Pharmacological and Chemical Analysis of Bauhinia divaricata L. Using an In Vitro Antiadipogenic Model
Abstract
1. Introduction
2. Results
2.1. Cell Viability Determined Using an MTT Assay in 3T3-1 Cells Treated with the Extracts Obtained from B. divaricata
2.2. Lipid Quantification with Oily Red Assay in the Three Extracts Evaluated from Bauhinia divaricata on Days 5, 7, and 9 of Differentiation in 3T3-L1 Cells
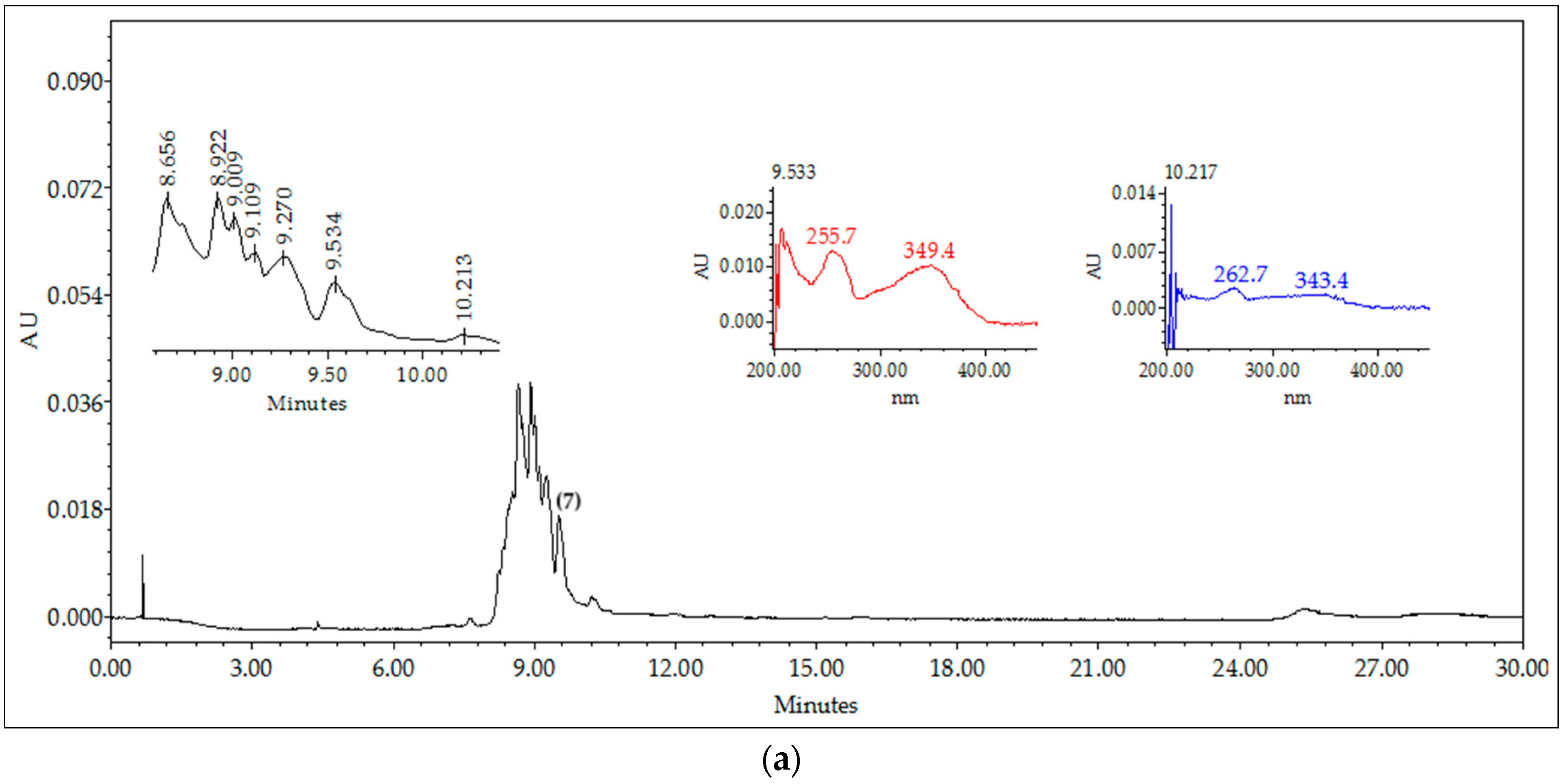
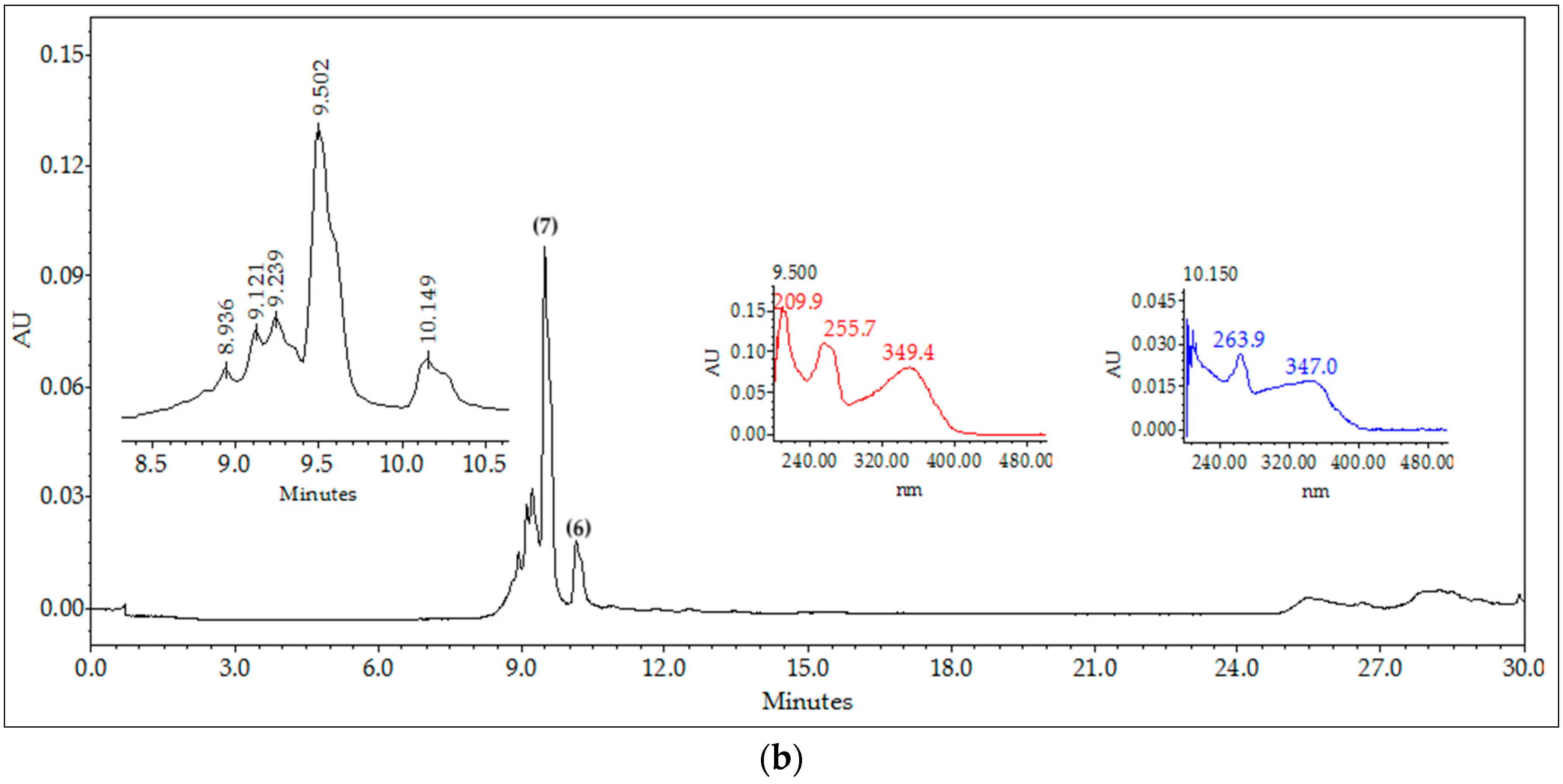
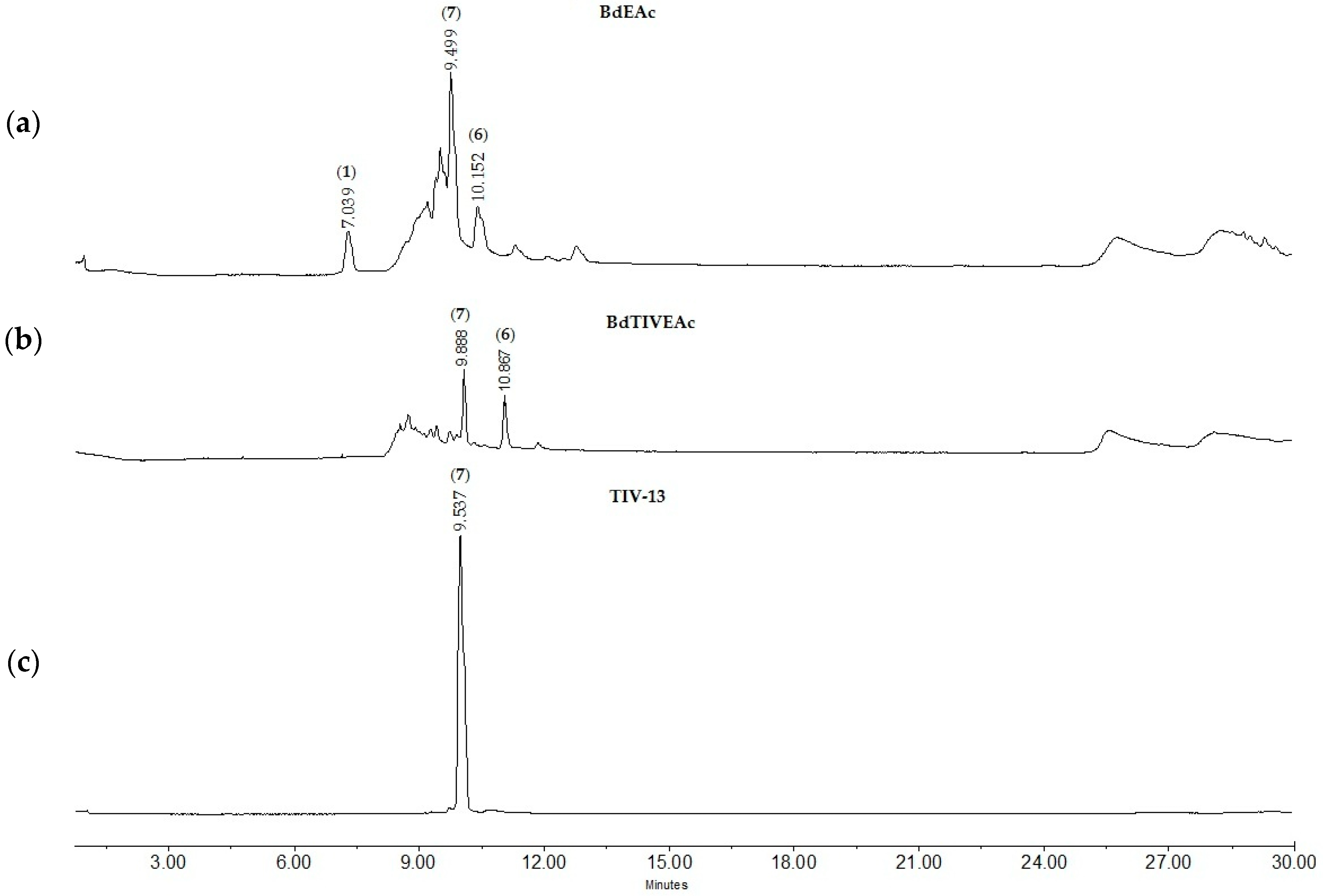
2.3. Chromatographic Fingerprints of Integrated Extracts
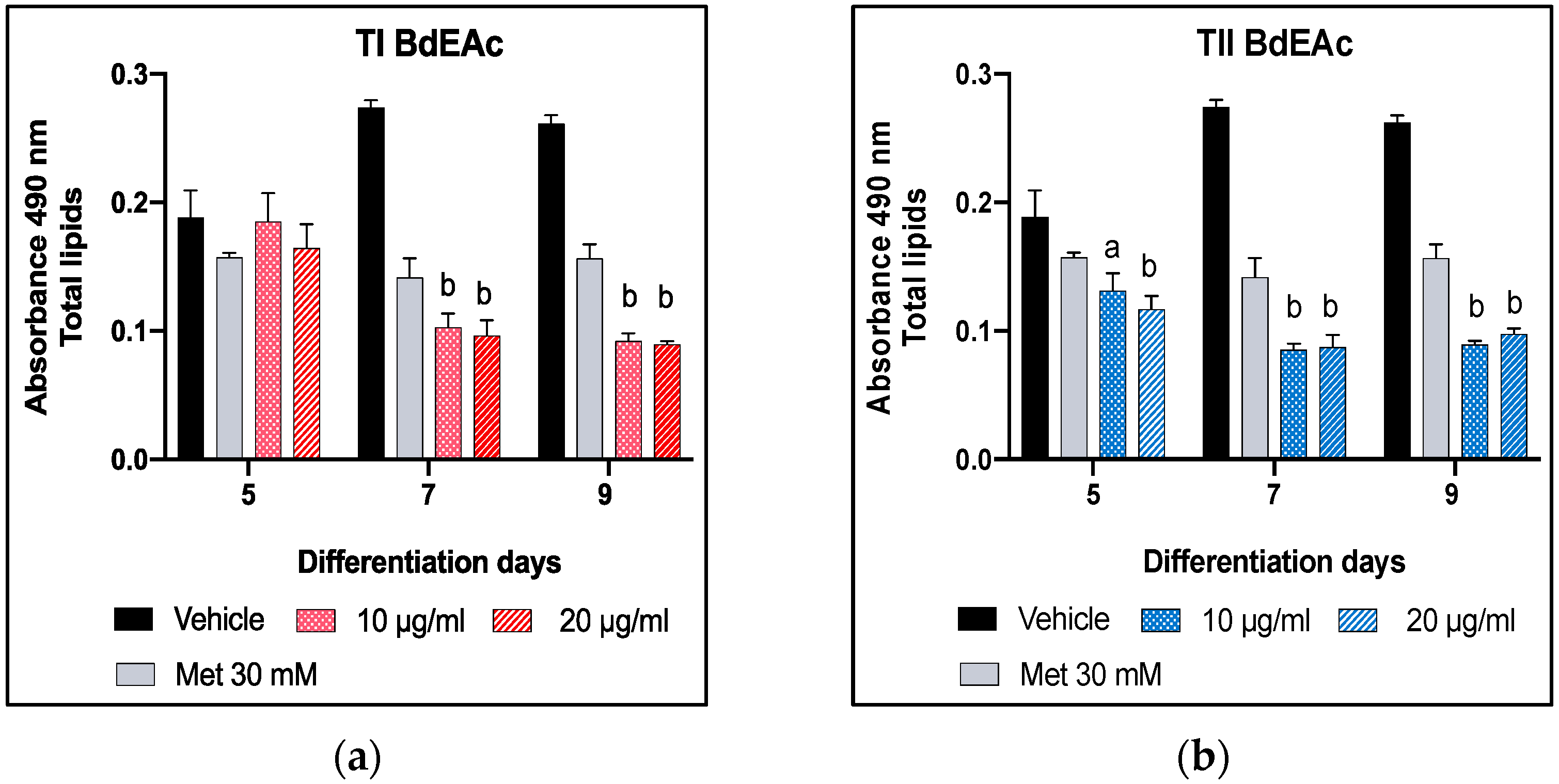
2.4. Lipid Quantification Using Oily Red Assay of BdEAc on Days 5, 7, and 9 of Differentiation in 3T3-L1 Cells
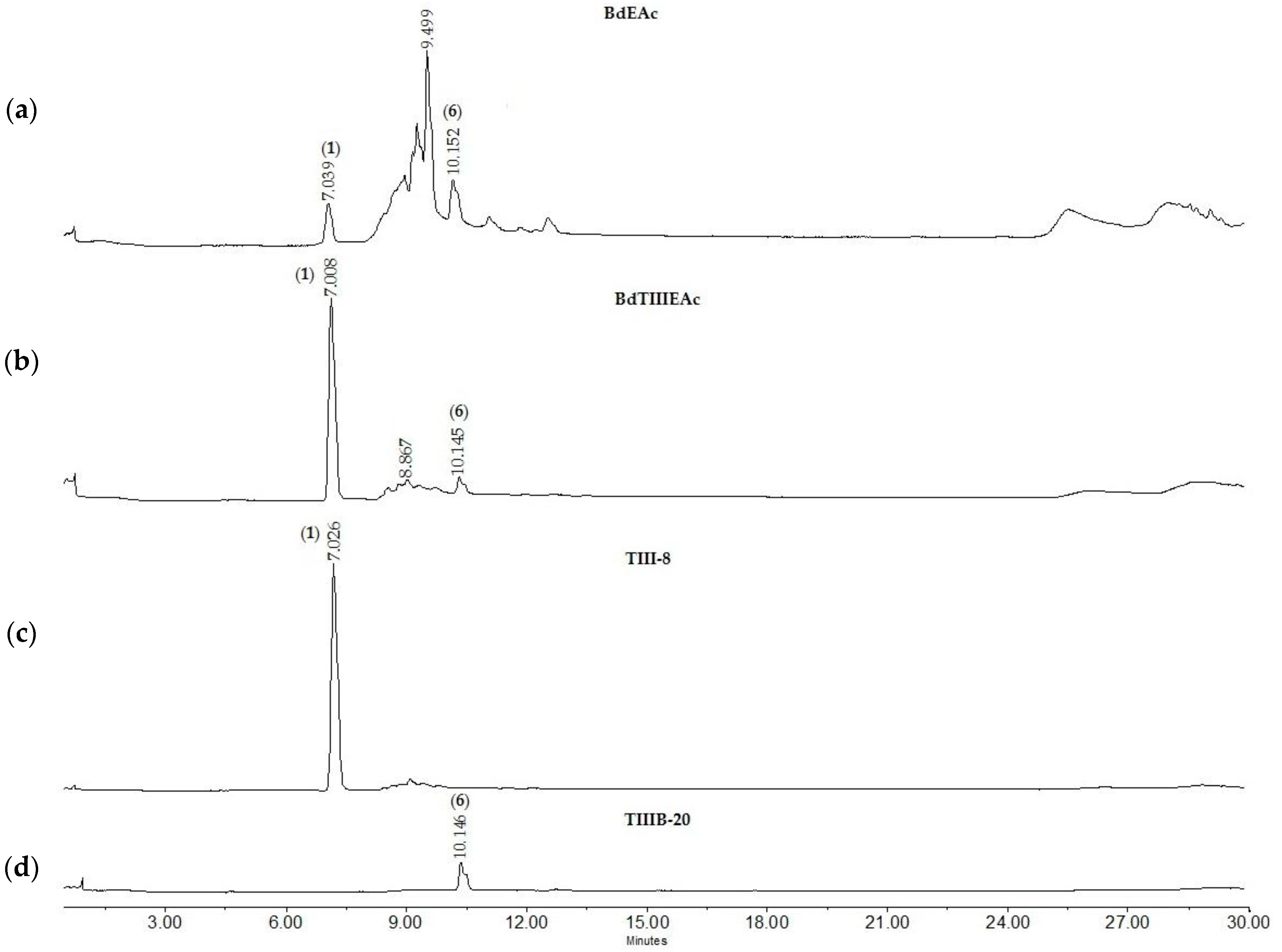
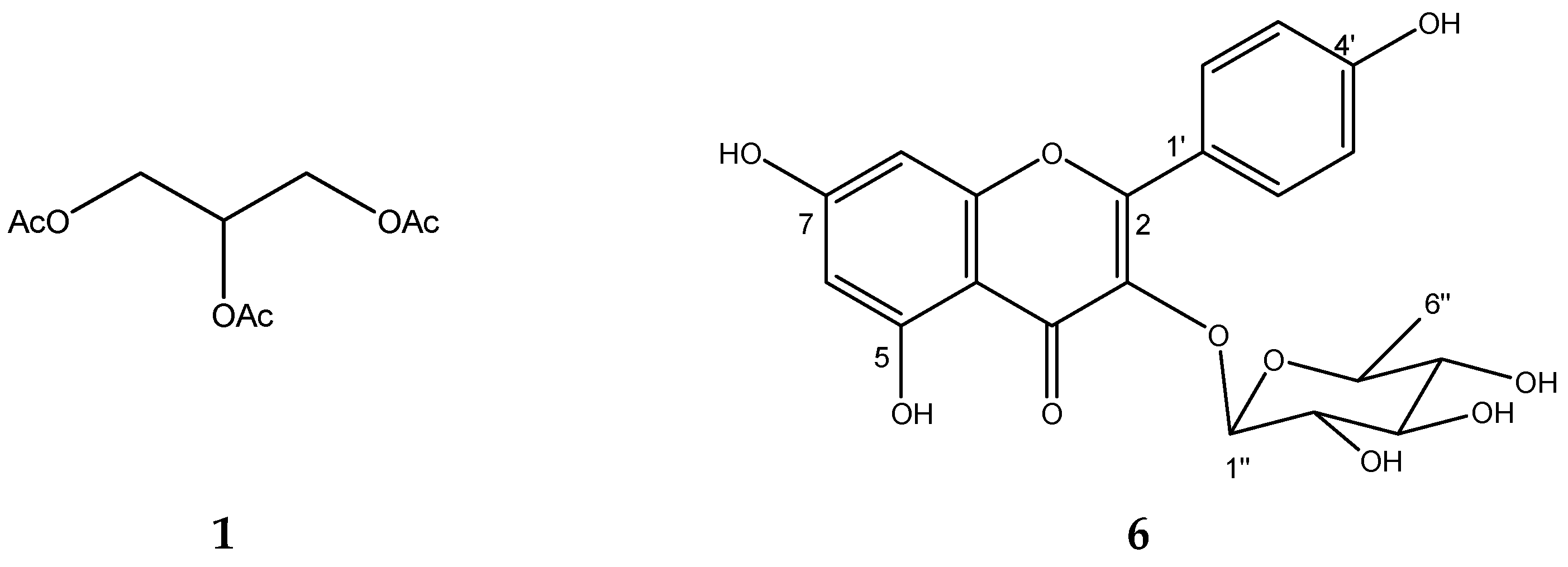
2.5. Chemical Characterization of TIII from BdEAc
2.6. GC-MS of Treatment TIII-8
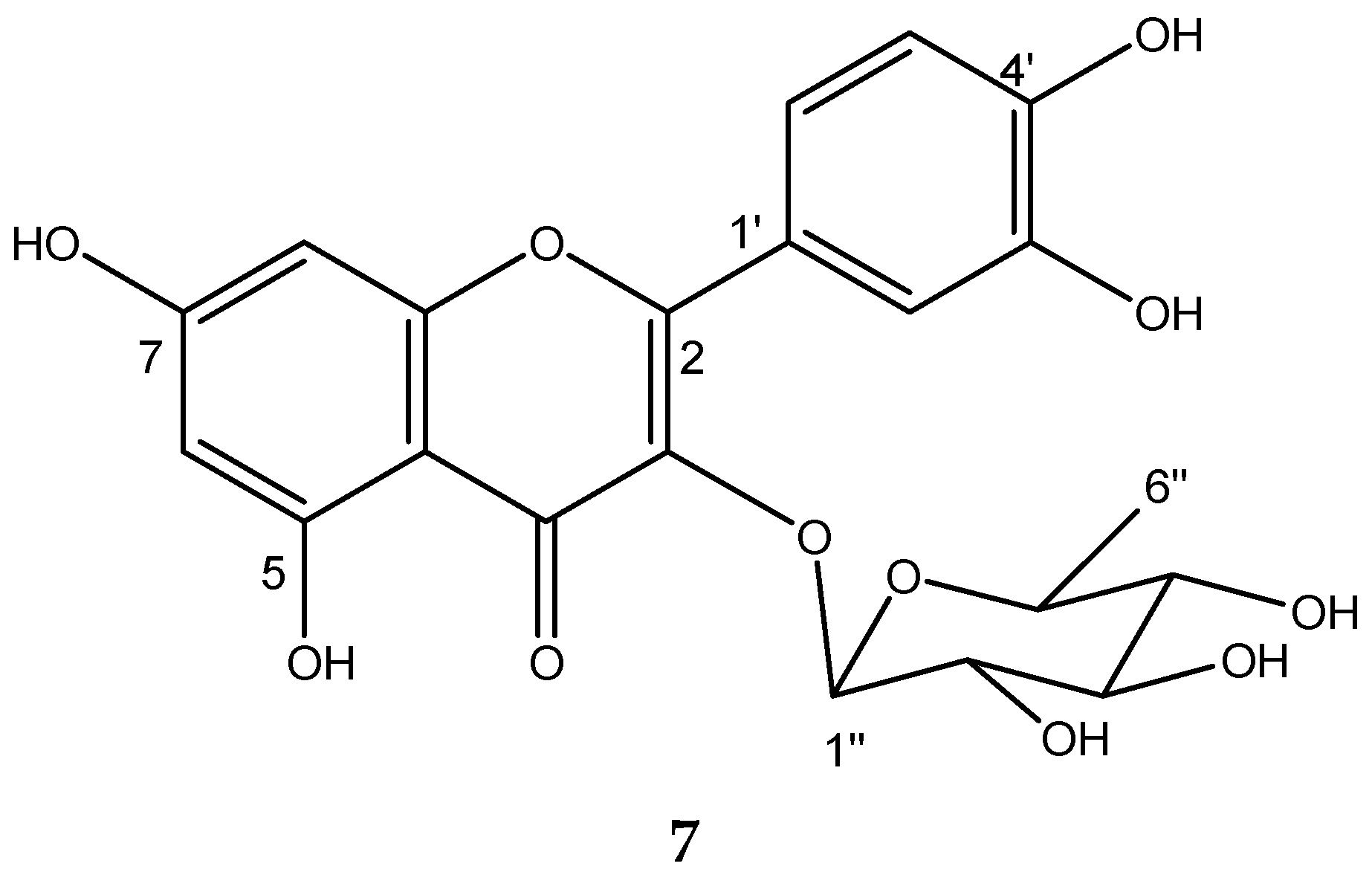
2.7. Chemical Characterization of TIV from BdEAc
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Extraction
4.3. Cell Line Differentiation: 3T3-L1
4.4. Cell Viability Determination Using MTT Assay
4.5. Quantification of Lipids Using Oily Red Assay
4.6. Chemical Characterization of the Extracts (BdEAc and BdHA) for HPLC
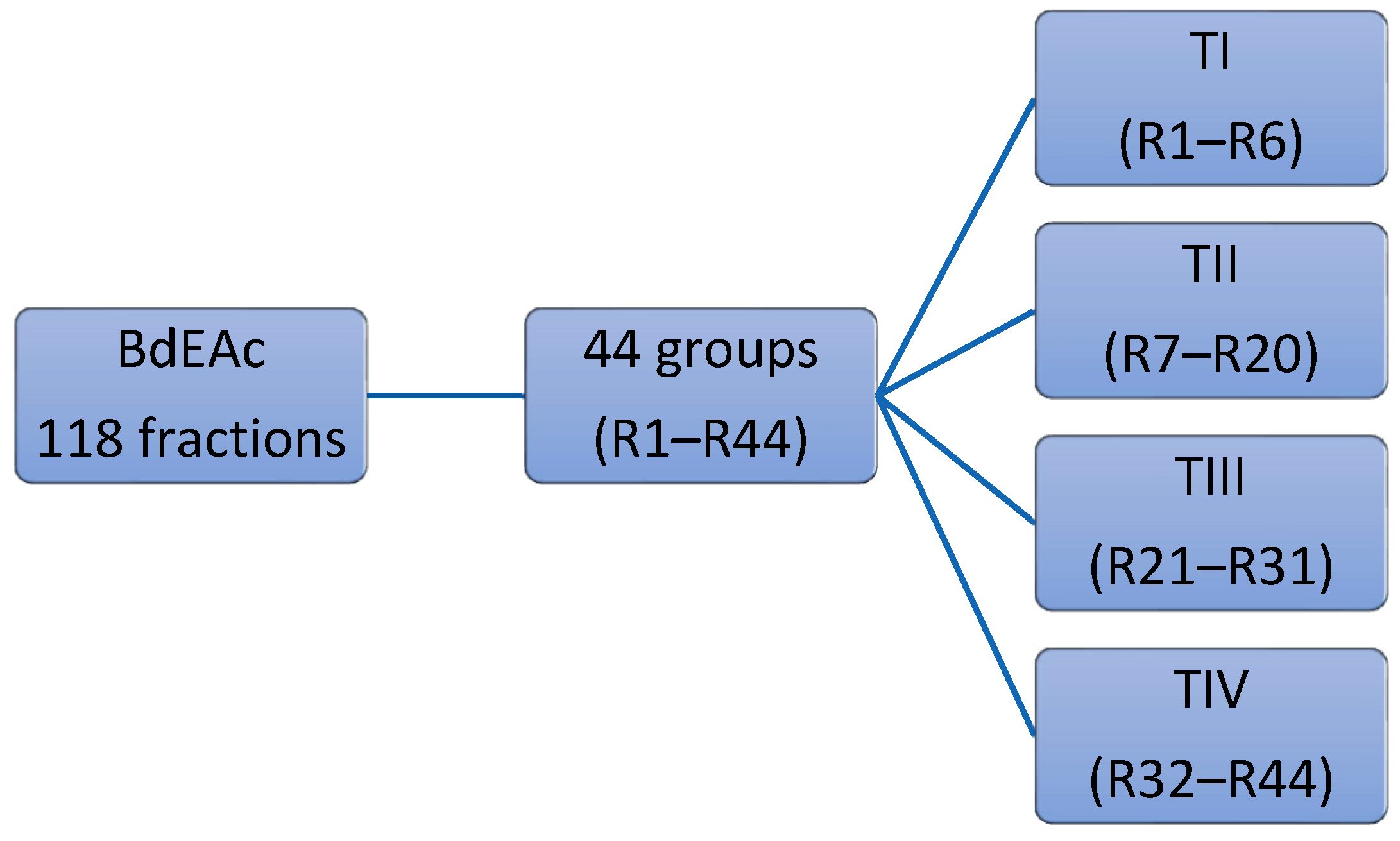
4.7. Fractionation of BdEAc
4.8. Obtaining the Triacetin, Kaempferol-3-O-rhamnoside, and Compounds (3–5) of Treatment III
4.9. Obtention of Quercetin-3-O-rhamnoside from Treatment IV
4.10. Quantitative Analyses of Kaempferol-3-rhamnoside and Quercetin 3-O-rhamnoside
4.11. GC-MS Analysis
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campos, I.; Galván, O.; Hernández, L.; Oviedo, C.; Barquera, S. Prevalence of obesity and associated risk factors in Mexican adults: Results of the Ensanut 2022. Salud Publica Mex. 2023, 65 (Suppl. S1), S238–S247. [Google Scholar] [CrossRef]
- Dávila, J.; González, J.; Barrera, A. Panorama de la obesidad en México. Rev. Med. Inst. Mex. Seguro Soc. 2015, 53, 240–249. [Google Scholar]
- Uribe, R.; Jiménez, A.; Morales, M.; Salazar, A.; Shamah, T. Percepción del peso corporal y de la probabilidad de desarrollar obesidad en adultos mexicanos. Salud Pública Méx. 2018, 60, 254–262. [Google Scholar] [CrossRef]
- Shamah, T.; Romero, M.; Barrientos, T.; Cuevas, L.; Bautista, S.; Colchero, M.A.; Gaona, E.B.; Lazcano, E.; Martínez, J.; Alpuche, C.; et al. Encuesta Nacional de Salud y Nutrición 2021 Sobre COVID-19; Resultados nacionales; Instituto Nacional de Salud Pública: Cuernavaca, Mexico, 2022.
- Esteve, M. Adipose tissue: Cell heterogeneity and functional diversity. Endocrinol. Nutr. 2014, 61, 100–112. [Google Scholar] [CrossRef]
- Sánchez, J.C.; Romero, C.R.; Arroyave, C.D.; Díaz, D.M.; Quiroz, A.F.; Quintero, J.F. El adipocito en el laboratorio: Historia, perspectivas y reporte de experiencia. Rev. CES Med. 2015, 29, 271–282. [Google Scholar]
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Perea, A.; López, G.; Padrón, M.; Lara, A.; Santamaría, C.; Ynga, M.; Peniche, J.; Espinosa, E.; Ballesteros, J. Evaluación, diagnóstico, tratamiento y oportunidades de prevención de la obesidad. Acta Pediátr. Méx. 2014, 35, 316–337. [Google Scholar]
- Rodrigo, S.; Soriano, J.M.; Merino, J.F. Obesity’s causes and treatment. Nutr. Clín. Diet. Hosp. 2017, 37, 87–92. [Google Scholar]
- Suárez, W.; Sánchez, A.; González, J. Fisiopatología de la obesidad: Perspectiva actual. Rev. Chil. Nutr. 2017, 44, 226–233. [Google Scholar] [CrossRef]
- Kishore, G.; Corby, M.; Hans-Rudolf, B.; Steven, H. Obesity Pathophysiology and Management. JACC 2018, 71, 69–84. [Google Scholar] [CrossRef]
- Ferreira, A.; Salame, L.; Cuenca, D. Tratamiento farmacológico de la obesidad. Rev. Medica Institutp Mex. Seguro Soc. 2018, 56, 395–409. [Google Scholar]
- Torres, R.; Duno, R.; y Can, L. El género Bauhinia (Fabaceae, Caesalpinioideae, Cercideae) en la península de Yucatán (México, Belice y Guatemala). Rev. Mex. Biodivers. 2009, 80, 293–301. [Google Scholar]
- Duno, R.; Cetzalix, W. La subfamilia Caesalpinioideae (Fabaceae) en la Península de Yucatán, México. Desde Herb. CICY 2016, 8, 181–188. [Google Scholar]
- García, R.; Chaires, L. Determinación de Actividad Antioxidante, Antimicrobiana y Anticancerígena de la Pata de Vaca Bauhinia divaricata L. Available online: www.covecyt.gob.mx/wp-content/uploads/2014/10/Ejemplo-abstract.pdf (accessed on 18 September 2023).
- Ankli, A.; Heinrich, M.; Bork, P.; Wolfram, L.; Bauerfeind, P.; Brun, R.; Schmid, C.; Weiss, C.; Bruggisser, R.; Gertsch, J.; et al. Yucatec Mayan medicinal plants: Evaluation based on indigenous uses. J. Ethnopharmacol. 2002, 79, 43–52. [Google Scholar] [CrossRef]
- Waizel, S.; Waizel, J. Algunas plantas utilizadas en México para el tratamiento del asma. An Orl Mex. 2009, 54, 45–71. [Google Scholar]
- Amer, A.; Mohammed, R.S.; Hussein, Y.; Ali, A.S.M.; Khalil, A. Development of Lepidium sativum Extracts/PVA Electrospun Nanofibers as Wound Healing Dressing. ACS Omega 2022, 7, 20683–20695. [Google Scholar] [CrossRef] [PubMed]
- Elloumi, W.; Maalej, A.; Ortiz, S.; Michel, S.; Chamkha, M.; Boutefnouchet, S.; Sayadi, S. Pistacia lentiscus L. Distilled Leaves as a Potential Cosmeceutical Ingredient: Phytochemical Characterization, Transdermal Diffusion, and Anti-Elastase and Anti-Tyrosinase Activities. Molecules 2022, 27, 855. [Google Scholar] [CrossRef] [PubMed]
- Regazzoni, L.; Arlandini, E.; Garzon, D.; Santagati, N.A.; Beretta, G.; Maffei Facino, R. A rapid profiling of gallotannins and flavonoids of the aqueous extract of Rhus coriaria L. by flow injection analysis with high-resolution mass spectrometry assisted with database searching. J. Pharm. Biomed. Anal. 2013, 72, 202–207. [Google Scholar] [CrossRef]
- Toloza, P.; Avello, M.; y Fernández, P. Determinación de rutina y trigonelina en extractos de hojas de Bauhinia forficata subsp. pruinosa y evaluación del efecto hipoglucemiante en humanos. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2015, 14, 21–32. [Google Scholar]
- De Sousa, F.; Barreto, A.; Siqueira, H.; Machado, R.; Sheridan, H.; Frankish, N. Hypoglycemic activity of two Brazilian Bauhinia species: Bauhinia forficata L. and Bauhinia monandra Kurz. Rev. Bras. Farmacogn. 2007, 17, 8–13. [Google Scholar]
- Herrera, M.; Santillán, M.; Romero, O.; Zamilpa, A.; Jiménez, E.; Tortoriello, J. Antidepressant-Like Effect of Bauhinia blakeana Dunn in a Neuroinflammation Model in Mice. Med. Princ. Pract. 2019, 29, 113–120. [Google Scholar] [CrossRef]
- Clavijo, M.; Goméz, D.; Gomez, C. Adipogénesis in vitro de células 3T3-L1. Rev. Med. 2007, 15, 170–176. [Google Scholar]
- Sharma, N.; Sharm, A.; Bhatia, G.; Landi, M.; Bikram, M.; Singh, J.; Satwinderjeet, K.; Bhardwaj, R. Isolation of Phytochemicals from Bauhinia variegata L. Bark and Their In Vitro Antioxidant and Cytotoxic Potential. Antioxidants 2019, 8, 492. [Google Scholar] [CrossRef]
- Kumar, T.; Chandrashekar, K.S. Chandrashekar. Bauhinia purpurea Linn. A review of its ethnobotany, phytochemical and pharmacological profile. Res. J. Med. Plant 2011, 5, 420–431. [Google Scholar]
- Farag, M.A.; Sakna, S.T.; El-Fiky, N.M.; Shabana, M.M.; Wessjohann, L.A. Phytochemical, antioxidant, and antidiabetic evaluation of eight Bauhinia L. species from Egypt using UHPLC-PDA-qTOF-MS and chemometrics. Phytochemistry 2015, 119, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Gaxiola, R.; Bitzer, O. Peroxidación de lípidos y la respuesta del sistema de defensa antioxidante en el diabético tipo 2 obeso en comparación al diabético tipo 2 sin obesidad. Nutr. Hosp. 2013, 28, 1905–1911. [Google Scholar] [CrossRef]
- Septembre, A.; Boumendjel, A.; Seteyen, A.-L.S.; Boina, C.; Gasque, P.; Guiraud, P.; Sélambarom, J. Focus on the high therapeutic potentials of quercetin and its derivatives. Phytomed. Plus 2022, 2, 100220. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.; Ding, G.; Ba, M.; Guo, Y.; Zou, Z. Two new derivatives of 2, 5-dihydroxyphenylacetic acid from the kernel of Entada phaseoloides. Molecules 2013, 18, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Rathore, K.; Jain, P.; y Ahmed, Z. Biological activity of Bauhinia racemosa against Diabetes and Interlinked Disorders like Obesity and Hyperlipidemia. Clin. Phytosci. 2017, 3, 7. [Google Scholar] [CrossRef]
- Ruiz, F.J.; Rupérez, A.I.; Gómez, C.; Gil, A.; Aguilera, C.M. Cell Models and Their Application for Studying Adipogenic Differentiation in Relation to Obesity: A Review. Int. J. Mol. Sci. 2016, 17, 1040. [Google Scholar] [CrossRef]
- Mossmann, T. Rapid colorimetric assay for celular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Gómez, A.; González, M.; Gallegos, A.J.; Escobar, A.; Flores, G.; Lobato, C.E. Spasmolytic, anti-inflammatory, and antioxidant activities of Salvia gesneriflora Lindley. Afr. J. Tradit. Complement. Altern. Med. 2018, 15, 72–82. [Google Scholar] [CrossRef][Green Version]


| Extracto | IC50 (μg/mL) |
|---|---|
| BdHex | >1000 |
| BdEAc | >1000 |
| BdHA | >1000 |
| Paclitaxel (+) | 14.32 |
| Position | 1H (δ, J in Hz) 1 | δ13C(δ) 1 |
|---|---|---|
| 1a b | 4.14, (dd, 6.3, 11.7) 4.19, dd (4.5, 11.6) | 65.52 |
| 2 | 3.93 (m) | 70.32 |
| 3a b | 3.70 (dd,3.9, 11.5) 3.60 (dd, 5.9, 11.5) | 63.45 |
| 4 | ----------------- | 171.6 |
| 5 | 2.1 (s) | 20.98 |
| Amer, A. A et al., 2022. [18] | Elloumi, W et al., 2022. [19] | |||||
|---|---|---|---|---|---|---|
| Position | 1H (δ, J in Hz) 6 | δ13C(δ) 6 | 1H (δ, J in Hz) 6 | δ13C(δ) 6 | 1H (δ, J in Hz) 6 | δ13C(δ) 6 |
| 2 | --- | 160.2 | --- | 157.2 | --- | --- |
| 3 | --- | 134.7 | --- | --- | --- | --- |
| 4 | --- | 178.1 | --- | 178.5 | --- | --- |
| 5 | --- | 164.1 | --- | 162.2 | --- | --- |
| 6 | 6.21 (1H, d, 1.9) | 98.6 | 6.25 (1H, d, 1.8) | --- | 6.19 (s) | --- |
| 7 | --- | 157.2 | --- | 164.2 | --- | --- |
| 8 | 6.38 (1H, d, 1.9) | 93.4 | 6.45 (1H, d, 2.3) | --- | 6.36 (s) | --- |
| 9 | --- | 157.8 | --- | 160.1 | --- | --- |
| 10 | --- | 104.3 | --- | --- | --- | --- |
| 1′ | --- | 122.8 | --- | --- | --- | --- |
| 2′ | 7.78 (1H, d, 8.7) | 130.4 | 7.84 (2H, dd, 8.6) | --- | 7.76 (d, 8.4) | --- |
| 3′ | 6.95 (1H, d, 8.7) | 115.1 | 7 (2H, dd, 8.6) | --- | 6.93 (d, 8.4) | --- |
| 4′ | --- | 161.8 | --- | 157.6 | --- | --- |
| 5′ | 6.95 (1H, d, 8.7) | 115.1 | 7 (2H, dd, 8.6) | --- | 6.93 (d, 8.4 | --- |
| 6′ | 7.78 (1H, d, 8.7) | 130.4 | 7 (2H, dd, 8.6) | --- | 7.76 (d, 8.4) | --- |
| 1′′ | 5.39 (1H, d, 1.4) | 102.1 | 5.52 (1H, d,1.4) | --- | 5.38 (d, 1.5) | --- |
| 2′′ | 4.23, (1H, dd, 1.4, 3.3) | 70.52 | 4.22 (1H, d, 1.4) | --- | 4.23 (dd, 3.3, 1.7) | --- |
| 3′′ | 3.72, (1H, dd, 3.3, 8.8) | 70.72 | 3.70 | --- | 3.72 (m) | --- |
| 4′′ | 3.32, (m) | 71.7 | --- | 3.30 | 3.34 (m) | --- |
| 5′′ | 3.32, (m) | 70.6 | --- | --- | 3.34 (m) | --- |
| 6′′ | 0.94, (1H, d, 5.5) | 16.2 | --- | 0.90 (3H, d, 6.0) | 0.93 (d, 5.7) | --- |
| RT (min) | Area (%) | Molecular Weight (g/mol) | Compound Name | Chemical Structure |
|---|---|---|---|---|
| 9.356 | 68.89 | 134.13 | 2,3-Dihydroxypropyl acetate | 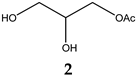 |
| 14.138 | 3.87 | 218.2 | Triacetin | 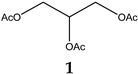 |
| 14.782 | 9.12% | 216.32 | (3R)-3-Hydroxydodecanoic acid | 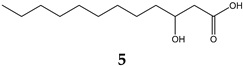 |
| 15.353 | 3.19% | 168.15 | 2,5-Dihydroxyphenylacetic acid | 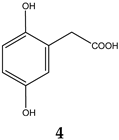 |
| 17.304 | 2% | 224.34 | (3E)-2-Methyl-4-(1,3,3-trimethyl-7-oxabicyclo[4.1.0]hept-2-yl)-3-buten-2-ol | 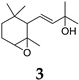 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islas-Garduño, A.L.; Romero-Cerecero, O.; Jiménez-Aparicio, A.R.; Tortoriello, J.; Montiel-Ruiz, R.M.; González-Cortazar, M.; Zamilpa, A. Pharmacological and Chemical Analysis of Bauhinia divaricata L. Using an In Vitro Antiadipogenic Model. Plants 2023, 12, 3799. https://doi.org/10.3390/plants12223799
Islas-Garduño AL, Romero-Cerecero O, Jiménez-Aparicio AR, Tortoriello J, Montiel-Ruiz RM, González-Cortazar M, Zamilpa A. Pharmacological and Chemical Analysis of Bauhinia divaricata L. Using an In Vitro Antiadipogenic Model. Plants. 2023; 12(22):3799. https://doi.org/10.3390/plants12223799
Chicago/Turabian StyleIslas-Garduño, Ana Laura, Ofelia Romero-Cerecero, Antonio Ruperto Jiménez-Aparicio, Jaime Tortoriello, Rosa Mariana Montiel-Ruiz, Manases González-Cortazar, and Alejandro Zamilpa. 2023. "Pharmacological and Chemical Analysis of Bauhinia divaricata L. Using an In Vitro Antiadipogenic Model" Plants 12, no. 22: 3799. https://doi.org/10.3390/plants12223799
APA StyleIslas-Garduño, A. L., Romero-Cerecero, O., Jiménez-Aparicio, A. R., Tortoriello, J., Montiel-Ruiz, R. M., González-Cortazar, M., & Zamilpa, A. (2023). Pharmacological and Chemical Analysis of Bauhinia divaricata L. Using an In Vitro Antiadipogenic Model. Plants, 12(22), 3799. https://doi.org/10.3390/plants12223799








