Unveiling the Impact of Drying Methods on Phytochemical Composition and Antioxidant Activity of Anthemis palestina
Abstract
1. Introduction
2. Results and Discussion
2.1. Composition of HDEO Obtained from the Plant Material Subjected to Different Drying Methods
2.2. GC/MS Analysis of HD-EOs Obtained from A. palestina Aerial Parts Subjected to Different Drying Methods
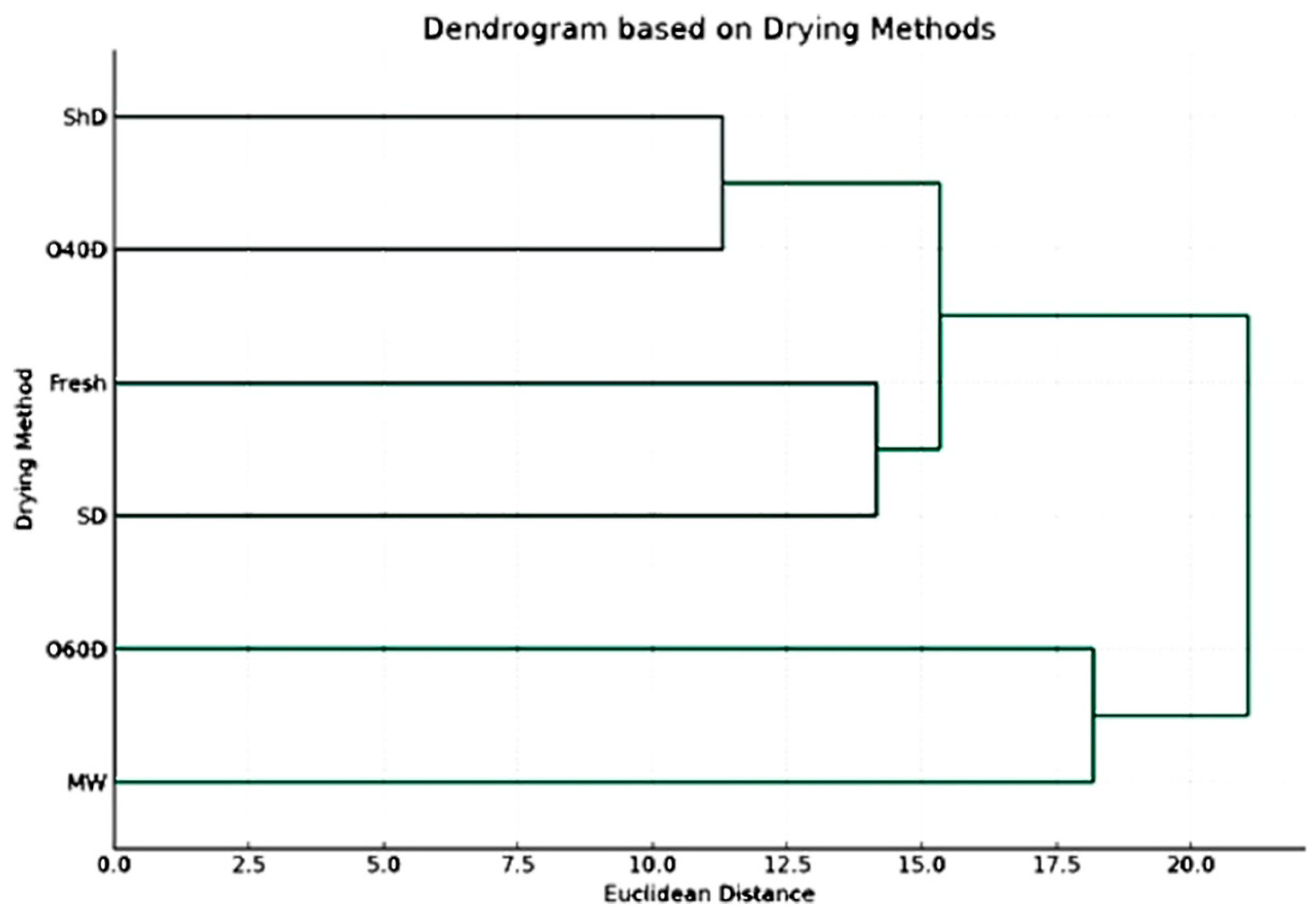
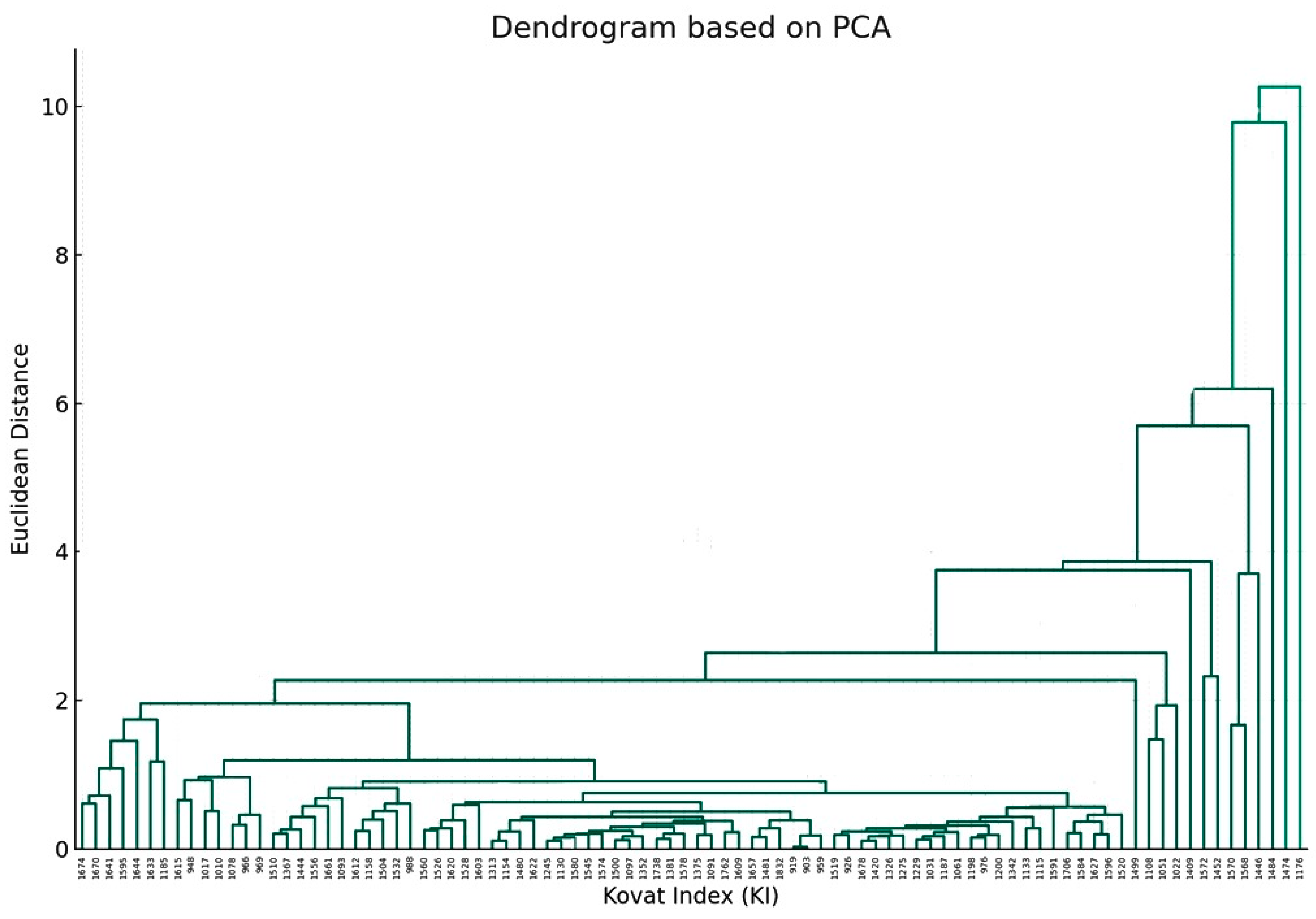
2.3. Principal Component Analysis and Cluster Analysis
2.4. Antioxidant Activities of HD-EOs
2.5. Total Phenol Content (TPC), Total Flavonoid Content (TFC) and Antioxidant Activity of APM Extracts
2.6. Correlation Studies: Antioxidant Activities, TPC, and TFC
2.7. LC-MS Analysis of Phytochemicals
3. Materials and Methods
3.1. Plant Material
3.2. Drying Conditions
3.3. Extraction of Essential Oil
3.4. Preparation of the Methanolic Extract
3.5. GC and GC-MS Analysis
3.6. Total Phenolic and Total Flavonoid Contents
3.7. Antioxidant Activity of HDEOs and Methanolic Extracts
3.8. LC-MS Analysis of Phytochemicals
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Bataineh, N.; Algethami, F.K.; Al-Jaber, H.I.; Alhamzani, A.G.; Bataineh, R.M.; Al-Dalahmeh, Y.; Bataineh, T.T.; Abu-Orabi, S.T.; Al-Qudah, M.A. Ballota saxatilis from Jordan: Evaluation of essential oil composition and phytochemical profiling of crude extracts and their in-vitro antioxidant Activity. Separations 2023, 10, 114. [Google Scholar] [CrossRef]
- Al-Qudah, M.A.; Al-Smadi, Z.M.; Al-Jaber, H.I.; Tashtoush, H.I.; Alkhatib, R.Q.; Bataineh, T.T.; Dalahmeh, Y.; Orabi, S.T.A. GC/MS and LC-MS/MS phytochemical evaluation of the essential oil and selected secondary metabolites of Ajuga orientalis from Jordan and its antioxidant activity. Arab. J. Chem. 2023, 16, 104641. [Google Scholar] [CrossRef]
- Al-Qudah, M.A. Antioxidant acitvity and chemical composition of essential oils of fresh and air-dried Jordanian Nepeta curviflora Boiss. J. Biol. Act. Prod. Nat. 2016, 6, 101–111. [Google Scholar]
- Diniz do Nascimento, L.; Moraes, A.A.; Costa, K.S.; Pereira Galúcio, J.M.; Taube, P.S.; Costa, C.M.; Neves Cruz, J.; de Aguiar Andrade, E.H.; Faria, L.J. Bioactive natural compounds and antioxidant activity of essential oils from spice plants: New findings and potential applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef]
- Al-Shuneigat, J.M.; Al-Tarawneh, I.N.; Al-Qudah, M.A.; Al-Sarayreh, S.A.; Al-Saraireh, Y.M.; Alsharafa, K.Y. The chemical composition and the antibacterial properties of Ruta graveolens L. essential oil grown in Northern Jordan. Jordan J. Biol. Sci. 2015, 8, 139–143. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Kameli, A.; Ferhat, M.A.; Saidi, F.; Tayebi, K. The food preservative potential of essential oils: Is lemongrass the answer? J. Verbraucherschutz Leb. 2013, 9, 13–21. [Google Scholar] [CrossRef]
- Al-Momani, L.A.; Abu-Orabi, S.T.; Hlail, H.M.; Alkhatib, R.Q.; Al-Dalahmeh, Y.; Al-Qudah, M.A. Anthemis cotula L. from Jordan: Essential oil composition, LC-ESI-MS/MS profiling of phenolic acids-flavonoids and in vitro antioxidant activity. Arab. J. Chem. 2023, 16, 104470. [Google Scholar] [CrossRef]
- Abu Zarga, M.H.; Al-Jaber, H.I.; Baba Amer, Z.Y.; Sakhrib, L.; Al-Qudah, M.A.; Al-humaidi, J.Y.; Abaza, I.F.; Afifi, F.U. Chemical composition, antimicrobial and antitumor activities of essential oil of Ammodaucus leucotrichus growing in Algeria. J. Biol. Act. Prod. Nat. 2013, 3, 224–231. [Google Scholar]
- da Costa, K.; Galúcio, J.; da Costa, C.; Santana, A.; dos Santos Carvalho, V.; do Nascimento, L. Exploring the potentiality of natural products from essential oils as inhibitors of odorant-binding proteins: A structure- and ligand-based virtual screening approach to find novel mosquito repellents. ACS Omega 2019, 4, 22475–22486. [Google Scholar] [CrossRef]
- Liang, J.-Y.; Guo, S.-S.; Zhang, W.-J.; Geng, Z.-F.; Deng, Z.-W.; Du, S.-S.; Zhang, J. Fumigant and repellent activities of essential oil extracted from Artemisia dubia and its main compounds against two stored product pests. Nat. Prod. Res. 2017, 32, 1234–1238. [Google Scholar] [CrossRef]
- Bertoli, A.; Conti, B.; Mazzoni, V.; Meini, L.; Pistelli, L. Volatile chemical composition and bioactivity of six essential oils against the stored food insect sitophilus zeamais motsch. (Coleoptera Dryophthoridae). Nat. Prod. Res. 2011, 26, 2063–2071. [Google Scholar] [PubMed]
- Boutebouhart, H.; Didaoui, L.; Tata, S.; Sabaou, N. Effect of extraction and drying method on chemical composition, and evaluation of antioxidant and antimicrobial activities of essential oils from Salvia officinalis. J. Essent. Oil Bear. Plants 2019, 22, 717–727. [Google Scholar] [CrossRef]
- Prusinowska, R.; Śmigielski, K. Losses of essential oils and antioxidants during the drying of herbs and spices. A review. Eng. Sci. Technol. 2015, 2, 49–62. [Google Scholar] [CrossRef]
- Figiel, A.; Szumny, A.; Gutiérrez-Ortíz, A.; Carbonell-Barrachina, Á.A. Composition of oregano essential oil (Origanum vulgare) as affected by drying method. J. Food Eng. 2010, 98, 240–247. [Google Scholar] [CrossRef]
- Sellami, I.H.; Wannes, W.A.; Bettaieb, I.; Berrima, S.; Chahed, T.; Marzouk, B.; Limam, F. Qualitative and quantitative changes in the essential oil of laurus nobilis L. leaves as affected by different drying methods. Food Chem. 2011, 126, 691–697. [Google Scholar] [CrossRef]
- Al-Qudah, M.A.; Onizat, M.A.; Alshamari, A.K.; Al-Jaber, H.I.; Bdair, O.M.; Muhaidat, R.; Al-Bataineh, N. Chemical composition and antioxidant activity of Jordanian Artemisia judaica L. as affected by different drying methods. Int. J. Food Prop. 2021, 24, 482–492. [Google Scholar] [CrossRef]
- Rohloff, J.; Dragland, S.; Mordal, R.; Iversen, T. Effect of harvest time and drying method on biomass production, essential oil yield, and quality of peppermint (Mentha piperita L.). J. Agric. Food Chem. 2005, 53, 4143–4148. [Google Scholar] [CrossRef]
- Asekun, O.T.; Grierson, D.S.; Afolayan, A.J. Effects of drying methods on the quality and quantity of the essential oil of Mentha longifolia L. subsp. capensis. Food Chem. 2007, 101, 995–998. [Google Scholar] [CrossRef]
- Rahimmalek, M.; Goli, S.A.H. Evaluation of six drying treatments with respect to essential oil yield, composition, and color characteristics of Thymys daenensis subsp. daenensis. Celak leaves. Ind. Crops Prod. 2013, 42, 613–619. [Google Scholar] [CrossRef]
- Sárosi, S.Z.; Sipos, L.; Kókai, Z.; Pluhár, Z.S.; Szilvássy, B.; Novák, I. Effect of different drying techniques on the aroma profile of Thymus vulgaris analyzed by GC-MS and sensory profile method. Ind. Crops Prod. 2013, 46, 210–216. [Google Scholar] [CrossRef]
- Europaea, F.; Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, A. (Eds.) Fernandes R: Genus Anthemis L. In Flora Europaea; Cambridge University Press: Cambridge, MA, USA; London, UK, 1976; Volume 4, pp. 145–159. [Google Scholar]
- Bardaweel, S.K.; Tawaha, K.A.; Hudaib, M.M. Antioxidant, antimicrobial and antiproliferative activities of Anthemis palestina essential oil. BMC Complement. Altern. Med. 2014, 14, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Bremer, K. Asteraceae, Cladistics and Classification; Timber Press: Portland, OR, USA, 1994. [Google Scholar]
- Al-Eisawi, D. List of Jordan vascular plants. Mitt. Bot. Munchen. 1982, 18, 79–182. [Google Scholar]
- Javidnia, K.; Miri, R.; Kamalinejad, M.; Sarkarzadeh, H.; Jamalian, A. Chemical composition of the essential oils of Anthemis altissima L. grown in Iran. Flavour Fragr. J. 2004, 19, 213–216. [Google Scholar] [CrossRef]
- Saroglou, V.; Dorizas, N.; Kypriotakis, Z.; Skaltsa, H.D. Analysis of the essential oil composition of eight Anthemis species from Greece. J. Chromatogr. A 2006, 1104, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Staneva, J.D.; Todorova, M.N.; Evstatieva, L.N. Sesquiterpene lactones as chemotaxonomic markers in genus Anthemis. Phytochemistry 2008, 69, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Bohlmann, F.; Arndt, C.; Bornowski, H.; Kleine, K.M. Polyacetylenverbindung XLVIII. Die Ployine der Gattung Anthemis L. Chem. Berichte 1963, 96, 1485–1494. [Google Scholar] [CrossRef]
- Bohlmann, F.; Kleine, K.M.; Arndt, C.; Köhn, S. Polyacetylenverbindungen, LXXVIII: Neue Inhaltsstoffe der Gattung Anthemis L. Chem. Berichte 1965, 98, 1616–1622. [Google Scholar] [CrossRef]
- Bohlmann, F.; Bohm, D.; Rybak, C. Polyacetylenverbindungen, LXXXVII: Über die Struktur und Biogenese eines aus Anthemis-Arten isolierten Thioäthers. Chem. Berichte 1965, 98, 3087–3091. [Google Scholar] [CrossRef]
- Bohlmann, F.; Kleine, K.M. Polyacetylenverbindungen, CVI. Über einige neue Acetylenverbindungen aus der Gattung Anthemis L. Chem. Berichte 1966, 99, 2096–2103. [Google Scholar] [CrossRef]
- Williams, C.A.; Greenham, J.; Harborne, J.B. The role of lipophilic and polar flavonoids in the classification of temperate members of the Anthemideae. Biochem. Syst. Ecol. 2001, 29, 929–945. [Google Scholar] [CrossRef]
- Bulatovic, V.M.; Menkovic, N.R.; Vajs, V.E.; Milosavijevic, S.M.; Djokovic, D.D. Essential oil of Anthemis carpatica. J. Essent. Oil Res. 1997, 9, 397–400. [Google Scholar] [CrossRef]
- Bulatovic, V.M.; Menkovic, N.R.; Vajs, V.E.; Milosavljevic, S.M.; Djokovic, D.D. Essential oil of Anthemis montana. J. Essent. Oil Res. 1998, 10, 223–226. [Google Scholar] [CrossRef]
- Grace, M.H. Chemical composition and biological activity of the volatiles of Anthemis melampodina and Pluchea dioscoridis. Phytother. Res. 2002, 16, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Bassols, F.; Thomas, A.F. The occurrence of 3-phenylpropyl isobutyrate in Roman Camomile oil. J. Essent. Oil Res. 1991, 3, 309–312. [Google Scholar] [CrossRef]
- Klimes, I.; Lamparsky, D.; Scholz, E. Vorkommen neuer bifunktioneller. Ester im Römisch-Kamillenöl (Anthemis nobilis L.). Helv. Chim. Acta 1981, 64, 2338–2349. [Google Scholar] [CrossRef]
- Thomas, A.F. The Occurrence of some novel diesters in Roman camomile oil. Helv. Chim. Acta 1981, 64, 2397–2400. [Google Scholar] [CrossRef]
- Khleifat, K.M.; Matar, S.A.; Jaafreh, M.; Qaralleh, H.; Al-limoun, M.O.; Alsharafa, K.Y. Essential oil of Centaurea damascena aerial parts, antibacterial and synergistic effect. J. Essent. Oil Bear. Plants 2019, 22, 356–367. [Google Scholar] [CrossRef]
- Khangholi, S.; Rezaeinodehi, A. Effect of drying temperature on essential oil content and composition of sweet wormwood (Artemisia annua) growing wild in Iran. Pak. J. Biol. Sci. 2008, 11, 934–937. [Google Scholar] [CrossRef]
- Hamrouni-Sellami, I.; Rahali, F.Z.; Rebey, I.B.; Bourgou, S.; Limam, F.; Marzouk, B. Total phenolics, flavonoids, and antioxidant activity of sage (Salvia officinalis L.) plants as affected by different drying methods. Food Bioprocess Technol. 2012, 6, 806–817. [Google Scholar] [CrossRef]
- Tomaino, A.; Cimino, F.; Zimbalatti, V.; Venuti, V.; Sulfaro, V.; De Pasquale, A.; Saija, A. Influence of heating on antioxidant activity and the chemical composition of some spice essential oils. Food Chem. 2005, 89, 549–554. [Google Scholar] [CrossRef]
- Martínez-Las Heras, R.; Heredia, A.; Castelló, M.L.; Andrés, A. Influence of drying method and extraction variables on the antioxidant properties of persimmon leaves. Food Biosci. 2014, 6, 1–8. [Google Scholar] [CrossRef]
- Capecka, E.; Marecczek, A.; Leja, M. Antioxidant activity of fresh and dry herbs of some Lamiaceae species. Food Chem. 2005, 93, 223–226. [Google Scholar] [CrossRef]
- Inchuen, S.; Narkrugsa, W.; Pornchaloempong, P. Effect of drying methods on chemical composition, color and antioxidant properties of Thai red curry powder. Kasetsart J. Nat. Sci. 2010, 44, 142–151. [Google Scholar]
- Annamalai, A. Effect of drying treatment on the contents of antioxidants in Cardiospermum halicacabum Linn. Int. J. Pharm. Biol. Sci. 2011, 2, 304–313. [Google Scholar]
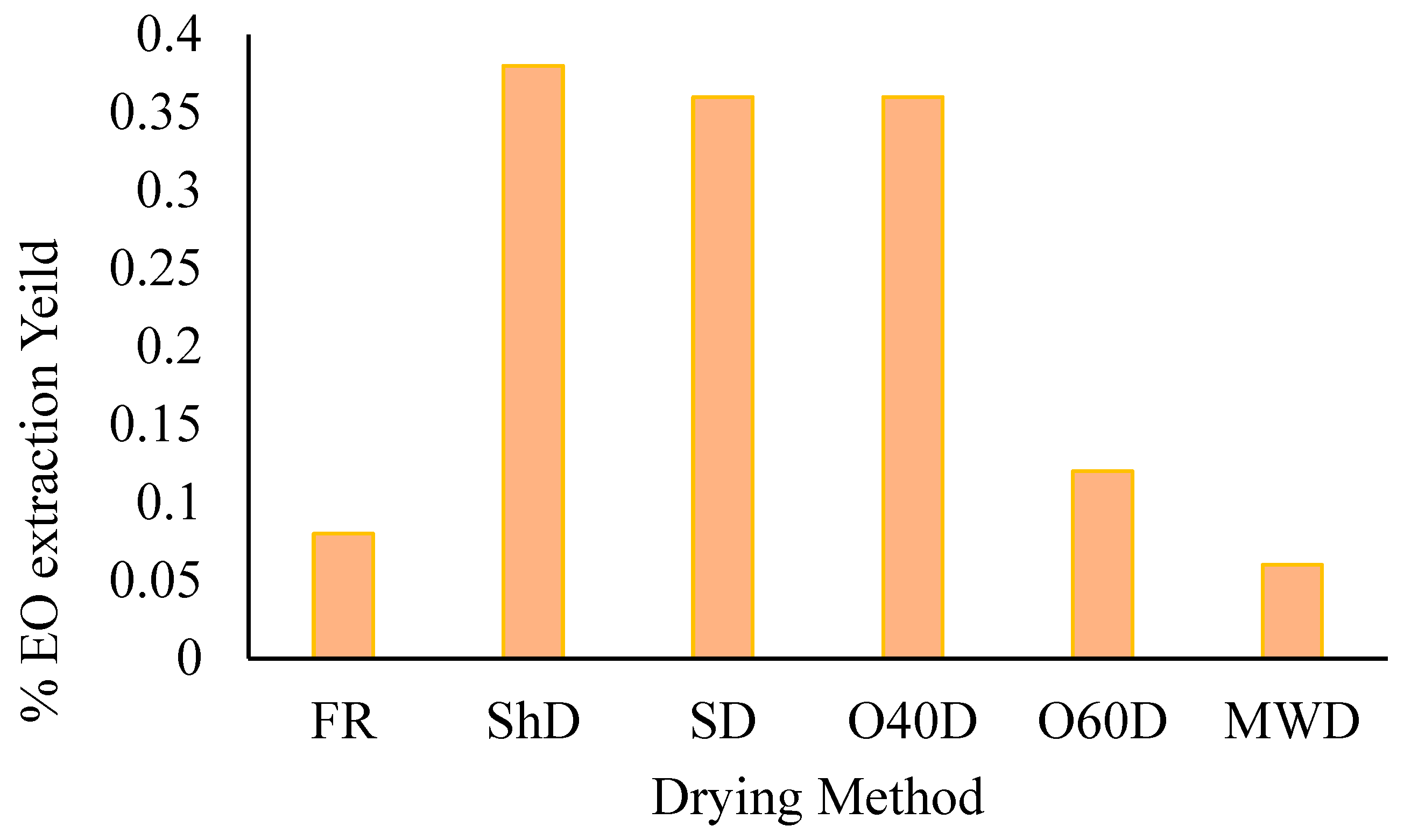

| No. | KI | Constituent | % Concentration | |||||
|---|---|---|---|---|---|---|---|---|
| FR | ShD | SD | O40D | O60D | MW | |||
| 1 | 816 | n-octane | - | - | - | - | - | 0.40 |
| 2 | 841 | (E)-2-Hexenal | 0.25 | 0.15 | - | - | - | - |
| 3 | 846 | (Z)-Salvene | 0.20 | 0.09 | - | - | - | - |
| 4 | 857 | Ethyl isovalerate | - | - | - | - | - | 0.13 |
| 5 | 859 | (Z)-2-Hexenol | 0.72 | - | - | - | - | - |
| 6 | 862 | n-Hexenol | 0.35 | - | - | - | - | - |
| 7 | 901 | Heptanal | - | 0.20 | - | - | - | 0.11 |
| 8 | 903 | 2-Ethoxy ethyl acetate | - | 0.60 | 0.81 | - | - | 0.35 |
| 9 | 919 | 2-methyl-4-Heptanone | - | 0.63 | 0.77 | - | - | 0.33 |
| 10 | 926 | Tricyclene | 0.29 | 0.48 | 0.13 | 0.15 | 0.19 | 0.79 |
| 11 | 927 | Artemisia triene | - | - | - | 1.36 | - | - |
| 12 | 939 | Allyl isovalerate | - | 0.14 | 0.16 | - | - | - |
| 13 | 941 | α-Pinene | - | - | - | 0.08 | - | - |
| 14 | 948 | Benzaldehyde | 0.19 | 2.68 | 0.44 | 1.26 | 1.26 | 0.16 |
| 15 | 959 | (E)-2-Heptenal | - | 0.73 | 1.06 | - | - | 0.47 |
| 16 | 966 | Sabinene | - | 0.74 | - | 1.38 | 0.09 | - |
| 17 | 969 | β-Pinene | 0.18 | 0.54 | - | 2.12 | 0.17 | 0.28 |
| 18 | 973 | trans-m-Mentha-2,8-diene | - | - | - | 0.09 | - | - |
| 19 | 976 | 6-methyl-5-Hepten-2-one | - | 0.25 | - | 0.14 | 0.17 | - |
| 20 | 983 | 3-ρ-Menthene | - | 0.16 | - | - | - | - |
| 21 | 988 | Myrcene | 1.57 | 0.19 | 0.39 | 0.67 | 0.16 | 1.20 |
| 22 | 989 | α-Phellandrene | - | - | - | 0.31 | - | - |
| 23 | 999 | δ-3-Carene | - | - | - | 0.15 | - | - |
| 24 | 1010 | α-Terpinene | 0.45 | 1.85 | 0.29 | 3.09 | 0.99 | 0.49 |
| 25 | 1017 | ρ-Cymene | 0.37 | 2.49 | 0.39 | 2.30 | 0.98 | 0.29 |
| 26 | 1022 | Sylvestrene | 3.19 | 2.37 | 0.68 | 3.21 | 1.09 | 2.41 |
| 27 | 1031 | Benzene acetaldehyde | 0.25 | - | - | 0.14 | 0.17 | - |
| 28 | 1042 | (E)-β-Ocimene | 0.26 | - | - | - | - | - |
| 29 | 1051 | γ-Terpinene | 1.03 | 4.24 | 0.58 | 5.62 | 2.01 | 0.94 |
| 30 | 1052 | Isobutyl acetoacetate | - | - | - | - | - | 0.46 |
| 31 | 1061 | cis-Sabinene hydrate | 0.33 | 0.53 | - | 0.23 | - | - |
| 32 | 1063 | cis-Linalool oxide | - | 0.28 | - | - | - | - |
| 33 | 1078 | Terpinolene | 0.30 | 1.12 | - | 1.40 | 0.47 | 0.21 |
| 34 | 1091 | Linalool | 0.31 | 0.24 | 0.63 | 0.14 | 0.35 | 0.46 |
| 35 | 1093 | trans-Sabinene hydrate | 0.97 | 1.40 | - | 1.23 | 0.30 | - |
| 36 | 1097 | n-Nonanal | 0.32 | - | 0.50 | - | - | 0.58 |
| 37 | 1098 | cis-Thujone | - | 0.69 | - | 0.63 | - | - |
| 38 | 1104 | 2-Methyl butyl isovalerate | - | - | - | 0.11 | - | - |
| 39 | 1108 | trans-Thujone | 2.46 | 5.02 | 0.46 | 4.11 | 0.44 | 0.37 |
| 40 | 1115 | cis-ρ-Menth-2-en-1-ol | 0.27 | 0.59 | 0.21 | 0.53 | 0.26 | - |
| 41 | 1130 | trans-Pinocarveol | 0.20 | 0.17 | 0.31 | 0.15 | - | - |
| 42 | 1133 | (E)-Tagetone | 0.47 | 0.9 | - | 0.78 | 0.46 | - |
| 43 | 1151 | Camphor | - | - | - | 0.10 | - | - |
| 44 | 1154 | neo-menthol | 0.41 | - | 1.17 | - | - | 0.40 |
| 45 | 1158 | cis-Chrysanthenol | 0.99 | 0.37 | - | 0.13 | - | - |
| 46 | 1176 | Terpinen-4-ol | 5.47 | 16.94 | 9.35 | 12.91 | 4.88 | 2.43 |
| 47 | 1178 | ρ-Cymen-8-ol | - | 0.21 | - | - | - | - |
| 48 | 1183 | trans-ρ-Mentha-1(7),8-dien-2-ol | - | - | - | - | - | - |
| 49 | 1184 | Thuj-3-en-10-al | - | - | - | 0.12 | - | - |
| 50 | 1185 | α-Terpinenol | 1.05 | 3.33 | 2.03 | 2.64 | 0.93 | 0.44 |
| 51 | 1187 | Myrtenol | 0.21 | 0.28 | - | - | 0.31 | - |
| 52 | 1189 | cis-Piperitol | - | - | - | 0.20 | - | - |
| 53 | 1198 | n-Decanal | - | - | 0.16 | 0.12 | 0.10 | - |
| 54 | 1200 | trans-Piperitol | - | 0.36 | 0.26 | 0.26 | 0.13 | - |
| 55 | 1204 | trans-Pulegol | - | - | - | 0.13 | 0.15 | - |
| 56 | 1209 | Nerol | - | 0.40 | - | 0.22 | - | - |
| 57 | 1214 | trans-Chrysanthenyl acetate | - | - | - | 0.09 | 0.13 | - |
| 58 | 1229 | (Z)-3-hexenyl-3-methyl Butanoate | 0.34 | 0.18 | - | 0.16 | - | - |
| 59 | 1236 | Hexyl isovalerate | 0.37 | - | - | - | - | - |
| 60 | 1245 | Geraniol | 0.26 | - | 0.29 | 0.14 | 0.16 | - |
| 61 | 1275 | Isobornyl acetate | 0.25 | 0.15 | 0.17 | 0.19 | 0.36 | 0.41 |
| 62 | 1288 | 3-Thujanol acetate | - | - | - | 0.09 | 0.10 | - |
| 63 | 1307 | (2E,4Z)-Decadienal | - | - | 0.20 | - | - | - |
| 64 | 1313 | Methyl geranate | 0.32 | - | 1.24 | 0.10 | - | 0.24 |
| 65 | 1326 | δ-Elemene | 0.35 | - | - | 0.11 | 0.13 | 0.47 |
| 66 | 1342 | dimethoxy-(E)-Citral | - | 0.59 | - | 0.38 | 0.27 | 0.72 |
| 67 | 1344 | 1-Phenyl pentan-3-one | 0.54 | - | 0.83 | - | - | - |
| 68 | 1348 | α-Terpinylacetate | 0.14 | - | - | - | - | - |
| 69 | 1352 | Nerylacetate | 0.15 | - | 0.34 | - | - | 0.34 |
| 70 | 1367 | α-Ylangene | 0.77 | 0.90 | 0.84 | 0.92 | 1.20 | 0.68 |
| 71 | 1372 | Geranyl acetate | 0.19 | - | - | - | - | - |
| 72 | 1375 | α-Copaene | 0.46 | 0.20 | 0.78 | 0.35 | 0.53 | 0.50 |
| 73 | 1380 | iso-Longifolene | - | - | - | - | 0.18 | - |
| 74 | 1381 | 7-epi-Sesquithujene | 0.53 | - | 0.31 | - | - | 0.34 |
| 75 | 1385 | (E)2-Octenol butanoate | 0.31 | - | 0.29 | - | - | - |
| 76 | 1389 | Methyl eugenol | - | - | - | 0.13 | - | - |
| 77 | 1402 | iso-Italicene | 0.48 | - | - | - | - | 0.21 |
| 78 | 1409 | (E)-Caryophyllene | 3.51 | 3.89 | 4.37 | 5.42 | 5.63 | 3.87 |
| 79 | 1420 | β-Copaene | 0.42 | 0.23 | - | 0.19 | 0.31 | 0.39 |
| 80 | 1434 | Iridolactone | 0.50 | - | - | - | - | - |
| 81 | 1441 | Aromadendrene | - | - | - | - | - | 0.16 |
| 82 | 1444 | α-Humulene | 0.96 | 0.76 | 0.81 | 1.19 | 1.31 | 0.58 |
| 83 | 1446 | (Z)-β-Farnesene | - | 5.11 | 5.91 | - | - | 9.26 |
| 84 | 1449 | epi-β-Santalene | - | 0.17 | 0.24 | - | - | - |
| 85 | 1452 | (E)-β-Farnesene | 3.20 | - | - | 5.08 | 10.92 | 0.20 |
| 86 | 1464 | allo-Aromadendrene | 0.39 | - | 0.21 | - | - | - |
| 87 | 1474 | γ-Muurolene | 11.2 | 6.82 | 6.00 | 7.95 | 18.69 | 18.73 |
| 88 | 1476 | γ-Gurjunene | - | 0.23 | 2.59 | - | - | - |
| 89 | 1480 | methyl-β-(E)-Ionol | - | - | - | 0.12 | - | - |
| 90 | 1479 | Amorpha-4,7(11)-diene | 2.11 | - | - | - | - | - |
| 91 | 1480 | Phenyl ethyl 2-methylbutanoate | 0.63 | - | 1.04 | - | - | 0.53 |
| 92 | 1481 | β-Selinene | 0.22 | - | 1.10 | 0.22 | 0.16 | - |
| 93 | 1482 | δ-Selinene | - | - | - | 0.12 | - | - |
| 94 | 1484 | α-Zingiberene | 3.10 | 5.46 | 1.70 | 4.49 | 8.60 | 14.70 |
| 95 | 1490 | Bicyclogermacrene | 2.27 | - | - | - | 0.34 | - |
| 96 | 1497 | Isodaucene | - | 1.84 | 0.61 | - | - | - |
| 97 | 1498 | α-Muurolene | 0.26 | - | - | - | 0.16 | - |
| 98 | 1499 | α-(E,E)-Farnesene | 1.85 | - | - | 1.11 | 2.22 | 5.12 |
| 99 | 1500 | β- Bisabolene | 0.34 | - | 0.45 | 0.08 | - | 0.32 |
| 100 | 1502 | β-Dihydro agarofuran | - | - | 0.79 | - | - | - |
| 101 | 1504 | δ-Amorphene | 1.11 | 0.25 | - | 0.36 | 0.61 | 0.84 |
| 102 | 1510 | δ-Cadinene | 0.72 | 0.75 | 0.60 | 0.81 | 1.43 | 0.85 |
| 103 | 1519 | (E)-dihydro-Apofarnesal | 0.41 | 0.44 | - | - | 0.21 | 0.46 |
| 104 | 1520 | β-Sesquiphellandrene | 0.36 | - | 0.56 | 0.39 | 1.26 | 0.25 |
| 105 | 1526 | (E)-iso-γ-Bisabolene | 0.82 | - | 1.58 | 0.12 | - | 1.00 |
| 106 | 1528 | Raspberry ketone | 0.29 | 0.33 | 1.53 | 0.50 | 0.38 | 0.70 |
| 107 | 1530 | α-Calacorene | - | - | - | - | 0.14 | - |
| 108 | 1532 | Hedycaryol | 1.08 | - | 0.42 | - | 1.19 | 0.94 |
| 109 | 1535 | Italicene epoxide | - | - | - | 0.49 | - | - |
| 110 | 1536 | (E)-Allyl cinnamate | - | 0.35 | - | - | - | - |
| 111 | 1545 | Elemol | 0.30 | 0.23 | 0.44 | 0.19 | 0.48 | - |
| 112 | 1551 | Germacrene B | - | - | - | - | - | 0.91 |
| 113 | 1556 | (E)-Nerolidol | 0.91 | 0.86 | 0.55 | 0.83 | 0.63 | - |
| 114 | 1560 | Santalenone | 0.63 | - | 1.72 | 0.18 | 0.47 | 0.87 |
| 115 | 1565 | Maaliol | - | - | - | - | - | 2.06 |
| 116 | 1566 | (Z)-3-Hexenyl benzoate | 0.55 | - | - | - | 0.38 | - |
| 117 | 1568 | Spathulenol | 1.65 | 0.74 | 7.96 | - | - | 1.47 |
| 118 | 1569 | (E)-α-isomethyl-Ionol acetate | - | 0.26 | - | 0.44 | - | - |
| 119 | 1570 | α-Cedrene epoxide | 1.14 | 3.73 | 6.65 | - | - | 0.35 |
| 120 | 1571 | Dendrolasin | - | 0.17 | - | - | - | - |
| 121 | 1572 | Caryophyllene oxide | 0.97 | - | 0.21 | 4.64 | 6.60 | - |
| 122 | 1573 | cis-β-Elemenone | - | 0.50 | - | - | - | - |
| 123 | 1574 | β-Copaen-4-α-ol | 0.40 | - | 0.31 | - | 0.17 | - |
| 124 | 1576 | Globulol | 0.32 | - | - | - | - | - |
| 125 | 1578 | ar-dihydro-Turmerone | 0.54 | - | 0.56 | - | - | 0.67 |
| 126 | 1580 | Salvial-4(14)-en-1-one | 0.16 | - | 0.33 | 0.33 | 0.27 | - |
| 127 | 1584 | Carotol | 0.52 | - | - | 0.25 | 0.35 | - |
| 128 | 1590 | Fokienol | - | 1.67 | 0.77 | - | - | - |
| 129 | 1591 | β-trans-Elemenone | 0.32 | - | - | 0.22 | 0.43 | 1.27 |
| 130 | 1595 | Khusimone | 1.91 | 0.55 | 1.28 | 1.36 | 1.35 | 3.22 |
| 131 | 1596 | Geranyl isovalerate | 0.75 | - | - | 0.57 | 0.89 | 0.38 |
| 132 | 1601 | (Z)-Sesquilavandulol | - | - | - | - | 0.18 | - |
| 133 | 1603 | β-Atlantol | 0.57 | 0.48 | 0.92 | 0.23 | 0.16 | 1.71 |
| 134 | 1609 | Humulene epoxide II | 0.76 | - | 0.71 | 0.42 | 0.46 | - |
| 135 | 1612 | Isolongifolan-7-α-ol | 1.21 | 0.19 | - | - | - | 0.39 |
| 136 | 1615 | 1,10-di-epi-Cubenol | - | 1.43 | 0.86 | 1.04 | 1.41 | 0.17 |
| 137 | 1620 | 10-epi-γ-Eudesmol | 0.73 | - | 1.45 | 0.49 | 0.45 | 0.64 |
| 138 | 1622 | Silphiperfol-6-en-5-one | 0.63 | 0.66 | 0.85 | - | - | 0.71 |
| 139 | 1625 | α-Acorenol | 0.35 | - | - | - | - | - |
| 140 | 1627 | Camphoric acid | 0.64 | - | - | 0.78 | 0.99 | - |
| 141 | 1629 | Gossonorol | 0.19 | - | 0.57 | - | - | - |
| 142 | 1633 | α-epi-Cadinol | 1.12 | 2.58 | 2.37 | 1.54 | 3.12 | 1.54 |
| 143 | 1634 | α-epi-Muurolol | 0.94 | - | 0.63 | - | - | - |
| 144 | 1640 | Cubenol | 0.14 | - | - | - | 0.15 | - |
| 145 | 1641 | β-Eudesmol | 2.08 | 0.40 | 1.21 | 0.32 | 1.60 | 0.58 |
| 146 | 1644 | α-Cadinol | 2.73 | 1.27 | 3.13 | 0.85 | 1.62 | 1.50 |
| 147 | 1644 | Citronellyl angelate | - | - | 0.82 | - | - | - |
| 148 | 1652 | 5-Hydroxy-isobornyl isobutanoate | 0.42 | - | 0.28 | - | - | - |
| 149 | 1657 | Selin-11-en-4-α-ol | 0.19 | - | 0.93 | - | 0.23 | - |
| 150 | 1661 | (E)-Bisabol-11-ol | 1.01 | 0.62 | - | 0.63 | 1.67 | 0.69 |
| 151 | 1670 | 14-hydroxy-9-epi-(E)-Caryophyllene | 1.53 | 0.75 | 1.26 | 1.20 | 0.87 | 1.23 |
| 152 | 1673 | 5-iso-Cedranol | - | - | - | - | - | 0.24 |
| 153 | 1674 | Eudesma-4(15),7-dien-1β-ol | 1.39 | 0.28 | 1.45 | 0.33 | 0.36 | 0.59 |
| 154 | 1678 | Shyobunol | 0.43 | 0.22 | - | 0.35 | 0.19 | 0.30 |
| 155 | 1695 | Eudesm-7(11)-en-4-ol | 0.47 | - | - | - | - | 0.30 |
| 156 | 1698 | n-Heptadecane | - | - | - | - | - | 0.23 |
| 157 | 1706 | Sesquicineol-2-one | 0.50 | - | 0.33 | 0.42 | 0.37 | - |
| 158 | 1734 | iso-Longifolol | 0.46 | - | - | - | - | - |
| 159 | 1738 | (E)-Pseudoisoeugenyl iso butyrate | 0.71 | - | 0.33 | - | - | 0.40 |
| 160 | 1745 | β-Acoradienol | - | - | - | 0.12 | - | - |
| 161 | 1752 | γ-Costol | - | - | - | - | 0.14 | - |
| 162 | 1762 | 14-oxy-α-Muurolene | 0.69 | - | 0.69 | - | 0.35 | - |
| 163 | 1832 | Cyclopentadecanolide | - | 0.14 | 0.68 | - | 0.37 | - |
| 164 | 1854 | cis-Thujopsenic acid | - | - | - | - | - | 0.16 |
| 165 | 1855 | (Z,Z)-Farnesyl acetone | - | - | - | 0.12 | 0.30 | - |
| Monoterpene hydrocarbons (MH) | 7.54 | 13.15 | 2.46 | 20.53 | 5.68 | 6.01 | ||
| Oxygenated monoterpenes (OM) | 16.31 | 33.57 | 16.74 | 27.68 | 10.69 | 6.02 | ||
| Sesquiterpene hydrocarbons (SH) | 35.43 | 26.61 | 28.66 | 29.04 | 53.69 | 59.38 | ||
| Oxygenated sesquiterpenes (OS) | 30.82 | 18.17 | 40.36 | 17.12 | 27.17 | 23.23 | ||
| Aliphatic compounds (AC) | 1.64 | 1.96 | 2.69 | 0.26 | 0.27 | 2.12 | ||
| Carboxylic acids and esters (CE) | 2.20 | 1.27 | 2.30 | 0.27 | - | 1.47 | ||
| Others | 1.27 | 3.01 | 2.80 | 2.03 | 2.19 | 0.86 | ||
| Total identified | 95.21 | 97.74 | 96.83 | 97.36 | 99.69 | 99.09 | ||
| HDEO | IC50 (μg/mL) | |
|---|---|---|
| DPPH | ABTS | |
| FR | (1.00 ± 0.03) × 10−2 | (8.61 ± 0.02) × 10−2 |
| ShD | (6.92 ± 0.02) × 0−2 | (4.81± 0.01) × 10−2 |
| SD | (1.31 ± 0.03) × 10−2 | (1.34 ± 0.01) × 10−2 |
| O40D | (1.66 ± 0.06) × 10−2 | (1.91 ± 0.01) × 10−2 |
| O60D | (2.18 ± 0.02) × 10−2 | (3.08 ± 0.01) × 10−2 |
| MWD | (2.84 ± 0.02) × 10−2 | (5.12 ± 0.04) × 10−2 |
| Ascorbic acid | (2.42 ± 3.21) × 10−3 | (1.92 ± 2.52) × 10−3 |
| α-tocopherol | (2.19 ± 9.45) × 10−3 | (2.07 ± 3.28) × 10−3 |
| Drying Method Used to Prepare the APM Extract | TPC (mg GA/g DE) | TFC (mg Q/g DE) | IC50 (μg/mL) | |
|---|---|---|---|---|
| DPPH | ABTS | |||
| ShD | 98.04 ± 0.14 | 289.84 ± 2.38 | (4.63 ± 0.06) × 10−2 | (4.46 ± 0.05) × 10−2 |
| SD | 105.37 ± 0.19 | 305.16 ± 3.93 | (4.42 ± 0.02) × 10−2 | (3.87 ± 0.02) × 10−2 |
| O40D | 82.98 ± 0.62 | 240.49 ±1.56 | (10.67 ± 0.04) × 10−2 | (7.47 ± 0.03) × 10−2 |
| O60D | 43.49 ± 0.57 | 52.94 ± 0.90 | (17.51 ± 1.72) × 10−2 | (23.99 ± 1.62) × 10−2 |
| MWD | 77.45 ± 0.57 | 179.70 ± 1.56 | (13.45 ± 0.24) × 10−2 | (7.64 ± 0.09) × 10−2 |
| Ascorbic acid | - | - | (1.80 ± 0.06) × 10−3 | (1.90 ± 0.06) × 10−3 |
| α-tocopherol | - | - | (2.30 ± 0.04) × 10−3 | (1.80 ± 0.01) × 10−3 |
| TPC | TFC | DPPH | ABTS | |
|---|---|---|---|---|
| TPC | 1 | 0.99027 | −0.95541 | −0.95987 |
| TFC | 0.99027 | 1 | −0.95703 | −0.94990 |
| DPPH | 0.95541 | −0.95703 | 1 | 0.84878 |
| ABTS | −0.95987 | −0.94990 | 0.84878 | 1 |
| No. | Rt | M/Z | M.WT | Mode of Ionization | Classification | Formula | Compound | ShD | SD | O40D | O60D | MWD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.97 | 117.0194 | 118.0267 | [M − H]− | Organic acid | C4H6O4 | Succinic acid | + | + | + | + | - |
| 2 | 1.04 | 171.0272 | 170.0199 | [M + H]+ | Phenolic acid | C7H6O5 | Gallic Acid | - | + | + | - | + |
| 3 | 1.18 | 125.0245 | 126.0318 | [M − H]− | Other | C6H6O3 | 4-Hydroxy-6-Methylpyran-2-one | + | + | + | - | + |
| 4 | 1.19 | 289.092 | 288.0847 | [M + H]+ | Other | C12H16O8 | Phlorin | + | - | - | - | + |
| 5 | 2.53 | 139.0393 | 138.032 | [M + H]+ | Phenolic acid | C7H6O3 | 4-Hydroxybenzoic acid | + | + | + | - | + |
| 6 | 2.89 | 191.0559 | 192.0632 | [M − H]− | Organic acid | C7H12O6 | Quinic acid | + | + | + | + | + |
| 7 | 2.90 | 353.0875 | 354.0948 | [M − H]− | phenolic acid | C16H18O9 | Chlorogenic acid | + | + | + | - | + |
| 8 | 2.92 | 163.0392 | 162.0319 | [M + H]+ | Organic acid | C6H10O3S | 2-Oxo-5-methylthiopentanoic acid | - | - | - | - | + |
| 9 | 3.22 | 179.0348 | 180.0421 | [M − H]− | Phenolic acid | C9H8O4 | Caffeic Acid | + | + | - | + | - |
| 10 | 3.30 | 307.0784 | 306.0712 | [M + H]+ | Flavonoid | C15H14O7 | 2,3-trans-3,4-trans-Leucocyanidin | - | + | - | - | + |
| 11 | 3.74 | 227.1276 | 226.1203 | [M + H]+ | Organic acid | C12H18O4 | 12-hydroxyjasmonic acid | - | - | - | - | + |
| 12 | 4.61 | 611.163 | 610.1558 | [M + H]+ | Flavonoid | C27H30O16 | Luteolin-7,3′-di-O-glucoside | - | + | - | - | - |
| 13 | 4.71 | 163.0392 | 162.032 | [M + H]+ | Other | C9H6O3 | Isofaurinone | - | - | - | + | - |
| 14 | 4.75 | 479.0825 | 480.0898 | [M − H]- | Flavonoid | C21H20O13 | Myricetin 3-glucoside | + | + | - | - | + |
| 15 | 4.81 | 565.156 | 564.1487 | [M + H]+ | Flavonoid | C26H28O14 | Apiin | - | + | - | - | - |
| 16 | 4.90 | 193.0505 | 192.0432 | [M + H]+ | Other | C10H8O4 | Scopoletin | - | + | + | - | - |
| 17 | 4.94 | 163.0394 | 162.0321 | [M + H]+ | Other | C9H6O3 | Faurinone | - | - | - | + | - |
| 18 | 5.29 | 621.1095 | 622.1167 | [M − H]− | Flavonoid | C27H26O17 | 4′-O-(GlcA(1-2)GlcA) Apigenin | + | + | + | - | - |
| 19 | 5.30 | 285.0404 | 286.0477 | [M − H]− | Flavonoid | C15H10O6 | 7,3′,4′,5′-Tetrahydroxyflavone | - | + | - | + | + |
| 20 | 5.58 | 465.1041 | 464.0968 | [M + H]+ | Flavonoid | C21H20O12 | Hyperoside | + | + | + | - | + |
| 21 | 5.59 | 463.0877 | 464.095 | [M − H]− | Flavonoid | C21H20O12 | Quercetin 3-O-glucoside | + | + | + | - | - |
| 22 | 5.87 | 447.0932 | 448.1001 | [M − H]− | Flavonoid | C21H20O11 | Luteolin 7-O-glucoside | - | + | + | - | + |
| 23 | 5.88 | 509.0937 | 508.0864 | [M + H]+ | Flavonoid | C22H20O14 | Patuletin 3-glucuronide | + | - | - | - | - |
| 24 | 5.95 | 493.0982 | 494.1055 | [M − H]− | Flavonoid | C22H22O13 | Laricitrin 3-galactoside | + | + | + | - | + |
| 25 | 6.28 | 499.1246 | 516.1278 | [M + H − H2O]+ | Organic acid | C25H24O12 | 1,3-Dicaffeoylquinic acid | + | + | + | - | + |
| 26 | 6.57 | 193.0506 | 194.0579 | [M − H]− | Phenolic acid | C10H10O4 | Isoferulic acid | - | - | + | - | - |
| 27 | 6.60 | 447.0928 | 448.1001 | [M − H]− | Flavonoid | C21H20O11 | Kaempferol-3-O-glucoside | + | - | + | - | - |
| 28 | 6.71 | 431.0971 | 432.1044 | [M − H]− | Flavonoid | C21H20O10 | Apigenin-7-O-glucoside | + | + | - | - | - |
| 29 | 6.81 | 445.0773 | 446.0846 | [M − H]− | Flavonoid | C21H18O11 | Genistein | + | + | + | + | + |
| 30 | 6.88 | 161.0244 | 162.0317 | [M − H]− | Other | C9H6O3 | 7-hydroxy-Coumarin | + | + | + | + | + |
| 31 | 6.90 | 163.0395 | 162.0322 | [M + H]+ | Other | C9H6O3 | 4-hydroxy-Coumarin | + | + | + | + | + |
| 32 | 6.91 | 515.119 | 516.1262 | [M − H]− | Other | C25H24O12 | 1,5-Dicaffeoylquinic acid | + | + | + | - | + |
| 33 | 6.94 | 477.1036 | 478.1109 | [M − H]− | Flavonoid | C22H22O12 | Isorhamnetin 3-glucoside | - | + | - | - | + |
| 34 | 7.17 | 507.1136 | 508.1209 | [M − H]− | Flavonoid | C23H24O13 | Syringetin 3-glucoside | + | + | - | - | + |
| 35 | 8.42 | 301.0353 | 302.0426 | [M − H]− | Flavonoid | C15H10O7 | Quercetin | - | + | - | - | - |
| 36 | 8.47 | 287.0559 | 286.0486 | [M + H]+ | Flavonoid | C15H10O6 | Luteolin | - | + | - | + | + |
| 37 | 8.75 | 317.0656 | 316.0583 | [M + H]+ | Flavonoid | C16H12O7 | 3-O-methyl Quercetin | - | + | + | - | - |
| 38 | 9.83 | 269.0462 | 270.0534 | [M − H]− | Flavonoid | C15H10O5 | Baicalein | - | - | + | - | - |
| 39 | 9.94 | 285.04 | 286.0473 | [M − H]− | Flavonoid | C15H10O6 | Kaempferol | - | + | - | - | - |
| 40 | 10.05 | 299.0556 | 300.0629 | [M − H]− | Flavonoid | C16H12O6 | Hispidulin | - | - | - | - | + |
| 41 | 10.32 | 315.0514 | 316.0587 | [M − H]− | Flavonoid | C16H12O7 | Isorhamnetin | - | + | + | - | - |
| 42 | 28.26 | 279.2322 | 278.2249 | [M + H]+ | Other | C18H30O2 | γ-Linolenic acid | + | - | - | + | + |
| 43 | 28.85 | 281.2472 | 280.2399 | [M + H]+ | Other | C18H32O2 | (9Z,12Z)-Linoleic acid | - | - | + | - | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Qudah, M.A.; Al-Jaber, H.I.; Abu Orabi, F.M.; Hasan, H.S.; Aldahoun, A.K.; Alhamzani, A.G.; Alakhras, A.I.; Bataineh, T.T.; Rawashdeh, A.M.M.; Abu-Orabi, S.T. Unveiling the Impact of Drying Methods on Phytochemical Composition and Antioxidant Activity of Anthemis palestina. Plants 2023, 12, 3914. https://doi.org/10.3390/plants12223914
Al-Qudah MA, Al-Jaber HI, Abu Orabi FM, Hasan HS, Aldahoun AK, Alhamzani AG, Alakhras AI, Bataineh TT, Rawashdeh AMM, Abu-Orabi ST. Unveiling the Impact of Drying Methods on Phytochemical Composition and Antioxidant Activity of Anthemis palestina. Plants. 2023; 12(22):3914. https://doi.org/10.3390/plants12223914
Chicago/Turabian StyleAl-Qudah, Mahmoud A., Hala I. Al-Jaber, Faten M. Abu Orabi, Hazem S. Hasan, Amal K. Aldahoun, Abdulrahman G. Alhamzani, Abbas I. Alakhras, Tareq T. Bataineh, Abdel Monem M. Rawashdeh, and Sultan T. Abu-Orabi. 2023. "Unveiling the Impact of Drying Methods on Phytochemical Composition and Antioxidant Activity of Anthemis palestina" Plants 12, no. 22: 3914. https://doi.org/10.3390/plants12223914
APA StyleAl-Qudah, M. A., Al-Jaber, H. I., Abu Orabi, F. M., Hasan, H. S., Aldahoun, A. K., Alhamzani, A. G., Alakhras, A. I., Bataineh, T. T., Rawashdeh, A. M. M., & Abu-Orabi, S. T. (2023). Unveiling the Impact of Drying Methods on Phytochemical Composition and Antioxidant Activity of Anthemis palestina. Plants, 12(22), 3914. https://doi.org/10.3390/plants12223914










