Diverse Bradyrhizobium spp. with Similar Symbiosis Genes Nodulate Peanut in Different Regions of China: Characterization of Symbiovar sv. Arachis
Abstract
:1. Introduction
2. Results
2.1. PCR-Based RFLP Analysis of IGS
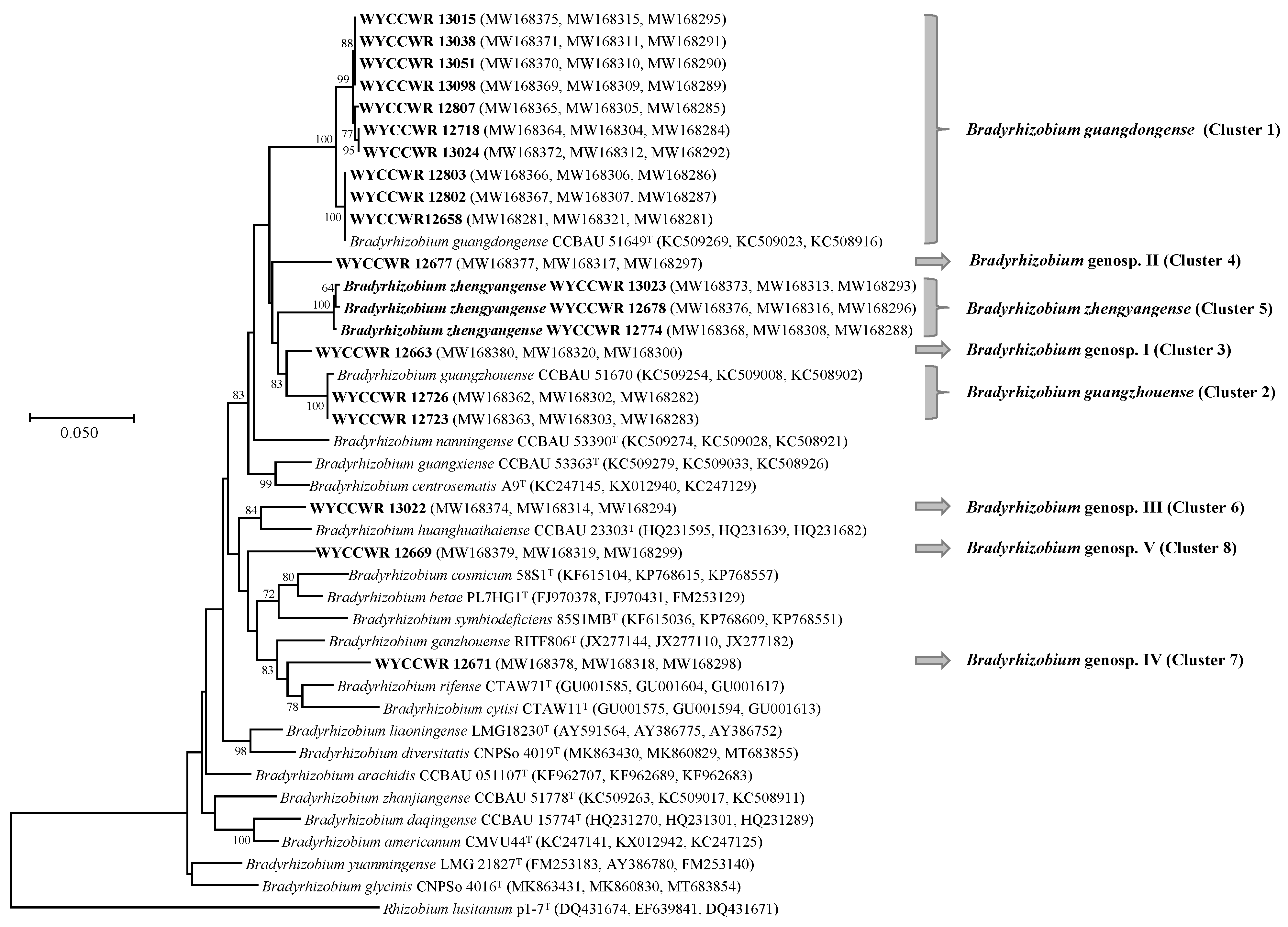
2.2. Phylogenetic Analysis of 16S rRNA and Housekeeping Genes
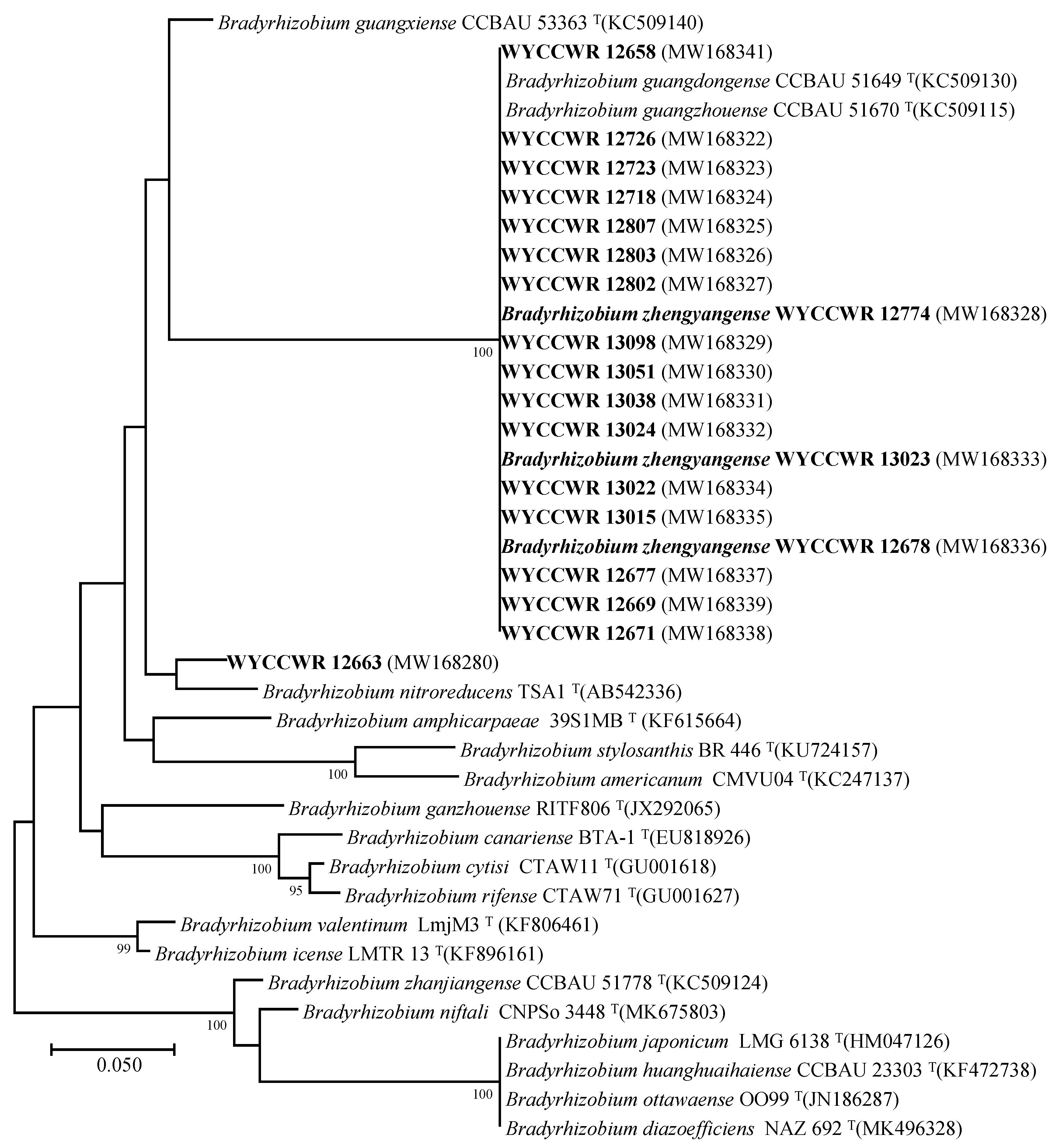
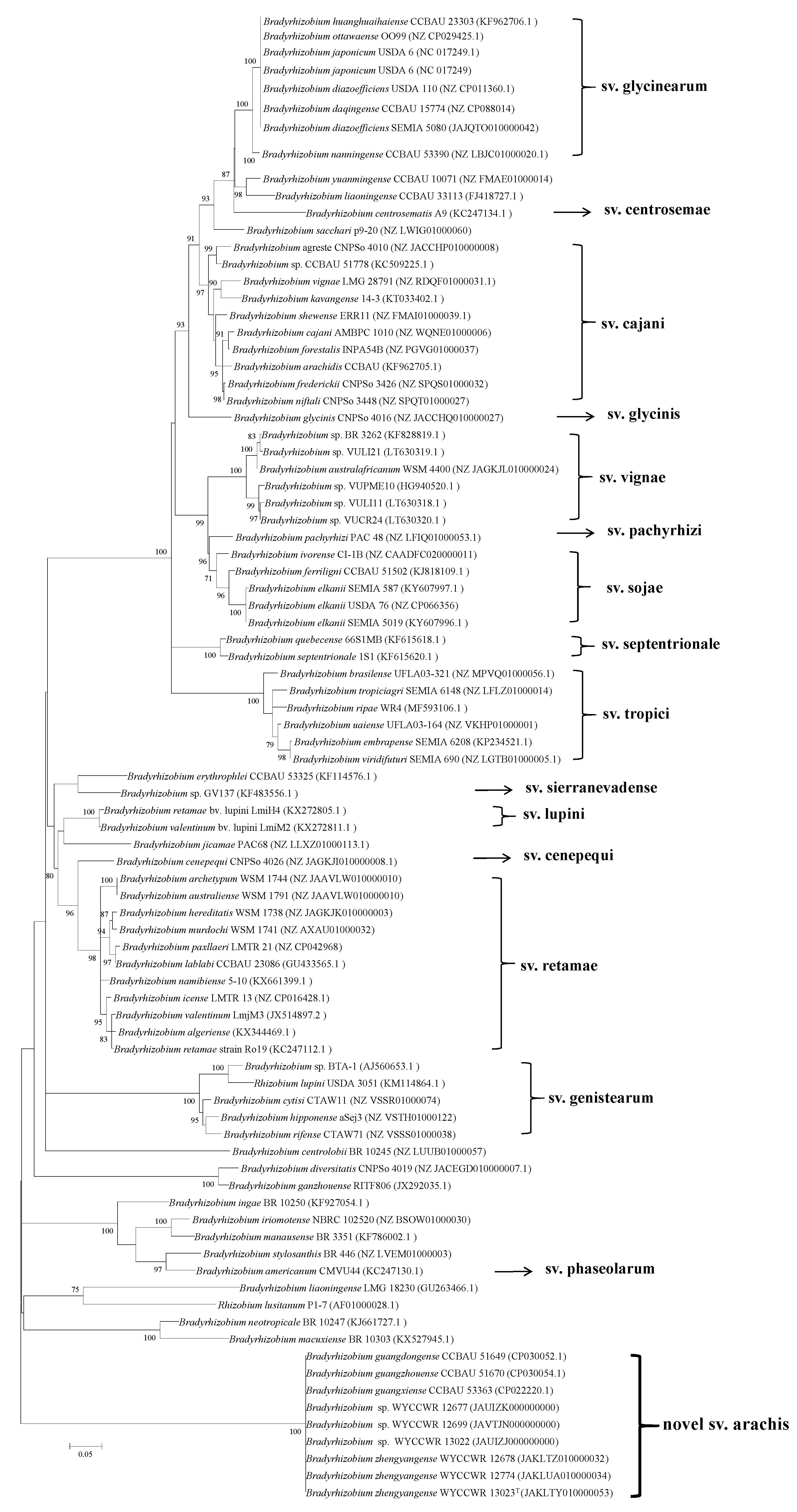
2.3. Phylogenies of Symbiosis Genes
3. Discussion
4. Materials and Methods
4.1. Soil Sampling and Physicochemical Characterization
4.2. Rhizobial Isolation and Nodulation Test
4.3. PCR-Based RFLP of 16S-23S rRNA Intergenic Spacer (IGS)
4.4. Phylogenetic Analysis of 16S rRNA Genes
4.5. Phylogenetic Analyses of Housekeeping and Symbiosis Genes
4.6. Genome Features
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrews, M.; Andrews, M.E. Specificity in legume-rhizobia symbioses. Int. J. Mol. Sci. 2017, 18, 705. [Google Scholar]
- Walker, L.; Lagunas, B.; Gifford, M.L. Determinants of host range specificity in legume-rhizobia symbiosis. Front. Microbiol. 2020, 11, 585749. [Google Scholar] [CrossRef] [PubMed]
- Delamuta, J.R.M.O.; Menna, P.; Ribeiro, R.A.; Hungria, M. Phylogenies of symbiotic genes of Bradyrhizobium symbionts of legumes of economic and environmental importance in Brazil support the definition of the new symbiovars pachyrhizi and sojae. Syst. Appl. Microbiol. 2017, 40, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Klepa, M.S.; Helene, L.C.F.; Hara, G.O.; Hungria, M. Bradyrhizobium cenepequi sp. nov., Bradyrhizobium semiaridum sp. nov., Bradyrhizobium hereditatis sp. nov and Bradyrhizobium australafricanum sp. nov., symbionts of different leguminous plants of Western Australia and South Africa and definition of three novel symbiovars. Int. J. Syst. Evol. Microbiol. 2022, 72, 005446. [Google Scholar]
- Andrews, M.; De Meyer, S.; James, E.K.; Stepkowski, T.; Hodge, S.; Simon, M.F.; Young, J.P.W. Horizontal transfer of symbiosis genes within and between rhizobial genera: Occurrence and importance. Genes 2018, 9, 321. [Google Scholar] [CrossRef]
- Wardell, G.E.; Hynes, M.F.; Young, P.J.; Harrison, E. Why are rhizobial symbiosis genes mobile? Philos. Trans. R. Soc. B Biol. Sci. 2022, 377, 20200471. [Google Scholar] [CrossRef]
- Rogel, M.A.; Ormeño-Orrillo, E.; Romero, E.M. Symbiovars in rhizobia reflect bacterial adaptation to legumes. Syst. Appl. Microbiol. 2011, 34, 96–104. [Google Scholar] [CrossRef]
- FAO. Production Year Book; FAO: Rome, Italy, 2023. [Google Scholar]
- Chang, Y.L.; Wang, J.Y.; Wang, E.T.; Liu, H.C.; Sui, X.H.; Chen, W.X. Bradyrhizobium lablabi sp. nov., isolated from effective nodules of Lablab purpureus and Arachis hypogaea. Int. J. Syst. Evol. Microbiol. 2011, 61, 2496–2502. [Google Scholar] [CrossRef]
- Wang, R.; Chang, Y.L.; Zheng, W.T.; Zhang, D.; Zhang, X.X.; Sui, X.H.; Wang, E.T.; Hu, J.Q.; Zhang, L.Y.; Chen, W.X. Bradyrhizobium arachidis sp nov., isolated from effective nodules Arachis hypogaea grown in China. Syst. Appl. Microbiol. 2013, 36, 101–105. [Google Scholar] [CrossRef]
- Li, Y.H.; Wang, R.; Zhang, X.X.; Young, J.P.W.; Wang, E.T.; Sui, X.H.; Chen, W.X. Bradyrhizobium guangdongense sp. nov. and Bradyrhizobium guangxiense sp. nov., isolated from effective nodules of peanut. Int. J. Syst. Evol. Microbiol. 2015, 65, 4655. [Google Scholar] [CrossRef]
- Li, Y.H.; Wang, R.; Sui, X.H.; Wang, E.T.; Chen, W.X. Bradyrhizobium nanningense sp. nov., Bradyrhizobium guangzhouense sp. nov. and Bradyrhizobium zhanjiangense sp. nov., isolated from effective nodules of peanut in Southeast China. Syst. Appl. Microbiol. 2019, 42, 126002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, N.; Li, S.; Peng, S.; Andrews, M.; Zhang, X.; Zhang, Y.; Yu, H.; Song, J.; Chen, W.; et al. Bradyrhizobium zhengyangense sp. nov., isolated from effective nodules of Arachis hypogaea L. in central China. Int. J. Syst. Evol. Microbiol. 2023, 73, 005723. [Google Scholar]
- Zhang, J.J.; Peng, S.S.; Li, S.; Song, J.C.; Brunel, B.; Wang, E.T.; James, E.K.; Chen, W.F.; Andrews, M. Arachis hypogaea L. from acid soils of Nanyang (China) is frequently associated with Bradyrhizobium guangdongense and occasionally with Bradyrhizobium ottawaense or three Bradyrhizobium genospecies. Microb. Ecol. 2021, 84, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.V.; De Meyer, S.E.; Simoes-Araujo, J.L.; Barbe, T.D.C.; Xavier, G.R.; O″Hara, G.; Ardley, J.K.; Rumjanek, N.G.; Willems, A.; Zilli, J.E. Bradyrhizobium manausense sp. nov., isolated from effective nodules of Vigna unguiculata grown in Brazilian Amazonian rainforest soils. Int. J. Syst. Evol. Microbiol. 2014, 64, 2358–2363. [Google Scholar] [CrossRef] [PubMed]
- Michel, D.C.; Costa, E.M.D.; Guimares, A.A.; Carvalho, T.S.D.; Moreira, F.M.D.S. Bradyrhizobium campsiandrae sp. nov., a nitrogen-fixing bacterial strain isolated from a native leguminous tree from the Amazon adapted to flooded conditions. Arch. Microbiol. 2021, 203, 233–240. [Google Scholar] [CrossRef]
- Lu, J.K.; Dou, Y.J.; Zhu, Y.J.; Wang, S.K.; Sui, X.H.; Kang, L.H. Bradyrhizobium ganzhouense sp. nov., an effective symbiotic bacterium isolated from Acacia melanoxylon R. Br. nodules. Int. J. Syst. Evol. Microbiol. 2014, 64, 1900–1905. [Google Scholar] [CrossRef]
- Jang, J.; Ashida, N.; Kai, A.; Isobe, K.; Nishizawa, T.; Otsuka, S.; Yokota, A.; Senoo, K.; Ishii, S. Presence of Cu-type (NirK) and cd1-type (NirS) nitrite reductase genes in the denitrifying bacterium Bradyrhizobium nitroreducens sp. nov. Microbes Environ. 2018, 33, 326–331. [Google Scholar] [CrossRef]
- Chen, J.; Hu, M.; Ma, H.; Wang, Y.; Wang, E.T.; Zhou, Z.; Gu, J. Genetic diversity and distribution of bradyrhizobia nodulating peanut in acid-neutral soils in Guangdong Province. Syst. Appl. Microbiol. 2016, 39, 418–427. [Google Scholar] [CrossRef]
- Shao, S.; Chen, M.; Liu, W.; Hu, X.; Li, Y. Long-term monoculture reduces the symbiotic rhizobial biodiversity of peanut. Syst. Appl. Microbiol. 2020, 43, 126101. [Google Scholar] [CrossRef]
- AFNOR. Soil Quality, Determination of pH; AFNOR: Paris, France, 2005. [Google Scholar]
- Han, L.; Wang, E.; Han, T.; Liu, J.; Sui, X.; Chen, W.; Chen, W. Unique community structure and biogeography of soybean rhizobia in the saline-alkaline soils of Xinjiang, China. Plant Soil 2009, 324, 291–305. [Google Scholar] [CrossRef]
- Vincent, J.M. A Manual for the Practical Study of Root Nodule Bacteria; International Biological Programme (By) Blackwell Scientific: Oxford, UK, 1970; Volume 15, pp. 1–164. [Google Scholar]
- Wei, G.H.; Chen, W.M.; Zhu, W.F.; Chen, C.; Young, J.P.W.; Bontemps, C. Invasive Robinia pseudoacacia in China is nodulated by Mesorhizobium and Sinorhizobium species that share similar nodulation genes with native American symbionts. FEMS Microbiol. Ecol. 2009, 68, 320–328. [Google Scholar] [CrossRef]
- Zhang, J.J.; Shang, Y.M.; Wang, E.T.; Chen, W.F.; de Lajudie, P.; Li, B.Y.; Guo, C.; Yang, X.; Zheng, J.Q.; Liu, C.Z. Mesorhizobium jarvisii sv. astragali as predominant microsymbiont for Astragalus sinicus L. in acidic soils, Xinyang, China. Plant Soil 2018, 433, 201–212. [Google Scholar] [CrossRef]
- Terefework, Z.; Kaijalainen, S.; Lindstrom, K. AFLP fingerprinting as a tool to study the genetic diversity of Rhizobium galegae isolated from Galega orientalis and Galega officinalis. J. Biotechnol. 2001, 91, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Laguerre, G.; Mavingui, P.; Allard, M.R.; Charnay, M.P.; Louvrier, P.; Mazurier, S.I.; Rigottier-Gois, L.; Amarger, N. Typing of rhizobia by PCR DNA fingerprinting and PCR-restriction fragment length polymorphism analysis of chromosomal and symbiotic gene regions: Application to Rhizobium leguminosarum and its different biovars. Appl. Environ. Microbiol. 1996, 62, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.T.; Van Berkum, P.; Sui, X.H.; Beyene, D.; Chen, W.X.; Martínez-Romero, E. Diversity of rhizobia associated with Amorpha fruticosa isolated from Chinese soils and description of Mesorhizobium amorphae sp. nov. Int. J. Syst. Bacteriol. 1999, 49, 51–65. [Google Scholar] [CrossRef]
- Vauterin, L.; Yang, P.; Hoste, B.; Pot, B.; Swings, J.; Kersters, K. Taxonomy of xanthomonads from cereals and grasses based on SDS-PAGE of proteins, fatty acid analysis and DNA hybridization. J. Gen. Microbiol. 1992, 138, 1467–1477. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2015, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Posada, D.; Crandall, K.A. MODELTEST: Testing the model of DNA substitution. Bioinformatics 1998, 14, 817–818. [Google Scholar] [CrossRef]
- Vinuesa, P.; Silva, C.; Lorite, M.J.; Izaguirre-Mayoral, M.L.; Bedmar, E.J.; Martinez-Romero, E. Molecular systematics of rhizobia based on maximum likelihood and Bayesian phylogenies inferred from rrs, atpD, recA and nifH sequences, and their use in the classification of Sesbania microsymbionts from Venezuelan wetlands. Syst. Appl. Microbiol. 2005, 28, 702–716. [Google Scholar] [CrossRef]
- Vinuesa, P.; Silva, C.; Werner, D.; Martínez-Romero, E. Population genetics and phylogenetic inference in bacterial molecular systematics: The roles of migration and recombination in Bradyrhizobium species cohesion and delineation. Mol. Phylogenet. Evol. 2005, 34, 29–54. [Google Scholar] [CrossRef]
- Haukka, K.; Lindstrom, K.; Young, J.P. Three phylogenetic groups of nodA and nifH genes in Sinorhizobium and Mesorhizobium isolates from leguminous trees growing in Africa and Latin America. Appl. Environ. Microbiol. 1998, 64, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Laguerre, G.; Nour, S.M.; Macheret, V.; Sanjuan, J.; Drouin, P.; Amarger, N. Classification of rhizobia based on nodC and nifH gene analysis reveals a close phylogenetic relationship among Phaseolus vulgaris symbionts. Microbiology 2001, 147, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Sarita, S.; Sharma, P.K.; Priefer, U.B.; Prell, J. Direct amplification of rhizobial nodC sequences from soil total DNA and comparison to nodC diversity of root nodule isolates. FEMS Microbiol. Ecol. 2010, 54, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tatiana, T.; Michael, D.C.; Azat, B.; Vyacheslav, C.; Nawrocki, E.P.; Leonid, Z.; Alexandre, L.; Pruitt, K.D.; Mark, B.; James, O. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar]


| Representative Isolate WYCCWR no. (Field Site) 1 | MLSA Similarity (%) with Species 3 | IGS Type | Isolate Number (% of Total Isolates) | |||||
|---|---|---|---|---|---|---|---|---|
| B.gd | B.gz | B.zh | B.gx | B.nn | B.ar | |||
| Bradyrhizobium guangdongense (Cluster 1, C1) 2 | ||||||||
| 13051 (ZC) | 98.7 | 94.6 | 93.6 | 93.5 | 94.0 | 94.1 | 2 | 155 (69.82) |
| 12658 (ZA) | 100 | 93.1 | 94.4 | 93.7 | 94.1 | 94.2 | ||
| 13038 (ZB) | 98.7 | 94.6 | 93.6 | 93.5 | 94.0 | 94.1 | ||
| 13098 (ZD) | 98.7 | 94.6 | 93.6 | 93.5 | 94.0 | 94.1 | ||
| 12802 (ZE) | 100 | 94.9 | 94.4 | 93.7 | 94.1 | 94.2 | ||
| 12718 (ZF) | 98.5 | 94.2 | 93.5 | 93.1 | 93.7 | 93.9 | ||
| 13015 (ZB) | 98.7 | 94.6 | 93.6 | 93.5 | 94.0 | 94.1 | 5 | 5 (2.25) |
| 12803 (ZE) | 100 | 94.9 | 94.4 | 93.7 | 94.1 | 94.2 | 9 | 3 (1.35) |
| 13024 (ZB) | 98.5 | 94.2 | 93.5 | 93.1 | 93.7 | 93.9 | 10 | 2 (0.90) |
| 12807 (ZE) | 98.7 | 94.4 | 93.6 | 93.3 | 93.7 | 93.8 | 12 | 2 (0.90) |
| Bradyrhizobium guangzhouense (C2) | ||||||||
| 12726 (ZF) | 94.9 | 99.7 | 95.0 | 94.2 | 94.1 | 93.7 | 3 | 4 (1.80) |
| 12723 (ZF) | 94.9 | 99.7 | 95.0 | 94.2 | 94.1 | 93.7 | 11 | 2 (0.90) |
| Bradyrhizobium genosp. I (C3) | ||||||||
| 12663 (ZA) | 95.4 | 96.7 | 96.0 | 94.9 | 94.9 | 94.2 | 13 | 2 (0.90) |
| Bradyrhizobium genosp. II (C4) | ||||||||
| 12677 (ZA) | 94.5 | 94.9 | 94.9 | 94.0 | 94.2 | 94.2 | 4 | 5 (2.25) |
| Bradyrhizobium zhengyangense (C5) | ||||||||
| 13023 (ZB) | 94.4 | 94.9 | 100 | 94.9 | 94.2 | 94.5 | 1 | 25 (11.26) |
| 12678 (ZA) | 94.5 | 95.0 | 99.7 | 94.9 | 94.3 | 94.5 | ||
| 12774 (ZE) | 94.5 | 95.1 | 99.7 | 95.1 | 94.4 | 94.7 | ||
| Bradyrhizobium genosp. III (C6) | ||||||||
| 13022 (ZB) | 93.6 | 94.9 | 93.7 | 93.7 | 93.6 | 94.4 | 8 | 7 (3.15) |
| Bradyrhizobium genosp. IV (C7) | ||||||||
| 12671 (ZA) | 92.1 | 94.0 | 92.4 | 92.2 | 92.4 | 93.0 | 7 | 3 (1.35) |
| Bradyrhizobium genosp. V (C8) | ||||||||
| 12669 (ZA) | 92.8 | 94.9 | 94.0 | 94.4 | 93.2 | 94.7 | 6 | 4 (1.80) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Feng, Y.; Wang, J.; Wang, E.; Andrews, M. Diverse Bradyrhizobium spp. with Similar Symbiosis Genes Nodulate Peanut in Different Regions of China: Characterization of Symbiovar sv. Arachis. Plants 2023, 12, 3776. https://doi.org/10.3390/plants12213776
Zhang J, Feng Y, Wang J, Wang E, Andrews M. Diverse Bradyrhizobium spp. with Similar Symbiosis Genes Nodulate Peanut in Different Regions of China: Characterization of Symbiovar sv. Arachis. Plants. 2023; 12(21):3776. https://doi.org/10.3390/plants12213776
Chicago/Turabian StyleZhang, Junjie, Yufeng Feng, Jingqi Wang, Entao Wang, and Mitchell Andrews. 2023. "Diverse Bradyrhizobium spp. with Similar Symbiosis Genes Nodulate Peanut in Different Regions of China: Characterization of Symbiovar sv. Arachis" Plants 12, no. 21: 3776. https://doi.org/10.3390/plants12213776
APA StyleZhang, J., Feng, Y., Wang, J., Wang, E., & Andrews, M. (2023). Diverse Bradyrhizobium spp. with Similar Symbiosis Genes Nodulate Peanut in Different Regions of China: Characterization of Symbiovar sv. Arachis. Plants, 12(21), 3776. https://doi.org/10.3390/plants12213776




