Diversity of Leaf Glands and Their Putative Functions in Rhamnaceae Species
Abstract
1. Introduction
2. Results
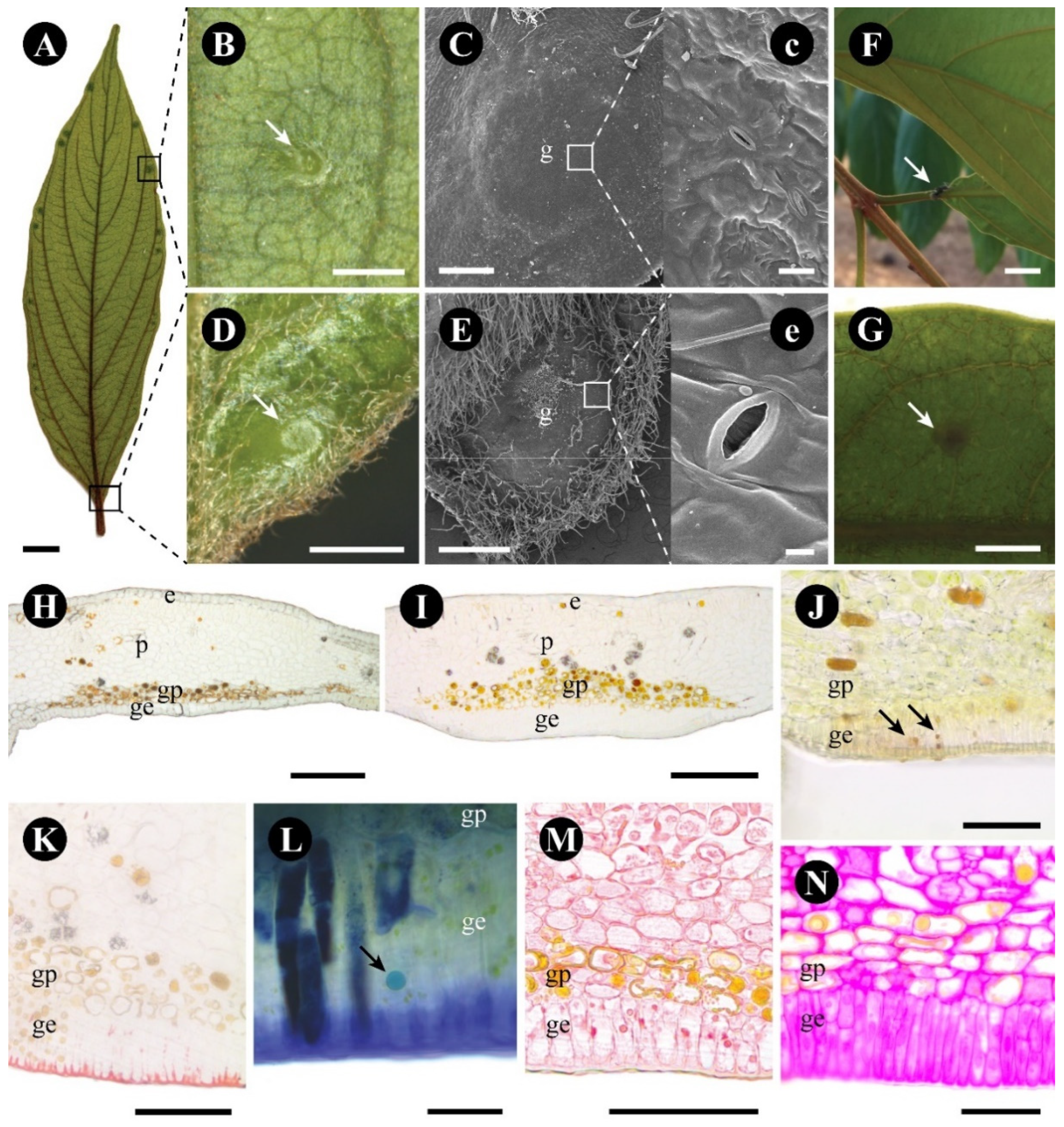
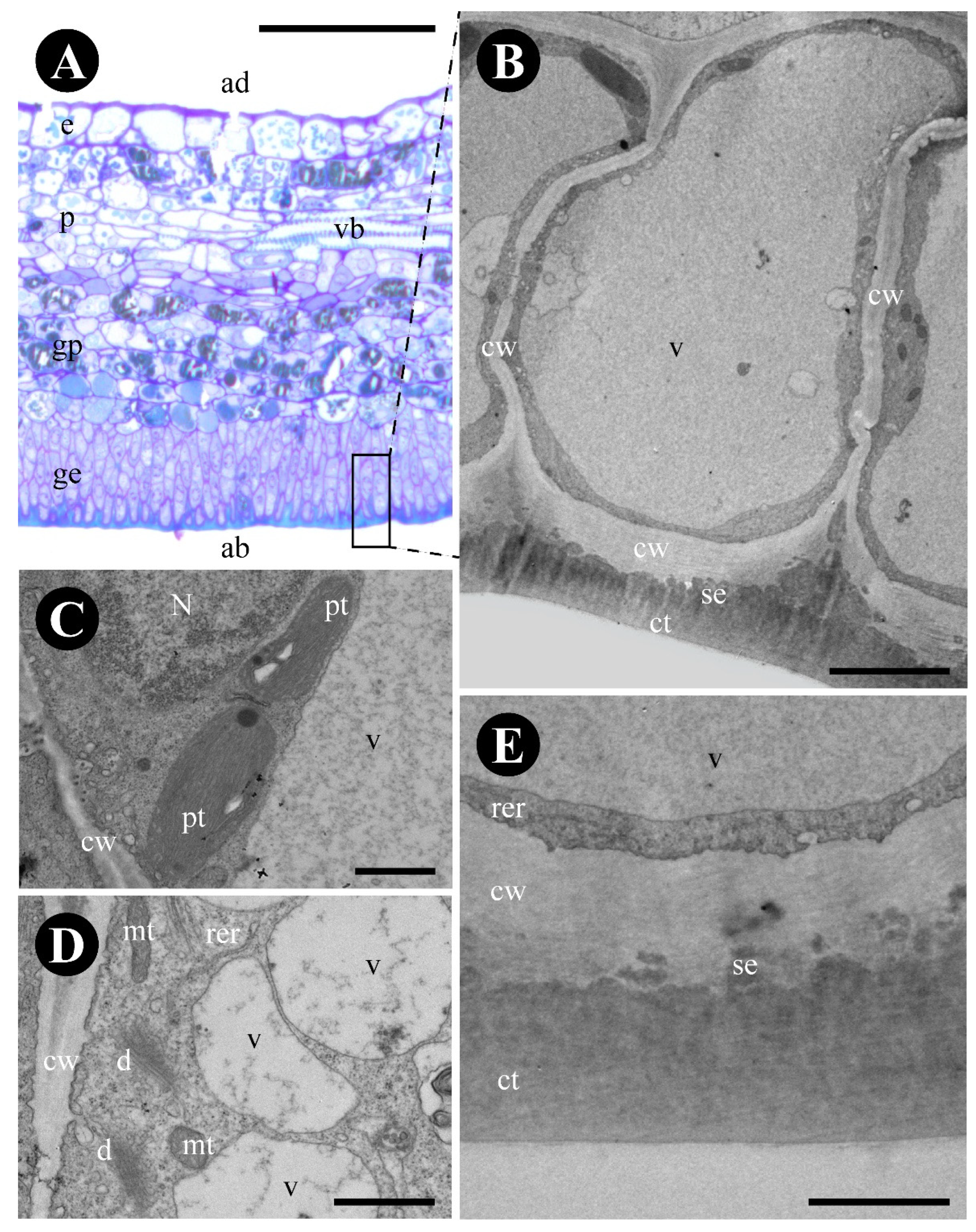
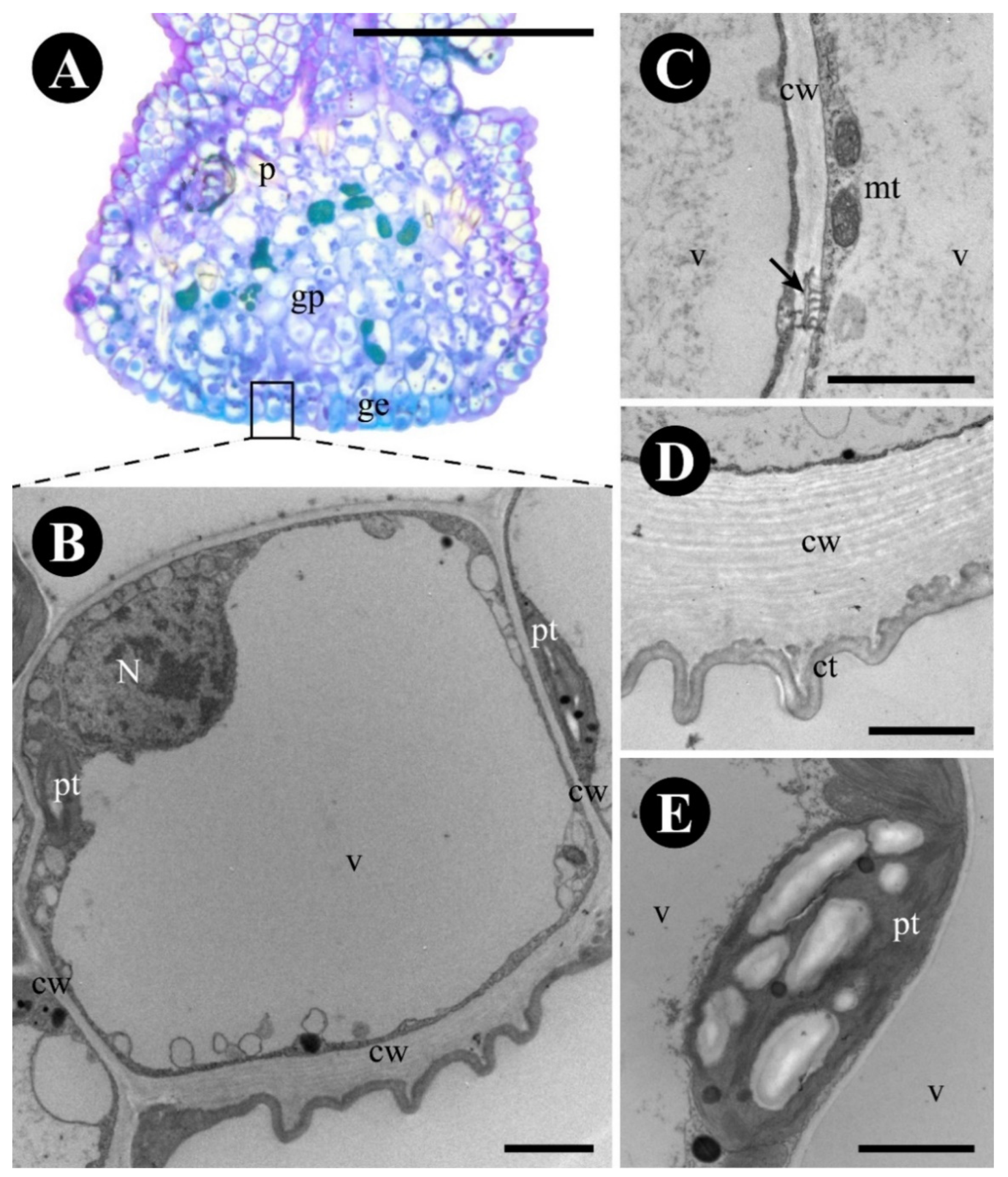
2.1. Colubrina glandulosa
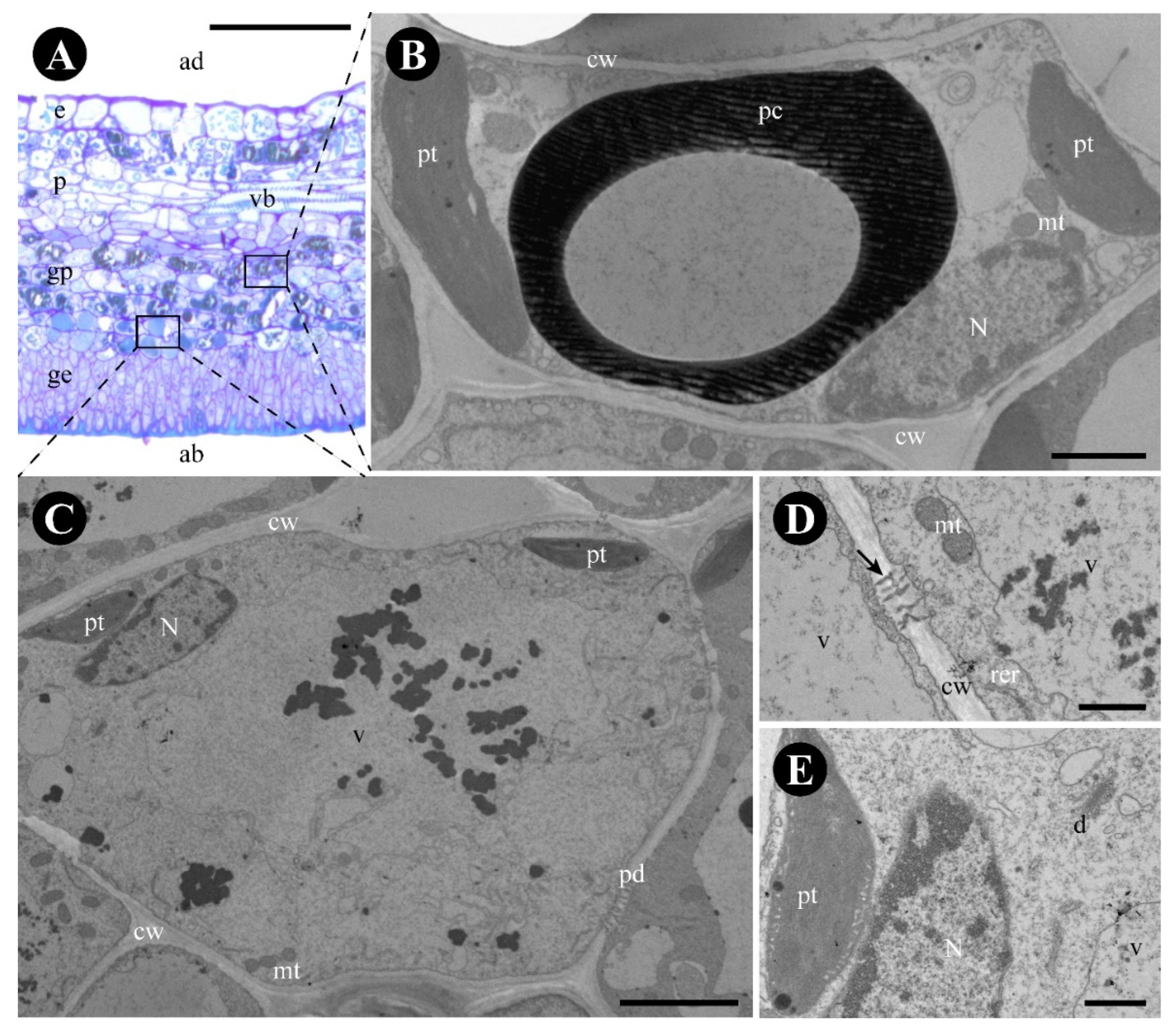
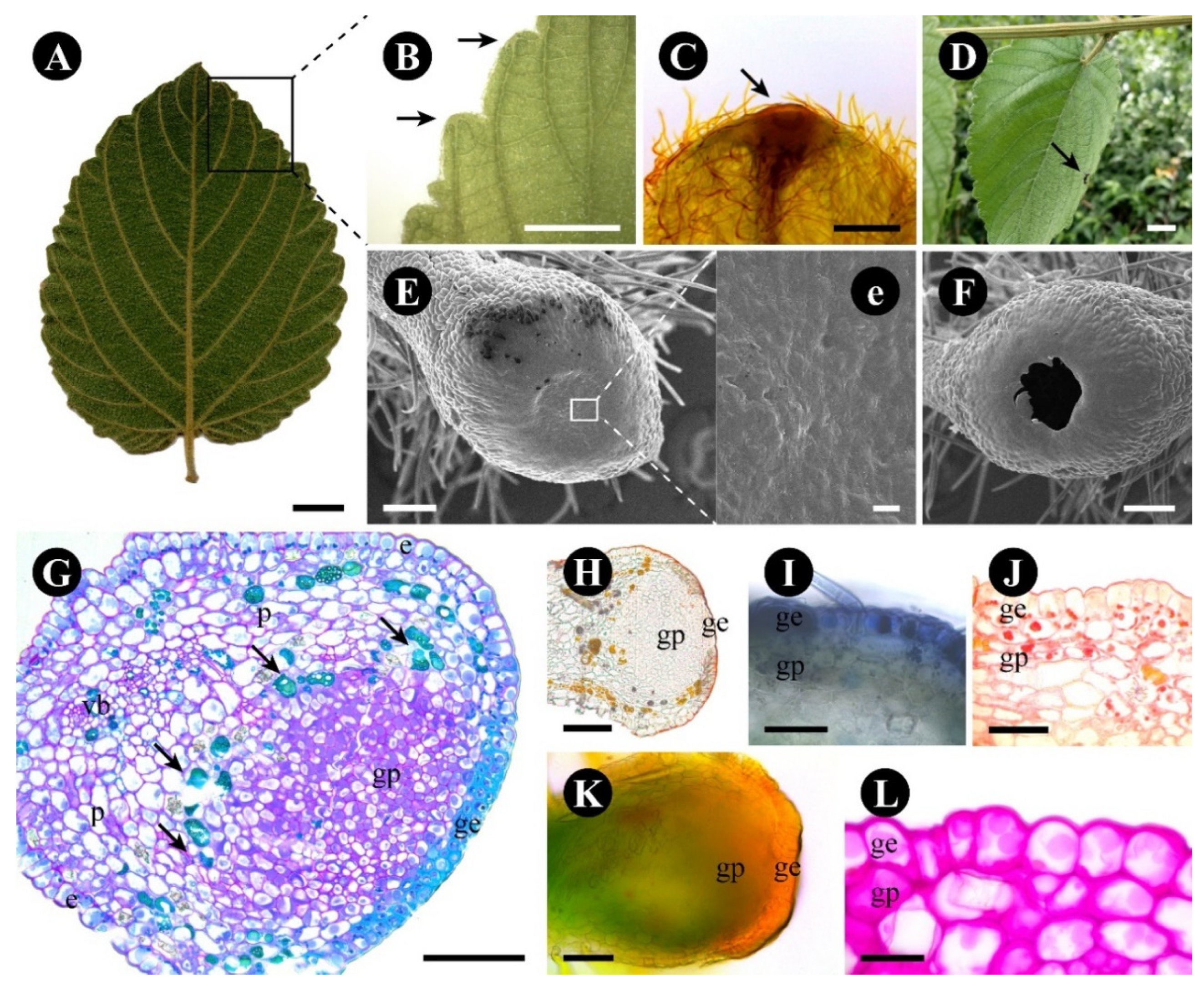
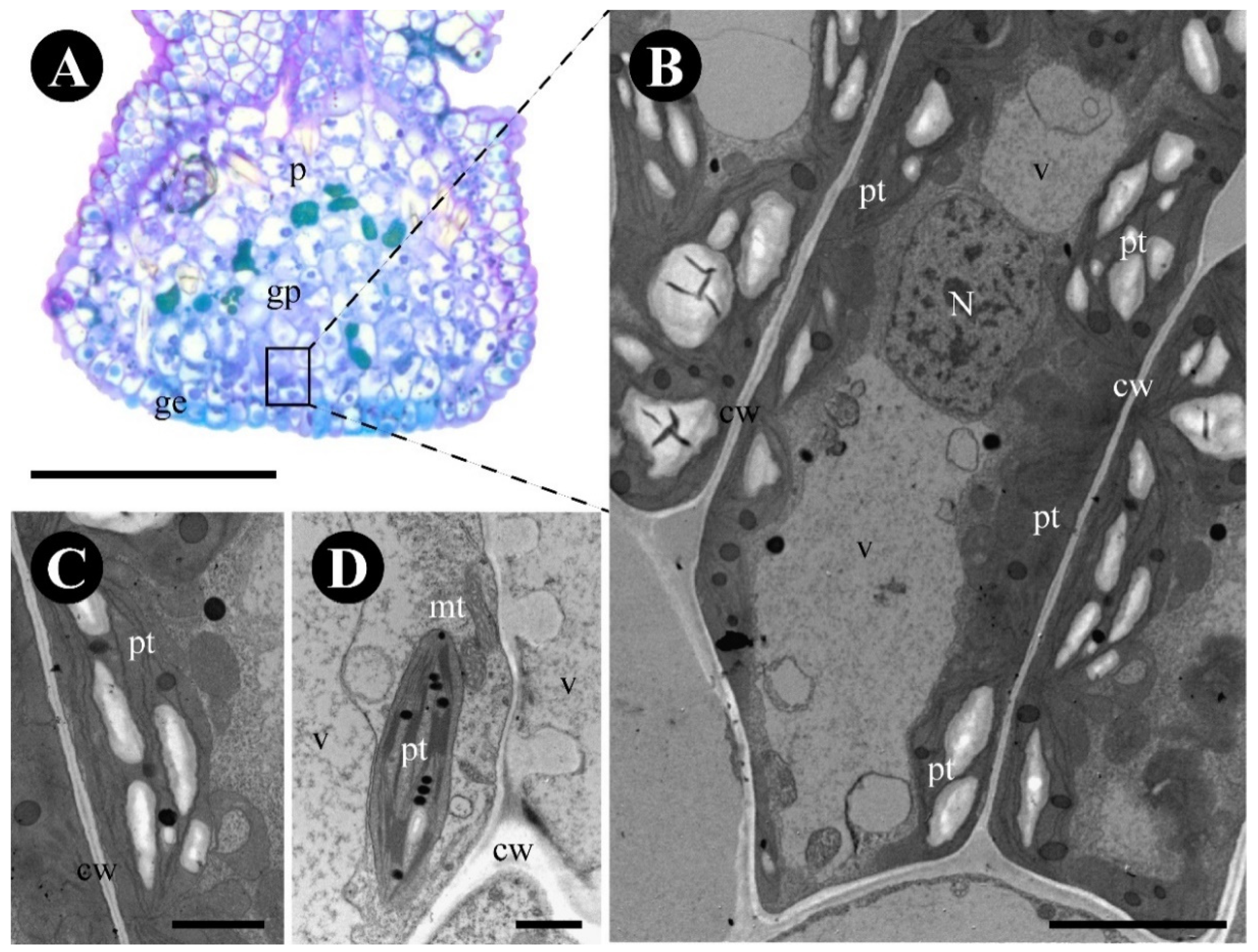
2.2. Gouania polygama
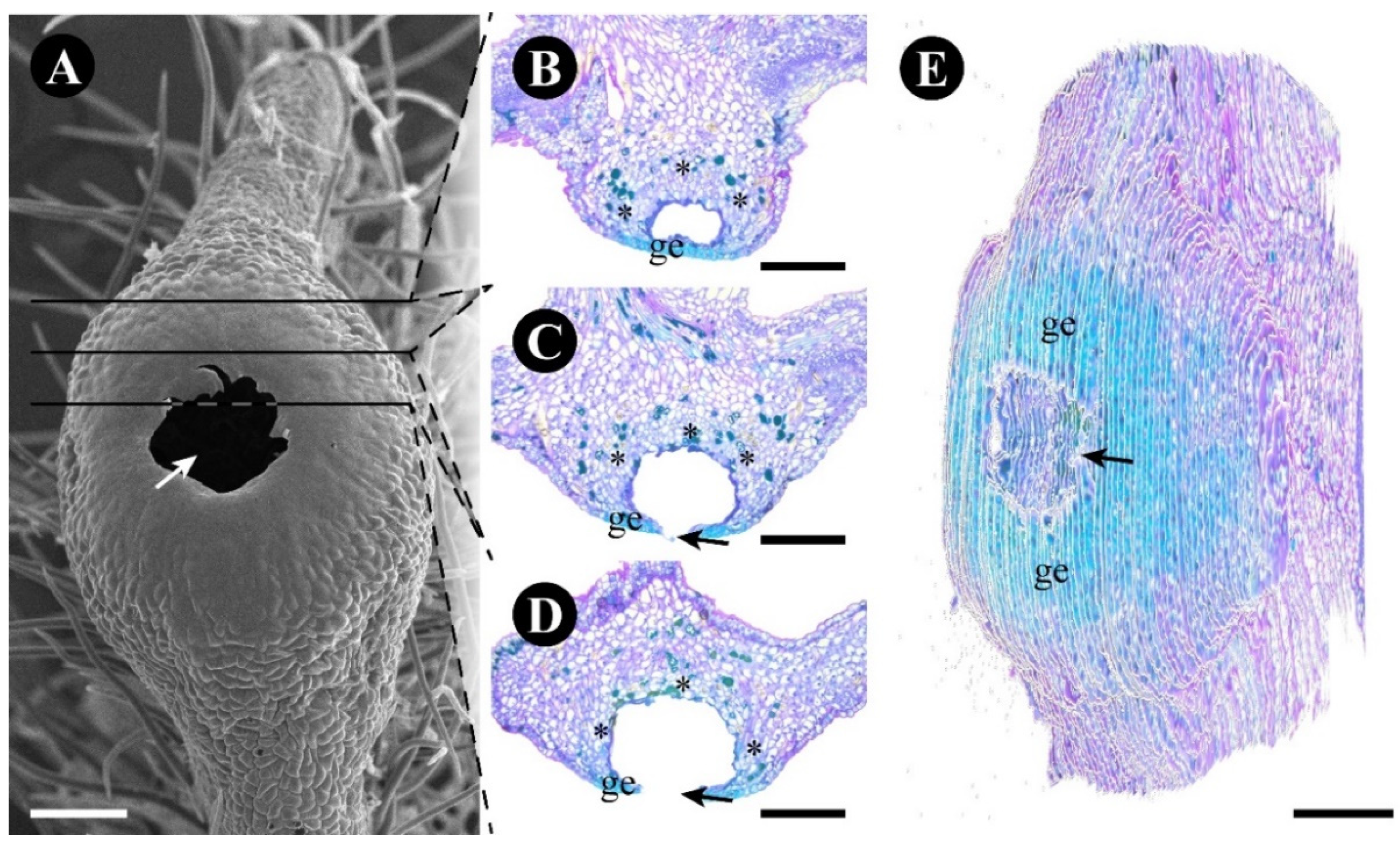
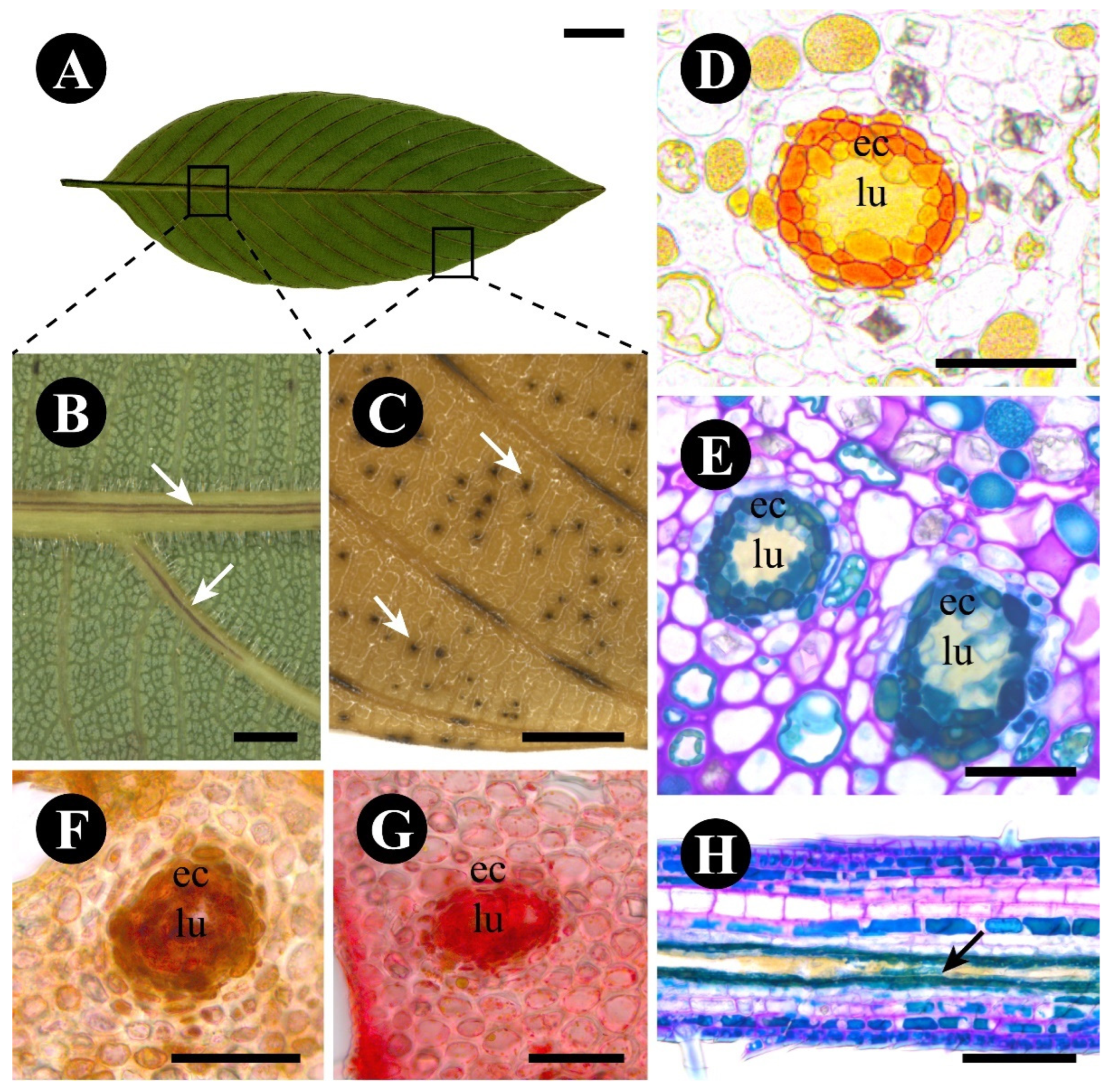
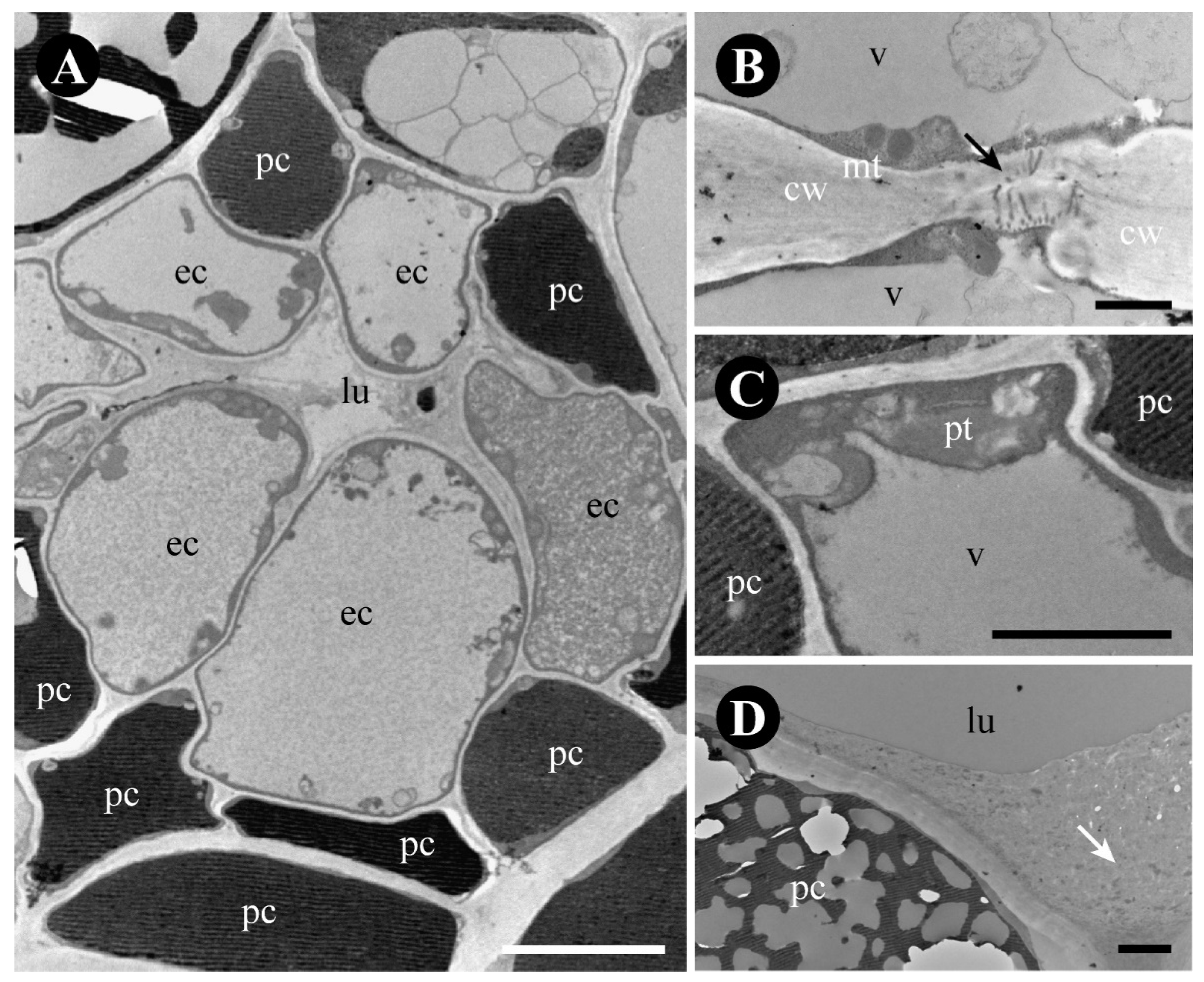
2.3. Rhamnidium elaeocarpum
3. Discussion
3.1. The Foliar Glands of Colubrina glandulosa and Gouania polygama Are Extrafloral Nectaries
3.2. The Foliar Glands of Gouania polygama and Those of Rosaceae Species
3.3. The Foliar Glands of Rhamnidium elaeocarpum Are Secretory Ducts and Cavities
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fahn, A. Secretory Tissues in Plants; Academic Press: London, UK, 1979. [Google Scholar]
- Ogura, Y. Comparative Anatomy of Vegetative Organs of the Pteridophytes, 2nd ed.; Encyclopedia of Plant Anatomy; Gebrüder Borntraeger Verlagsbuchhandlung: Berlin, Germany, 1972. [Google Scholar]
- Gerson, U. The association between pteridophytes and arthropods. Fern Gaz. 1979, 12 Pt 1, 29–45. [Google Scholar]
- Yatskievych, G.; Windham, M.D. Polypodiaceae Polypody Family. Canotia 2009, 5, 34–38. [Google Scholar]
- Fahn, A. Secretory tissues in vascular plants. New Phytol. 1988, 108, 229–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Fujii, T.; Abe, H.; Fujiwara, T.; Fujita, M.; Takabe, K. Anatomical Features of Radial Resin Canals in Pinus Densiflora. IAWA J. 2008, 29, 179–187. [Google Scholar] [CrossRef]
- Tölke, E.; Lacchia, A.; Lima, E.; Demarco, D.; Ascensão, L.; Carmello-Guerreiro, S. Secretory ducts in Anacardiaceae revisited: Updated concepts and new findings based on histochemical evidence. S. Afr. J. Bot. 2021, 138, 394–405. [Google Scholar] [CrossRef]
- Nicolson, S.W.; Nepi, M.; Pacini, E. Nectaries and Nectar; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Caspary, R. De Nectariis. Commentationem Batanicam; Adolphum Marcum: Bonn, Germany, 1948; 56p. [Google Scholar]
- Del-Claro, K.; Rico-Gray, V.; Torezan-Silingardi, H.M.; Alves-Silva, E.; Fagundes, R.; Lange, D.; Dáttilo, W.; Vilela, A.A.; Aguirre, A.; Rodriguez-Morales, D. Loss and gains in ant–plant interactions mediated by extrafloral nectar: Fidelity, cheats, and lies. Insectes Sociaux 2016, 63, 207–221. [Google Scholar] [CrossRef]
- Schmid, R. Reproductive versus extra-reproductive nectaries—Historical perspective and terminological recommendations. Bot. Rev. 1988, 54, 179–227. [Google Scholar] [CrossRef]
- Zimmermann, J. Über Die Extrafloralen Nektarien Der Angiospermen. Bot. Tidsskr. 1932, 49, 99–196. [Google Scholar]
- Buono, R.A.; de Oliveira, A.B.; Paiva, E.A.S. Anatomy, Ultrastructure and Chemical Composition of Food Bodies of Hovenia dulcis (Rhamnaceae). Ann. Bot. 2008, 101, 1341–1348. [Google Scholar] [CrossRef]
- Rickson, F.R. Developmental anatomy and ultrastructure of the ant-food bodies (beccariian bodies) of Macaranga triloba and M. hypoleuca (euphorbiaceae). Am. J. Bot. 1980, 67, 285–292. [Google Scholar] [CrossRef]
- O’Dowd, D.J. Pearl Bodies as Ant Food: An Ecological Role for Some Leaf Emergences of Tropical Plants. Biotropica 1982, 14, 40. [Google Scholar] [CrossRef]
- Vázquez-González, C.; Zas, R.; Erbilgin, N.; Ferrenberg, S.; Rozas, V.; Sampedro, L. Resin ducts as resistance traits in conifers: Linking dendrochronology and resin-based defences. Tree Physiol. 2020, 40, 1313–1326. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Erbilgin, N. Larger Resin Ducts Are Linked to the Survival of Lodgepole Pine Trees During Mountain Pine Beetle Outbreak. Front. Plant Sci. 2019, 10, 1459. [Google Scholar] [CrossRef] [PubMed]
- Simpson, M.G. Plant Systematics, 3rd ed.; Academic Press: Cambridge, MA, USA, 2019; ISBN 9780128126288. [Google Scholar]
- WFO. Rhamnaceae Juss. Available online: http://www.worldfloraonline.org/taxon/wfo-7000000524 (accessed on 25 February 2023).
- Chaia, E.E.; Huss-Danell, K.; Wall, L.G.; Myrold, D.D. Nitrogen fixation by riparian plants belonging to Coriariaceae, Rhamnaceae, and Gunneraceae in Northwest Patagonia. Symbiosis 2019, 77, 237–247. [Google Scholar] [CrossRef]
- Souza, V.C.; Lorenzi, H. Botânica Sistemática: Guia Ilustrado Para Identificação Das Famílias de Fanerógamas Nativas e Exóticas No Brasil, Baseado Em APG III, 3rd ed.; Instituto Plantarum: Nova Odessa, Brazil, 2012. [Google Scholar]
- Sytsma, K.J.; Robertson, K.R. Rosales. Available online: https://www.britannica.com/plant/Rosales (accessed on 3 October 2022).
- Maas, P.A. Indian Medicine And Ayurveda. In The Cambridge History of Science; Ancient Science; University Press: Cambridge, UK, 2018; Volume 1, ISBN 9781108682626. [Google Scholar]
- Kartina, D.; Wahab, A.W.; Ahmad, A.; Irfandi, R.; Raya, I. In vitro antibacterial and anticancer activity of Zn(II)Valinedithiocarbamate complexes. J. Phys. Conf. Ser. 2019, 1341, 032042. [Google Scholar] [CrossRef]
- Gemoll, K. Anatomisch-Systematische Untersuchung Des Blattes Der Rhamneen Aus Den Triben: Rhamneen, Colletieen und Gouanieen. Bot. Tidsskr. 1902, 12, 351–424. [Google Scholar]
- Solereder, H. Systematic Anatomy of the Dicotyledons: A Handbook for Laboratories of Pure and Applied Botany; Clarendon Press: Oxford, UK, 1908; Volume 1–2. [Google Scholar]
- Sivasankari, M.P.; Sankaravadivoo, A. Leaf Anatomical Studies of Ziziphus mauritiana Lam. Int. J. Curr. Res. Biosci. Plant Biol. 2017, 4, 67–72. [Google Scholar] [CrossRef]
- Arsenijević, J.; Drobac, M.; Slavkovska, V.; Kovačević, N.; Lakušić, B. Anatomical Analysis and Phytochemical Screening of Frangula rupestris (Scop.) Schur (Rhamnaceae). Bot. Serbica 2018, 42, 231–239. [Google Scholar]
- Ribeiro, C.; Marinho, C.; Teixeira, S. Uncovering the Neglected Floral Secretory Structures of Rhamnaceae and Their Functional and Systematic Significance. Plants 2021, 10, 736. [Google Scholar] [CrossRef]
- Ribeiro, C.C.; Marinho, C.R.; Mansano, V.F.; Teixeira, S.P. The structural diversity of floral nectaries does not mean ontogenic diversity in Rhamnaceae species. Flora Morphol. Distrib. Funct. Ecol. Plants 2022, 290, 152048. [Google Scholar] [CrossRef]
- Wollenweber, E.; Dörr, M.; Bohm, B.A.; Roitman, J.N. Exudate Flavonoids of Eight Species of Ceanothus (Rhamnaceae). Z. Fur Naturforschung J. Biosci. 2004, 59, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.C. Revision of Colubrina (Rhamnaceae). Brittonia 1971, 23, 2–53. [Google Scholar] [CrossRef]
- Lima, R.B.; Giulietti, A.M. Rhamnaceae. In Flora Fanerogâmica do Estado de São Paulo; Instituto de Botânica: São Paulo, Brazil, 2005; pp. 331–342. [Google Scholar]
- Palacios, W. A new species of Colubrina (Rhamnaceae) of the Amazon region of Ecuador. Phytotaxa 2015, 224, 296. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Wäckers, F. Recruitment of Predators and Parasitoids by Herbivore-Injured Plants. In Advances in Insect Chemical Ecology; Cambridge University Press: Cambridge, UK, 2009; pp. 21–75. [Google Scholar]
- Scrivanti, L.R.; Bernardello, G.; Anton, A.M. The foveola of Bothriochloa alta (Poaceae: Andropogoneae): Extrafloral nectary or secretory gland of essential oils? Flora 2008, 203, 55–59. [Google Scholar] [CrossRef]
- Bentley, B.L. Plants Bearing Extrafloral Nectaries and the Associated Ant Community: Interhabitat Differences in the Reduction of Herbivore Damage. Ecology 1976, 57, 815–820. [Google Scholar] [CrossRef]
- Tölke, E.D.; Capelli, N.d.V.; Pastori, T.; Alencar, A.C.; Cole, T.C.H.; Demarco, D. Diversity of Floral Glands and Their Secretions in Pollinator Attraction. In Co-Evolution of Secondary Metabolites; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2020; pp. 709–754. [Google Scholar] [CrossRef]
- Singsaas, E.L. Terpenes and the thermotolerance of photosynthesis. New Phytol. 2000, 146, 1–4. [Google Scholar] [CrossRef]
- Gotelli, M.M.; Galati, B.G.; Medan, D. Morphological and ultrastructural studies of floral nectaries in Rhamnaceae. J. Torrey Bot. Soc. 2017, 144, 63–73. [Google Scholar] [CrossRef]
- Lersten, N.R.; Curtis, J.D. Foliar anatomy of Polygonum (Polygonaceae): Survey of epidermal and selected internal structures. Plant Syst. Evol. 1992, 182, 71–106. [Google Scholar] [CrossRef]
- Sawidis, T. The subglandular tissue of Hibiscus rosa-sinensis nectaries. Flora 1998, 193, 327–335. [Google Scholar] [CrossRef]
- Stpiczynska, M.; Davies, K.L.; Gregg, A. Nectary Structure and Nectar Secretion in Maxillaria coccinea (Jacq.) L.O. Williams ex Hodge (Orchidaceae). Ann. Bot. 2004, 93, 87–95. [Google Scholar] [CrossRef]
- Cortez, P.A.; Caetano, A.P.d.S.; Carmello-Guerreiro, S.M.; Teixeira, S.P. Elucidating the mechanism of poricidal anther dehiscence in Miconia species (Melastomataceae). Flora 2014, 209, 571–579. [Google Scholar] [CrossRef]
- Chin, S.; Lutz, S.; Wen, J.; Potter, D. The Bitter and the Sweet: Inference of Homology and Evolution of Leaf Glands in Prunus (Rosaceae) through Anatomy, Micromorphology, and Ancestral–Character State Reconstruction. Int. J. Plant Sci. 2013, 174, 27–46. [Google Scholar] [CrossRef]
- Kumachova, T.K.; Babosha, A.V.; Ryabchenko, A.S.; Voronkov, A.S. Colleters in Leaves of Mespilus Germanica L. (Rosaceae): Micromorphology, Histochemistry and Fluorescence. Micron 2023, 175, 103537. [Google Scholar] [CrossRef] [PubMed]
- Dalvi, V.C.; de Sousa Silva, M.; Rios, A.B.M.; Coutinho, Í.A.C. Leaf Secretory Structures in Rosa lucieae (Rosaceae): Two Times of Secretion—Two Ecological Functions? Protoplasma 2023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-D.; Soltis, D.E.; Yang, Y.; Li, D.-Z.; Yi, T.-S. Multi-gene analysis provides a well-supported phylogeny of Rosales. Mol. Phylogenetics Evol. 2011, 60, 21–28. [Google Scholar] [CrossRef]
- Lersten, N.R. Crystals of calcium compounds in gramineae. New Phytol. 1983, 93, 633–637. [Google Scholar] [CrossRef]
- Guignard, M.L. Colin Sur La Présence De Réservoirs A Gomme Chez Les Rhamnées. B Soc. Bot. Fr. 1888, 35, 325–327. [Google Scholar] [CrossRef]
- Bouchet, P. Étude ultrastructurale des cellules constituant les poches “lysigènes” à mucilage de la bourdaine: Rhamnus, Frangula L. C R Hebd. Seances Acad. Sci. 1974, 279, 1073–1076. [Google Scholar]
- Serdar, B.; Çoşkunçelebi, K.; Terzioğlu, S.; Hampe, A. Anatomical notes on Turkish Frangula alnus Mill. (Rhamnaceae). Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2007, 141, 69–74. [Google Scholar] [CrossRef]
- da Silva, L.A.; Sales, J.D.F.; Guimarães, R.M.; Oliveira, J.A.; Filho, S.C.V. Morphological aspects of fruits, seeds and seedlings of Rhamnidium elaeocarpum Reissek. Semina Cienc. Agrar. 2015, 36, 1179–1190. [Google Scholar] [CrossRef][Green Version]
- Herzog, T.H. Anatomisch-Systematische Untersuchung Des Blattes Der Rhamneen Aus Den Triben: Ventilagineen, Zizypheen Und Rhamneen. Bot. Tidsskr. 1903, 15, 95–207. [Google Scholar]
- Lima, R.B.; Barbosa, M.R.V.; Giulietti, A.M. Rhamnaceae in Flora e Funga Do Brasil. Available online: https://floradobrasil.jbrj.gov.br/FB207 (accessed on 3 April 2023).
- Kraus, J.E.; Arduin, M. Manual Básico de Métodos Em Morfologia Vegetal; EDUR: Seropédica, Brazil, 1997. [Google Scholar]
- O’Brien, T.P.; Feder, N.; McCully, M.E. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 1964, 59, 368–373. [Google Scholar] [CrossRef]
- Purvis, M.J.; Collier, D.C.; Walls, D. Laboratory Techniques in Botany; Butterworths: London, UK, 1964. [Google Scholar]
- O’Brien, T.P.; McCully, M.E. The Study of Plant Structure Principles and Selected Methods; Termarcarphi Pty. Ltd.: Melbourne, Australia, 1981. [Google Scholar]
- Pearse, A.G.E. Histochemistry: Theoretical and Applied, 3rd ed.; JA Churchill, Ltd.: London, UK, 1968; Volume 2. [Google Scholar]
- Johansen, D.A. Plant Microtechnique, 1st ed.; McGraw-Hill Publishing Company, Ltd.: London, UK, 1940. [Google Scholar]
- Ventrella, M.C.; Almeida, A.L.; Nery, L.A.; Coelho, V.P.M. Métodos Histoquímicos Aplicados Às Sementes [Recurso Eletrônico]; UFV: Viçosa, Brazil, 2013. [Google Scholar]
- Mace, M.E.; Howell, C.R. Histochemistry and identification of condensed tannin precursors in roots of cotton seedlings. Can. J. Bot. 1974, 52, 2423–2426. [Google Scholar] [CrossRef]
- Vidal, B.D.C. Dichroism in collagen bundles stained with Xylidine-Ponceau 2R. Ann. Histochem. 1970, 15, 289–296. [Google Scholar]
- Livingston, D.P.; Tuong, T.D. Three-dimensional reconstruction of frozen and thawed plant tissues from microscopic images. In Plant Cold Acclimation; Hincha, D., Zuther, E., Eds.; Humana Press: New York, NY, USA, 2014; pp. 117–137. [Google Scholar]
| Stain | Target Compound | Colubrina glandulosa | Gouania polygama | Rhamnidium elaeocarpum | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glandular Epid | Glandular Parench | Glandular Epid | Glandular Parench | Epithelial Cell | Lumen | |||||||
| cyt | cw | cyt | cw | cyt | cw | cyt | cw | cyt | cw | |||
| Toluidine blue | Phenolic compounds | - | - | + | - | - | - | + | - | - | - | + |
| Fehling reagent | Reducing sugar | + | - | + | - | + | - | + | - | - | - | - |
| Lugol reagent | Starch grains | - | - | - | - | - | - | - | - | - | - | - |
| NADI reagent | Terpenes | + | - | - | - | + | - | + | - | - | - | - |
| PAS reagent | Neutral polysaccharides | + | + | + | + | + | + | + | + | + | + | - |
| Sudan IV | Lipids | - | + | - | - | - | - | - | - | + | - | + |
| Xilidine Ponceau | Protein bodies | + | - | - | - | + | - | - | - | + | - | + |
| Vanillin-hydrochloric acid | Tannin | - | - | - | - | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwamoto, L.; Vicentini, T.A.; Ramos, F.P.; Ribeiro, C.C.; Teixeira, S.P. Diversity of Leaf Glands and Their Putative Functions in Rhamnaceae Species. Plants 2023, 12, 3732. https://doi.org/10.3390/plants12213732
Iwamoto L, Vicentini TA, Ramos FP, Ribeiro CC, Teixeira SP. Diversity of Leaf Glands and Their Putative Functions in Rhamnaceae Species. Plants. 2023; 12(21):3732. https://doi.org/10.3390/plants12213732
Chicago/Turabian StyleIwamoto, Lucas, Thales Augusto Vicentini, Felipe Paulino Ramos, Carimi Cortez Ribeiro, and Simone Pádua Teixeira. 2023. "Diversity of Leaf Glands and Their Putative Functions in Rhamnaceae Species" Plants 12, no. 21: 3732. https://doi.org/10.3390/plants12213732
APA StyleIwamoto, L., Vicentini, T. A., Ramos, F. P., Ribeiro, C. C., & Teixeira, S. P. (2023). Diversity of Leaf Glands and Their Putative Functions in Rhamnaceae Species. Plants, 12(21), 3732. https://doi.org/10.3390/plants12213732








