Eugenol: In Vitro and In Ovo Assessment to Explore Cytotoxic Effects on Osteosarcoma and Oropharyngeal Cancer Cells
Abstract
1. Introduction
2. Results
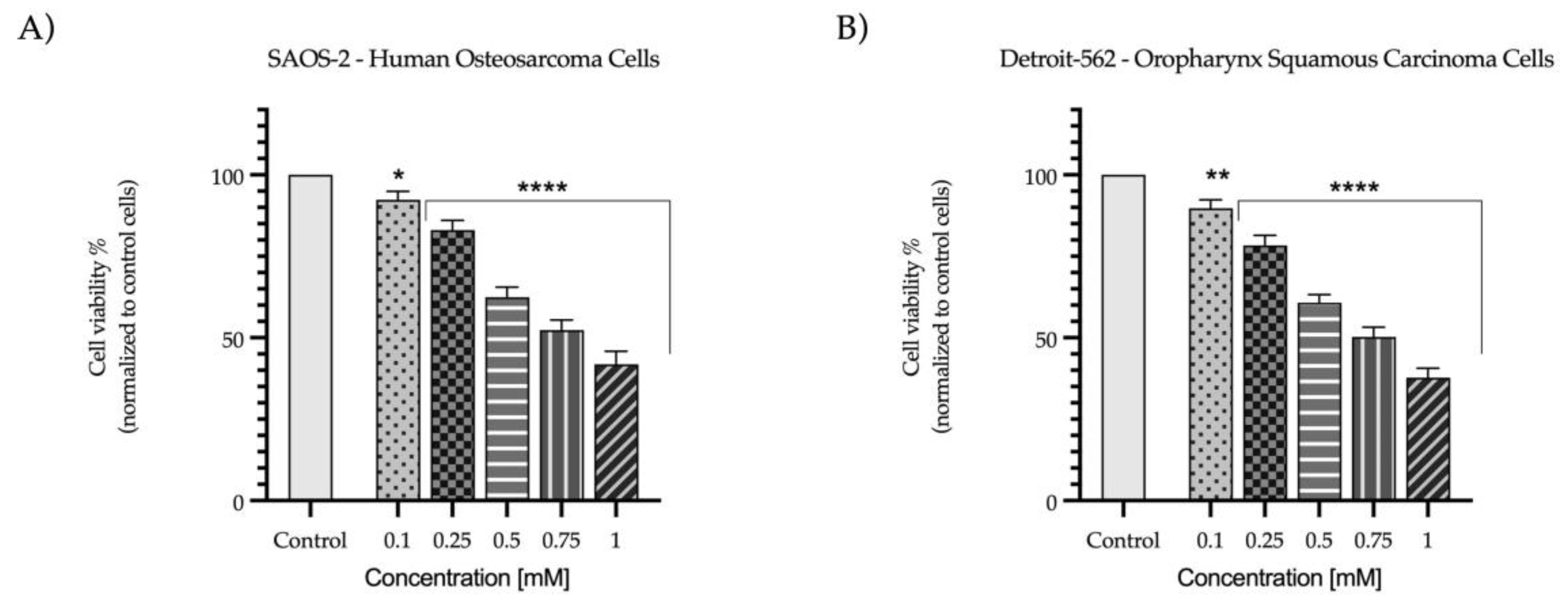
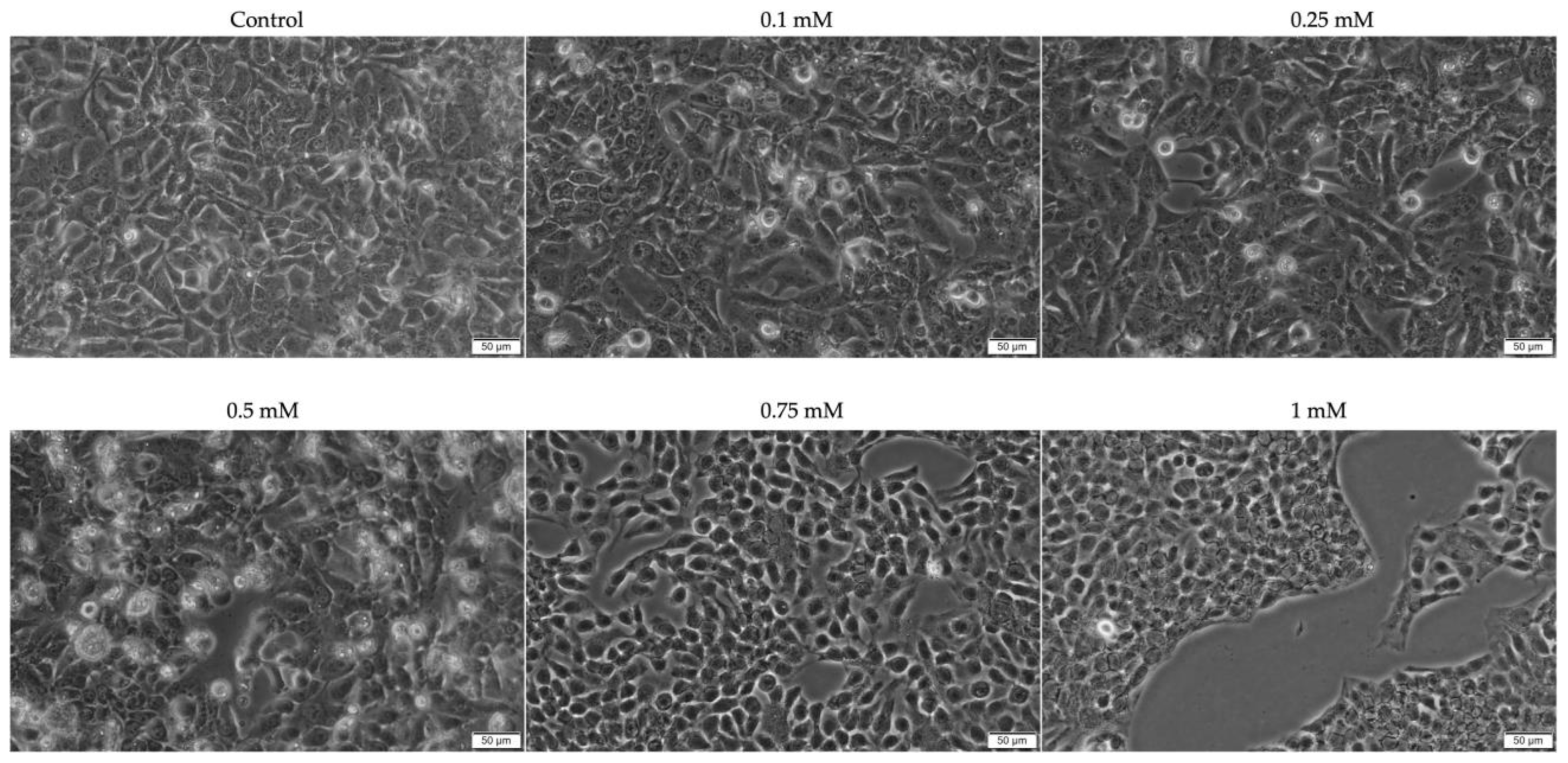
2.1. Evaluation of the Cytotoxic Profile
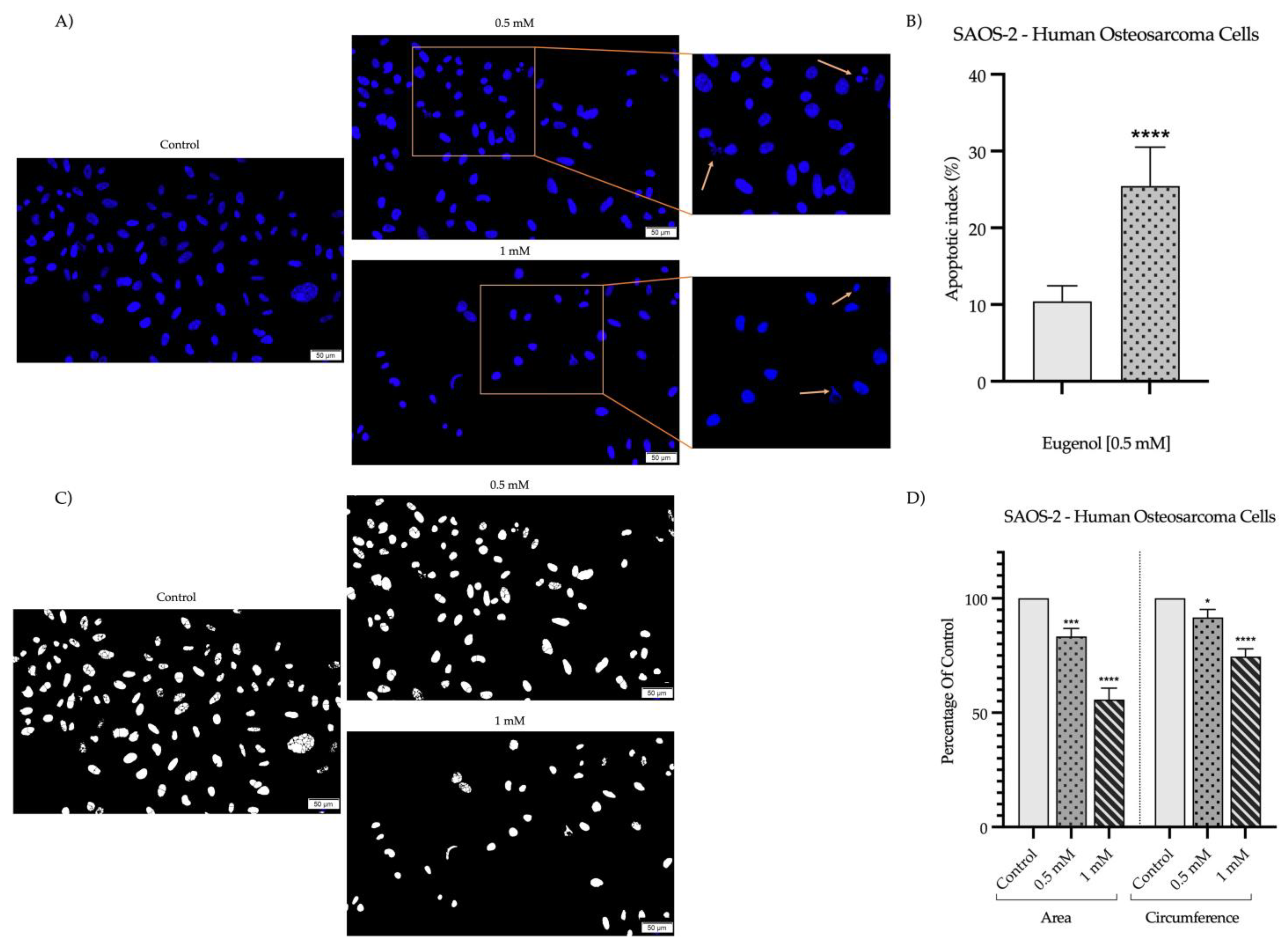
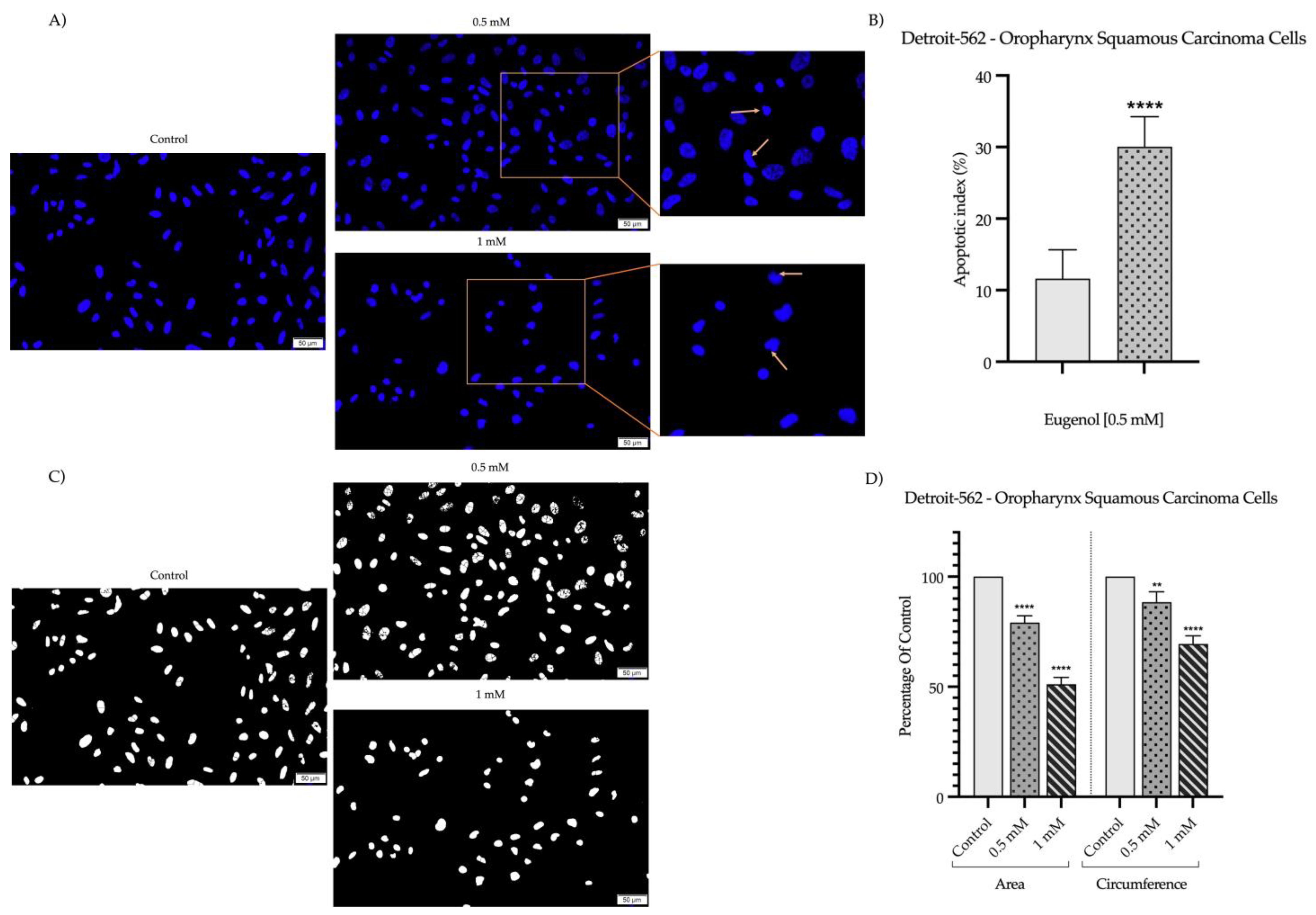
2.2. Detection and Quantification of Nuclear Morphology
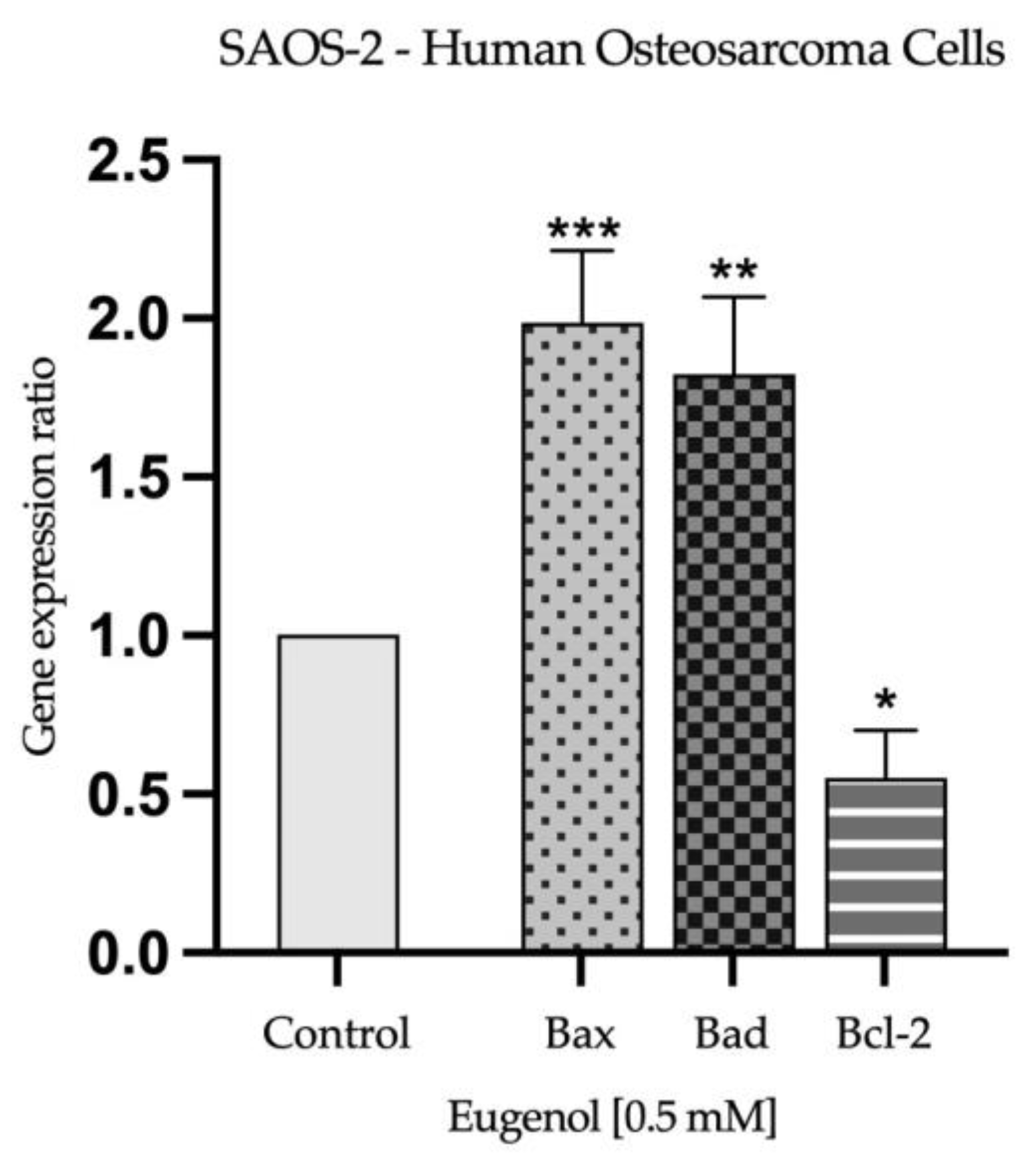
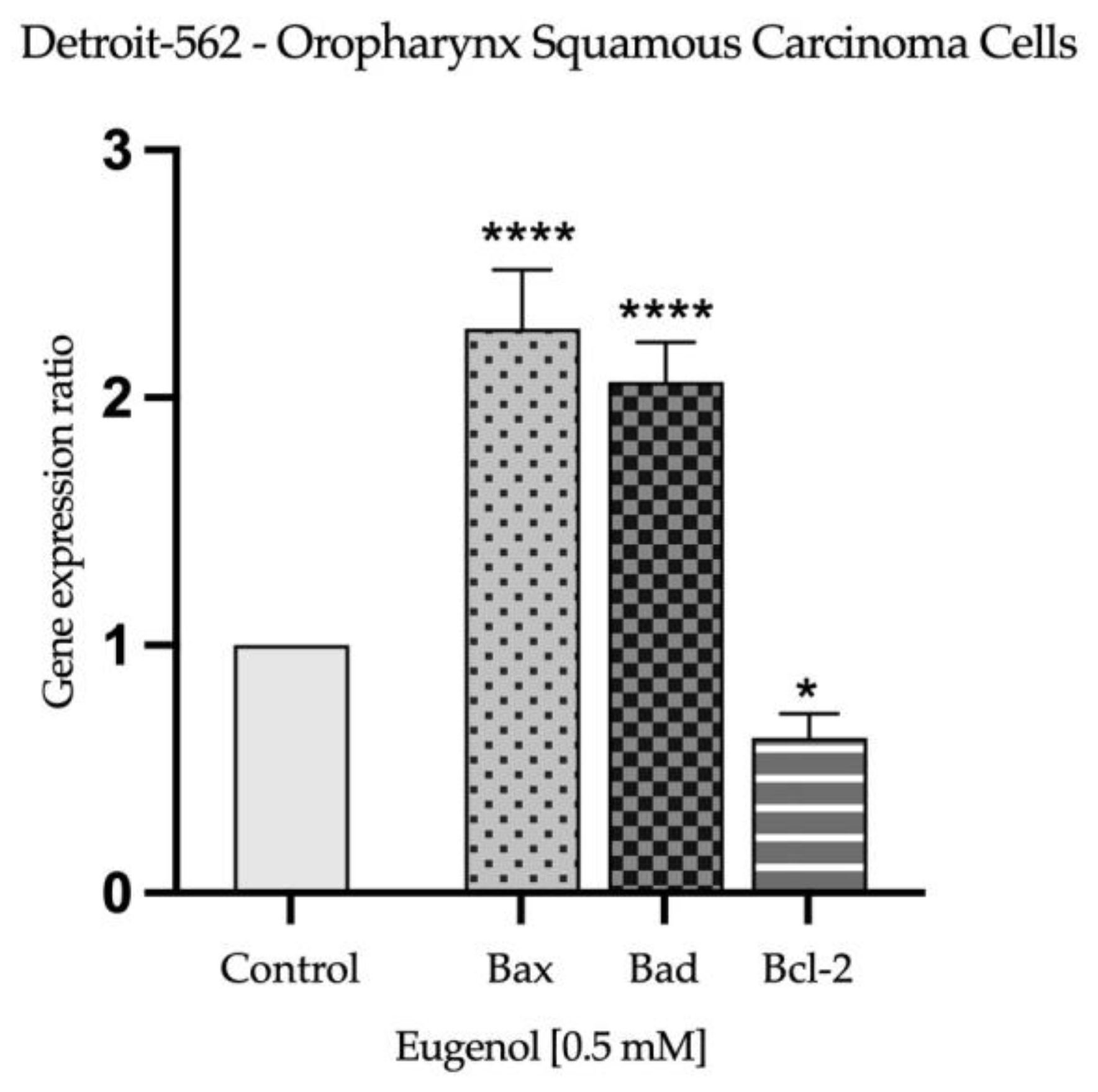
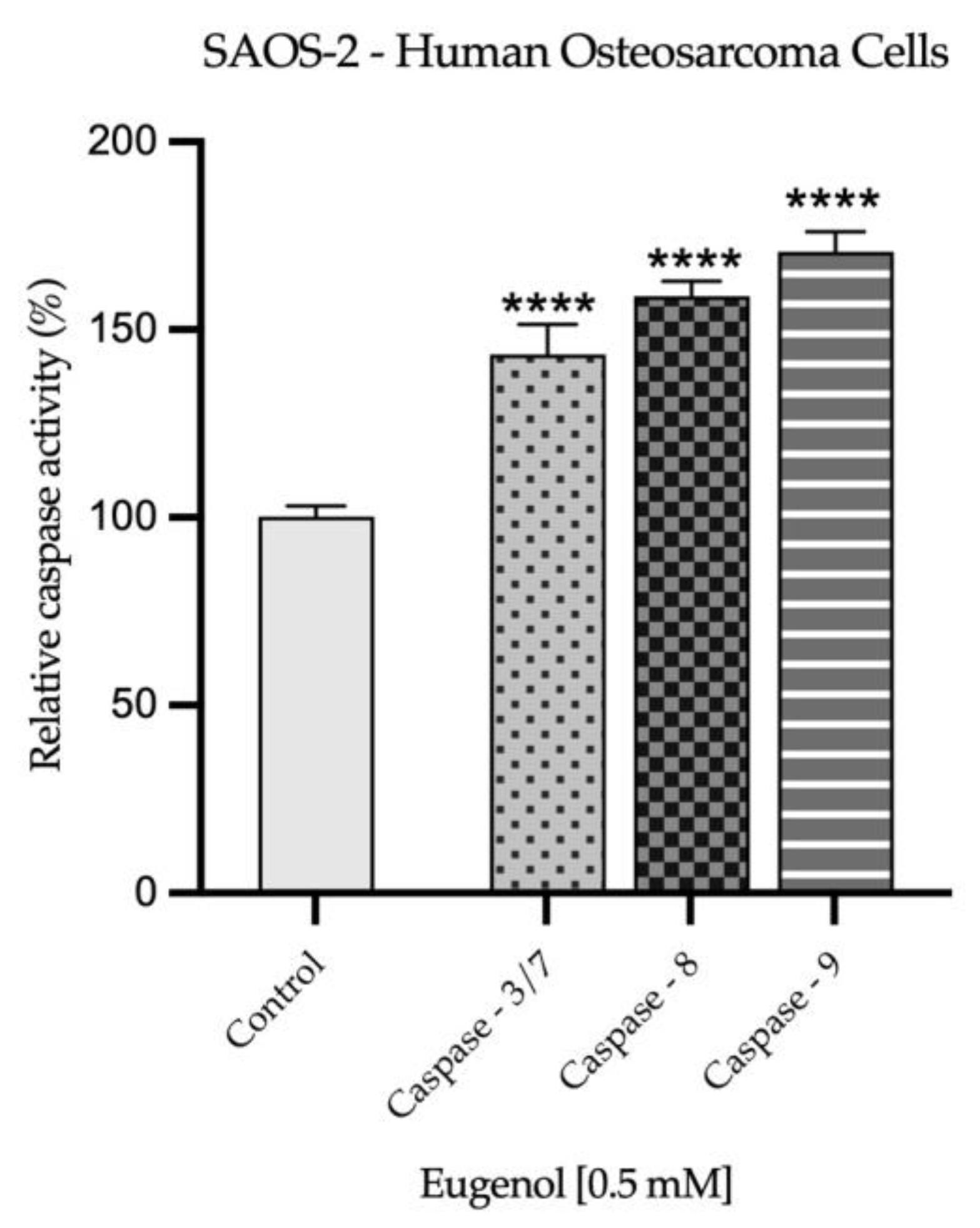
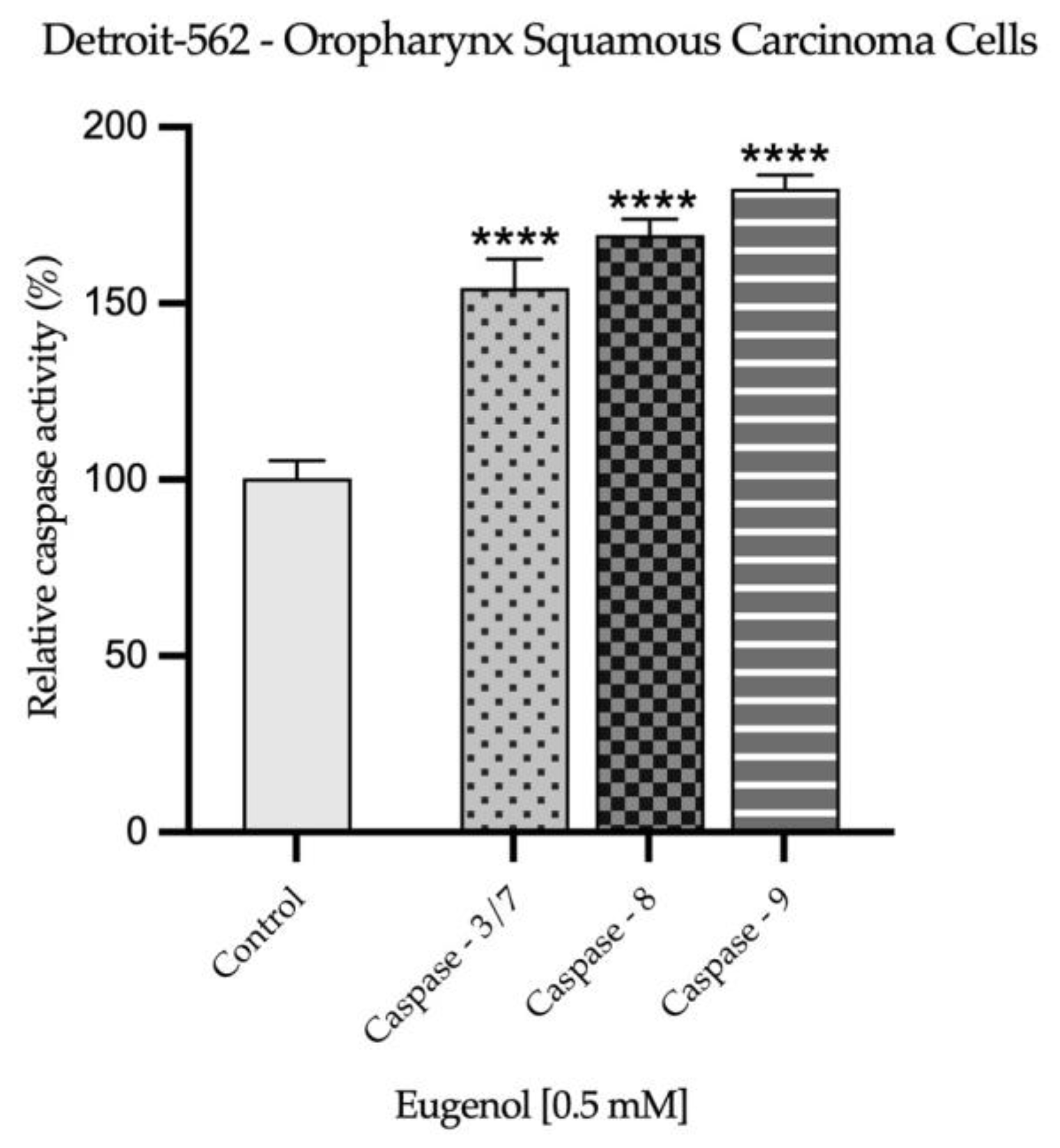
2.3. Real Time PCR Study and Caspases Activity
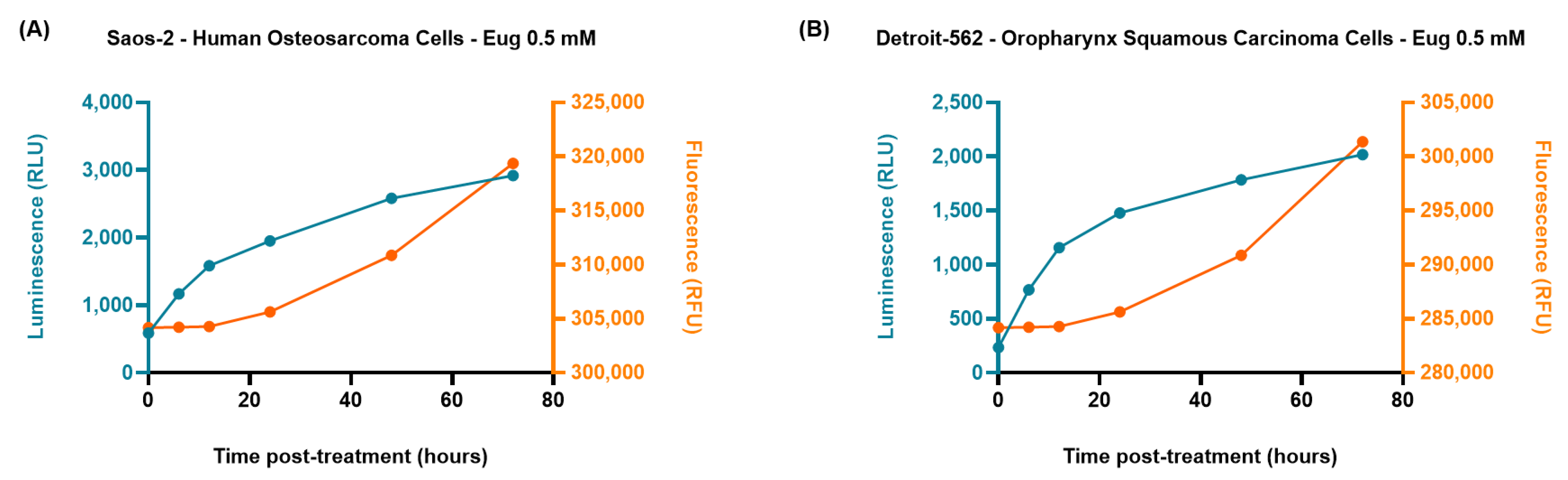
2.4. The RealTime-Glo™ Annexin V Apoptosis and Necrosis Assay
2.5. Hen’s Egg Test—Chorioallantoic Membrane (HET-CAM)
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Cellular Viability Evaluation
4.4. Cellular Morphology Evaluation
4.5. Nuclear Morphology Evaluation
4.6. Gene Expression Ratio
4.7. Caspase-3/7, -8, and -9 Activity
4.8. The RealTime-Glo™ Annexin V Apoptosis and Necrosis Assay
4.9. Hen’s Egg Test—Chorioallantoic Membrane (HET-CAM)
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cragg, G.M.; Pezzuto, J.M. Natural Products as a Vital Source for the Discovery of Cancer Chemotherapeutic and Chemopreventive Agents. Med. Princ. Pract. 2016, 25, 41–59. [Google Scholar] [CrossRef]
- Tobeiha, M.; Rajabi, A.; Raisi, A.; Mohajeri, M.; Yazdi, S.M.; Davoodvandi, A.; Aslanbeigi, F.; Vaziri, M.S.; Hamblin, M.R.; Mirzaei, H. Potential of Natural Products in Osteosarcoma Treatment: Focus on Molecular Mechanisms. Biomed. Pharmacother. 2021, 144, 112257. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, Y.; Gupta, V.K.; Jaitak, V. Anticancer Activity of Essential Oils: A Review. J. Sci. Food Agric. 2013, 93, 3643–3653. [Google Scholar] [CrossRef] [PubMed]
- Blowman, K.; Magalhães, M.; Lemos, M.F.L.; Cabral, C.; Pires, I.M. Anticancer Properties of Essential Oils and Other Natural Products. Evid.-Based Complement. Altern. Med. 2018, 2018, 3149362. [Google Scholar] [CrossRef] [PubMed]
- Barboza, J.N.; da Silva Maia Bezerra Filho, C.; Silva, R.O.; Medeiros, J.V.R.; de Sousa, D.P. An Overview on the Anti-Inflammatory Potential and Antioxidant Profile of Eugenol. Oxid. Med. Cell Longev. 2018, 2018, 3957262. [Google Scholar] [CrossRef]
- Park, S.H.; Sim, Y.B.; Lee, J.K.; Kim, S.M.; Kang, Y.J.; Jung, J.S.; Suh, H.W. The Analgesic Effects and Mechanisms of Orally Administered Eugenol. Arch. Pharm. Res. 2011, 34, 501–507. [Google Scholar] [CrossRef]
- Benencia, F.; Courrèges, M.C. In Vitro and in Vivo Activity of Eugenol on Human Herpesvirus. Phytother. Res. 2000, 14, 495–500. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant Activity of Eugenol: A Structure–Activity Relationship Study. J. Med. Food. 2011, 14, 975–985. [Google Scholar] [CrossRef]
- Zari, A.T.; Zari, T.A.; Hakeem, K.R. Anticancer Properties of Eugenol: A Review. Molecules 2021, 26, 7407. [Google Scholar] [CrossRef]
- Khalil, A.A.; Rahman, U.U.; Khan, M.R.; Sahar, A.; Mehmood, T.; Khan, M. Essential Oil Eugenol: Sources, Extraction Techniques and Nutraceutical Perspectives. RSC Adv. 2017, 7, 32669–32681. [Google Scholar] [CrossRef]
- Fadilah, F.; Yanuar, A.; Arsianti, A.; Andrajati, R. Phenylpropanoids, Eugenol Scaffold, and Its Derivatives as Anticancer. Asian J. Pharm. Clin. Res. 2017, 10, 41–46. [Google Scholar] [CrossRef]
- Begum, S.N.; Ray, A.S.; Rahaman, C.H. A Comprehensive and Systematic Review on Potential Anticancer Activities of Eugenol: From Pre-Clinical Evidence to Molecular Mechanisms of Action. Phytomedicine 2022, 107, 154456. [Google Scholar] [CrossRef] [PubMed]
- Beird, H.C.; Bielack, S.S.; Flanagan, A.M.; Gill, J.; Heymann, D.; Janeway, K.A.; Livingston, J.A.; Roberts, R.D.; Strauss, S.J.; Gorlick, R. Osteosarcoma. Nat. Rev. Dis. Primers 2022, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. International Osteosarcoma Incidence Patterns in Children and Adolescents, Middle Ages, and Elderly Persons. Int. J. Cancer 2009, 125, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Walsh, M.F.; Wu, G.; Edmonson, M.N.; Gruber, T.A.; Easton, J.; Hedges, D.; Ma, X.; Zhou, X.; Yergeau, D.A.; et al. Germline Mutations in Predisposition Genes in Pediatric Cancer. N. Engl. J. Med. 2015, 373, 2336–2346. [Google Scholar] [CrossRef]
- Cundy, T. Paget’s Disease of Bone. Metabolism 2018, 80, 5–14. [Google Scholar] [CrossRef]
- Tucker, M.A.; D’Angio, G.J.; Boice, J.D.; Strong, L.C.; Li, F.P.; Stovall, M.; Stone, B.J.; Green, D.M.; Lombardi, F.; Newton, W.; et al. Bone Sarcomas Linked to Radiotherapy and Chemotherapy in Children. N. Engl. J. Med. 1987, 317, 588–593. [Google Scholar] [CrossRef]
- Martins-Neves, S.R.; Sampaio-Ribeiro, G.; Gomes, C.M.F. Self-Renewal and Pluripotency in Osteosarcoma Stem Cells’ Chemoresistance: Notch, Hedgehog, and Wnt/β-Catenin Interplay with Embryonic Markers. Int. J. Mol. Sci. 2023, 24, 8401. [Google Scholar] [CrossRef]
- Ferrari, D.; Moneghini, L.; Allevi, F.; Bulfamante, G.; Biglioli, F. Osteosarcoma of the Jaw: Classification, Diagnosis and Treatment. In Osteosarcoma-Biology, Behavior and Mechanisms; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, Q.; Gong, X.; Liu, J.; Ma, Y. Osteosarcoma: A Review of Current and Future Therapeutic Approaches. Biomed. Eng. Online 2021, 20, 24. [Google Scholar] [CrossRef]
- Smeland, S.; Bielack, S.S.; Whelan, J.; Bernstein, M.; Hogendoorn, P.; Krailo, M.D.; Gorlick, R.; Janeway, K.A.; Ingleby, F.C.; Anninga, J.; et al. Survival and Prognosis with Osteosarcoma: Outcomes in More than 2000 Patients in the EURAMOS-1 (European and American Osteosarcoma Study) Cohort. Eur. J. Cancer 2019, 109, 36–50. [Google Scholar] [CrossRef]
- Jamal, Z.; Anjum, F. Oropharyngeal Squamous Cell Carcinoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK563268/ (accessed on 27 April 2023).
- Panarese, I.; Aquino, G.; Ronchi, A.; Longo, F.; Montella, M.; Cozzolino, I.; Roccuzzo, G.; Colella, G.; Caraglia, M.; Franco, R. Oral and Oropharyngeal Squamous Cell Carcinoma: Prognostic and Predictive Parameters in the Etiopathogenetic Route. Expert Rev. Anticancer Ther. 2019, 19, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Fauzi, F.H.; Hamzan, N.I.; Rahman, N.A.; Suraiya, S.; Mohamad, S. Detection of Human Papillomavirus in Oropharyngeal Squamous Cell Carcinoma. J. Zhejiang Univ. Sci. B 2020, 21, 961–976. [Google Scholar] [CrossRef] [PubMed]
- Stern, P.L.; Dalianis, T. Oropharyngeal Squamous Cell Carcinoma Treatment in the Era of Immune Checkpoint Inhibitors. Viruses 2021, 13, 1234. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Tseng, Y.H. The Potential of Phytochemicals in Oral Cancer Prevention and Therapy: A Review of the Evidence. Biomolecules 2020, 10, 1150. [Google Scholar] [CrossRef]
- Padhy, I.; Paul, P.; Sharma, T.; Banerjee, S.; Mondal, A. Molecular Mechanisms of Action of Eugenol in Cancer: Recent Trends and Advancement. Life 2022, 12, 1795. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Costello, L.; Toner, M.; Pierse, D.; Stassen, L.F.A. Osteosarcoma (Osteogenic Sarcoma) of the Jaws Presenting in General Dental Practice—A Series of Four Cases. Br. Dent. J. 2021, 230, 583–586. [Google Scholar] [CrossRef]
- Golusiński, W.; Golusińska-Kardach, E. Current Role of Surgery in the Management of Oropharyngeal Cancer. Front. Oncol. 2019, 9, 388. [Google Scholar] [CrossRef]
- Ali Abdalla, Y.O.; Subramaniam, B.; Nyamathulla, S.; Shamsuddin, N.; Arshad, N.M.; Mun, K.S.; Awang, K.; Nagoor, N.H. Natural Products for Cancer Therapy: A Review of Their Mechanism of Actions and Toxicity in the Past Decade. J. Trop. Med. 2022, 2022, 5794350. [Google Scholar] [CrossRef]
- Nisar, M.F.; Khadim, M.; Rafiq, M.; Chen, J.; Yang, Y.; Wan, C.C. Pharmacological Properties and Health Benefits of Eugenol: A Comprehensive Review. Oxid. Med. Cell. Longev. 2021, 2021, 2497354. [Google Scholar] [CrossRef]
- Ulanowska, M.; Olas, B. Biological Properties and Prospects for the Application of Eugenol—A Review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef]
- Luiz De Sá Júnior, P.; Aparecida, D.; Câmara, D.; Santos Costa, A.; Luis, J.; Ruiz, M.; Levy, D.; Azevedo, R.A.; Fernanda, K.; Pasqualoto, M.; et al. Apoptotic Effect of Eugenol Envolves G2/M Phase Abrogation Accompanied by Mitochondrial Damage and Clastogenic Effect on Cancer Cell in Vitro. Phytomedicine 2016, 23, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Al Wafai, R.; El-Rabih, W.; Katerji, M.; Safi, R.; El Sabban, M.; El-Rifai, O.; Usta, J. Chemosensitivity of MCF-7 Cells to Eugenol: Release of Cytochrome-c and Lactate Dehydrogenase. Sci. Rep. 2017, 7, 43730. [Google Scholar] [CrossRef] [PubMed]
- Ramazani, E.; YazdFazeli, M.; Ahmad Emami, S.; Mohtashami, L.; Javadi, B.; Asili, J.; Tayarani-Najaran, Z. Protective Effects of Cinnamomum Verum, Cinnamomum Cassia and Cinnamaldehyde against 6-OHDA-Induced Apoptosis in PC12 Cells. Mol. Biol. Rep. 2020, 47, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Park, J.H.; Kim, G.C.; Park, B.S.; Gil, Y.G.; Kim, C.H. The Mechanism of Apoptosis Induced by Eugenol in Human Osteosarcoma Cells. J. Korean Assoc. Oral Maxillofac. Surg. 2007, 33, 20–27. [Google Scholar]
- Surducan, D.A.; Racea, R.C.; Cabuta, M.; Olariu, I.; Macasoi, I.; Rusu, L.C.; Chiriac, S.D.; Chioran, D.; Dinu, S.; Pricop, M.O. Eugenol Induces Apoptosis in Tongue Squamous Carcinoma Cells by Mediating the Expression of Bcl-2 Family. Life 2023, 13, 22. [Google Scholar] [CrossRef]
- Racea, R.C.; Merghes, P.E.; Gurgus, D.; Macasoi, I.; Rusu, L.C.; Chioran, D.; Dinu, S.; Breban-Schwarzkopf, D.; Szuhanek, C.; Rivis, M. Eugenol: In Vitro Characterization Of The Cytotoxic Profile At The Level Of Colorectal Carcinoma Cells. Farmacia 2023, 71, 288–295. [Google Scholar] [CrossRef]
- ISO 10993-5:2009(En); Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009. Available online: https://www.iso.org/obp/ui/#iso:std:iso:10993:-5:ed-3:v1:en (accessed on 25 July 2023).
- Razak, M.A.I.A.; Hamid, H.A.; Othman, R.N.I.R.; Moktar, S.A.M.; Miskon, A. Improved Drug Delivery System for Cancer Treatment by D-Glucose Conjugation with Eugenol From Natural Product. Curr. Drug Deliv. 2020, 18, 312–322. [Google Scholar] [CrossRef]
- Rothzerg, E.; Pfaff, A.L.; Koks, S. Innovative Approaches for Treatment of Osteosarcoma. Exp. Biol. Med. 2022, 247, 310–316. [Google Scholar] [CrossRef]
- Wen, Y.; Grandis, J.R. Emerging Drugs for Head and Neck Cancer. Expert Opin. Emerg. Drugs 2015, 20, 313–329. [Google Scholar] [CrossRef]
- Kis, A.M.; Macasoi, I.; Paul, C.; Radulescu, M.; Buzatu, R.; Watz, C.G.; Cheveresan, A.; Berceanu, D.; Pinzaru, I.; Dinu, S.; et al. Methotrexate and Cetuximab—Biological Impact on Non-Tumorigenic Models: In Vitro and In Ovo Assessments. Medicina 2022, 58, 167. [Google Scholar] [CrossRef] [PubMed]
- Sramek, M.; Neradil, J.; Sterba, J.; Veselska, R. Non-DHFR-Mediated Effects of Methotrexate in Osteosarcoma Cell Lines: Epigenetic Alterations and Enhanced Cell Differentiation. Cancer Cell Int. 2016, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, A.; Shanehbandi, D.; Yousefi, B.; Soleimanpour, J. Thymoquinone Potentiates Methotrexate Mediated-Apoptosis in Saos-2 Osteosarcoma Cell Line. Drug Res. 2022, 72, 390–395. [Google Scholar]
- Young, N.R.; Soneru, C.; Liu, J.; Grushko, T.A.; Hardeman, A.; Olopade, O.I.; Baum, A.; Solca, F.; Cohen, E.E.W. Afatinib Efficacy against Squamous Cell Carcinoma of the Head and Neck Cell Lines in Vitro and in Vivo. Target Oncol. 2015, 10, 501–508. [Google Scholar] [CrossRef]
- Lin, S.R.; Chang, C.H.; Hsu, C.F.; Tsai, M.J.; Cheng, H.; Leong, M.K.; Sung, P.J.; Chen, J.C.; Weng, C.-F. Natural Compounds as Potential Adjuvants to Cancer Therapy: Preclinical Evidence. Br. J. Pharmacol. 2020, 177, 1409–1423. [Google Scholar] [CrossRef]
- Duan, Y.; Huang, X.; Qiao, B.; Ma, R.; Li, J. Eugenol Inhibits the Biological Activities of an Oral Squamous Cell Carcinoma Cell Line SCC9 via Targeting MIF. Anticancer Agents Med. Chem. 2022, 22, 2799–2806. [Google Scholar] [CrossRef]
- Kim, Y.H.; Park, B.S. The Effect of Eugenol on the Induction of Apoptosis in HSC-2 Human Oral Squamous Cell Carcinoma. Orig. Artic. J. Korean Soc. Dent. Hyg. 2015, 15, 523–532. [Google Scholar] [CrossRef][Green Version]
- Yoo, C.-B.; Han, K.-T.; Cho, K.-S.; Ha, J.; Park, H.-J.; Nam, J.-H.; Kil, U.-H.; Lee, K.-T. Eugenol Isolated from the Essential Oil of Eugenia Caryophyllata Induces a Reactive Oxygen Species-Mediated Apoptosis in HL-60 Human Promyelocytic Leukemia Cells. Cancer Lett. 2005, 225, 41–52. [Google Scholar] [CrossRef]
- das Chagas Pereira de Andrade, F.; Mendes, A.N. Computational Analysis of Eugenol Inhibitory Activity in Lipoxygenase and Cyclooxygenase Pathways. Sci. Rep. 2020, 10, 16204. [Google Scholar] [CrossRef]
- Fangjun, L.; Zhijia, Y. Tumor Suppressive Roles of Eugenol in Human Lung Cancer Cells. Thorac. Cancer 2018, 9, 25–29. [Google Scholar] [CrossRef]
- Fathy, M.; Fawzy, M.A.; Hintzsche, H.; Nikaido, T.; Dandekar, T.; Othman, E.M. Eugenol Exerts Apoptotic Effect and Modulates the Sensitivity of HeLa Cells to Cisplatin and Radiation. Molecules 2019, 24, 3979. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, U.; Groscurth, P. Morphological Features of Cell Death. Physiology 2004, 19, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Menyhárt, O.; Harami-Papp, H.; Sukumar, S.; Schäfer, R.; Magnani, L.; de Barrios, O.; Győrffy, B. Guidelines for the Selection of Functional Assays to Evaluate the Hallmarks of Cancer. Biochim. Biophys. Acta Rev. Cancer. 2016, 1866, 300–319. [Google Scholar] [CrossRef] [PubMed]
- Doonan, F.; Cotter, T.G. Morphological Assessment of Apoptosis. Methods 2008, 44, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Vidhya, N.; Devaraj, N. Induction of Apoptosis by Eugenol in Human Breast Cancer Cell. Indian J. Exp. Biol. 2011, 49, 871–878. [Google Scholar]
- Das, A.; Harshadha, K.; Dhinesh Kannan, S.K.; Hari Raj, K.; Jayaprakash, B. Evaluation of Therapeutic Potential of Eugenol-A Natural Derivative of Syzygium aromaticum on Cervical Cancer. Asian Pac. J. Cancer Prev. 2018, 19, 1977–1985. [Google Scholar]
- Mandelkow, R.; GüMBEL, D.; Ahrend, H.; Kaul, A.; Zimmermann, U.; Burchardt, M.; Stope, M.B. Detection and Quantification of Nuclear Morphology Changes in Apoptotic Cells by Fluorescence Microscopy and Subsequent Analysis of Visualized Fluorescent Signals. Anticancer Res. 2017, 37, 2239–2244. [Google Scholar] [CrossRef]
- Permatasari, H.K.; Effendi, A.B.; Qhabibi, F.R.; Fawwaz, F.; Dominique, A. Eugenol Isolated from Syzygium aromaticum Inhibits HeLa Cancer Cell Migration by Altering Epithelial-Mesenchymal Transition Protein Regulators. J. Appl. Pharm. Sci. 2021, 11, 49–53. [Google Scholar]
- Kim, G.C.; Choi, D.S.; Lim, J.S.; Jeong, H.C.; Kim, I.R.; Lee, M.H.; Park, B.S. Caspases-Dependent Apoptosis in Human Melanoma Cell by Eugenol. Korean J. Anat. 2016, 39, 245–253. [Google Scholar] [CrossRef]
- Lindenboim, L.; Zohar, H.; Worman, H.J.; Stein, R. The Nuclear Envelope: Target and Mediator of the Apoptotic Process. Cell Death Discov. 2020, 6, 29. [Google Scholar] [CrossRef]
- Islam, S.S.; Al-Sharif, I.; Sultan, A.; Al-Mazrou, A.; Remmal, A.; Aboussekhra, A. Eugenol Potentiates Cisplatin Anti-Cancer Activity through Inhibition of ALDH-Positive Breast Cancer Stem Cells and the NF-ΚB Signaling Pathway. Mol. Carcinog. 2018, 57, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Hemaiswarya, S.; Doble, M. Combination of Phenylpropanoids with 5-Fluorouracil as Anti-Cancer Agents against Human Cervical Cancer (HeLa) Cell Line. Phytomedicine 2013, 20, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Panda, C.K.; Das, S. Clove (Syzygium aromaticum L.), a Potential Chemopreventive Agent for Lung Cancer. Carcinogenesis 2006, 27, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Bhattacharjee, S.; Mandal, D.P. Induction of Apoptosis by Eugenol and Capsaicin in Human Gastric Cancer AGS Cells-Elucidating the Role of P53. Asian Pac. J. Cancer Prev. 2015, 16, 6753–6759. [Google Scholar] [CrossRef]
- Dhani, S.; Zhao, Y.; Zhivotovsky, B. A Long Way to Go: Caspase Inhibitors in Clinical Use. Cell Death Dis. 2021, 12, 949. [Google Scholar] [CrossRef] [PubMed]
- Kupcho, K.; Shultz, J.; Hurst, R.; Hartnett, J.; Zhou, W.; Machleidt, T.; Grailer, J.; Worzella, T.; Riss, T.; Lazar, D.; et al. A Real-Time, Bioluminescent Annexin V Assay for the Assessment of Apoptosis. Apoptosis 2019, 24, 184–197. [Google Scholar] [CrossRef]
- RealTime-GloTM Annexin V Apoptosis Assay|Annexin V Staining|Apoptosis Assay. Available online: https://www.promega.ro/products/cell-health-assays/apoptosis-assays/realtime-glo-annexin-v-apoptosis-assay/?catNum=JA1011 (accessed on 5 October 2023).
- Winter, G.; Koch, A.B.F.; Löffler, J.; Jelezko, F.; Lindén, M.; Li, H.; Abaei, A.; Zuo, Z.; Beer, A.J.; Rasche, V. In Vivo PET/MRI Imaging of the Chorioallantoic Membrane. Front. Phys. 2020, 8, 151. [Google Scholar] [CrossRef]
- Budai, P.; Kormos, É.; Buda, I.; Somody, G.; Lehel, J. Comparative Evaluation of HET-CAM and ICE Methods for Objective Assessment of Ocular Irritation Caused by Selected Pesticide Products. Toxicol. Vitr. 2021, 74, 105150. [Google Scholar] [CrossRef]
- Ahmad, N.; Jalees Ahmad, F.; Bedi, S.; Sharma, S.; Umar, S.; Azam Ansari, M. A Novel Nanoformulation Development of Eugenol and Their Treatment in Inflammation and Periodontitis. Saudi Pharm. J. 2019, 27, 778–790. [Google Scholar] [CrossRef]
- Thanekar, D.; Dhodi, J.; Gawali, N.; Raju, A.; Deshpande, P.; Degani, M.; Juvekar, A. Evaluation of Antitumor and Anti-Angiogenic Activity of Bioactive Compounds from Cinnamomum Tamala: In Vitro, in Vivo and in Silico Approach. S. Afr. J. Bot. 2016, 104, 6–14. [Google Scholar] [CrossRef]
- Gad El-Hak, H.; Gerges, M. Evaluating the teratogenic effect of Eugenol in the development of the chick embryos. J. Biol. Stud. 2018, 1, 59–75. [Google Scholar]
- Gag, O.; Macasoi, I.; Pinzaru, I.; Dinu, S.; Popovici, R.; Cosoroaba, M.R.; Buzatu, R.; Cabuta, M.; Chiriac, S.D. In Vitro Assessment of the Impact of Ultraviolet B Radiation on Oral Healthy and Tumor Cells. Photonics 2023, 10, 464. [Google Scholar] [CrossRef]
- Eidet, J.R.; Pasovic, L.; Maria, R.; Jackson, C.J.; Utheim, T.P. Objective Assessment of Changes in Nuclear Morphology and Cell Distribution Following Induction of Apoptosis. Diagn. Pathol. 2014, 9, 92. [Google Scholar] [CrossRef]
- Rednic, R.; Macasoi, I.; Pinzaru, I.; Dehelean, C.A.; Tomescu, M.C.; Susan, M.; Feier, H. Pharmaco-Toxicological Assessment of the Combined Cytotoxic Effects of Digoxin and Betulinic Acid in Melanoma Cells. Life 2022, 12, 1855. [Google Scholar] [CrossRef] [PubMed]

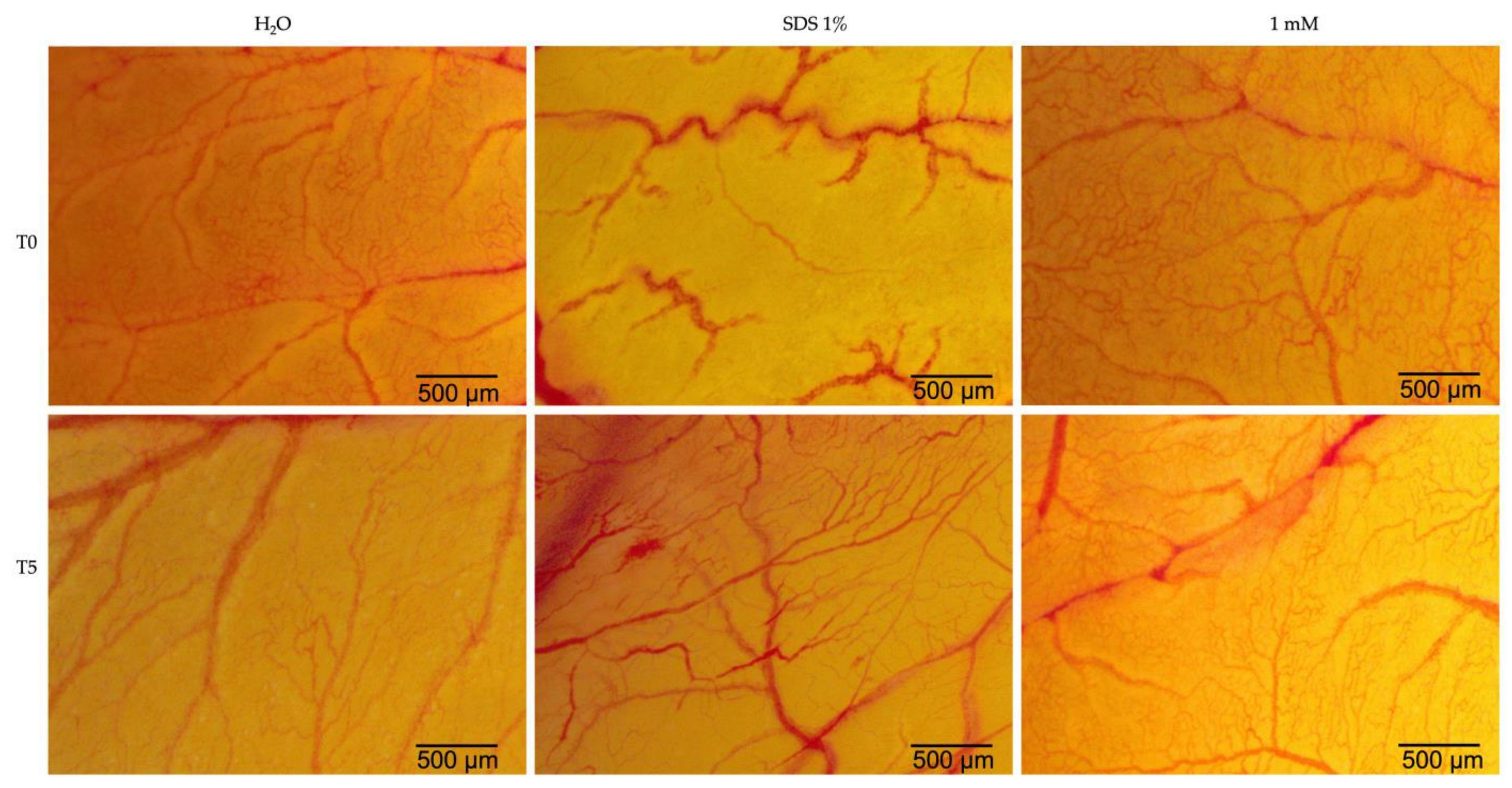
| H2O | SDS 1% | Eug 1 mM | |
|---|---|---|---|
| IS | 0.15 | 19.87 | 1.69 |
| tH | 300 | 21 | 300 |
| tL | 299 | 17 | 278 |
| tC | 298 | 15 | 263 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Racea, R.-C.; Macasoi, I.-G.; Dinu, S.; Pinzaru, I.; Marcovici, I.; Dehelean, C.; Rusu, L.-C.; Chioran, D.; Rivis, M.; Buzatu, R. Eugenol: In Vitro and In Ovo Assessment to Explore Cytotoxic Effects on Osteosarcoma and Oropharyngeal Cancer Cells. Plants 2023, 12, 3549. https://doi.org/10.3390/plants12203549
Racea R-C, Macasoi I-G, Dinu S, Pinzaru I, Marcovici I, Dehelean C, Rusu L-C, Chioran D, Rivis M, Buzatu R. Eugenol: In Vitro and In Ovo Assessment to Explore Cytotoxic Effects on Osteosarcoma and Oropharyngeal Cancer Cells. Plants. 2023; 12(20):3549. https://doi.org/10.3390/plants12203549
Chicago/Turabian StyleRacea, Robert-Cosmin, Ioana-Gabriela Macasoi, Stefania Dinu, Iulia Pinzaru, Iasmina Marcovici, Cristina Dehelean, Laura-Cristina Rusu, Doina Chioran, Mircea Rivis, and Roxana Buzatu. 2023. "Eugenol: In Vitro and In Ovo Assessment to Explore Cytotoxic Effects on Osteosarcoma and Oropharyngeal Cancer Cells" Plants 12, no. 20: 3549. https://doi.org/10.3390/plants12203549
APA StyleRacea, R.-C., Macasoi, I.-G., Dinu, S., Pinzaru, I., Marcovici, I., Dehelean, C., Rusu, L.-C., Chioran, D., Rivis, M., & Buzatu, R. (2023). Eugenol: In Vitro and In Ovo Assessment to Explore Cytotoxic Effects on Osteosarcoma and Oropharyngeal Cancer Cells. Plants, 12(20), 3549. https://doi.org/10.3390/plants12203549












