Mechanisms of Copper Toxicity and Tolerance in the Aquatic Moss Taxiphyllum barbieri
Abstract
1. Introduction
2. Results
2.1. Cu and K Content
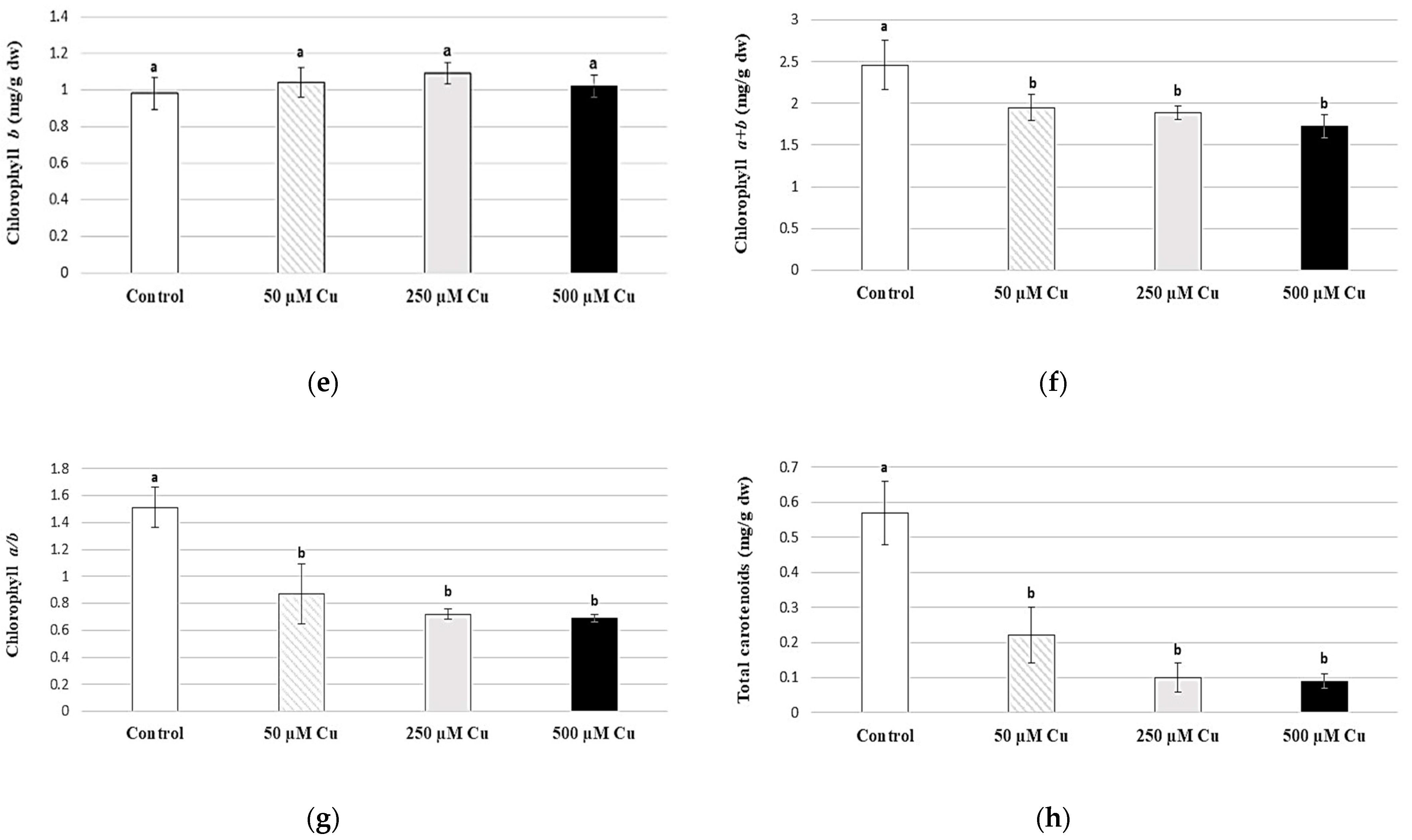
2.2. Composition of Assimilation Pigments and Chlorophyll a Fluorescence
2.3. Soluble Proteins and TBARS
2.4. Content of Antioxidants
2.5. Content of Selected Amino Acids and Related Compounds, Determination of Phenolic Acids
3. Discussion
3.1. Cu and K Content
3.2. Composition of Assimilation Pigments and Chlorophyll a Fluorescence
3.3. Content of Soluble Proteins and TBARS
3.4. Role of Antioxidants in Cu Tolerance/Toxicity
3.5. Role of Amino Acids and Related Compounds, Determination of Phenolic Acids in Cu Tolerance/Toxicity
4. Materials and Methods
4.1. Sample Cultivation, Collection and Cu Treatment
4.2. Determination of Cu and K Content
4.3. Determination of Assimilation Pigments
4.4. Determination of Soluble Proteins and TBARS
4.5. Determination of Glutathione (GSH, GSSG), Ascorbic Acid (AsA), Selected Amino Acids and Related Compounds as Well as Determination of Phenolic Acids
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rice, J. Environmental health indicators. Ocean Coast. Manag. 2003, 46, 235–259. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology: Volume 3: Environmental Toxicology; Springer: Geneva, Switzerland, 2012; pp. 133–164. [Google Scholar]
- Sahu, V.; Asthana, A.; Nath, V.; Yunus, M. Bryophytes: A useful tool in heavy metal monitoring. EnviroNews 2007, 13, 1–2. [Google Scholar]
- Pearson, J.; Wells, D.; Seller, K.; Bennett, A.; Soares, A.; Woodall, J.; Ingrouille, M. Traffic exposure increases natural 15N and heavy metal concentrations in mosses. New Phytol. 2000, 147, 317–326. [Google Scholar] [CrossRef]
- Bruns, I.; Siebert, A.; Baumbach, R.; Miersch, J.; Günther, D.; Markert, B.; Krauss, G.-J. Analysis of heavy metals and sulphur-rich compounds in the water moss Fontinalis antipyretica L. ex Hedw. Fresenius’ J. Anal. Chem. 1995, 353, 101–104. [Google Scholar] [CrossRef]
- Glime, J.M. Economic and ethnic uses of bryophytes. Flora N. Am. 2007, 27, 14–41. [Google Scholar]
- Siebert, A.; Bruns, I.; Krauss, G.-J.; Miersch, J.; Markert, B. The use of the aquatic moss Fontinalis antipyretica L. ex Hedw. as a bioindicator for heavy metals: 1. Fundamental investigations into heavy metal accumulation in Fontinalis antipyretica L. ex Hedw. Sci. Total Environ. 1996, 177, 137–144. [Google Scholar] [CrossRef]
- Brinkman, A.H. Hepatics and sites: A short study in the ecology of hepatics. Bryologist 1929, 32, 29–30. [Google Scholar] [CrossRef]
- Whitton, B.; Say, P.J.; Wehr, J.D. Use of Plants to Monitor Heavy Metals in Rivers; Durham University: Durham, UK, 1981. [Google Scholar]
- Mouvet, C. Accumulation of chromium and copper by the aquatic moss Fontinalis antipyretica L. ex hedw transplanted in a metal-contaminated river. Environ. Technol. 1984, 5, 541–548. [Google Scholar]
- Kelly, M.; Girton, C.É.; Whitton, B. Use of moss-bags for monitoring heavy metals in rivers. Water Res. 1987, 21, 1429–1435. [Google Scholar] [CrossRef]
- Gonçalves, E.P.; Soares, H.M.; Boaventura, R.A.; Machado, A.A.; da Silva, J.C.E. Seasonal variations of heavy metals in sediments and aquatic mosses from the Cávado river basin (Portugal). Sci. Total Environ. 1994, 142, 143–156. [Google Scholar] [CrossRef]
- Calevro, F.; Campani, S.; Filippi, C.; Batistoni, R.; Deri, P.; Bucci, S.; Ragghianti, M.; Mancino, G. Bioassays for testing effects of Al, Cr and Cd using development in the amphibian Pleurodeles waltl and regeneration in the planarian Dugesia etrusca. Aquat. Ecosyst. Health Manag. 1999, 2, 281–288. [Google Scholar] [CrossRef]
- Amor, L.; Kennes, C.; Veiga, M.C. Kinetics of inhibition in the biodegradation of monoaromatic hydrocarbons in presence of heavy metals. Bioresour. Technol. 2001, 78, 181–185. [Google Scholar] [CrossRef]
- Kawanishi, S.; Inoue, S.; Oikawa, S.; Yamashita, N.; Toyokuni, S.; Kawanishi, M.; Nishino, K. Oxidative DNA damage in cultured cells and rat lungs by carcinogenic nickel compounds. Free Radic. Biol. Med. 2001, 31, 108–116. [Google Scholar] [CrossRef]
- Monni, S.; Uhlig, C.; Hansen, E.; Magel, E. Ecophysiological responses of Empetrum nigrum to heavy metal pollution. Environ. Pollut. 2001, 112, 121–129. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Pokrovsky, O. Metal adsorption on mosses: Toward a universal adsorption model. J. Colloid Interface Sci. 2014, 415, 169–178. [Google Scholar] [CrossRef]
- Bargabli, R.; Battisti, E.; Cardaioli, E.; Formichi, P.; Nelli, L. La deposizione atmosferica di elementi in tracce in Italia. Prime rilevazioni mediante i muschi. Inquinamento 1994, 36, 48–58. [Google Scholar]
- Zechmeister, H. Growth rates of five pleurocarpous moss species under various climatic conditions. J. Bryol. 1995, 18, 455–468. [Google Scholar] [CrossRef]
- Samecka-Cymerman, A.; Kosior, G.; Kempers, A. Comparison of the moss Pleurozium schreberi with needles and bark of Pinus sylvestris as biomonitors of pollution by industry in Stalowa Wola (southeast Poland). Ecotoxicol. Environ. Saf. 2006, 65, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Figueira, R.; Ribeiro, T. Transplants of aquatic mosses as biomonitors of metals released by a mine effluent. Environ. Pollut. 2005, 136, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, B.; Dhal, N.K.; Dash, A.K.; Panda, B.P.; Panigrahi, K.C.S.; Pradhan, A. Perspective of mitigating atmospheric heavy metal pollution: Using mosses as biomonitoring and indicator organism. Environ. Sci. Pollut. Res. 2019, 26, 29620–29638. [Google Scholar] [CrossRef]
- Lang, I.; Wernitznig, S. Sequestration at the cell wall and plasma membrane facilitates zinc tolerance in the moss Pohlia drummondii. Environ. Exp. Bot. 2011, 74, 186–193. [Google Scholar] [CrossRef]
- Tipping, E.; Vincent, C.; Lawlor, A.; Lofts, S. Metal accumulation by stream bryophytes, related to chemical speciation. Environ. Pollut. 2008, 156, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.H. Mineral nutrition. In Bryophyte Ecology; Springer: Berlin/Heidelberg, Germany, 1982; pp. 383–444. [Google Scholar]
- Harmens, H.; Norris, D.A.; Koerber, G.R.; Buse, A.; Steinnes, E.; Rühling, Å. Temporal trends in the concentration of arsenic, chromium, copper, iron, nickel, vanadium and zinc in mosses across Europe between 1990 and 2000. Atmos. Environ. 2007, 41, 6673–6687. [Google Scholar] [CrossRef]
- AH-Peng, C.; Rausch de Traubenberg, C. Bryophytes aquatiques bioaccumulateurs de polluants et indicateurs écophysiologiques de stress: Synthèse bibliographique1. Cryptogam. Bryol. 2004, 25, 205–248. [Google Scholar]
- Whitton, B.A. Use of plants for monitoring heavy metals in freshwaters. In Modern Trends in Applied Aquatic Ecology; Springer: Boston, MA, USA, 2003; pp. 43–63. [Google Scholar]
- Hart, B.; Scaife, B. Toxicity and bioaccumulation of cadmium in Chlorella pyrenoidosa. Environ. Res. 1977, 14, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Tyler, G. Bryophytes and heavy metals: A literature review. Bot. J. Linn. Soc. 1990, 104, 231–253. [Google Scholar] [CrossRef]
- Pätsikkä, E.; Kairavuo, M.; Šeršen, F.; Aro, E.M.; Tyystjärvi, E. Excess copper predisposes photosystem II to photoinhibition in vivo by outcompeting iron and causing decrease in leaf chlorophyll. Plant Physiol. 2002, 129, 1359–1367. [Google Scholar] [CrossRef]
- Wilkie, D.; La Farge, C. Bryophytes as heavy metal biomonitors in the Canadian High Arctic. Arct. Antarct. Alp. Res. 2011, 43, 289–300. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Maggini, R.; Incrocci, L.; Pardossi, A.; Tzortzakis, N. Copper tolerance and accumulation on Pelargonium graveolens L’Hér. grown in hydroponic culture. Plants 2021, 10, 1663. [Google Scholar] [CrossRef]
- Sabovljević, A.; Vujičić, M.; Stanković, J.; Sabovljević, M. Effects of zinc and copper on development and survival of the moss Atrichum undulatum in controlled conditions. Bot. Serbica 2018, 42, 181–184. [Google Scholar]
- Figueira, R.; Sérgio, C.; Sousa, A. Distribution of trace metals in moss biomonitors and assessment of contamination sources in Portugal. Environ. Pollut. 2002, 118, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Bačkor, M.; Kováčik, J.; Dzubaj, A.; Bačkorová, M. Physiological comparison of copper toxicity in the lichens Peltigera rufescens (Weis) Humb. and Cladina arbuscula subsp. mitis (Sandst.) Ruoss. Plant Growth Regul. 2009, 58, 279–286. [Google Scholar] [CrossRef][Green Version]
- Bačkorová, M.; Maslaňáková, I.; Bačkor, M. Copper uptake and copper-induced physiological changes in the marine alga Cladophora prolifera (Roth.) Kütz. (Chlorophyta, Ulvophyceae). Braz. J. Bot. 2016, 39, 447–452. [Google Scholar] [CrossRef]
- Bačkor, M.; Klejdus, B.; Vantová, I.; Kováčik, J. Physiological adaptations in the lichens Peltigera rufescens and Cladina arbuscula var. mitis, and the moss Racomitrium lanuginosum to copper-rich substrate. Chemosphere 2009, 76, 1340–1343. [Google Scholar] [CrossRef]
- Bačkor, M.; Loppi, S. Interactions of lichens with heavy metals. Biol. Plant. 2009, 53, 214–222. [Google Scholar] [CrossRef]
- Jose, A. A Study of Heavy Metal Bioaccumulation Effects on Chlorophyll Content of the Two Aquatic Bryophytes Taxiphyllum barbieri (Java Moss) and Vesicularia montagnei (Christmas Moss). Ph.D. Thesis, St. Teresa’s College, Ernakulam, India, 2022. [Google Scholar]
- Aydoğan, S.; Erdağ, B.; Aktaş, L. Bioaccumulation and oxidative stress impact of Pb, Ni, Cu, and Cr heavy metals in two bryophyte species, Pleurochaete squarrosa and Timmiella barbuloides. Turk. J. Bot. 2017, 41, 464–475. [Google Scholar] [CrossRef]
- Yruela, I. Copper in plants. Braz. J. Plant Physiol. 2005, 17, 145–156. [Google Scholar] [CrossRef]
- Stanković, J.D.; Sabovljević, A.D.; Sabovljević, M.S. Bryophytes and heavy metals: A review. Acta Bot. Croat. 2018, 77, 109–118. [Google Scholar] [CrossRef]
- Maresca, V.; Bellini, E.; Landi, S.; Capasso, G.; Cianciullo, P.; Carraturo, F.; Pirintsos, S.; Sorbo, S.; di Toppi, L.S.; Esposito, S. Biological responses to heavy metal stress in the moss Leptodictyum riparium (Hedw.) Warnst. Ecotoxicol. Environ. Saf. 2022, 229, 113078. [Google Scholar] [CrossRef]
- Fatoba, P.; Udoh, E.G. Effects of some heavy metals on chlorophyll accumulation in Barbula lambarenensis. Ethnobot. Leafl. 2008, 2008, 107. [Google Scholar]
- Garty, J. Biomonitoring atmospheric heavy metals with lichens: Theory and application. Crit. Rev. Plant Sci. 2001, 20, 309–371. [Google Scholar] [CrossRef]
- Ronen, R.; Galun, M. Pigment extraction from lichens with dimethyl sulfoxide (DMSO) and estimation of chlorophyll degradation. Environ. Exp. Bot. 1984, 24, 239–245. [Google Scholar] [CrossRef]
- Bačkor, M.; Zetikova, J. Effects of copper, cobalt and mercury on the chlorophyll content of lichens Cetraria islandica and Flavocetraria cucullata. J. Hattori Bot. Lab. 2003, 93, 175–187. [Google Scholar]
- Misra, M.; Tandon, P. Heavy metal accumulation and chlorophyll content in moss samples collected from heavy traffic sites. Res. Environ. Life Sci. 2014, 7, 111–114. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Vavilin, D.V.; Ducruet, J.-M.; Matorin, D.N.; Venediktov, P.S.; Rubin, A.B. Membrane lipid peroxidation, cell viability and photosystem II activity in the green alga Chlorella pyrenoidosa subjected to various stress conditions. J. Photochem. Photobiol. B Biol. 1998, 42, 233–239. [Google Scholar] [CrossRef]
- Cu, C.; Cr, Z. Heavy-metal phytotoxicity induces oxidative stress in a moss, Taxithellium sp. Curr. Sci. 2003, 84, 63. [Google Scholar]
- Esposito, S.; Sorbo, S.; Conte, B.; Basile, A. Effects of heavy metals on ultrastructure and HSP70s induction in the aquatic moss Leptodictyum riparium Hedw. Int. J. Phytoremed. 2012, 14, 443–455. [Google Scholar] [CrossRef]
- Esposito, S.; Loppi, S.; Monaci, F.; Paoli, L.; Vannini, A.; Sorbo, S.; Maresca, V.; Fusaro, L.; Asadi Karam, E.; Lentini, M. In-field and in-vitro study of the moss Leptodictyum riparium as bioindicator of toxic metal pollution in the aquatic environment: Ultrastructural damage, oxidative stress and HSP70 induction. PLoS ONE 2018, 13, e0195717. [Google Scholar] [CrossRef]
- Stohs, S.J.; Bagchi, D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef]
- Borsani, O.; Valpuesta, V.; Botella, M.A. Evidence for a role of salicylic acid in the oxidative damage generated by NaCl and osmotic stress in Arabidopsis seedlings. Plant Physiol. 2001, 126, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Mateo, A.; Funck, D.; Mühlenbock, P.; Kular, B.; Mullineaux, P.M.; Karpinski, S. Controlled levels of salicylic acid are required for optimal photosynthesis and redox homeostasis. J. Exp. Bot. 2006, 57, 1795–1807. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Bao, Z.; Zhu, Z.; Ying, Q.; Qian, Q. Effects of different treatments of salicylic acid on heat tolerance, chlorophyll fluorescence, and antioxidant enzyme activity in seedlings of Cucumis sativa L. Plant Growth Regul. 2006, 48, 127–135. [Google Scholar] [CrossRef]
- Pinto, E.; Sigaud-kutner, T.C.; Leitao, M.A.; Okamoto, O.K.; Morse, D.; Colepicolo, P. Heavy metal-induced oxidative stress in algae 1. J. Phycol. 2003, 39, 1008–1018. [Google Scholar] [CrossRef]
- Varela Río, Z.; Debén García, S.; Saxena, D.K.; Aboal Viñas, J.R.; Fernández Escribano, J.Á. Levels of Antioxidant Compound Glutathione in Moss from Industrial Areas. Atmosphere 2018, 9, 284. [Google Scholar] [CrossRef]
- Bellini, E.; Maresca, V.; Betti, C.; Castiglione, M.R.; Fontanini, D.; Capocchi, A.; Sorce, C.; Borsò, M.; Bruno, L.; Sorbo, S. The moss Leptodictyum riparium counteracts severe cadmium stress by activation of glutathione transferase and phytochelatin synthase, but slightly by phytochelatins. Int. J. Mol. Sci. 2020, 21, 1583. [Google Scholar] [CrossRef]
- Yadav, S. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Mäkinen, K.; De, S. The significance of methionine cycle enzymes in plant virus infections. Curr. Opin. Plant Biol. 2019, 50, 67–75. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Brosnan, M.E. The sulfur-containing amino acids: An overview. J. Nutr. 2006, 136, 1636S–1640S. [Google Scholar] [CrossRef]
- Siu, K.K.; Asmus, K.; Zhang, A.N.; Horvatin, C.; Li, S.; Liu, T.; Moffatt, B.; Woods, V.L., Jr.; Howell, P.L. Mechanism of substrate specificity in 5′-methylthioadenosine/S-adenosylhomocysteine nucleosidases. J. Struct. Biol. 2011, 173, 86–98. [Google Scholar] [CrossRef][Green Version]
- Torricelli, E.; Gorbi, G.; Pawlik-Skowronska, B.; Di Toppi, L.S.; Corradi, M.G. Cadmium tolerance, cysteine and thiol peptide levels in wild type and chromium-tolerant strains of Scenedesmus acutus (Chlorophyceae). Aquat. Toxicol. 2004, 68, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Bottari, E.; Festa, M.R. Asparagine as a ligand for cadmium (II), lead (II) and zinc (II). Chem. Speciat. Bioavailab. 1996, 8, 75–83. [Google Scholar] [CrossRef]
- Lea, P.J.; Sodek, L.; Parry, M.A.; Shewry, P.R.; Halford, N.G. Asparagine in plants. Ann. Appl. Biol. 2007, 150, 1–26. [Google Scholar] [CrossRef]
- Salbitani, G.; Maresca, V.; Cianciullo, P.; Bossa, R.; Carfagna, S.; Basile, A. Non-Protein Thiol Compounds and Antioxidant Responses Involved in Bryophyte Heavy-Metal Tolerance. Int. J. Mol. Sci. 2023, 24, 5302. [Google Scholar] [CrossRef]
- Bačkor, M.; Váczi, P.; Barták, M.; Buďová, J.; Dzubaj, A. Uptake, photosynthetic characteristics and membrane lipid peroxidation levels in the lichen photobiont Trebouxia erici exposed to copper and cadmium. Bryologist 2007, 110, 100–107. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Bacova, R.; Klejdus, B.; Ryant, P.; Cernei, N.; Adam, V.; Huska, D. The effects of 5-azacytidine and cadmium on global 5-methylcytosine content and secondary metabolites in the freshwater microalgae Chlamydomonas reinhardtii and Scenedesmus quadricauda. J. Phycol. 2019, 55, 329–342. [Google Scholar] [CrossRef]
- Kolackova, M.; Chaloupsky, P.; Cernei, N.; Klejdus, B.; Huska, D.; Adam, V. Lycorine and UV-C stimulate phenolic secondary metabolites production and miRNA expression in Chlamydomonas reinhardtii. J. Hazard. Mater. 2020, 391, 122088. [Google Scholar] [CrossRef]



| µg/g DW | Control | 50 µM Cu | 250 µM Cu | 500 µM Cu |
|---|---|---|---|---|
| MTA | 2.49 ± 0.8b | 4.01 ± 0.94b | 3.65 ± 1.28b | 8.43 ± 1.29a |
| SAH | 1.26 ± 0.09b | 2.05 ± 0.39ab | 1.87 ± 1.0b | 3.57 ± 0.45a |
| Asn | 151 ± 46.1a | 96.5 ± 28.9ab | 35.3 ± 42.4b | 32.5 ± 21.2b |
| Betaine | 27.7 ± 12.1a | 32.7 ± 17.3a | 14.3 ± 8.53a | 15.9 ± 6.31a |
| Cys | 2.13 ± 0.16a | 0.10 ± 0.05b | 0.06 ± 0.04b | 0.04 ± 0.01b |
| Cystathionine | 0.11 ± 0.02ab | 0.13 ± 0.02a | 0.07 ± 0.02bc | 0.06 ± 0.01c |
| Glu | 98.4 ± 12.1a | 57.6 ± 12.4b | 75.6 ± 13.6ab | 51.4 ± 7.3b |
| Met | 10.4 ± 1.6a | 10.72 ± 2.76a | 7.9 ± 3.7a | 7.6 ± 1.1a |
| Pro | 50.9 ± 11.9b | 56.8 ± 8.6b | 58.6 ± 9.8b | 85.4 ± 8.5a |
| Ser | 282 ± 109a | 312 ± 56a | 268 ± 40.0a | 331 ± 82.1a |
| Taurine | 1.75 ± 0.09a | 1.85 ± 0.83a | 2.81 ± 0.47a | 2.71 ± 1.84a |
| 3,4diOH Benzaldehyde | 48.8 ± 34.3b | 449 ± 219a | 327 ± 132a | 902 ± 183a |
| Caffeic acid | 44.8 ± 24.8a | 55.2 ± 52.7a | 56.3 ± 4.43a | 82.5 ± 52.8a |
| Chlorogenic acid | 425 ± 264b | 717 ± 30.5ab | 681 ± 65.6ab | 960 ± 160a |
| Sinapic acid | 2324 ± 796a | 1612 ± 187a | 1450 ± 486a | 1197 ± 202a |
| Syringic acid | 1493 ± 854a | 1070 ± 523a | 815 ± 280a | 591 ± 178a |
| Neochlorogenic acid | 127 ± 31.4a | 198 ± 65.3a | 176 ± 31.2a | 218 ± 65.7a |
| p-Coumaric acid | 465 ± 72.7b | 3094 ± 1833ab | 2424 ± 1725ab | 4994 ± 296a |
| pOH Benzaldehyde | 5613 ± 1008a | 7203 ± 2095a | 4893 ± 738a | 5567 ± 1295a |
| pOH Benzoic acid | 282 ± 60.1a | 155 ± 71.3a | 127 ± 51.5a | 176 ± 72.2a |
| Protocatechuic acid | 60.1 ± 18.2a | 82.6 ± 7.93a | 113 ± 48.3a | 92.9 ± 12.0a |
| Salicylic acid | 188 ± 33.0a | 171 ± 40.6a | 43.2 ± 33.3b | 158 ± 50.0a |
| Vanillic acid | 411 ± 72.5ab | 350 ± 84.2b | 358 ± 31.4b | 560 ± 87.6a |
| Vanillin | 973 ± 244a | 977 ± 62.2a | 569 ± 100b | 788 ± 138ab |
| Cinnamic acid | 5395 ± 472b | 16,664 ± 6817ab | 10,507 ± 5189b | 22,272 ± 2552a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bačkor, M.; Goga, M.; Singh, P.; Tuptová, V. Mechanisms of Copper Toxicity and Tolerance in the Aquatic Moss Taxiphyllum barbieri. Plants 2023, 12, 3607. https://doi.org/10.3390/plants12203607
Bačkor M, Goga M, Singh P, Tuptová V. Mechanisms of Copper Toxicity and Tolerance in the Aquatic Moss Taxiphyllum barbieri. Plants. 2023; 12(20):3607. https://doi.org/10.3390/plants12203607
Chicago/Turabian StyleBačkor, Martin, Michal Goga, Pragya Singh, and Viktória Tuptová. 2023. "Mechanisms of Copper Toxicity and Tolerance in the Aquatic Moss Taxiphyllum barbieri" Plants 12, no. 20: 3607. https://doi.org/10.3390/plants12203607
APA StyleBačkor, M., Goga, M., Singh, P., & Tuptová, V. (2023). Mechanisms of Copper Toxicity and Tolerance in the Aquatic Moss Taxiphyllum barbieri. Plants, 12(20), 3607. https://doi.org/10.3390/plants12203607








