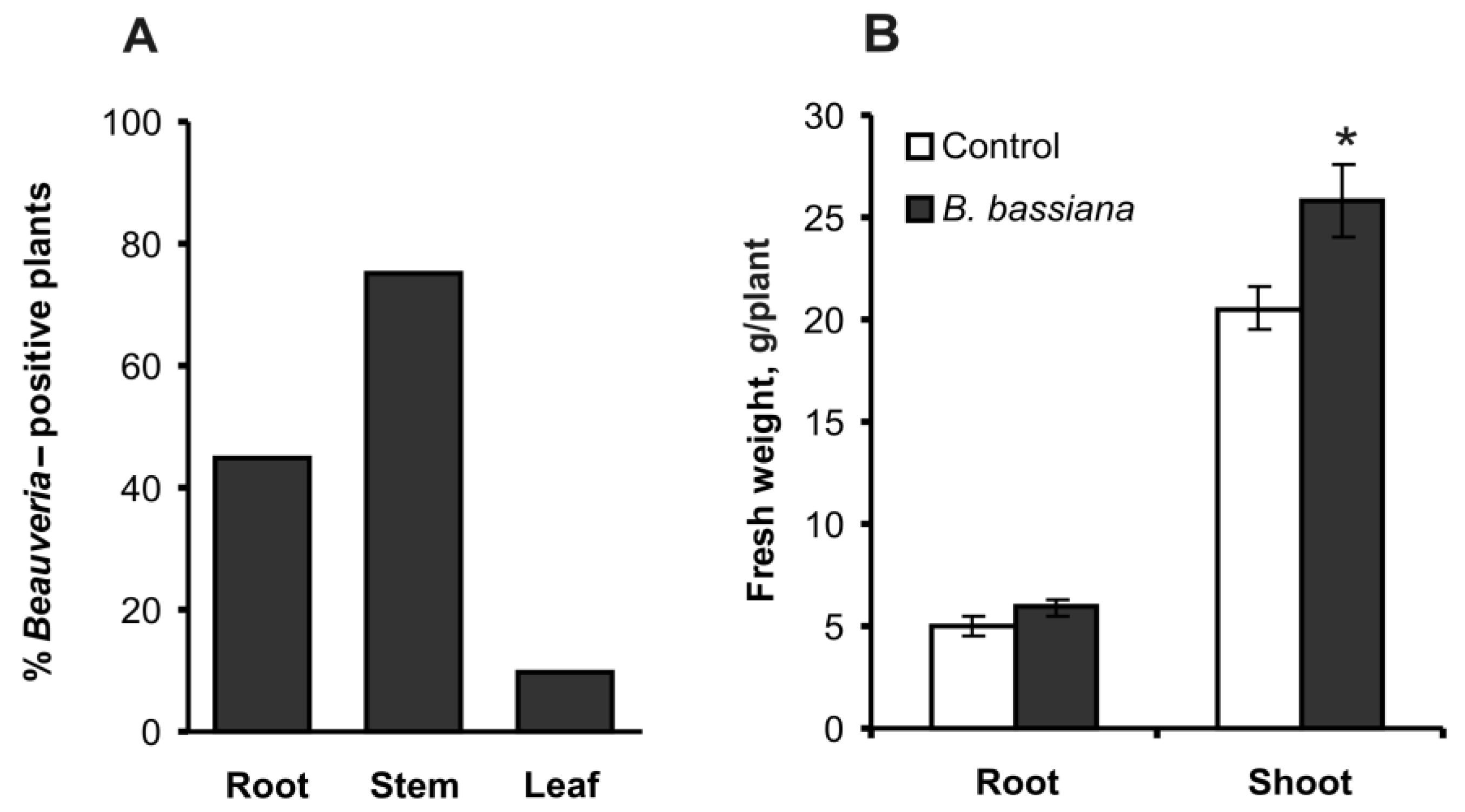
2.1. Colonization and Growth Parameters
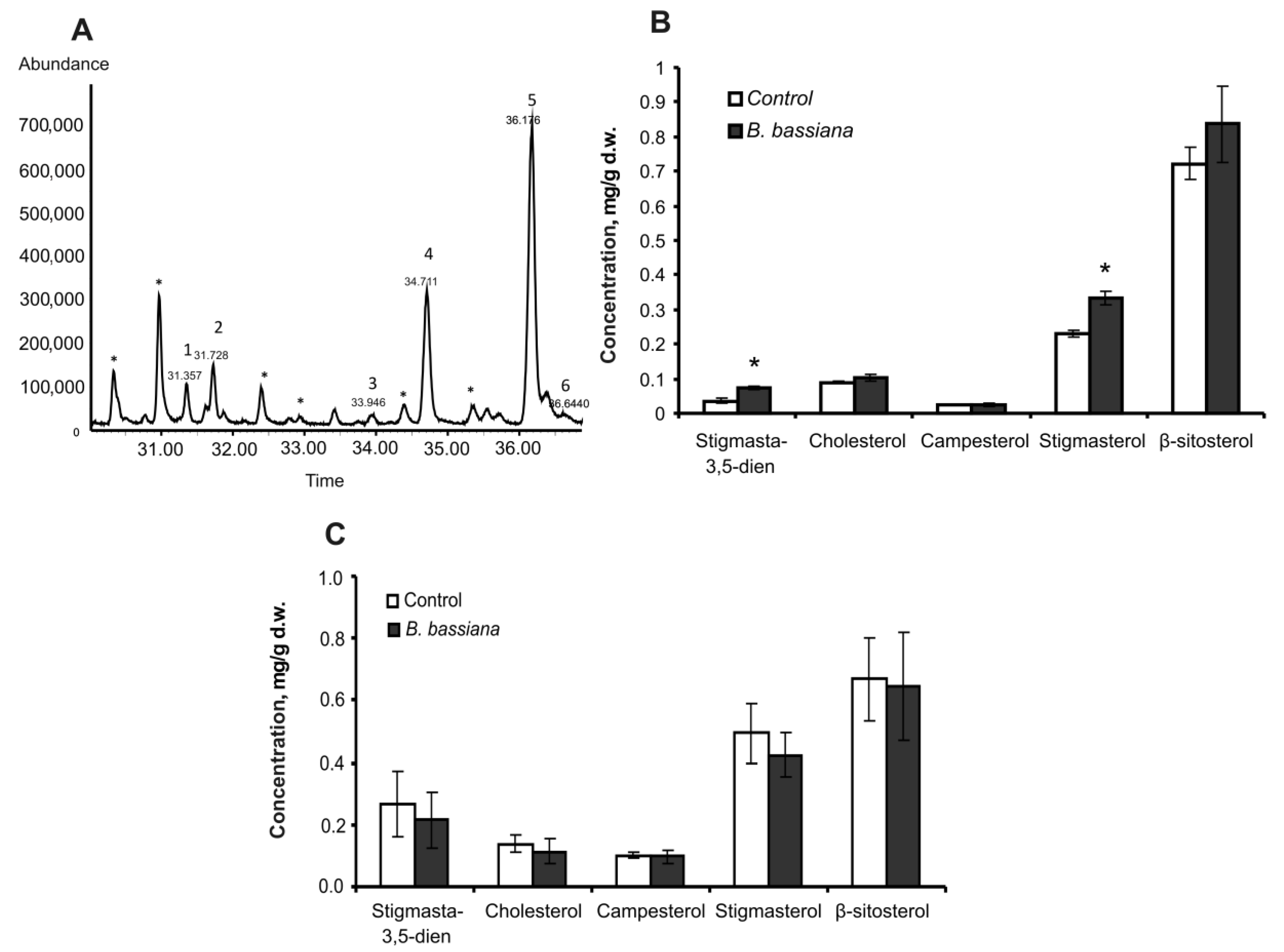
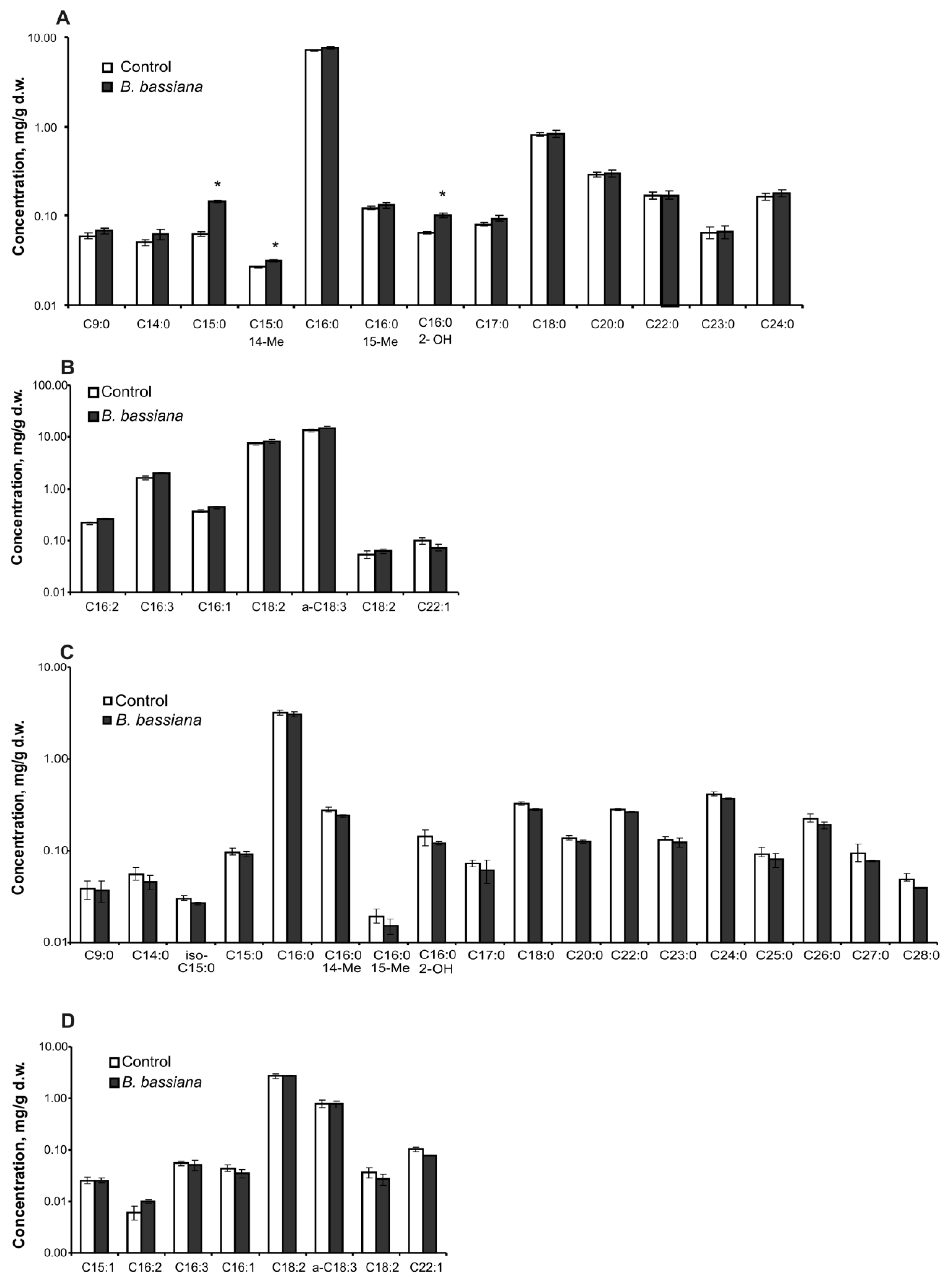
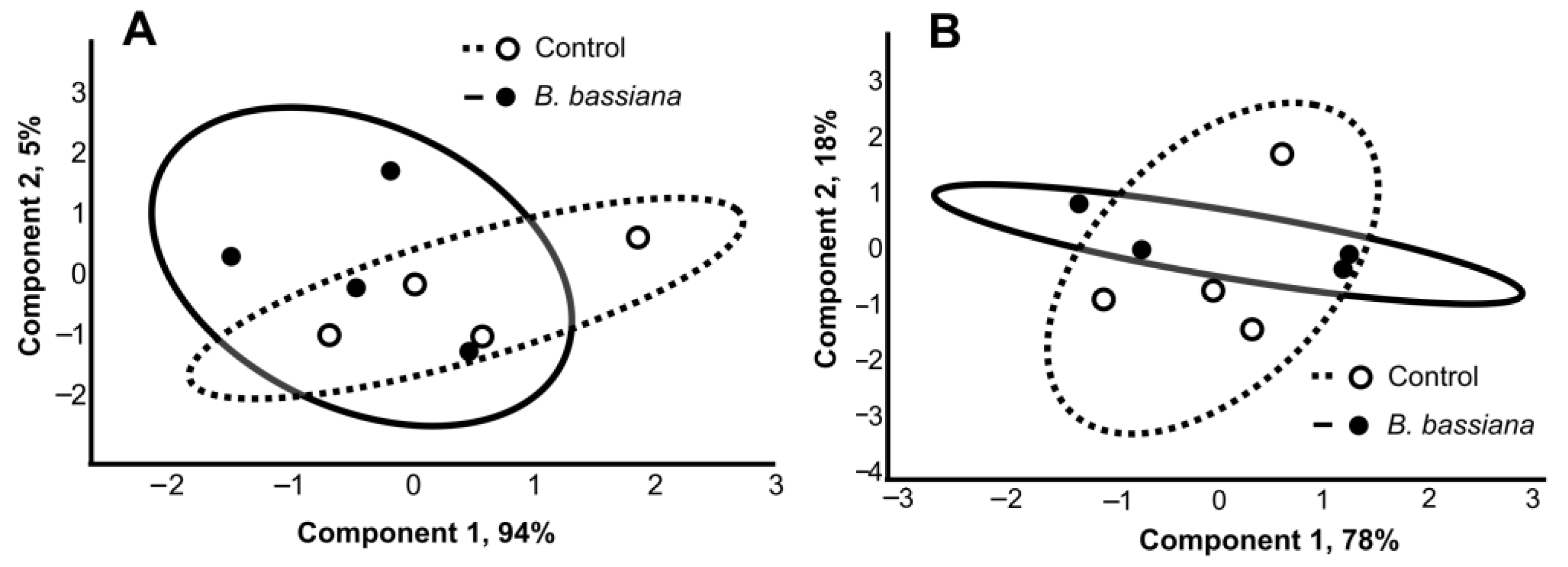
2.2. Amounts of Sterols and Fatty Acids
2.3. Amounts of Hydroxycinnamic Acids
3. Discussion
4. Material and Methods
4.1. The Fungus and Its Cultivation
4.2. Plant Cultivation and Inoculation with the Fungus
4.3. The Extent of Colonization and Plant Weight
4.4. PCR-Based Confirmation of Reisolate Identity
4.5. Plant Material for Lipid and Phenolic Acid Quantification
4.6. Extraction of Lipid Compounds and Derivatization
4.7. Extraction of Phenolic Acids
4.8. GC/MS and HPLC Analyses
4.9. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vega, F. The use of fungal entomopathogens as endophytes in biological control: A review. Mycologia 2018, 110, 4–30. [Google Scholar] [CrossRef]
- Ortiz-Urquiza, A. The Split Personality of Beauveria bassiana: Understanding the Molecular Basis of Fungal Parasitism and Mutualism. mSystems 2021, 6, e0076621. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; Garrido-Jurado, I.; González-Mas, N.; Yousef-Yousef, M. Ecosystem services of entomopathogenic ascomycetes. J. Invertebr. Pathol. 2023, 201, 108015. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Bidochka, M.J. Root colonization by endophytic insect-pathogenic fungi. J. Appl. Microbiol. 2021, 130, 570–581. [Google Scholar] [CrossRef]
- St Leger, R.J.; Wang, J.B. Metarhizium: Jack of all trades, master of many. Open Biol. 2020, 10, 200307. [Google Scholar] [CrossRef] [PubMed]
- Raad, M.; Glare, T.R.; Brochero, H.L.; Müller, C.; Rostás, M. Transcriptional Reprogramming of Arabidopsis thaliana Defence Pathways by the Entomopathogen Beauveria bassiana Correlates with Resistance Against a Fungal Pathogen but Not Against Insects. Front. Microbiol. 2019, 10, 615. [Google Scholar] [CrossRef] [PubMed]
- Proietti, S.; Falconieri, G.S.; Bertini, L.; Pascale, A.; Bizzarri, E.; Morales-Sanfrutos, J.; Sabidó, E.; Ruocco, M.; Monti, M.M.; Russo, A.; et al. Beauveria bassiana rewires molecular mechanisms related to growth and defense in tomato. J. Exp. Bot. 2023, 74, 4225–4243. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, G.; Ownley, B.H.; Auge, R.M.; Toler, H.; Dee, M.; Vu, A.; Kollner, T.G.; Chen, F. Colonization by arbuscular mycorrhizal and endophytic fungi enhanced terpene production in tomato plants and their defense against a herbivorous insect. Symbiosis 2015, 65, 65–74. [Google Scholar] [CrossRef]
- Wei, Q.Y.; Li, Y.Y.; Xu, C.; Wu, Y.X.; Zhang, Y.R.; Liu, H. Endophytic colonization by Beauveria bassiana increases the resistance of tomatoes against Bemisia tabaci. Arthropod-Plant Interact. 2020, 14, 289–300. [Google Scholar] [CrossRef]
- Ríos-Moreno, A.; Garrido-Jurado, I.; Resquín-Romero, G.; Arroyo-Manzanares, N.; Arce, L.; Quesada-Moraga, E. Destruxin A production by Metarhizium brunneum strains during transient endophytic colonisation of Solanum tuberosum. Biocontrol. Sci. Technol. 2016, 26, 1574–1585. [Google Scholar] [CrossRef]
- Krell, V.; Unger, S.; Jakobs-Schoenwandt, D.; Patel, A.V. Importance of phosphorus supply through endophytic Metarhizium brunneum for root:shoot allocation and root architecture in potato plants. Plant Soil 2018, 430, 87–97. [Google Scholar] [CrossRef]
- Krell, V.; Unger, S.; Jakobs-Schoenwandt, D.; Patel, A.V. Endophytic Metarhizium brunneum mitigates nutrient deficits in potato and improves plant productivity and vitality. Fungal Ecol. 2018, 34, 43–49. [Google Scholar] [CrossRef]
- Tomilova, O.G.; Kryukova, N.A.; Efimova, M.V.; Kovtun, I.S.; Kolomeichuk, L.V.; Kryukov, V.Y.; Glupov, V.V. Early Physiological Response of Potato Plants to Entomopathogenic Fungi under Hydroponic Conditions. Horticulturae 2021, 7, 217. [Google Scholar] [CrossRef]
- Tyurin, M.; Kabilov, M.R.; Smirnova, N.; Tomilova, O.G.; Yaroslavtseva, O.; Alikina, T.; Glupov, V.V.; Kryukov, V.Y. Can Potato Plants Be Colonized with the Fungi Metarhizium and Beauveria under Their Natural Load in Agrosystems? Microorganisms 2021, 9, 1373. [Google Scholar] [CrossRef]
- Tomilova, O.G.; Shaldyaeva, E.M.; Kryukova, N.A.; Pilipova, Y.V.; Schmidt, N.S.; Danilov, V.P.; Kryukov, V.Y.; Glupov, V.V. Entomopathogenic fungi decrease Rhizoctonia disease in potato in field conditions. PeerJ 2020, 8, e9895. [Google Scholar] [CrossRef]
- Welling, M.T.; Liu, L.; Rose, T.J.; Waters, D.L.; Benkendorff, K. Arbuscular mycorrhizal fungi: Effects on plant terpenoid accumulation. Plant Biol. 2016, 18, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh, S.; Pirzad, A. Pseudomonas and mycorrhizal fungi co-inoculation alter seed quality of flax under various water supply conditions. Ind. Crops Prod. 2019, 129, 518–524. [Google Scholar] [CrossRef]
- Igiehon, N.O.; Babalola, O.O.; Cheseto, X.; Torto, B. Effects of rhizobia and arbuscular mycorrhizal fungi on yield, size distribution and fatty acid of soybean seeds grown under drought stress. Microbiol. Res. 2021, 242, 126640. [Google Scholar] [CrossRef]
- Gholinezhad, E.; Darvishzadeh, R. Influence of arbuscular mycorrhiza fungi and drought stress on fatty acids profile of sesame (Sesamum indicum L.). Field Crops Res. 2021, 262, 108035. [Google Scholar] [CrossRef]
- Aboobucker, S.I.; Suza, W.P. Why Do Plants Convert Sitosterol to Stigmasterol? Front. Plant Sci. 2019, 10, 354. [Google Scholar] [CrossRef]
- Hartmann, M.A. Plant sterols and the membrane environment. Trends Plant Sci. 1998, 3, 170–175. [Google Scholar] [CrossRef]
- Wang, K.; Senthil-Kumar, M.; Ryu, C.-M.; Kang, L.; Mysore, K.S. Phytosterols play a key role in plant innate immunity against bacterial pathogens by regulating nutrient efflux into the apoplast. Plant Physiol. 2012, 158, 1789–1802. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Ding, N.Z. Plant Unsaturated Fatty Acids: Multiple Roles in Stress Response. Front. Plant Sci. 2020, 11, 562785. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.F.; Oraby, H.F. Fatty Acids and Bioactive Lipids of Potato Cultivars: An Overview. J. Oleo Sci. 2016, 65, 459–470. [Google Scholar] [CrossRef]
- Haruma, T.; Yamaji, K.; Ogawa, K.; Masuya, H.; Sekine, Y.; Kozai, N. Root-endophytic Chaetomium cupreum chemically enhances aluminium tolerance in Miscanthus sinensis via increasing the aluminium detoxicants, chlorogenic acid and oosporein. PLoS ONE 2019, 14, e0212644. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef] [PubMed]
- Akyol, H.; Riciputi, Y.; Capanoglu, E.; Caboni, M.F.; Verardo, V. Phenolic Compounds in the Potato and Its Byproducts: An Overview. Int. J. Mol. Sci. 2016, 17, 835. [Google Scholar] [CrossRef] [PubMed]
- Narukawa, M. Chlorogenic Acid Facilitates Root Hair Formation in Lettuce Seedlings. Plant Cell Physiol. 2009, 50, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Denaxa, N.-K.; Roussos, P.; Kostelenos, G.; Vemmos, S. Chlorogenic Acid: A Possible Cofactor in the Rooting of ‘Kalamata’ Olive Cultivar. J. Plant Growth Regul. 2021, 40, 2017–2027. [Google Scholar] [CrossRef]
- Tsafouros, A.; Roussos, P.A. Dopamine, Chlorogenic Acid, and Quinones as Possible Cofactors of Increasing Adventitious Rooting Potential of In Vitro Krymsk 5 Cherry Rootstock Explants. Agronomy 2022, 12, 1154. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Feng, S.; Yang, Z. Characteristics and bioactivity of a chlorogenic acid-producing endophytic bacterium isolated from Lonicera japonicae. Intl. J. Agric. Biol. 2019, 21, 743–749. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A. Role of Polyphenols in the Resistance Mechanisms of Plants Against Fungal Pathogens and Insects. In Phytochemistry: Advances in Research; Imperato, F., Ed.; Recearch Signpost: Thiruvananthapuram, India, 2006; pp. 23–67. [Google Scholar]
- Martínez, G.; Regente, M.; Jacobi, S.; Del Rio, M.; Pinedo, M.; de la Canal, L. Chlorogenic acid is a fungicide active against phytopathogenic fungi. Pestic. Biochem. Phys. 2017, 40, 30–35. [Google Scholar] [CrossRef]
- Desmedt, W.; Mangelinckx, S.; Kyndt, T.; Vanholme, B. A Phytochemical Perspective on Plant Defense Against Nematodes. Front. Plant Sci. 2020, 11, 602079. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Vidal, S.; Salinas, J.; Tena, M.; Lopez-Llorca, L.V. Proteomic analysis of date palm (Phoenix dactylifera L.) responses to endophytic colonization by entomopathogenic fungi. Electrophoresis 2009, 30, 2996–3005. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Lovett, B.; Fang, W.; St Leger, R.J. Metarhizium robertsii produces indole-3-acetic acid, which promotes root growth in Arabidopsis and enhances virulence to insects. Microbiology 2017, 163, 980–991. [Google Scholar] [CrossRef]
- Morikawa, T.; Mizutani, M.; Aoki, N.; Watanabe, B.; Saga, H.; Saito, S.; Oikawa, A.; Suzuki, H.; Sakurai, N.; Shibata, D.; et al. Cytochrome P450 CYP710A encodes the sterol C-22 desaturase in Arabidopsis and tomato. Plant Cell 2006, 18, 1008–1022. [Google Scholar] [CrossRef]
- Griebel, T.; Zeier, J. A role for β-sitosterol to stigmasterol conversion in plant–pathogen interactions. Plant J. 2010, 63, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Hirsch-Hoffmann, M.; Hennig, L.; Gruissem, W. GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol. 2004, 136, 2621–2632. [Google Scholar] [CrossRef] [PubMed]
- Fabro, G.; Di Rienzo, J.A.; Voigt, C.A.; Savchenko, T.; Dehesh, K.; Somerville, S.; Alvarez, M.E. Genome-wide expression profiling Arabidopsis at the stage of Golovinomyces cichoracearum haustorium formation. Plant Physiol. 2008, 146, 1421–1439. [Google Scholar] [CrossRef]
- Gupta, R.; Keppanan, R.; Leibman-Markus, M.; Rav-David, D.; Elad, Y.; Ment, D.; Bar, M. The Entomopathogenic Fungi Metarhizium brunneum and Beauveria bassiana Promote Systemic Immunity and Confer Resistance to a Broad Range of Pests and Pathogens in Tomato. Phytopathology 2022, 112, 784–793. [Google Scholar] [CrossRef]
- Pose, D.; Castanedo, I.; Borsani, O.; Nieto, B.; Rosado, A.; Taconnat, L.; Ferrer, A.; Dolan, L.; Valpuesta, V.; Botella, M.A. Identification of the Arabidopsis dry2⁄sqe1-5 mutant reveals a central role for sterols in drought tolerance and regulation of reactive oxygen species. Plant J. 2009, 59, 63–76. [Google Scholar] [CrossRef]
- Spanu, P.; Kämper, J. Genomics of biotrophy in fungi and oomycetes--emerging patterns. Curr. Opin. Plant Biol. 2010, 13, 409–414. [Google Scholar] [CrossRef]
- Cao, J.L.; He, W.X.; Zou, Y.N.; Wu, Q.S. An endophytic fungus, Piriformospora indica, enhances drought tolerance of trifoliate orange by modulating the antioxidant defense system and composition of fatty acids. Tree Physiol. 2023, 43, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Iida, Y.; Higashi, Y.; Nishi, O.; Kouda, M.; Maeda, K.; Yoshida, K.; Asano, S.; Kawakami, T.; Nakajima, K.; Kuroda, K.; et al. Entomopathogenic fungus Beauveria bassiana-based bioinsecticide suppresses severity of powdery mildews of vegetables by inducing the plant defense responses. Front. Plant Sci. 2023, 14, 1211825. [Google Scholar] [CrossRef]
- Pohl, C.H.; Kock, J.L.F.; Thibane, V.S. Antifungal free fatty acids: A review. In Science Against Microbial Pathogens: Communicating Current Research and Technological Advances; Mendez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2011; pp. 61–71. [Google Scholar]
- Muola, A.; Birge, T.; Helander, M.; Mathew, S.; Harazinova, V.; Saikkonen, K.; Fuchs, B. Endophytic Beauveria bassiana induces biosynthesis of flavonoids in oilseed rape following both seed inoculation and natural colonization. Pest Manag. Sci. 2023. [Google Scholar] [CrossRef]
- Homayoonzadeh, M.; Esmaeily, M.; Talebi, K.; Allahyari, H.; Reitz, S.; Michaud, J.P. Inoculation of cucumber plants with Beauveria bassiana enhances resistance to Aphis gossypii (Hemiptera: Aphididae) and increases aphid susceptibility to pirimicarb. Eur. J. Entomol. 2022, 119, 1–11. [Google Scholar] [CrossRef]
- Mei, Y.; Sun, H.; Du, G.; Wang, X.; Lyu, D. Exogenous chlorogenic acid alleviates oxidative stress in apple leaves by enhancing antioxidant capacity. Sci. Hortic. 2020, 274, 109676. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, S.; Zhang, Y.; Wang, X.; Li, D.; Wang, R. PbHCT4 regulates growth through affecting chlorogenic acid (cga) content in pear. Sci. Hortic. 2022, 303, 111225. [Google Scholar] [CrossRef]
- Franklin, G.; Dias, A.C. Chlorogenic acid participates in the regulation of shoot, root and root hair development in Hypericum perforatum. Plant Physiol. Biochem. Plant Physiol. Biochem. 2011, 49, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Pilet, P.E. Effect of chlorogenic acid on the auxin catabolism and the auxin content of root tissues. Phytochemistry 1964, 3, 617–621. [Google Scholar] [CrossRef]
- Imbert, M.P.; Wilson, L.A. Effects of chlorogenic and caffeic acids on IAA oxidase preparations from sweet potato roots. Phytochemistry 1972, 11, 2671–2676. [Google Scholar] [CrossRef]
- Valle, E.; Werner, I.; Frydman, M.; Silen, L.G. On Anti-Fungal Factors in Potato Leaves. Acta Chem. Scand. 1957, 11, 395–397. [Google Scholar] [CrossRef][Green Version]
- Lattanzio, V.; Di Venere, D.; Linsalata, V.; Bertolini, P.; Ippolito, A.; Salerno, M. Low temperature metabolism of apple phenolics and quiescence of Phlyctaena vagabunda. J. Agric. Food Chem. 2001, 49, 5817–5821. [Google Scholar] [CrossRef]
- Sung, W.; Lee, D.G. Antifungal action of chlorogenic acid against pathogenic fungi. Pure Appl. Chem. 2010, 82, 219–226. [Google Scholar] [CrossRef]
- Bischoff, J.F.; Rehner, S.A.; Humber, R.A. A multilocus phylogeny of the Metarhizium anisopliae lineage. Mycologia 2009, 101, 512–530. [Google Scholar] [CrossRef]
- Posadas, J.B.; Comerio, R.M.; Mini, J.I.; Nussenbaum, A.L.; Lecuona, R.E. A novel dodine-free selective medium based on the use of cetyl trimethyl ammonium bromide (CTAB) to isolate Beauveria bassiana, Metarhizium anisopliae sensu lato and Paecilomyces lilacinus from soil. Mycologia 2012, 104, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Kepler, R.M.; Ugine, T.A.; Maul, J.E.; Cavigelli, M.A.; Rehner, S.A. Community composition and population genetics of insect pathogenic fungi in the genus Metarhizium from soils of a long-term agricultural research system. Environ. Microbiol. 2015, 17, 2791–2804. [Google Scholar] [CrossRef] [PubMed]
- Rasool, S.; Markou, A.; Hannula, S.E.; Biere, A. Effects of tomato inoculation with the entomopathogenic fungus Metarhizium brunneum on spider mite resistance and the rhizosphere microbial community. Front. Microbiol. 2023, 14, 1197770. [Google Scholar] [CrossRef]
- Siqueira, A.C.O.; Mascarin, G.M.; Gonçalves, C.R.N.C.B.; Marcon, J.; Quecine, M.C.; Figueira, A.; Delalibera, Í., Jr. Multi-Trait Biochemical Features of Metarhizium Species and Their Activities That Stimulate the Growth of Tomato Plants. Front. Sustain. Food Syst. 2020, 4, 137. [Google Scholar] [CrossRef]
- Baron, N.C.; de Souza Pollo, A.; Rigobelo, E.C. Purpureocillium lilacinum and Metarhizium marquandii as plant growth-promoting fungi. PeerJ 2020, 8, e9005. [Google Scholar] [CrossRef]
- Posada, F.; Aime, M.C.; Peterson, S.W.; Rehner, S.A.; Vega, F.E. Inoculation of coffee plants with the fungal entomopathogen Beauveria bassiana (Ascomycota: Hypocreales). Mycol. Res. 2007, 111, 748–757. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, A.C.; Saari, S.; Moran-Diez, M.E.; Meyling, N.V.; Raad, M.; Glare, T.R. Beauveria bassiana as an endophyte: A critical review on associated methodology and biocontrol potential. BioControl 2017, 62, 1–17. [Google Scholar] [CrossRef]
- Castro-Vásquez, R.M.; Molina-Bravo, R.; Hernández-Villalobos, S.; Vargas-Martínez, A.; González-Herrera, A.; Montero-Astúa, M. Identification and phylogenetic analysis of a collection of Beauveria spp. Isolates from Central America and Puerto Rico. J. Invertebr. Pathol. 2021, 184, 107642. [Google Scholar] [CrossRef] [PubMed]
- Rehner, S.A.; Minnis, A.M.; Sung, G.H.; Luangsa-ard, J.J.; Devotto, L.; Humber, R.A. Phylogeny and systematics of the anamorphic, entomopathogenic genus Beauveria. Mycologia 2011, 103, 1055–1073. [Google Scholar] [CrossRef] [PubMed]
- Levchenko, M.; Gerus, A.; Malysh, S.; t Orazova, S.; Lednev, G. The effect of endophytic colonization of wheat plants by the fungus Beauveria bassiana on the development of the nymphs of the migratory and desert locusts. BIO Web Conf. 2020, 18, 1–4. [Google Scholar] [CrossRef]
- Tomilova, O.G.; Kryukov, V.Y.; Kryukova, N.A.; Tolokonnikova, K.P.; Tokarev, Y.S.; Rumiantseva, A.S.; Alekseev, A.A.; Glupov, V.V. Effects of passages through an insect or a plant on virulence and physiological properties of the fungus Metarhizium robertsii. PeerJ 2023, 11, e15726. [Google Scholar] [CrossRef]
- Vogelstein, B.; Gillespie, D. Preparative and analytical purification of DNA from agarose. Proc. Natl. Acad. Sci. USA 1979, 76, 615–619. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Shang, X.; Keum, Y.-S. Advances in Lipid Extraction Methods—A Review. Int. J. Mol. Sci. 2021, 22, 13643. [Google Scholar] [CrossRef]
- Dove, H.; Mayes, R.W. Protocol for the Analysis of N-Alkanes and Other Plant-Wax Compounds and for Their Use as Markers for Quantifying the Nutrient Supply of Large Mammalian Herbivores. Nat. Protoc. 2006, 1, 1680–1697. [Google Scholar] [CrossRef]
- Laskos, K.; Czyczyło-Mysza, I.M.; Dziurka, M.; Noga, A.; Goralska, M.; Bartyzel, J.; Myskow, B. Correlation between Leaf Epicuticular Wax Composition and Structure, Physio-biochemical Traits and Drought Resistance in Glaucous and Non-glaucous Near-isogenic Lines of Rye. Plant J. 2021, 108, 93–119. [Google Scholar] [CrossRef] [PubMed]
- Boeing, J.S.; Barizão, E.O.; Silva, B.C.E.; Montanher, P.F.; de Cinque Almeida, V.; Visentainer, J.V. Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: Application of principal component analysis. Chem. Cent. J. 2014, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, M.A.Z.; Roehrs, M.; de Pereira, C.M.P.; Freitag, R.A.; de Bairros, A.V. Analysis of phytosterols in plants and derived products by gas chromatography—A short critical review. Austin Chromatogr. 2014, 1, 1021. [Google Scholar]
- Lagarda, M.J.; García-Llatas, G.; Farré, R. Analysis of phytosterols in foods. J. Pharm. Biomed. Anal. 2006, 41, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Z.; Kao, S.Y.; Jian, H.C.; Yu, Y.M.; Li, J.Y.; Wang, W.H.; Tsai, C.W. Determination of cholesterol and four phytosterols in foods without derivatization by gas chromatography-tandem mass spectrometry. J. Food. Drug Anal. 2015, 23, 636–644. [Google Scholar] [CrossRef]
- Chamorro, S.; Cueva-Mestanza, R.; Pascual-Teresa, S. Effect of spray drying on the polyphenolic compounds present in purple sweet potato roots: Identification of new cinnamoylquinic acids. Food Chem. 2021, 345, 128679. [Google Scholar] [CrossRef]
- Levene, H. Robust tests for equality of variances. In Contributions to Probability and Statistics; Olkin, 1st ed.; Stanford University Press: Palo Alto, CA, USA, 1960; pp. 278–292. [Google Scholar]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyurin, M.; Chernyak, E.; Tomilova, O.; Tolokonnikova, K.; Malysh, S.M.; Khramova, E.; Morozov, S.; Kryukov, V. The Fungus Beauveria bassiana Alters Amounts of Sterols, Fatty Acids, and Hydroxycinnamic Acids in Potato Solanum tuberosum. Plants 2023, 12, 3938. https://doi.org/10.3390/plants12233938
Tyurin M, Chernyak E, Tomilova O, Tolokonnikova K, Malysh SM, Khramova E, Morozov S, Kryukov V. The Fungus Beauveria bassiana Alters Amounts of Sterols, Fatty Acids, and Hydroxycinnamic Acids in Potato Solanum tuberosum. Plants. 2023; 12(23):3938. https://doi.org/10.3390/plants12233938
Chicago/Turabian StyleTyurin, Maksim, Elena Chernyak, Oksana Tomilova, Khristina Tolokonnikova, Svetlana M. Malysh, Elena Khramova, Sergey Morozov, and Vadim Kryukov. 2023. "The Fungus Beauveria bassiana Alters Amounts of Sterols, Fatty Acids, and Hydroxycinnamic Acids in Potato Solanum tuberosum" Plants 12, no. 23: 3938. https://doi.org/10.3390/plants12233938
APA StyleTyurin, M., Chernyak, E., Tomilova, O., Tolokonnikova, K., Malysh, S. M., Khramova, E., Morozov, S., & Kryukov, V. (2023). The Fungus Beauveria bassiana Alters Amounts of Sterols, Fatty Acids, and Hydroxycinnamic Acids in Potato Solanum tuberosum. Plants, 12(23), 3938. https://doi.org/10.3390/plants12233938







