Genome-Wide Identification and Characterization of the HAK Gene Family in Quinoa (Chenopodium quinoa Willd.) and Their Expression Profiles under Saline and Alkaline Conditions
Abstract
:1. Introduction
2. Results
2.1. Identification of the HAK Family Genes in C. quinoa
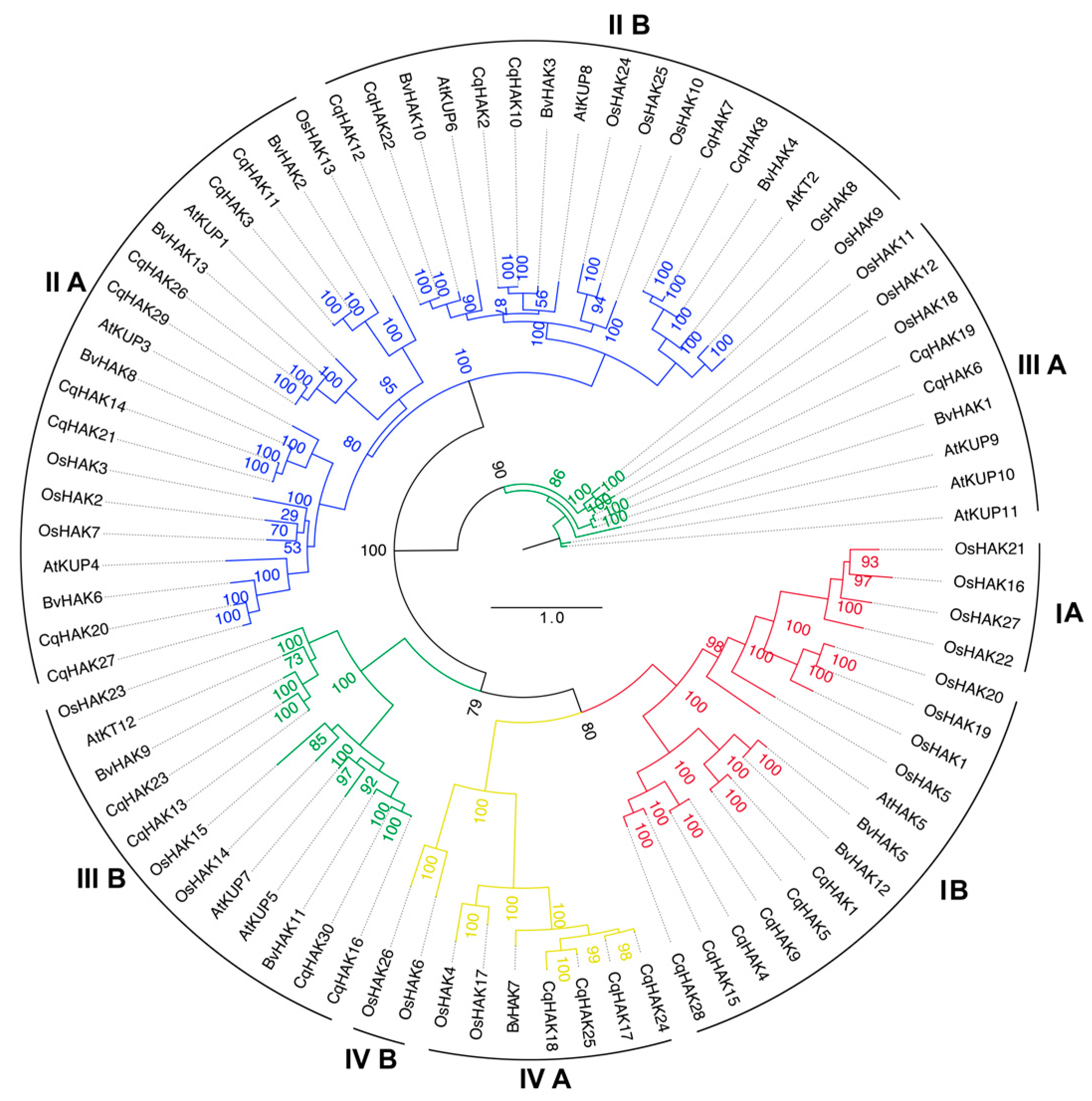
2.2. Phylogenetic Analysis of the CqHAK Transporters
2.3. Motif, Domain, and Gene Structure Analyses of CqHAKs
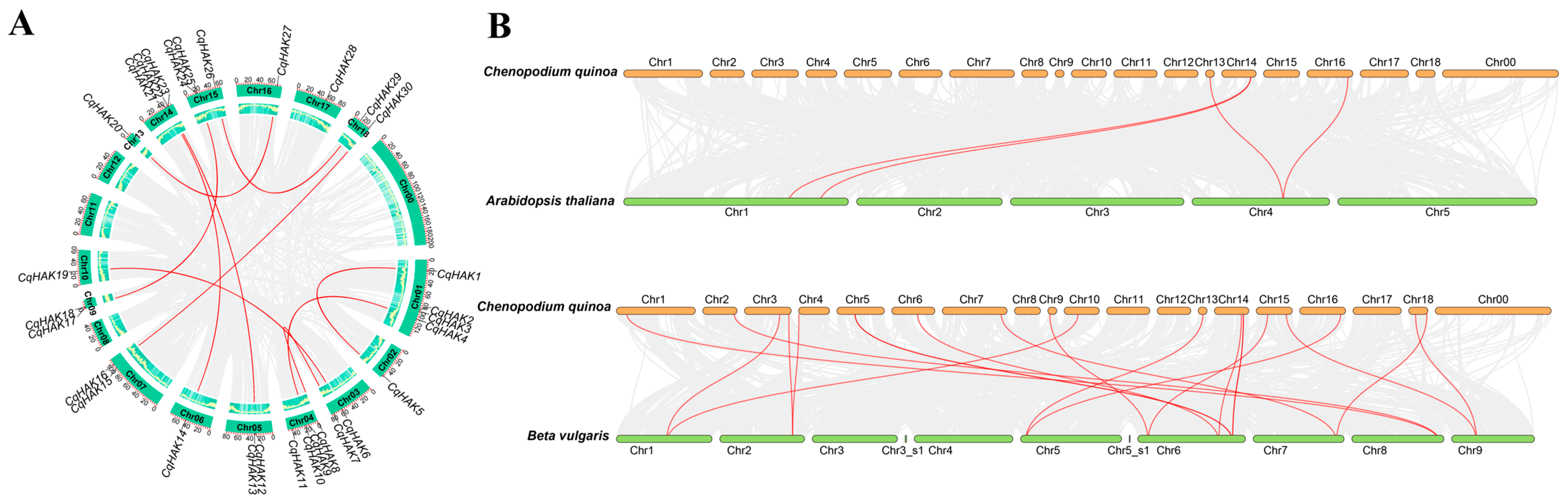
2.4. Chromosomal Distribution and Synteny Analysis of the CqHAK Genes
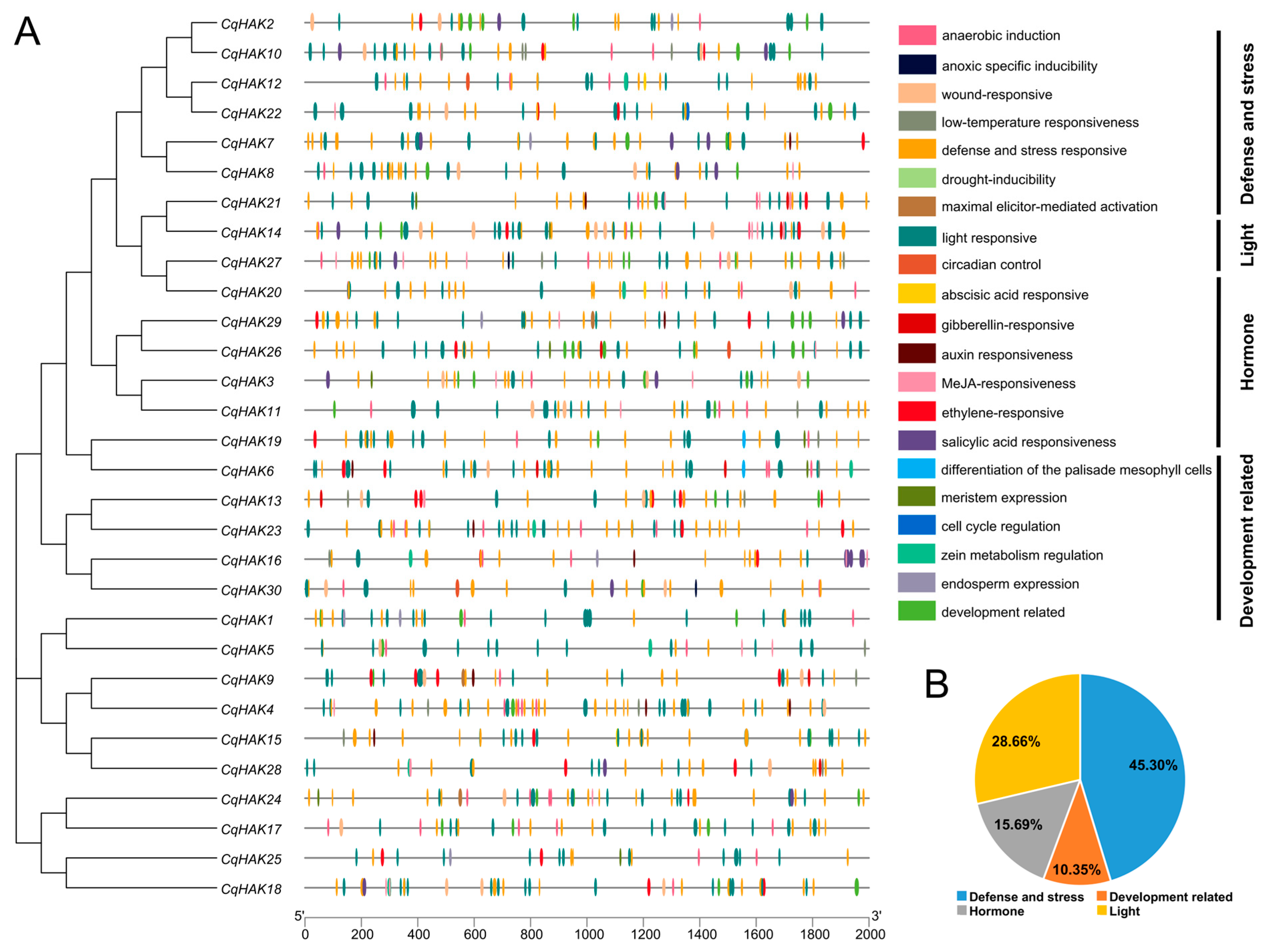
2.5. Cis-Regulatory Elements Analysis of the CqHAK Genes
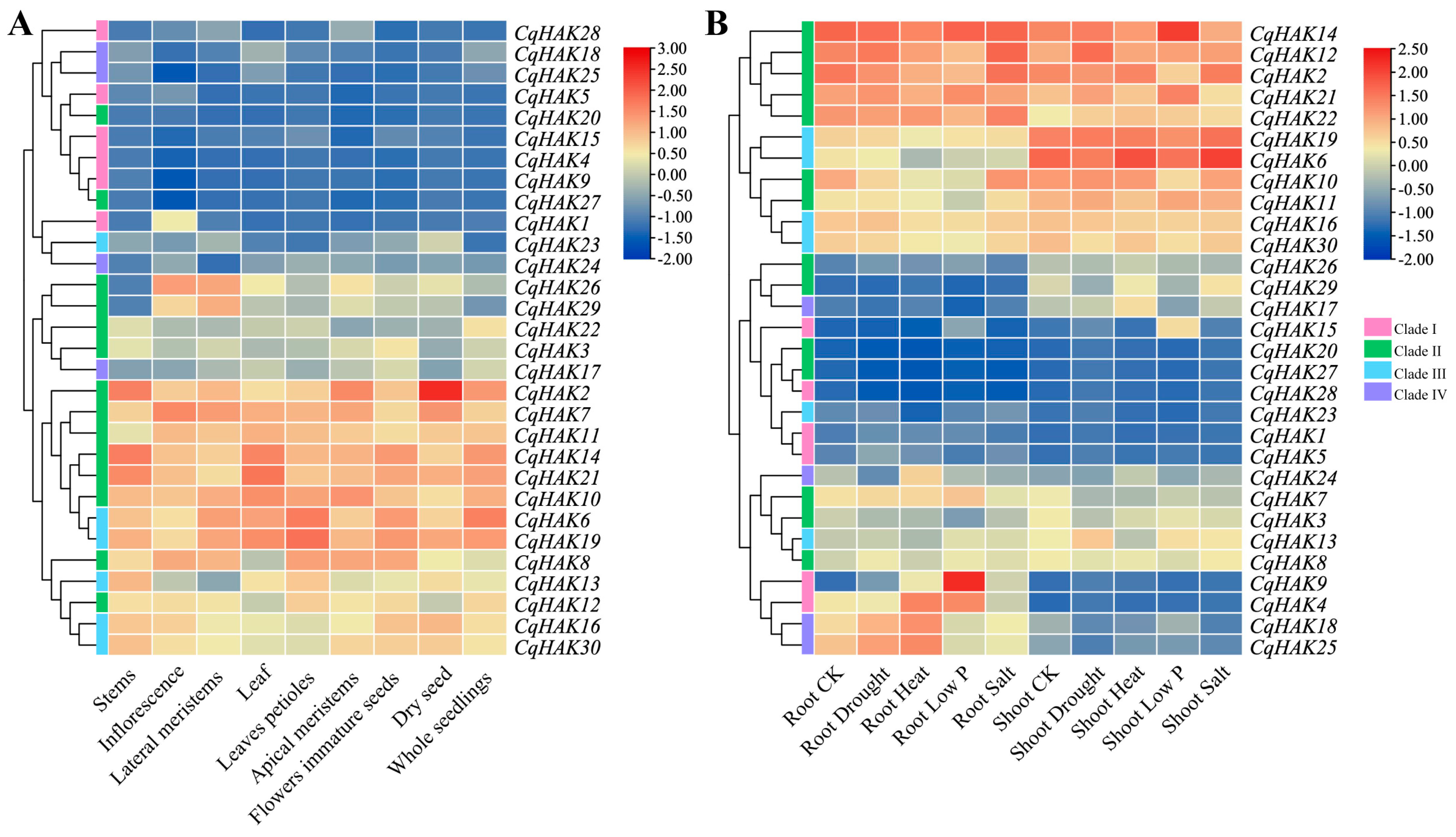
2.6. Expression Pattern Analysis of the CqHAK Genes in Various Tissues and under Abiotic Stresses
2.7. Validation of the Expression Profiles of the HAK Genes under Salt and Alkali Stress by Quantitative RT-PCR (qRT-PCR)
3. Discussion
4. Summary
5. Materials and Methods
5.1. Identification of HAK Family Members in Quinoa
5.2. Analyses of Sequence, Conserved Motif and Structural Characterization
5.3. Analyses of Phylogenetic Relationship
5.4. Analysis of Chromosome Locations and Collinearity
5.5. Analysis of Expression Level of HAK Genes
5.6. Plant Materials and Treatments
5.7. RNA Extraction, Analysis of the Gene Expression by RT-qPCR, and Correlation Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Leigh, R.A.; Wyn Jones, R.G. A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytol. 1984, 97, 1–13. [Google Scholar] [CrossRef]
- Hu, W.; Yang, J.; Meng, Y.; Wang, Y.; Chen, B.; Zhao, W.; Oosterhuis, D.M.; Zhou, Z.J. Potassium application affects carbohydrate metabolism in the leaf subtending the cotton (Gossypium hirsutum L.) boll and its relationship with boll biomass. Field Crops Res. 2015, 179, 120–131. [Google Scholar] [CrossRef]
- Ye, T.; Li, Y.; Zhang, J.; Hou, W.; Zhou, W.; Lu, J.; Xing, Y.; Li, X. Nitrogen, phosphorus, and potassium fertilization affects the flowering time of rice (Oryza sativa L.). Glob. Ecol. Conserv. 2019, 20, e00753. [Google Scholar] [CrossRef]
- Chambi-Legoas, R.; Chaix, G.; Castro, V.R.; Franco, M.P.; Tomazello-Filho, M.J. Inter-annual effects of potassium/sodium fertilization and water deficit on wood quality of Eucalyptus grandis trees over a full rotation. For. Ecol. Manag. 2021, 496, 119415. [Google Scholar] [CrossRef]
- Ali, M.M.; Petropoulos, S.A.; Selim, D.A.; Elbagory, M.; Othman, M.M.; Omara, A.E.; Mohamed, M.H. Plant growth, yield and quality of potato crop in relation to potassium fertilization. Agronomy 2021, 11, 675. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S.J. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Hossen, M.S.; Masud, A.A.; Moumita; Fujita, M.J. Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.H. Regulation of potassium transport and signaling in plants. Curr. Opin. Plant Biol. 2017, 39, 123–128. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.F.; Wu, W.H. Potassium and phosphorus transport and signaling in plants. J. Integr. Plant Biol. 2021, 63, 34–52. [Google Scholar] [CrossRef]
- Corratgé-Faillie, C.; Jabnoune, M.; Zimmermann, S.; Véry, A.A.; Fizames, C.; Sentenac, H.J. Potassium and sodium transport in non-animal cells: The Trk/Ktr/HKT transporter family. Cell. Mol. Life Sci. 2010, 67, 2511–2532. [Google Scholar] [CrossRef]
- Li, W.; Xu, G.; Alli, A.; Yu, L. Plant HAK/KUP/KT K+ transporters: Function and regulation. Semin. Cell Dev. Biol. 2018, 74, 133–141. [Google Scholar] [CrossRef]
- Gierth, M.; Mäser, P.; Schroeder, J.I. The potassium transporter AtHAK5 functions in K+ deprivation-induced high-affinity K+ uptake and AKT1 K+ channel contribution to K+ uptake kinetics in Arabidopsis roots. Plant Physiol. 2005, 137, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Wu, W.; Wu, W.H.; Wang, Y. Potassium transporter KUP7 is involved in K+ acquisition and translocation in Arabidopsis root under K+-limited conditions. Mol. Plant 2016, 9, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhang, S.; Hu, Y.; Wu, F.; Hu, Q.; Chen, G.; Cai, J.; Wu, T.; Moran, N.; Yu, L.J. The role of a potassium transporter OsHAK5 in potassium acquisition and transport from roots to shoots in rice at low potassium supply levels. Plant Physiol. 2014, 166, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Hu, Q.; Luo, L.; Yang, T.; Zhang, S.; Hu, Y.; Yu, L.; Xu, G.J. Rice potassium transporter OsHAK1 is essential for maintaining potassium-mediated growth and functions in salt tolerance over low and high potassium concentration ranges. Plant Cell Environ. 2015, 38, 2747–2765. [Google Scholar] [CrossRef]
- Chen, G.; Liu, C.; Gao, Z.; Zhang, Y.; Jiang, H.; Zhu, L.; Ren, D.; Yu, L.; Xu, G.; Qian, Q.J. OsHAK1, a high-affinity potassium transporter, positively regulates responses to drought stress in rice. Front. Plant Sci. 2017, 8, 1885. [Google Scholar] [CrossRef]
- Zhang, M.; Liang, X.; Wang, L.; Cao, Y.; Song, W.; Shi, J.; Lai, J.; Jiang, C.J. A HAK family Na+ transporter confers natural variation of salt tolerance in maize. Nat. Plants 2019, 5, 1297–1308. [Google Scholar] [CrossRef]
- Elumalai, R.P.; Nagpal, P.; Reed, J.W. A mutation in the Arabidopsis KT2/KUP2 potassium transporter gene affects shoot cell expansion. Plant Cell 2002, 14, 119–131. [Google Scholar] [CrossRef]
- Rigas, S.; Ditengou, F.A.; Ljung, K.; Daras, G.; Tietz, O.; Palme, K.; Hatzopoulos, P.J. Root gravitropism and root hair development constitute coupled developmental responses regulated by auxin homeostasis in the Arabidopsis root apex. New Phytol. 2013, 197, 1130–1141. [Google Scholar] [CrossRef]
- Daras, G.; Rigas, S.; Tsitsekian, D.; Iacovides, T.A.; Hatzopoulos, P.J. Potassium transporter TRH1 subunits assemble regulating root-hair elongation autonomously from the cell fate determination pathway. Plant Sci. 2015, 231, 131–137. [Google Scholar] [CrossRef]
- Jarvis, D.E.; Ho, Y.S.; Lightfoot, D.J.; Schmöckel, S.M.; Li, B.; Borm, T.J.; Ohyanagi, H.; Mineta, K.; Michell, C.T.; Saber, N.J. The genome of Chenopodium quinoa. Nature 2017, 542, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa, L.; González, J.A.; Barrios-Masias, F.H.; Fuentes, F.; Murphy, K.M. Quinoa abiotic stress responses: A review. Plants 2018, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Grabov, A.J. Plant KT/KUP/HAK potassium transporters: Single family-multiple functions. Ann. Bot. 2007, 99, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Shen, L.; Shen, Z.; Jing, W.; Ge, H.; Zhao, J.; Zhang, W.J. The potassium transporter OsHAK21 functions in the maintenance of ion homeostasis and tolerance to salt stress in rice. Plant Cell Environ. 2015, 38, 2766–2779. [Google Scholar] [CrossRef]
- Tahir, M.M.; Tong, L.; Xie, L.; Wu, T.; Ghani, M.I.; Zhang, X.; Li, S.; Gao, X.; Tariq, L.; Zhang, D.J. Identification of the HAK gene family reveals their critical response to potassium regulation during adventitious root formation in apple rootstock. Hortic. Plant J. 2023, 9, 45–59. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, H.J.; Chen, P.; Zhang, L.L.; Zhu, J.T.; Li, P.F.; Yang, J.; Ke, Y.Z.; Zhou, Y.H.; Li, J.N. Genome-wide survey and expression analysis of the KT/HAK/KUP family in Brassica napus and its potential roles in the response to K+ deficiency. Int. J. Mol. Sci. 2020, 21, 9487. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, X.; Mao, W.; Zhang, X.; Chen, S.; Zhan, K.; Bi, H.; Xu, H.J. Genome-wide identification and analysis of HAK/KUP/KT potassium transporters gene family in wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2018, 19, 3969. [Google Scholar] [CrossRef]
- Rehman, H.M.; Nawaz, M.A.; Shah, Z.H.; Daur, I.; Khatoon, S.; Yang, S.H.; Chung, G.J. In-depth genomic and transcriptomic analysis of five K+ transporter gene families in soybean confirm their differential expression for nodulation. Front. Plant Sci. 2017, 8, 804. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, Y.; Zhang, N.; Wu, Z.; Zeng, Q.; Wu, J.; Wu, X.; Wang, L.; Zhang, J.; Qi, Y.J. Genome-wide systematic characterization of the HAK/KUP/KT gene family and its expression profile during plant growth and in response to low-K+ stress in Saccharum. BMC Plant Biol. 2020, 20, 20. [Google Scholar] [CrossRef]
- Zou, C.; Chen, A.; Xiao, L.; Muller, H.M.; Ache, P.; Haberer, G.; Zhang, M.; Jia, W.; Deng, P.; Huang, R.J. A high-quality genome assembly of quinoa provides insights into the molecular basis of salt bladder-based salinity tolerance and the exceptional nutritional value. Cell Res. 2017, 27, 1327–1340. [Google Scholar] [CrossRef]
- Gupta, M.; Qiu, X.; Wang, L.; Xie, W.; Zhang, C.; Xiong, L.; Lian, X.; Zhang, Q.J. Genomics. KT/HAK/KUP potassium transporters gene family and their whole-life cycle expression profile in rice (Oryza sativa). Mol. Genet. Genom. 2008, 280, 437–452. [Google Scholar] [CrossRef]
- Liu, J.; Chen, N.; Chen, F.; Cai, B.; Dal Santo, S.; Tornielli, G.B.; Pezzotti, M.; Cheng, Z.M. Genome-wide analysis and expression profile of the bZIP transcription factor gene family in grapevine (Vitis vinifera). BMC Genom. 2014, 15, 281. [Google Scholar] [CrossRef]
- Zhang, P.; Yan, H.; Liu, Y.; Chai, Y.J. Genome-wide identification and functional characterization of wheat Brassinazole-resistant transcription factors in response to abiotic stresses and stripe rust infection. Front. Plant Sci. 2023, 14, 1144379. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Shin, R.; Schachtman, D.P. Expression of KT/KUP genes in Arabidopsis and the role of root hairs in K+ uptake. Plant Physiol. 2004, 134, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Sun, K.; Mackaluso, J.D.; Seddon, A.E.; Jin, R.; Thomashow, M.F.; Shiu, S.H. Cis-regulatory code of stress-responsive transcription in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2011, 108, 14992–14997. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, Y.W.; Zhou, J.M.; Zhao, S.P.; Zhang, X.H.; Min, D.H. Genome-wide analysis of the lectin receptor-like kinase family in foxtail millet (Setaria italica L.). Plant Cell Tissue Organ Cult. 2016, 127, 335–346. [Google Scholar] [CrossRef]
- Shabala, S.J. Learning from halophytes: Physiological basis and strategies to improve abiotic stress tolerance in crops. Ann. Bot. 2013, 112, 1209–1221. [Google Scholar] [CrossRef]
- Bartels, D.; Sunkar, R.J. Drought and salt tolerance in plants. Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Wang, H.; Takano, T.; Liu, S.J. Screening and evaluation of saline-alkaline tolerant germplasm of rice (Oryza sativa L.) in soda saline–alkali soil. Agronomy 2018, 8, 205. [Google Scholar] [CrossRef]
- Amirinejad, A.A.; Sayyari, M.; Ghanbari, F.; Kordi, S.J. Salicylic acid improves salinity-alkalinity tolerance in pepper (Capsicum annuum L.). Adv. Hortic. Sci. 2017, 31, 157–164. [Google Scholar]
- Li, J.; Long, Y.; Qi, G.N.; Li, J.; Xu, Z.J.; Wu, W.H.; Wang, Y. The Os-AKT1 channel is critical for K+ uptake in rice roots and is modulated by the rice CBL1-CIPK23 complex. Plant Cell 2014, 26, 3387–3402. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Hampton, C.R.; Shin, R.; Barkla, B.J.; White, P.J.; Schachtman, D.P. The high affinity K+ transporter AtHAK5 plays a physiological role in planta at very low K+ concentrations and provides a caesium uptake pathway in Arabidopsis. J. Exp. Bot. 2008, 59, 595–607. [Google Scholar] [CrossRef]
- Rubio, F.; Nieves-Cordones, M.; Alemán, F.; Martínez, V. Relative contribution of AtHAK5 and AtAKT1 to K+ uptake in the high-affinity range of concentrations. Physiol. Plant. 2008, 134, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Mäser, P.; Thomine, S.; Schroeder, J.I.; Ward, J.M.; Hirschi, K.; Sze, H.; Talke, I.N.; Amtmann, A.; Maathuis, F.J.; Sanders, D.J. Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 2001, 126, 1646–1667. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R.J. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.h.; Jin, H.; Marler, B.; Guo, H.J. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]


| Name | Gene ID | Length (aa) | Intron | Molecular Weight (Da) | Theoretical PI | Subcellular Localization | Location |
|---|---|---|---|---|---|---|---|
| CqHAK1 | AUR62021456 | 650 | 6 | 72,965.38 | 8.18 | Plasma membrane | Chr01: 18278493–18282659 |
| CqHAK2 | AUR62042411 | 774 | 7 | 87,036.04 | 6.74 | Plasma membrane | Chr01: 88160907–88166983 |
| CqHAK3 | AUR62040746 | 731 | 9 | 81,227.69 | 8.83 | Plasma membrane | Chr01: 90527306–90533106 |
| CqHAK4 | AUR62028032 | 742 | 8 | 83,030.02 | 7.32 | Plasma membrane | Chr01: 104924582–104937024 |
| CqHAK5 | AUR62010943 | 640 | 7 | 71,619.97 | 8.21 | Plasma membrane | Chr02: 54650085–54654440 |
| CqHAK6 | AUR62034910 | 744 | 7 | 83,398.35 | 6.78 | Plasma membrane | Chr03: 61032690–61040255 |
| CqHAK7 | AUR62012363 | 756 | 8 | 84,438.67 | 6.74 | Plasma membrane | Chr03: 77154455–77160490 |
| CqHAK8 | AUR62031491 | 910 | 12 | 10,2229.37 | 6.93 | Plasma membrane | Chr04: 71229–79662 |
| CqHAK9 | AUR62020693 | 768 | 7 | 86,037.89 | 7.88 | Plasma membrane | Chr04: 17190576–17199015 |
| CqHAK10 | AUR62035954 | 687 | 6 | 77,129.18 | 6.48 | Plasma membrane | Chr04: 21795245–21801387 |
| CqHAK11 | AUR62026895 | 720 | 8 | 80,136.43 | 8.96 | Plasma membrane | Chr04: 45774326–45780518 |
| CqHAK12 | AUR62014006 | 698 | 6 | 77,969.08 | 6.91 | Plasma membrane | Chr05: 31254125–31259655 |
| CqHAK13 | AUR62014007 | 835 | 8 | 92,889.21 | 6.22 | Plasma membrane | Chr05: 31289460–31294418 |
| CqHAK14 | AUR62017474 | 784 | 8 | 87,191.16 | 8.7 | Plasma membrane | Chr06: 44873117–44879731 |
| CqHAK15 | AUR62033186 | 770 | 7 | 86,317.46 | 6.4 | Plasma membrane | Chr07: 95200544–95206655 |
| CqHAK16 | AUR62001622 | 786 | 9 | 88,068.4 | 7.88 | Plasma membrane | Chr07: 102724390–102734985 |
| CqHAK17 | AUR62003489 | 681 | 6 | 75,594.81 | 8.66 | Plasma membrane | Chr09: 2936029–2942420 |
| CqHAK18 | AUR62003490 | 590 | 7 | 65,465.15 | 6.1 | Plasma membrane | Chr09: 2945466–2951051 |
| CqHAK19 | AUR62026619 | 809 | 8 | 90,741.81 | 7.88 | Plasma membrane | Chr10: 23716177–23724950 |
| CqHAK20 | AUR62010772 | 655 | 10 | 72,550.63 | 9.17 | Plasma membrane | Chr13: 7774288–7788227 |
| CqHAK21 | AUR62042858 | 784 | 8 | 87,071.95 | 8.56 | Plasma membrane | Chr14: 46675721–46682205 |
| CqHAK22 | AUR62005353 | 696 | 6 | 77,683.74 | 7.08 | Plasma membrane | Chr14: 50083331–50089025 |
| CqHAK23 | AUR62005354 | 826 | 9 | 91,509.16 | 6.43 | Plasma membrane | Chr14: 50115909–50122274 |
| CqHAK24 | AUR62017798 | 754 | 8 | 83,549.36 | 8.45 | Plasma membrane | Chr15: 19349048–19362387 |
| CqHAK25 | AUR62017799 | 708 | 7 | 78,514.06 | 6.52 | Plasma membrane | Chr15: 19364004–19370291 |
| CqHAK26 | AUR62025122 | 737 | 8 | 81,976.91 | 6.92 | Plasma membrane | Chr15: 52273038–52277536 |
| CqHAK27 | AUR62019774 | 706 | 8 | 79,121.86 | 9.15 | Plasma membrane | Chr16: 70971581–70976287 |
| CqHAK28 | AUR62036631 | 476 | 3 | 54,185.71 | 6.8 | Plasma membrane | Chr17: 53383724–53386568 |
| CqHAK29 | AUR62037170 | 741 | 8 | 82,669.67 | 6.92 | Plasma membrane | Chr18: 11688609–11693135 |
| CqHAK30 | AUR62020155 | 745 | 9 | 83,356.3 | 8.66 | Plasma membrane | Chr18: 31295458–31306880 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Lin, Y.; Zhang, S.; Lin, Z.; Chen, S.; Wang, Z. Genome-Wide Identification and Characterization of the HAK Gene Family in Quinoa (Chenopodium quinoa Willd.) and Their Expression Profiles under Saline and Alkaline Conditions. Plants 2023, 12, 3747. https://doi.org/10.3390/plants12213747
Chen Y, Lin Y, Zhang S, Lin Z, Chen S, Wang Z. Genome-Wide Identification and Characterization of the HAK Gene Family in Quinoa (Chenopodium quinoa Willd.) and Their Expression Profiles under Saline and Alkaline Conditions. Plants. 2023; 12(21):3747. https://doi.org/10.3390/plants12213747
Chicago/Turabian StyleChen, Yanqiong, Yingfeng Lin, Shubiao Zhang, Zhongyuan Lin, Songbiao Chen, and Zonghua Wang. 2023. "Genome-Wide Identification and Characterization of the HAK Gene Family in Quinoa (Chenopodium quinoa Willd.) and Their Expression Profiles under Saline and Alkaline Conditions" Plants 12, no. 21: 3747. https://doi.org/10.3390/plants12213747
APA StyleChen, Y., Lin, Y., Zhang, S., Lin, Z., Chen, S., & Wang, Z. (2023). Genome-Wide Identification and Characterization of the HAK Gene Family in Quinoa (Chenopodium quinoa Willd.) and Their Expression Profiles under Saline and Alkaline Conditions. Plants, 12(21), 3747. https://doi.org/10.3390/plants12213747







