Ecological Stoichiometric Characteristics in Organs of Ammopiptanthus mongolicus in Different Habitats
Abstract
1. Introduction
2. Results
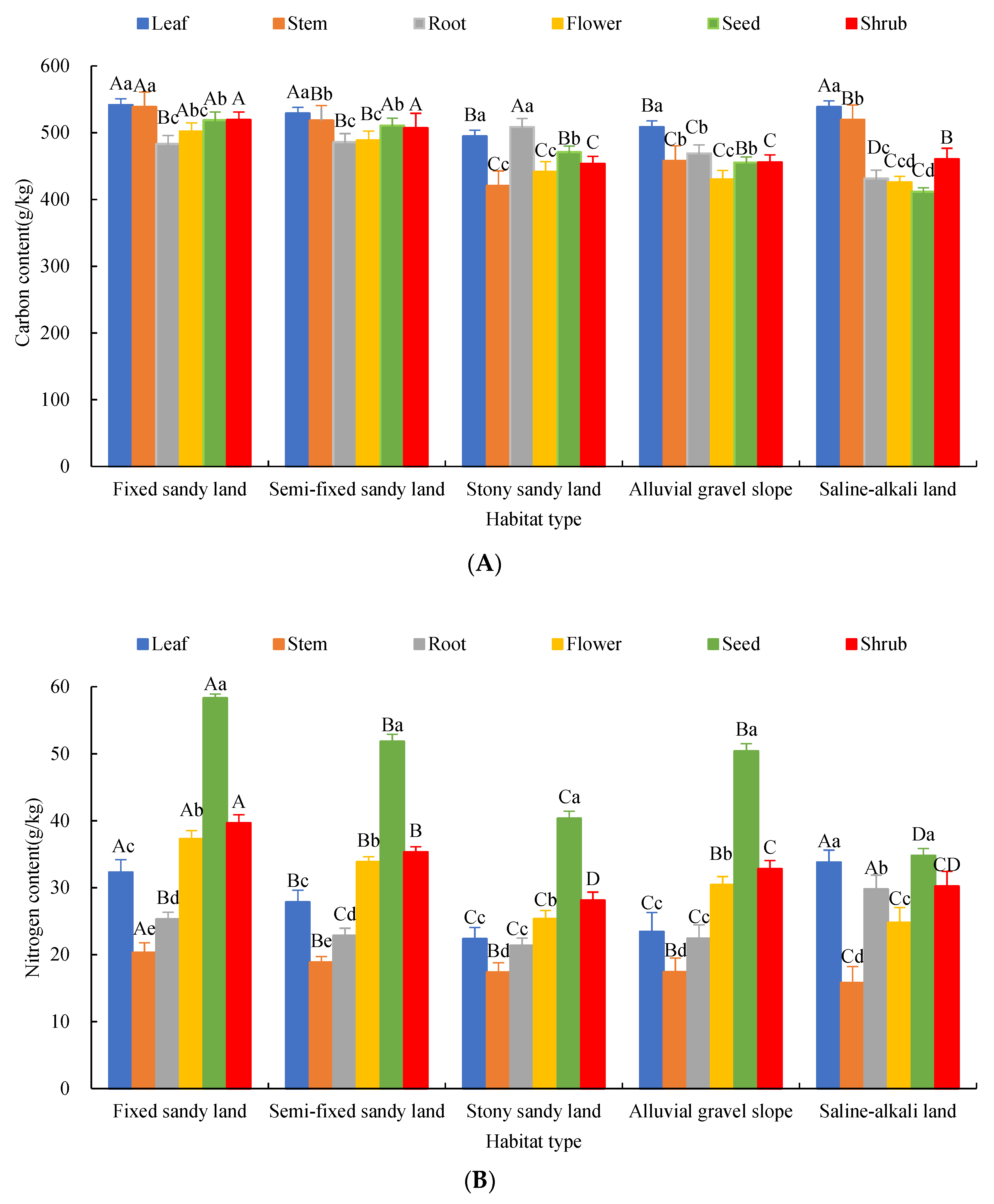
2.1. Changes of C, N, P and K Content of Each Organ of A. mongolicus
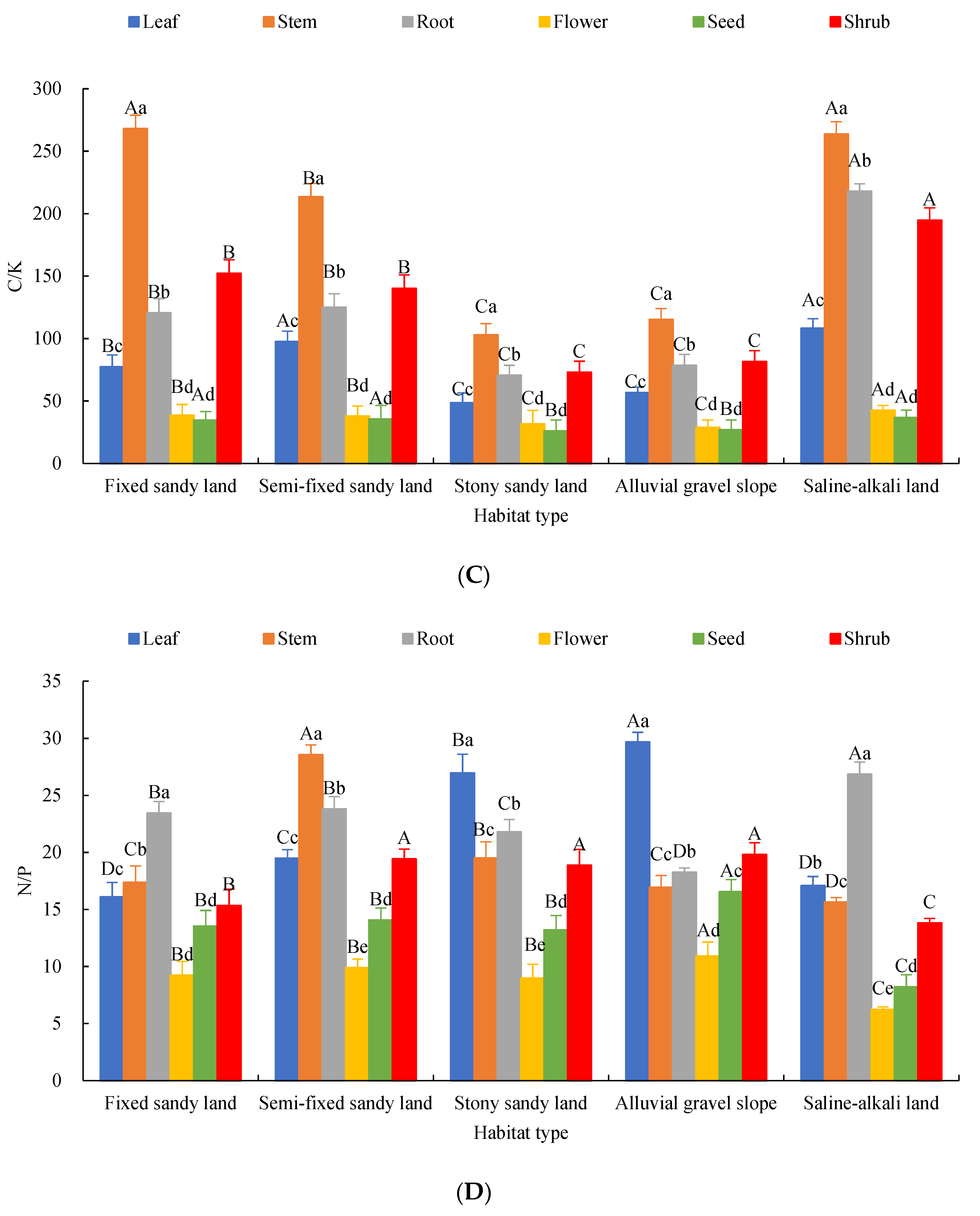
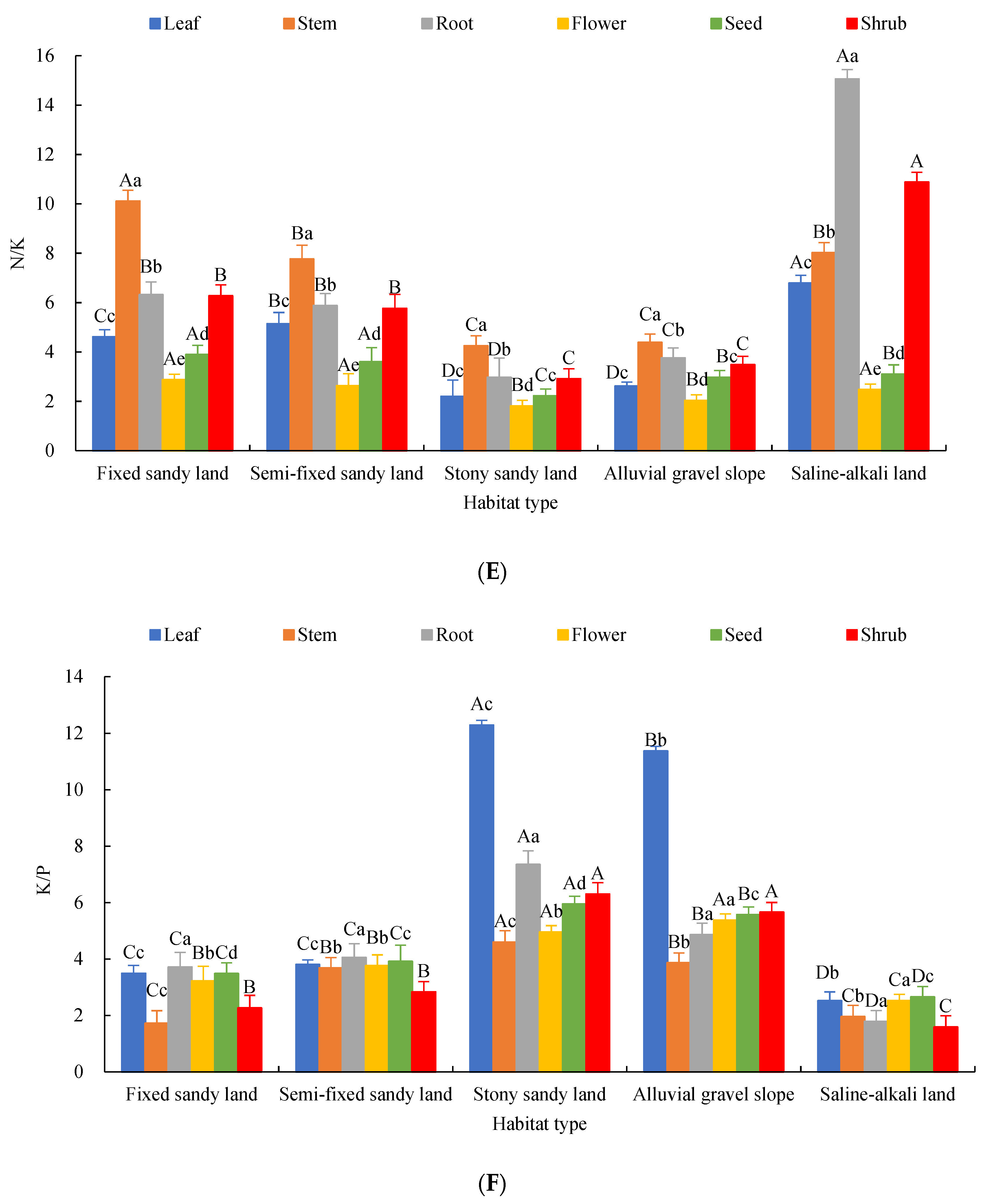
2.2. Characteristics of Chemical Element Stoichiometry in Each Organ of A. mongolicus
2.3. Effects of Organs and Habitats on the Elemental Content and Stoichiometric Ratios of A. mongolicus
3. Discussion
3.1. Variation Characteristics of C, N, P and K Contents and Stoichiometric Ratios in Each Organ of A. mongolicus
3.2. Response of Stoichiometric Characteristics of A. mongolicus Organs to Habitat
4. Materials and Methods
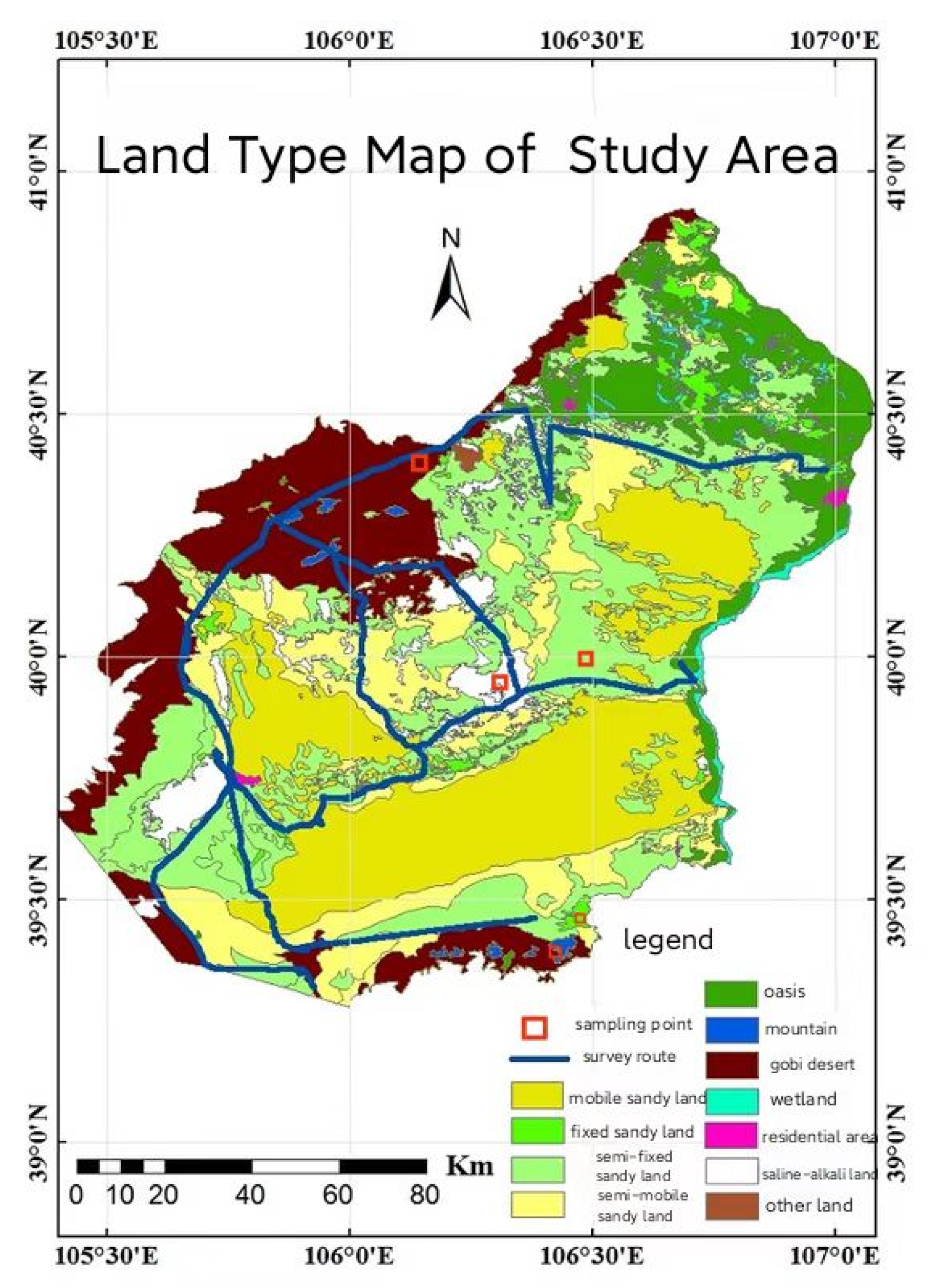
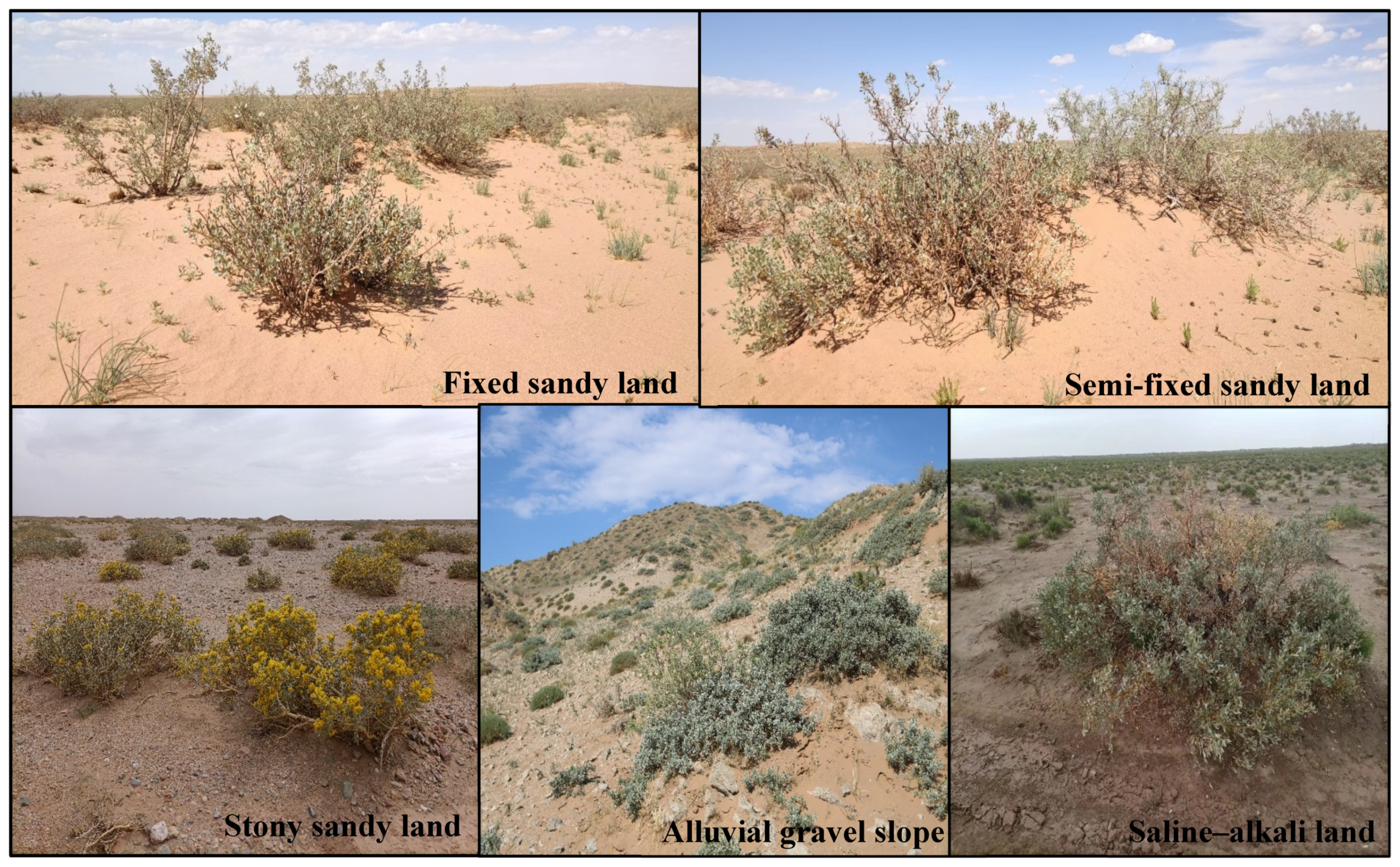
4.1. Sample Area Description
4.2. Plot Selection and Setting
4.3. Sample Collection and Determination Methods
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chapin, F.S. The mineral nutrition of wild plants. Annu. Rev. Ecol. Syst. 1980, 11, 233–260. [Google Scholar] [CrossRef]
- Akram, M.A.; Zhang, Y.; Wang, X.; Shrestha, N.; Malik, K.; Khan, I.; Ma, W.; Sun, Y.; Li, F.; Ran, J.; et al. Phylogenetic independence in the variations in leaf functional traits among different plant life forms in an arid environment. J. Plant Physiol. 2022, 272, 153671. [Google Scholar] [CrossRef] [PubMed]
- Güsewell, S. N:P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.J.; Sterner, R.W.; Gorokhova, E.; Fagan, W.F.; Markow, T.A.; Cotner, J.B.; Harrison, J.F.; Hobbie, S.E.; Odell, G.M.; Weider, L.W. Biological stoichiometry from genes to ecosystems. Ecol. Lett. 2000, 3, 540–550. [Google Scholar] [CrossRef]
- Akram, M.; Wang, X.; Hu, W.; Xiong, J.; Zhang, Y.; Deng, Y.; Ran, J.; Deng, J. Convergent Variations in the Leaf Traits of Desert Plants. Plants 2020, 9, 990. [Google Scholar] [CrossRef] [PubMed]
- Aronsson, A.; Elowson, S. Effects of irrigation and fertilization on mineral nutrients in scots pine needles. Ecol. Bull. 1980, 32, 219–228. [Google Scholar]
- Wang, F.; Guo, S.J.; Zhang, W.X. Seasonal variations of C, N and P stoichiometry characteristics in leaves of main shrubs in Minqin arid desert area. Acta Bot. Boreali-Occident. Sin. 2020, 40, 121–129. [Google Scholar]
- Chen, C.; Wang, G.J.; Zhao, Y.; Zhou, G.X.; Li, L.; Gao, J.Q. Seasonal dynamics and allometric growth relationships of C, N, and P stoichiometry in the organs of Cunninghamia lanceolata from Huitong. Acta Ecol. Sin. 2016, 36, 7614–7623. [Google Scholar]
- Zhang, T.L.; Qiu, Z.J.; Wu, Z.M.; Chen, Z.H.; Hu, H.; Zhou, G.Y.; Zhao, H.B.; Li, Z.J.; Cai, Z.L. Stoichiometric characteristics of carbon, nitrogen, phosphorus and potassium in organs of coniferous-broadleaved mixed forest in Northern Guangdong. For. Res. 2021, 34, 149–157. [Google Scholar]
- Sardans, J.; Rivas-Ubach, A.; Peñuelas, J. The elemental stoichiometry of aquatic and terrestrial ecosystems and its relationships with organismic lifestyle and ecosystem structure and function: A review and perspectives. Biogeochemistry 2011, 111, 1–39. [Google Scholar] [CrossRef]
- Touati, S.; Ayadi, J.; Bouajila, A.; Acila, S.; Rahmani, R.; Bouajila, J.; Debouba, M. Leaf morpho-physiology and phytochemistry of olive trees as affected by cultivar type and increasing aridity. J. Arid Land 2022, 14, 1159–1179. [Google Scholar] [CrossRef]
- Zhang, J.; He, N.; Liu, C.; Xu, L.; Yu, Q.; Yu, G. Allocation strategies for nitrogen and phosphorus in forest plants. Oikos 2018, 127, 1506–1514. [Google Scholar] [CrossRef]
- Wang, H.; Su, H.; Biswas, A.; Cao, J. Leaf stoichiometry of Leontopodium lentopodioides at high altitudes on the northeastern Qinghai-Tibetan Plateau, China. J. Arid Land 2022, 14, 1124–1137. [Google Scholar] [CrossRef]
- Wang, K.; Gao, S.; Liu, H.B.; Lv, L.Y.; Jiao, X.L. Effects of nitrogen and water addition on C, N, P stoichiometry in different organs of poplar seedlings. Chin. J. Ecol. 2021, 40, 3870–3880. [Google Scholar]
- Duan, Y.Z.; Wang, C.; Wang, H.T.; Du, Z.Y.; He, Y.M.; Chai, G.Q. Predicting the potential distribution of Ammopiptanthus species in China under different climates using ecological niche models. Acta Ecol. Sin. 2020, 40, 7668–7680. [Google Scholar]
- Niu, R.K.; Gao, R.H.; Hou, Y.Q.; Wang, X.D.; Wang, C.X. Predictionof the geographic distribution of Ammopiptanthus mongolicus under climate change. J. Northwest For. Univ. 2021, 36, 102–107. [Google Scholar]
- Wang, Y.; Shangguan, Z. Formation mechanisms and remediation techniques for low-efficiency artificial shelter forests on the Chinese Loess Plateau. J. Arid Land 2022, 14, 837–848. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, J.-M.; Xing, Z. Spatial patterns of leaf nitrogen and phosphorus stoichiometry across southeast to central Tibet. J. Mt. Sci. 2022, 19, 2651–2663. [Google Scholar] [CrossRef]
- Elser, J.J.; Fagan, W.F.; Denno, R.F.; Dobberfuhl, D.R.; Folarin, A.; Huberty, A.; Interlandi, S.; Kilham, S.S.; McCauley, E.; Schulz, K.L.; et al. Nutritional constraints in terrestrial and freshwater food webs. Nature 2000, 408, 578–580. [Google Scholar] [CrossRef]
- Ren, S.-J.; Yu, G.-R.; Jiang, C.-M.; Fang, H.-J.; Sun, X.-M. Stoichiometric characteristics of leaf carbon, nitrogen, and phosphorus of 102 dominant species in forest ecosystems along the North-South Transect of East China. Chin. J. Appl. Ecol. 2012, 23, 581–586. [Google Scholar]
- He, J.-S.; Fang, J.; Wang, Z.; Guo, D.; Flynn, D.F.B.; Geng, Z. Stoichiometry and large-scale patterns of leaf carbon and nitrogen in the grassland biomes of China. Oecologia 2006, 149, 115–122. [Google Scholar] [CrossRef]
- Zhang, K.; He, M.Z.; Li, X.R.; Tan, H.J.; Gao, Y.H.; Li, G.; Han, G.J.; Wu, Y.Y. Foliar carbon, nitrogen and phosphorus stoichiometry of typical desert plants across the Alashan Desert. Acta Ecol. Sin. 2014, 34, 6538–6547. [Google Scholar]
- Zheng, S.; Shangguan, Z. Spatial patterns of leaf nutrient traits of the plants in the Loess Plateau of China. Trees 2007, 21, 357–370. [Google Scholar] [CrossRef]
- Liu, P.; Ma, H.; Zhi, Y.B.; Cui, Y.; Sun, A.A.; Guo, Y.N.; Li, Q.; Gao, T.Y.; Zhang, H.L.; Liu, H.Y. Ecological stoichiometric differences of nine typical eremophyte species. Arid Zone Res. 2018, 35, 207–216. [Google Scholar]
- Han, W.; Fang, J.; Guo, D.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.B.; Shangguan, Z.P. Seasonal variations in leaf C, N, and P stoichiometry of typical plants in the Yangou watershed in the loess hilly gully region. Acta Ecol. Sin. 2011, 31, 4985–4991. [Google Scholar]
- Tian, D.; Yan, Z.-B.; Fang, J.-Y. Review on characteristics and main hypotheses of plant ecological stoichiometry. Chin. J. Plant Ecol. 2021, 45, 682–713. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Z.H.; Yang, J.M.; Chai, X.T.; Zeng, F.J. Stoichiometric characteristics of leaves and fine roots in Alhagi sparsifolia in response to the addition of nitrogen and water. Arid Zone Res. 2022, 39, 551–559. [Google Scholar]
- He, H.B.; Hao, Y.G.; Ding, Q.; Jia, G.X. Characteristics of plant community of Ammopiptanthus mongolicus and the diversity of its nodules. J. Beijing For. Univ. 2006, 28, 123–128. [Google Scholar]
- Minden, V.; Kleyer, M. Internal and external regulationof plant organ stoichiometry. Plant Biol. 2014, 16, 897–907. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Potassium: A neglected nutrient in global change. Glob. Ecol. Biogeogr. 2015, 24, 261–275. [Google Scholar] [CrossRef]
- Ågren, G.I. The C:N:P stoichiometry of autotrophs—Theory and observations. Ecol. Lett. 2004, 7, 185–191. [Google Scholar] [CrossRef]
- Niklas, K.J.; Cobb, E.D. N, P, and C stoichiometry of Eranthis hyemalis (Ranunculaceae) and the allometry of plant growth. Am. J. Bot. 2005, 92, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Xu, X.W.; Sun, Y.Q.; Qiu, Y.Z.; Li, S.Y.; Gao, P.; Zhong, X.B.; Yan, J.; Wang, G.F. Stoichiometric characteristics of C, N, P for three desert plants leaf and soil at different habitats. Arid Land Geogr. 2014, 37, 996–1004. [Google Scholar]
- Elser, J.J.; Fagan, W.F.; Kerkhoff, A.J.; Swenson, N.G.; Enquist, B.J. Biological stoichiometry of plant production: Metabolism, scaling and ecological response to global change. New Phytol. 2010, 186, 593–608. [Google Scholar] [CrossRef]
- Bista, D.R.; Heckathorn, S.A.; Jayawardena, D.M.; Mishra, S.; Boldt, J.K. Effects of drought on nutrient uptake and the levels of nutrient-uptake proteins in roots of drought-sensitive and -tolerant grasses. Plants 2018, 7, 28. [Google Scholar] [CrossRef]
- Bao, H.; Qing, H.; Wang, L.X.; Duan, Y.X.; Liu, D.W.; Zhuo, Y.; Liu, H.M. A study on ecological stoichiometry of plants on lakeside zone of Wuliangsuhai Lake. J. Inn. Mong. Univ. (Nat. Sci. Ed.) 2014, 45, 404–409. [Google Scholar]
- Luo, Y.; Gong, L. Stoichiometric characteristics in root, stem and leaf of Phragmites australis in different habitats in the southern marginal zone of Tarim Basin. Chin. J. Ecol. 2016, 35, 684–691. [Google Scholar]
- Li, H.S. Modern Plant Physiology; Higher Education Press: Beijing, China, 2002. [Google Scholar]
- Kerkhoff, A.J.; Fagan, W.F.; Elser, J.J.; Enquist, B.J. Phylogenetic and Growth Form Variation in the Scaling of Nitrogen and Phosphorus in the Seed Plants. Am. Nat. 2006, 168, 103–122. [Google Scholar] [CrossRef]
- Fortunel, C.; Fine, P.V.A.; Baraloto, C. Leaf, stem and root tissue strategies across 758 Neotropical tree species. Funct. Ecol. 2012, 26, 1153–1161. [Google Scholar] [CrossRef]
- Chen, X.P.; Guo, B.Q.; Zhong, Q.L.; Wang, M.T.; Li, M.; Yang, F.C.; Cheng, D.L. Response of fine root carbon, nitrogen, and phosphorus stoichiometry to soil nutrients in Pinus taiwanensis along an elevation gradient in the Wuyi mountains. Acta Ecol. Sin. 2018, 38, 273–281. [Google Scholar]
- Schreeg, L.A.; Santiago, L.S.; Wright, S.J.; Turner, B.L. Stem, root, and older leaf N:P ratios are more responsive indicators of soil nutrient availability than new foliage. Ecology 2014, 95, 2062–2068. [Google Scholar] [CrossRef] [PubMed]
- Song, S.M.; Zhang, D.J.; Zhang, J.; Yang, W.Q.; Zhang, Y.; Zhou, Y.; Li, X. Edge effects of forest gap in Pinus massoniana plantations on the ecological stoichiometry of Cinnamomum longepaniculatum. Chin. J. Plant Ecol. 2017, 41, 1081–1090. [Google Scholar]
- Dong, X.; Xin, Z.M.; Huang, Y.R.; Li, X.L.; Hao, Y.G.; Liu, F.; Liu, M.H.; Li, W. Soil stoichiometry in typical shrub communities in the Ulan Buh Desert. Acta Ecol. Sin. 2019, 39, 6247–6256. [Google Scholar]
- Bao, S.D. Agrochemical Analysis of Soil, 3rd ed.; China Agricultural Publishing House Press: Beijing, China, 2000. [Google Scholar]


| Variables | Organ | Habitat | Interaction |
|---|---|---|---|
| C content | 3.68 * | 4.88 * | 10.27 ** |
| N content | 79.81 ** | 15.26 ** | 8.91 ** |
| P content | 113.01 ** | 9.98 ** | 2.18 |
| K content | 399.00 ** | 147.24 ** | 4.87 ** |
| C/N | 157.81 ** | 3.45 | 14.49 |
| C/P | 42.14 ** | 2.59 | 3.59 * |
| C/K | 101.28 ** | 55.15 ** | 11.95 ** |
| N/P | 37.39 ** | 3.60 | 5.24 ** |
| N/K | 42.11 ** | 52.29 ** | 18.63 ** |
| K/P | 17.51 ** | 116.37 ** | 13.43 ** |
| Habitat | Height (cm) | Diameter (mm) | The Crown Area (m2) | Vegetation Coverage (%) | Soil Moisture (%) | Soil Organic Carbon (g/kg) | Soil Total Nitrogen (g/kg) | Soil Total Phosphorus (g/kg) |
|---|---|---|---|---|---|---|---|---|
| Fixed sandy land | 118.23 ± 19.65 | 24.39 ± 2.89 | 12.51 ± 1.89 | 25–40% | 3.92 ± 0.75 | 3.92 ± 1.75 | 0.38 ± 0.17 | 0.34 ± 0.06 |
| Semi-fixed sandy land | 89.5 ± 3.7 | 22.69 ± 2.33 | 8.23 ± 0.45 | 10–25% | 3.36 ± 1.02 | 3.36 ± 1.07 | 0.31 ± 0.08 | 0.32 ± 0.05 |
| Stony sandy land | 67.6 ± 5.6 | 15.72 ± 1.05 | 5.68 ± 0.56 | 15–30% | 3.25 ± 0.83 | 3.35 ± 0.93 | 0.26 ± 0.04 | 0.27 ± 0.03 |
| Alluvial gravel slope | 34.5 ± 2.9 | 7.89 ± 1.03 | 2.09 ± 0.42 | 10–25% | 2.91 ± 0.72 | 2.99 ± 0.82 | 0.29 ± 0.07 | 0.31 ± 0.02 |
| Saline–alkali land | 78.6 ± 6.6 | 18.75 ± 1.19 | 6.48 ± 0.56 | 20–35% | 4.39 ± 0.85 | 4.19 ± 0.45 | 0.41 ± 0.12 | 0.36 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Zhang, J.; Xin, Z.; Huang, Y.; Han, C.; Li, Y.; Lu, Q. Ecological Stoichiometric Characteristics in Organs of Ammopiptanthus mongolicus in Different Habitats. Plants 2023, 12, 414. https://doi.org/10.3390/plants12020414
Dong X, Zhang J, Xin Z, Huang Y, Han C, Li Y, Lu Q. Ecological Stoichiometric Characteristics in Organs of Ammopiptanthus mongolicus in Different Habitats. Plants. 2023; 12(2):414. https://doi.org/10.3390/plants12020414
Chicago/Turabian StyleDong, Xue, Jinbo Zhang, Zhiming Xin, Yaru Huang, Chunxia Han, Yonghua Li, and Qi Lu. 2023. "Ecological Stoichiometric Characteristics in Organs of Ammopiptanthus mongolicus in Different Habitats" Plants 12, no. 2: 414. https://doi.org/10.3390/plants12020414
APA StyleDong, X., Zhang, J., Xin, Z., Huang, Y., Han, C., Li, Y., & Lu, Q. (2023). Ecological Stoichiometric Characteristics in Organs of Ammopiptanthus mongolicus in Different Habitats. Plants, 12(2), 414. https://doi.org/10.3390/plants12020414






