Abstract
The root microbiome structure ensures optimal plant host health and fitness, and it is, at least in part, defined by the plant genotype. It is well documented that root-secreted amino acids promote microbial chemotaxis and growth in the rhizosphere. However, whether the plant-mediated re-uptake of amino acids contributes to maintaining optimal levels of amino acids in the root exudates, and, in turn, microbial growth and metabolism, remains to be established. Here, we show that Lysine-Histidine Transporter-1 (LHT1), an amino acid inward transporter expressed in Arabidopsis thaliana roots, limits the growth of the plant-growth-promoting bacteria Pseudomonas simiae WCS417r (Ps WCS417r). The amino acid profiling of the lht1 mutant root exudates showed increased levels of glutamine, among other amino acids. Interestingly, lht1 exudates or Gln-supplemented wild-type exudates enhance Ps WCS417r growth. However, despite promoting bacterial growth and robust root colonization, lht1 exudates and Gln-supplemented wild-type exudates inhibited plant growth in a Ps WCS417r-dependent manner. The transcriptional analysis of defense and growth marker genes revealed that plant growth inhibition was not linked to the elicitation of plant defense but likely to the impact of Ps WCS417r amino acids metabolism on auxin signaling. These data suggest that an excess of amino acids in the rhizosphere impacts Ps WCS417r metabolism, which, in turn, inhibits plant growth. Together, these results show that LHT1 regulates the amino-acid-mediated interaction between plants and Ps WCS417r and suggest a complex relationship between root-exuded amino acids, root colonization by beneficial bacteria, bacterial metabolism, and plant growth promotion.
Keywords:
Arabidopsis; LHT1; rhizosphere; amino acid homeostasis; beneficial microbes; plant fitness 1. Introduction
Crop agriculture has been fueled by an over reliance on chemical fertilizers that have environmentally damaging consequences. Proposed alternative approaches to enhancing plant growth include the use of plant-growth-promoting bacteria (PGPB), such as Pseudomonas simiae WCS417r, formerly known as Pseudomonas fluorescens WCS417r [1]. In addition to factors that impact soil quality, metabolites exuded by roots have a major impact on the establishment of root associations with PGPB. Plants exude nutrient-rich fluids through their roots. These fluids, known as root exudates, influence the rhizosphere by inhibiting the growth of harmful microbes while promoting the growth of beneficial ones. Amino acids (AAs) are among the most represented metabolites in the root exudates of the model plant Arabidopsis thaliana [2,3,4] Microbes in the rhizosphere use AAs, or their derivatives, as food sources and perhaps to communicate with other microbes (e.g., quorum sensing) [5,6,7] In addition, microbes use AA concentration gradients to navigate the soil toward roots. For example, in the colonization of tomato root tips, wild-type Pseudomonas fluorescens Pf0–1 outcompetes a triple-mutant derivative strain with impaired chemotaxis towards AAs [8]. Microbes also use rhizospheric AAs to communicate with the plant. For instance, some microbial products trigger the release of AAs from plant roots [9], creating a self-reinforcing microbe–plant interaction. Microbes can also convert AAs into bioactive compounds with opposing effects on plant physiology. For example, microbes can convert AAs into plant-growth-promoting substances, such as the auxin hormone indole-3-acetic acid (IAA) [10]. In contrast, on the other side of the spectrum, microbes have the capacity to metabolize AAs present in the rhizosphere (e.g., glutamine) into derivatives that can inhibit plant growth (e.g., ammonium) [11,12].
On the plant side, the uptake of inorganic and organic nitrogen (including AAs) through the plant roots has been thoroughly studied, and AA importers mediating this uptake have been reported [13,14,15,16,17]. There are at least 61 transporters with confirmed or putative AA uptake activity encoded in the genome of Arabidopsis thaliana [18,19,20]. However, to our knowledge, only three of these transporters localize to the plasma membrane of root cortex cells and can take up AAs at the low concentrations at which they are present in the soil. These three AA importers are LHT1 of the LHT family (lysine–histidine-like transporters), AAP5 of the AAP family (amino acid permeases), and ProT2 of the ProT family (proline transporters) [21,22,23,24,25] These AA importers have broad and overlapping specificities. However, they also show increased affinity for AAs that share molecular geometries and charges [21,26,27]. Notwithstanding their detailed characterization, it is still unclear to which extent AA importers expressed in the root cortex modulate the concentration of plant-derived AAs in the rhizosphere and how their activities contribute to building associations with beneficial bacteria [8]. Answering this question is important as the concentration and balance of different AAs in the rhizosphere may impact the growth of belowground microbes and hence the growth of plants themselves. For example, the colonization of cucumber roots by Bacillus amyloliquefaciens SQR9 enhances tryptophan secretion from the roots and promotes IAA biosynthesis by B. amyloliquefaciens SQR9, which, in turn, boosts plant growth [10]. Amino acids also serve as chemoattractants for different soil microbes, such as Pseudomonas fluorescens Pf0–1 [8], Ralstonia pseudosolanacearum [28], and Sinorhizobium meliloti [29]. To address if and how AA importers contribute to maintaining the amino-acidic composition of the root exudates, the composition and concentration of individual AAs were assessed in root exudates of Arabidopsis wild-type and loss-of-function mutants for the amino acid transporters AAP5, ProT2, and LHT1. Data obtained from AAs profiling, root colonization, plant growth assessment, and Arabidopsis gene expression indicate that LHT1 modulates the concentration of AAs in the root exudates, which, in turn, controls the growth and metabolism of Ps WCS417r and its ability to promote plant growth.
2. Results
2.1. The Amino Acid Transporter LHT1 Modulates Amino Acid Content in Arabidopsis Root Exudates
Plants can modulate amino acid (AA) content in the rhizosphere by controlling the expression and activity of exporters that exude AAs out of the roots or importers that take up AAs present in the soil [30]. However, it is less clear to what extent plants use AA importers to take up AAs that the plants themselves exude into the rhizosphere. This putative retrieval of AAs from the exudates could contribute to modulating AA content in the rhizosphere and hence the growth of PGPB. To establish whether plants control the accumulation of plant-derived amino acids in the rhizosphere through the activity of AA importers, loss-of-function mutants for AA transporters with confirmed uptake activity and expressed in the root [21,22,23,24,25], were tested. The loss-of-function mutants aap5, prot2, and lht1 were chosen because: (i) they represent three families of AA importers, (ii) are expressed in the cortex of the root [23,25,27], and (iii) LHT1 and AAP5, at least, can take up AAs at the concentrations present in the soil [24].
The experimental strategy was based on the method described by Haney et al. [31] with some modifications (Figure S1A). Briefly, Arabidopsis seedlings were initially grown in full-strength (1X) MS medium with sucrose (0.5%) for 12 days and then transferred to half-strength (0.5X) MS without sucrose for 3 days. To enable the collection of root exudates for further analysis, the seedlings were grown with the roots submerged in liquid culture and separated from the shoots by a sterilized polytetrafluoroethylene (PTFE) mesh that floats at the surface of the liquid. After 15 days of growth, the root exudates were collected, and the total AA content was assessed using a previously described method [32]. Two aap5 and one prot2 mutant lines showed no differences in total AA content in the root exudates when compared to Col-0 wild-type plants (Figure S1B). By contrast, the root exudates of the lht1 mutants showed increased levels of AAs when compared to wildtype (Figure 1A). Since LHT1 localizes to the plasma membrane of the rhizodermis [21], where it acts as an AA importer [22], and the culture media was not supplemented with AAs, the AAs that accumulate in the lht1 root exudates must be of plant origin. Hence, the changes in the composition of the lht1 exudates are most likely due to deficient rhizospheric AA reuptake by LHT1. Together, the results indicate that the loss of the AA importer LHT1 can alter the AA profile of root exudates and that not all AA importers expressed in the root serve the function of maintaining the AA concentrations of the exudates. Even though the biochemical activities and localization of LHT1 have been known to be compatible with regulating the rhizosphere’s AA composition through re-uptake, this specific function had not been previously reported.
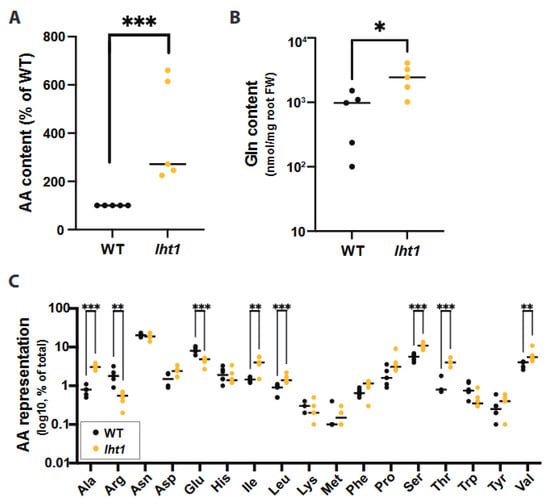
Figure 1.
LHT1 modulates amino acid content of Arabidopsis root exudates. Across all panels in this figure, unless otherwise stated, data are from at least 3 independent biological replicates. In each biological replicate, at least 5 seedlings/plants or their products (e.g., exudates) were analyzed. Each dot in the scattered dot plots shows the median of the 5 or more seedlings/plants, and the median of all replicates is depicted as a bar. A two-sided Student’s t-test was performed for statistical comparison of two means, or a Welch’s t-test was used for two means with unequal variances when relevant. The p-values are represented as * ≤0.05, ** ≤0.01, and *** ≤0.005. Further experimental and analysis details are described in Section 5. (A) The lht1 mutant root exudates have higher AA content than wild-type (WT) exudates. Exudates obtained from 15-day-old lht1 or WT seedlings grown on 1X MS liquid medium containing 0.5% sucrose were filter-sterilized, and their total AA content was assessed using the L-Amino Acid Quantitation Colorimetric/Fluorometric Kit (see Methods). Each data point represents the median of 6 wells in each of 5 independent biological replicates. Data originally scored as nM of total L-AAs per mg of fresh root tissue are presented as a percentage of WT to be able to compare independent biological replicates in the same graph. (B) The lht1 mutant root exudates have higher glutamine (Gln) content than wild-type exudates. Root exudates obtained in (A) were treated with formic acid and profiled via LC-MS. Data is shown as a percentage of total AAs in lht1 and WT exudates. (C) The lht1 mutant root exudates show imbalanced AA content. Glycine data was omitted because unplanted MS medium contained background levels of glycine (2 mg/L). LC-MS analysis was performed on 6 independent samples per condition.
Liquid chromatography coupled to mass spectrometry (LC-MS) analysis was used to define the specific AA changes in the root exudates of the lht1 plant. This analysis revealed that glutamine (Gln) was the most abundant amino acid that had significantly elevated concentrations in the root exudates of lht1 compared to those of wild-type plants (Figure 1B). Nonetheless, in line with the broad AA substrate specificity of LHT1 [21], the analysis revealed that several other AAs were enriched in lht1 root exudates, including alanine, asparagine, aspartic acid, leucine, isoleucine, methionine, phenylalanine, proline, serine, threonine, and valine when compared to the root exudates of wild-type plants (Figure 1C). The rationale to assign LHT1 a role in AA re-uptake from the root exudates is based on its thoroughly established importer function [33]. Since LHT1 moves AAs in an inward direction only, and our root exudate assays were performed on AA-free media, the most parsimonious model to explain the overaccumulation of AAs in the lht1 root exudates is that LHT1 modulates the levels of AAs in the exudates through the re-uptake of previously exuded amino acids.
2.2. The lht1 Root Exudates Enhance Ps WCS417r Growth
Plant-growth-promoting bacteria (PGPB), such as Ps WCS417r, colonize the surface of Arabidopsis roots and promote plant growth [1]. Since the growth of PGPB depends on the biophysical properties and composition of the rhizosphere, which are modulated by the plant itself, testing whether the changes in lht1 root exudates affected the growth of Ps WCS417r would provide important information to define which plant-made AAs could favor bacterial and plant growth. To this end, the growth of Ps WCS417r in wild-type and lht1 root exudates, as well as in unplanted MS media (MS medium not conditioned by plants) as a control, was longitudinally measured using absorbance OD600nm over a 24 h period with intermittent shaking in a microtiter plate reader. In line with their higher AA content, lht1 root exudates supported more bacterial growth than wild-type root exudates (Figure 2A). Considering that wild-type and lht1 root exudates may differ in the content and abundance of metabolites other than AAs, testing the effect of AA abundance on PGPB growth would at least address if AAs that are over-represented in lht1 exudates could augment bacterial growth. The supplementation of wild-type exudates with Gln was sufficient to boost Ps WCS417r growth (Figure 2B), suggesting that plant-derived Gln is a limiting factor for Ps WCS417r growth in the Arabidopsis rhizosphere. Similarly, supplementing wild-type root exudates with serine (Ser), another AA that accumulates in the lht1 root exudates, promoted Ps WCS417r growth (Figure 2C), suggesting an overall positive correlation between amino acid content in the root exudates and the growth of beneficial microbes in the rhizosphere.
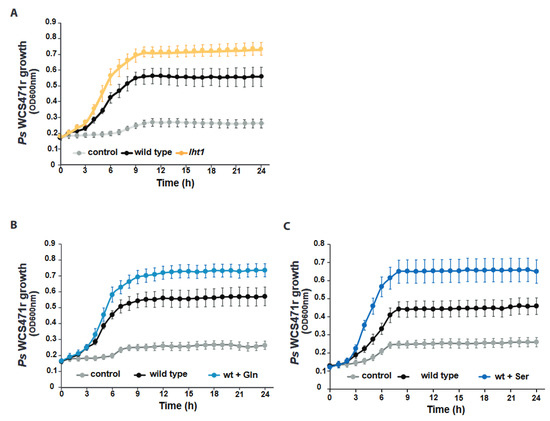
Figure 2.
The lht1-derived root exudates enhance Ps WCS417r growth. Across all panels in this figure, data represent 3 independent biological replicates. Exudates were obtained, as described in Figure 1A and Section 5. In each biological replicate, at least 6 wells per condition were seeded with exudates or control media and longitudinally scored for growth every hour using OD600nm as the readout. Plates were incubated and scored in a temperature-controlled plate reader with shaking. Control corresponds to MS medium without exudate. Each dot in the curves represents the median growth of 6 wells at a given time point. Error bars are SEM. Statistical analysis was performed using the CGGC (comparison of groups of growth curves) permutation test to compare pairs of samples (e.g., wild type vs. lht1) over the course of growth (24 h). All differences between conditions were statistically significant. Further experimental and analysis details are described in Section 5. (A) Ps WCS417r grows better in lht1-derived than WT exudates. (B) Ps WCS417r grows better in Gln-supplemented WT exudates. WT exudates were supplemented with 1 mM Gln (final concentration) or an equal volume of water as control. (C) Ps WCS417r grows better in Ser-supplemented WT exudates. WT exudates were supplemented with 1 mM Ser (final concentration) or an equal volume of water as control.
2.3. Loss of LHT1 Promotes Root Colonization by Ps WCS417r
Having uncovered that lht1 root exudates promote more Ps WCS417r growth, the next question to address was whether this enhanced in vitro growth would translate to better root colonization. To this end, Arabidopsis seedlings were grown for two weeks in MS-PhytoAgar plates incubated vertically and then transferred to sterile plates with autoclaved 3 MM paper as the substrate (Figure S2A,B). One day later, roots were flood-inoculated with Ps WCS417r. After inoculation, the plates were incubated horizontally, and root colonization proceeded for 72 h. At that time, roots were weighed and processed to assess colonization as colony-forming units (CFU) per root weight. In line with the in vitro growth assays, the roots of lht1 supported more bacterial growth than the roots of wild-type seedlings (Figure 3A). These data support the hypothesis that the higher concentrations of AAs present in the rhizosphere of lht1 plants have a positive impact on the growth of PGPB, such as Ps WCS417r. Next, to investigate the mechanisms underlying the enhanced bacterial colonization of lht1 roots, two processes known to contribute to the establishment of a successful root–bacteria interaction, chemotaxis [8,34,35,36] and biofilm formation [37], were tested. The chemotaxis index was assessed using a modified capillary assay previously described [34,38] (Figure S3). Unplanted MS media (MS medium that has not been conditioned by roots) was used to assess the background swarming motility of Ps WCS417r. The data showed a significant preference for lht1 root exudates in both non-competitive (Figure 3B) and competitive chemotaxis assays (Figure 3C–E). To define whether the chemotaxis-enhancing capacity of lht1 exudates was driven, at least in part, by the AAs that promote bacterial growth, wild-type exudates supplemented with Gln were also tested. Indeed, wild-type exudates supplemented with Gln showed higher chemotaxis-promoting capacity and hence higher Ps WCS417r titers than non-supplemented wild-type exudates (Figure 3F). The capacity of Ps WCS417r to form biofilms in wild-type and lht1 root exudates, as well as in unplanted media as a control, was assessed using the crystal violet staining method [37]. We hypothesized that the increased accumulation of AAs observed in the root exudates obtained from lht1 plants could facilitate biofilm formation and, in this way, the growth of Ps WCS417r in the rhizosphere. Consistent with this hypothesis, Ps WCS417r showed an enhanced capacity to form biofilm in the lht1 root exudates compared to wild-type root exudates (Figure 3G). Therefore, altogether, the AA transporter LHT1 defines the AA content of the root exudates, the chemotaxis, the biofilm formation, and the growth capacity of Ps WCS417r.
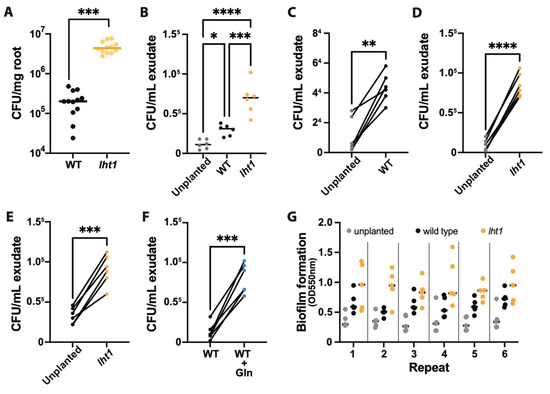
Figure 3.
Ps WCS417r more effectively colonizes lht1 than wild-type roots. Across all panels in this figure, data are from at least 5–6 independent biological replicates. In each biological replicate, at least 5 seedlings/plants or their products (e.g., exudates) were analyzed. Each dot represents the median of 5 seedlings, 5 independently collected exudates, or 3 chemotaxis syringes per condition. In panels (A,B) the median of all biological replicates is shown as a bar. A two-sided Student’s t-test was performed for statistical comparison of two means or a Welch’s t-test for two means with unequal variances. For comparison of more than two means, a one-way ANOVA followed by Tukey’s posthoc test or a Kruskal–Wallis test for unequal variances followed by Dunn’s posthoc test was performed. The p-values are represented as * ≤0.05, ** ≤0.01, *** ≤0.005, and **** ≤0.001. Further experimental and analysis details are described in Section 5. (A) Loss of LHT1 promotes root colonization by Ps WCS417r. The 1X MS-wetted 3MM paper in which 6 seedlings were growing was inoculated with Ps WCS417r at OD600nm = 0.00002. Seventy-two hours later, the roots were harvested, weighed, and ground; platting of serial dilution was used to assess colony-forming units (CFU) on LB agar with 50 µg/mL of rifampicin. Colonization is presented as CFU per mg of root fresh weight. (B) AAs-rich lht1 exudates attract Ps WCS417r more effectively than WT exudates. For this non-competitive chemotaxis assay, 200 µL of filter-sterilized exudates of each condition were loaded in 1 mL syringes without the needles. The tip of each syringe was then dipped in an independent Petri dish containing chemotaxis buffer inoculated with Ps WCS417r at a final OD600nm = 0.002. After 1 h, bacterial cells inside the syringe were harvested and diluted to count CFUs. Data are depicted as total CFUs per mL of exudate. (C–F) For competitive chemotaxis assays, 200 µL of filter-sterilized exudates of each condition were loaded in 1 mL syringes. Then, the tip of two syringes of different conditions was dipped in the same Petri dish containing chemotaxis buffer inoculated with Ps WCS417r at a final OD600nm = 0.002. After 30 min, bacterial cells inside each of the two syringes were harvested and diluted to measure CFU. Data are presented as total CFU per mL of each exudate. Competitive chemotaxis results are presented as follows: (C) Control 0.5X MS medium (without sucrose) versus WT exudate, (D) Control 0.5X MS medium (without sucrose) versus lht1 exudate, (E) WT versus lht1 exudate, and (F) WT exudate versus WT exudate supplemented with 1 mM Glutamine. (G) The lht1 exudates better support Ps WCS417r capacity to produce biofilm. Six aliquots of 0.5X MS medium (without sucrose), wild-type root exudates, or lht1 root exudates were loaded on independent wells of a 96-well round-bottom plate and then inoculated with 2 µL freshly harvested Ps WCS417r grown overnight in LB medium. After 48 h of static growth, biofilms were stained with crystal violet, and absorbance at 550 nm was measured in a microplate reader, as described in Methods. Each depicted dot represents the absorbance in each of 6 wells per condition. Biofilm formation was independently tested 6 times.
2.4. Rhizospheric Amino Acids Stimulate Robust Root Colonization but Compromise Plant Growth in a Microbial-Dependent Manner
Ps WCS417r colonizes the root surface and promotes the growth of wild-type plants with a wild-type rhizospheric composition. Since the AA-enriched exudates of lht1 support more bacterial growth than wild-type exudates, we hypothesized that wild-type plants exposed to lht1 exudates and Ps WCS417r (lht1 + Ps) would grow larger than plants exposed to wild-type exudates and Ps WCS417r (WT + Ps). Unexpectedly, this analysis showed the opposite outcome: lht1 exudates and Ps WCS417r inhibited the growth of wild-type seedlings (Figure 4A). Importantly, as expected, when wild-type seedlings were treated with Ps WCS417r only, they grew larger than untreated seedlings. Similarly, lht1 exudates alone did not inhibit the growth of wild-type seedlings (Figure 4A). Therefore, the growth inhibition depends on both lht1 root exudates and Ps WCS417r. To narrow down the mediators of this plant growth inhibitory effect, Arabidopsis growth was assessed after exposure to Gln-supplemented wild-type root exudates and Ps WCS417r (WT-Gln + Ps). Like lht1 + Ps, WT-Gln + Ps also inhibited the growth of wild-type Arabidopsis seedlings (Figure 4B). Ruling out a direct toxic effect of Gln, seedling growth was not inhibited by 1 mM or 10 mM Gln (Figure 4B), implying a microbe-mediated inhibition of plant growth that is dependent on Ps WCS417r Gln metabolism. Importantly, a high Ps WCS417r inoculation titer (OD600nm = 1.6) did not compromise plant growth, suggesting that the high bacterial colonization in lht1 + Ps and WT-Gln + Ps does not contribute to plant growth inhibition (Figure 4B). To test the impact of other AAs that accumulate in lht1 root exudates, plant growth in wild-type exudates supplemented with 1 mM or 10 mM serine (Ser) was assessed. Interestingly, an excess of Ser significantly inhibited plant growth at both concentrations tested and in the absence of Ps WCS417r (Figure 4C). These data demonstrate that Ser does not contribute to the plant growth inhibitory effect of lht1 exudates mediated by Ps WCS417r.
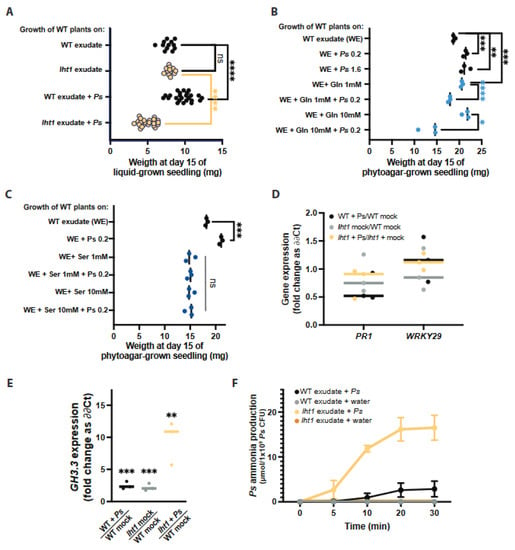
Figure 4.
Excess AAs in lht1 exudates combined with Ps WCS417r inhibit wild-type Arabidopsis growth. Across all panels, each dot represents an independent biological replicate. The median of all biological replicates is depicted as a bar. A two-sided Student’s t-test was performed for statistical comparison of two means or a Welch’s t-test for two means with unequal variances. For comparison of more than two means, a one-way ANOVA followed by Tukey’s posthoc test, or a Kruskal–Wallis test for unequal variances followed by Dunn’s posthoc test was performed. The p-values are represented as * ≤0.05, ** ≤0.01, *** ≤0.005, and **** ≤0.001. Further experimental and analysis details are described in Section 5. (A) The lht1 exudates inhibit the growth of wild-type Arabidopsis when inoculated with Ps WCS417r. Fifteen-day-old wild-type seedlings growing on 12-well plates with floating mesh on WT or lht1 exudate with or without Ps WCS417r OD600nm = 0.2. Each dot represents the median weight of ≥3 seedlings per condition. The experiment was repeated 6 times; the median of all biological replicates is depicted as a bar. (B) Gln-supplemented WT exudates combined with Ps WCS417r inhibit the growth of wild-type Arabidopsis. Assay was carried out as in (A), except that two different Ps WCS417r inoculum titters were used (OD600nm = 0.2 or 1.6) and that WT exudates were supplemented with Gln 1mM or 10 mM and either inoculated or not with Ps WCS417r (OD600nm = 0.2). (C) Ser-supplemented WT exudates directly inhibit the growth of wild-type Arabidopsis. Assay and controls are shown in (B), except for WT exudates that were supplemented with 1 mM or 10 mM Ser (instead of Gln). (D) Pathogen-responsive genes are not induced in plants concomitantly exposed to Ps WCS417r and lht1 exudates. Wild-type seedlings were exposed for 24 h to WT exudate (depicted as WT mock), WT exudate + Ps WCS417r (final OD600nm = 0.2), lht1 exudate (depicted as lht1 mock), or lht1 exudate + Ps WCS417r (final OD600nm = 0.2), as described here in (A) and in Methods. Gene expression in the roots was assessed using RT-qPCR. Fold change was calculated using ∂∂Ct. The specific comparisons are depicted in the X-axis. Each dot corresponds to an independent biological replicate (calculated based on 2 experimental replicates), and the median of the 3 biological replicates is depicted as a bar. (E) GH3.3, a mediator of the stress-triggered growth inhibition response, is induced in plants concomitantly exposed to Ps WCS417r and lht1 exudates. Expression of the gene encoding the auxin-conjugating enzyme GH3.3 is measured in the conditions described in (D). (F) Ps WCS417r produces more ammonia from lht1 than from wild-type exudates. Ammonia excreted by Ps WCS417r while incubated on WT or lht1 exudates was assessed at 0, 5, 10, 20, and 30 min. Exudates obtained in three independent experiments were assessed for ammonia independently but simultaneously. After the indicated times, the incubation media supernatants were harvested, filter sterilized, and their ammonia content was assessed using an ammonia assay kit (AbCam; Cat # ab102509) following manufacturer’s guidelines. Each dot represents the median ammonia concentration (µmol/mL), and error bars correspond to the SEM.
Across plant species, the response to abiotic and biotic stressors usually includes the inhibition of plant growth [39,40,41]. To identify the underlying mechanisms associated with plant growth inhibition when Ps WCS417r colonizes an AA-rich rhizosphere, the expression of Arabidopsis genes induced in response to various stressors that produce plant growth inhibition was tested. First, the elicitation of plant immunity is typically accompanied by the inhibition of plant growth [42,43,44]. To test if plant immunity was elicited by the combination of lht1 exudates and Ps WCS417r, the expression levels of PR1 and WRKY29 were assessed by RT-qPCR. The expression of these genes is induced in response to a broad range of pathogenic cues [45,46]. Arguing against a generalized activation of the plant defense in response to larger-than-normal root colonization that could negatively impact plant growth, the data showed similar levels of PR1 or WRKY29 expression in wild-type roots colonized with Ps WCS417r, regardless of the origin of the plant exudates (Figure 4D). The levels and potency of the plant growth hormone indole-3-acetic acid (IAA) are partially controlled by the activity of enzymes that conjugate amino acids to IAA, producing IAA derivatives less capable of promoting plant growth [47]. Some members of the GH3 family of IAA-amido synthetases whose encoding genes transcriptionally respond to stress are necessary and sufficient for promoting stress-induced plant growth inhibition, presumably by lowering the effective concentration of active IAA [47]. GH3.3, in particular, has been used as a marker of stress-mediated growth inhibition [48]. The expression of GH3.3 in wild-type plants exposed to lht1 exudates and Ps WCS417r was significantly higher than that of wild-type plants exposed to WT exudates and Ps WCS417r (Figure 4E). Lastly, as excessive ammonia is known to inhibit plant growth [11,12,49], we tested the hypothesis that Ps WCS417r may convert the excess Gln present in lht1 exudates or Gln-supplemented WT exudates into ammonia. Thus, the capacity of Ps WCS417r to produce ammonia when grown in wild-type or lht1 exudates was assessed. In line with the ammonia toxicity hypothesis, the levels of ammonia produced by Ps WCS417r were higher in the lht1 exudates than in the WT exudates (Figure 4F). Notwithstanding the lack of direct evidence, the increased levels of AAs in the lht1 root exudates could enhance Ps WCS417r-mediated production of ammonia, which could, in turn, contribute to inhibiting plant growth. Overall, the data presented in this section suggest that LHT1-mediated regulation of the root exudate’s amino-acidic composition is critical to maintaining the concentration and representation of AAs, which is optimal for the beneficial effect of root colonization by PGPB.
3. Discussion
3.1. LHT1 Activity Is Essential to Maintain a Balanced Composition of Amino Acids in the Root Exudates
Plants release a fraction of their metabolites into the rhizosphere as root exudates. Since some of the exudate-dependent microbes present in the rhizosphere promote plant growth, the metabolites present in root exudates represent an investment rather than an expenditure for the plant. It has been proposed that, like seeds or growing organs (e.g., new leaves), belowground microorganisms are additional sinks of plant photosynthates [50]. Therefore, as with other plant sinks, it is reasonable to hypothesize that plants would regulate the transport of metabolites to and from the rhizosphere. Furthermore, it had been hypothesized that active recycling of low molecular weight compounds from the rhizosphere is particularly important for plants to control the growth of rhizosphere microbes and to ward off the pathogen invasion of root tissues [51]. Yet, whether plants exert control over the accumulation of plant-derived metabolites in the rhizosphere through AA uptake was unclear. The present study was based on two related hypotheses: (i) plants would regulate the levels of AAs in the rhizosphere, including the AAs released by the plants themselves, and (ii) some of the AA importers that contribute to the uptake of AAs from the growth medium and the soil may also contribute to retrieving plant-derived AAs back into the plant. In support of these hypotheses, the data showed that a loss-of-function mutant of the AA importer LHT1 leads to an increased accumulation of AAs in the root exudates of Arabidopsis (Figure 1A). Furthermore, our search for specific root-exudate metabolites able to change the structure of the rhizosphere allowed us to identify Gln as an AA enriched in the root-exudates of the lht1 (Figure 1B) mutant plants whose sole supplementation is sufficient to boost the growth of the PGPB Ps WCS417r (Figure 2A,B). Unexpectedly though, this increased PGPB growth (Figure 3A) did not translate into increased plant growth (Figure 4A). Together, the data suggest that wild-type plants maintain a balanced concentration of AAs in the exudates that is optimal to promote PGPB growth and, in turn, to maximize plant growth, as well as that LHT1 contributes to the maintaining of this optimal concentration of AAs in the exudates. In line with an optimized plant–microbe interaction that is, at least in part, modulated by LHT1, one hour after the inoculation of Arabidopsis roots with Ps WCS417r, LHT1 expression increases in root tissues [52], suggesting a mechanism by which microbes and plants communicate to maintain levels of AAs in the rhizosphere that sustain optimized PGPB growth and metabolism and hence optimal growth of the plant itself.
3.2. The Re-Uptake of Root-Exuded Amino Acids Is Necessary to Modulate Bacterial Metabolism and Promote Plant Growth
The root-associated microbiome is mainly located in the rhizosphere, the narrow (1–3 mm) region of soil surrounding the root that is rich in plant-made metabolites exuded by the roots [53]. Several PGPB species that live in the rhizosphere have been shown to either produce plant hormones or regulate the levels of plant hormones that have a positive effect on plant growth [54,55]. The mechanisms by which Ps WCS417r promotes plant growth, however, are not fully understood. To explain the detrimental effects of Gln-supplemented Ps WCS417r on plant growth, several hypotheses can be formulated, some of which would be worth testing in future studies. One hypothesis would be that excessive levels of AAs could have a direct detrimental effect on the physiology of the plant itself. This may be proven correct for some AAs, including Ser, which was sufficient to inhibit Arabidopsis root and shoot growth (Figure 4C). Gln has been reported to inhibit root growth of the Ler (Lansberg erecta) but not of the Sha (Shakdara) ecotypes of Arabidopsis [56], suggesting that the root growth of the Col-0 ecotype used in our experiments could also be inhibited by an excess of Gln. However, in the experimental setup used in our study, the detrimental effect of Gln was only evident in the presence of Ps WCS417r (Figure 4B). For instance, even relatively high concentrations (10 mM) of Gln alone did not inhibit plant growth (Figure 4B). In contrast, the combination of Ps WCS417r with 1 mM Gln causes subtle growth inhibition and 10 mM robustly inhibits plant growth (Figure 4B). This observation suggests that the microbe-mediated bioconversion of Gln is required to inhibit plant growth. Bioconversion by soil-dwelling microbes has been extensively studied for chemicals of anthropogenic origin (e.g., pesticides) [57,58], but much less is known about the PGPB bioconversion of plant-derived compounds. Nevertheless, it is well-documented that microbes convert Gln into ammonium and that high ammonium levels inhibit the growth of roots and shoots in Arabidopsis, especially in conditions of low potassium levels [11,12,49]. Furthermore, the genome of P. simiae encodes enzymes that use Gln as substrate (locus tag PS417_15505 and PS417_18410) and produce glutamate and ammonia. The activation of ammonia-producing reaction, together with the inhibition of the ammonia-consuming reaction carried out by glutamine synthase (locus tag PS417_01655), would be favored in the Gln-rich environment of the lht1 rhizosphere. In agreement with the literature, Ps WCS417r produced high ammonia levels when lht1 exudates were used as growth medium (Figure 4F). However, in the conditions used for assessing plant growth in this study, the high availability of potassium in the MS medium may alleviate ammonia toxicity. Considering that the high ammonia level present in the MS medium (20 mM) does not inhibit plant growth, the local concentrations of ammonia produced by Ps WCS417r Gln metabolism would need to be higher than at least 20 mM to be able to inhibit plant growth. Thus, in future studies, it would be relevant to test whether Ps WCS417r strains with reduced capacity to release ammonium from glutamine (e.g., a glsA mutant; locus tag PS417_15505) would maintain the capacity to grow robustly in exudates supplemented with Gln and perhaps promote plant growth to a larger extent compared to the wild-type strain. Other byproducts of PGPB metabolism may also affect plant growth; thus, in future studies, it would be informative to perform the metabolic profiling of root exudates post-PGPB growth to identify metabolites that are only present at high levels in the supernatant of Ps WCS417r grown in lht1 exudates. A complementary hypothesis would be that Ps WCS417r-generated byproducts of Gln metabolism activate a stress response in the plant that includes growth inhibition. Reduced plant growth in response to abiotic and biotic stress is well documented. The metabolism of plant growth hormones, such as IAA, mediates the growth inhibitory response to stress [47]. The GH3 family of proteins are important players in the plant inhibitory metabolism of IAA upon stress. In line with the notion that the Gln metabolism by Ps WCS417r described here may promote the formation of IAA derivatives that suppress plant growth, Gln-supplemented Ps WCS417r, but not Gln or bacteria alone, induced the induction of the Arabidopsis gene encoding the IAA-amido synthetase, GH3.3. This result provides a clue for future dissection of the mechanism by which the Gln metabolism in Ps WCS417r inhibits plant growth.
4. Conclusions
Data presented in this study establishes that Arabidopsis uses AAs reuptake to control the organic composition of the rhizosphere as a relevant strategy to foster enduring beneficial interactions with their root microbiota. The modulation of the concentration of AAs in the exudates is, at least in part, executed by LHT1. In the absence of LHT1-mediated re-uptake, the excessive levels of AAs in the rhizosphere cause microbe-mediated inhibition of plant growth. Future studies will define how Gln metabolism in Ps WCS417r leads to plant growth inhibition. Among other possibilities, the alkalinization of the rhizosphere and the impact on auxin levels as potential sources of plant growth inhibition need to be further investigated.
5. Methods
5.1. Plant Materials
For all experiments, Arabidopsis wild-type plants were Col-0 and mutants were of Col-0 background. All T-DNA lines belong to the SALK Collection [59] and were obtained from the Arabidopsis Biological Resource Center, Ohio State University. Homozygous mutant lines were selected by PCR genotyping, as previously described [32]. Seeds were always surface-sterilized using 10% bleach three times for two minutes, followed by three washes with sterile water, and resuspended in 0.1% PhytoAgar (PlantMedia, Dublin, OH, USA; Cat#40100072-2), as well as being stratified in the dark at 4 °C for at least two days.
5.2. Growth of Seedlings in Vertical Plates
Stratified seeds were germinated on square plates (100 mm × 100 mm square plates; Thermo-Fisher Scientific, Waltham, MA, USA; Cat#FB0875711A) containing sterile full-strength (1X) Murashige and Skoog (MS) basal medium with vitamins (PhytoTech, Lenexa, KS, USA; Cat# M519), supplemented with 0.5% sucrose (Millipore Sigma, Darmstadt, Germany; Cat#S7903), 0.5 g/L MES (Millipore Sigma, Darmstadt, Germany; Cat#M8250), and 0.7% PhytoAgar (PlantMedia, Dublin, OH, USA; Cat#40100072-1). The pH was corrected to 5.7 with KOH. Plates were sealed with parafilm and incubated vertically in a reach-in plant growth incubator (Conviron Adaptis 1000, Winnipeg, MB, Canada) at 25 ± 0.2 °C, 75% RH, 16 h Light/8 h Dark, and 100 µmoles/m2/s light intensity for the times indicated in the relevant figure legends. Uniformly growing seedlings were then selected and transferred to new plates containing autoclaved-3 MM paper cut to fit 100 mm × 100 mm square plates and wetted to saturation with 0.5X liquid MS medium pH 5.7 without sucrose. These plates were incubated horizontally under the same conditions as above for one day to allow roots to attach to the 3 MM paper.
5.3. Root Exudate Collection Assays
Root exudates were collected using a modification of a previously published method [31]. Arabidopsis seeds were sown on an autoclaved polytetrafluoroethylene (PTFE) mesh (McMaster-Carr, Elmhurst, IL, USA; Cat#1100t41) floating on the surface of 1 mL 1X MS liquid medium containing 0.5% sucrose in 12-well tissue culture plates (USA Scientific, Ocala, FL, USA; Cat#CC7682-7512). Plates were incubated in reach-in plant growth incubators (Conviron Adaptis A1000) at 25 ± 0.2 °C, 75% RH, 16 h Light/8 h Dark, and 100 µmoles/m2/s light intensity. Sterilized and stratified seeds were sown on the floating mesh. Twelve days after sowing, the medium was replaced with 0.5X MS liquid medium without sucrose, and plants were allowed to grow for three additional days. Importantly, in these conditions, roots grow submerged in medium while shoots remain on the air space of the well. Root exudates were then collected and filter-sterilized through 0.22 µm filter for further processing.
5.4. Colorimetric/Fluorometric Quantitation of Amino Acids
Total amino acid content of root exudates was assessed using the L-Amino Acid Quantitation Colorimetric/Fluorometric Kit (BioVision #K639-100) following the manufacturer’s instructions with modifications. Briefly, a 12.5 μL reaction mix containing 11.5 μL L-amino acid assay buffer, 0.5 μL L-amino acid probe, and 0.5 μL L-amino acid enzyme mix was added to each well containing 12.5 µL of the test samples, L-amino acid standards to generate a standard curve, or unplanted media to estimate and later subtract the background signal. The reactions were incubated in the microtiter plate reader (SpectraMax® i3x, Molecular Devices) for 30 min at 37 °C. The fluorescent signal (Ex/Em = 535/587 nm) was recorded every 5 min. AA quantification was performed on 5–6 independent samples per condition.
5.5. Liquid Chromatography–Mass Spectrometry Analysis of Amino Acids
Root exudates were vacuum-dried and reconstituted in 100 µL of 0.1% formic acid. Samples were analyzed at the University of Virginia-Biomolecular Analysis Facility Core following a standard protocol [60]. Serial dilutions of standards of every AA were run for every analysis. An amount of 5 µL of samples were injected into a UHPLC system (Ultimate 3000, Thermo, San Jose, CA, USA) and separated through a 3 min isocratic elution (5% acetonitrile, 95% water, 0.1% formic acid) on a 1.7 µm C18 column (Kinetex XB-C18, Phenomenex, Torrance, CA, USA) at 250 µL/min and 25 °C. High-resolution mass spectrometry analysis was performed using a triple quadrupole orbitrap mass spectrometer (Q-Exactive HF-X, Thermo Fisher Scientific, Waltham, MA, USA). Glycine data was omitted from final analysis as unplanted MS medium contained background levels of glycine (2 mg/L). Data analysis was performed with the proprietary XCalibur software (Thermo Fisher Scientific, Cat# OPTON-30965), and targeted peak detection was achieved using ICIS peak integration algorithm. Thermo Fisher Scientific quantitative analysis software (Quan Browser) was then used to generate calibration curves, followed by determining the concentration of the amino acids in the root exudate and unplanted samples. LC-MS analysis was performed on 6 independent samples per condition.
5.6. Bacterial Growth on Root Exudates
Pseudomonas simiae WCS417r, formerly known as Pseudomonas fluorescens WCS417r, was maintained on LB plates supplemented with 50 µg mL−1 rifampicin. A single colony was randomly picked from a plate and grown overnight in 100 mL of LB at 28 °C and 230 rpm until the cultures reached OD600 = 0.4–0.8. The bacteria were then harvested by centrifugation, washed three times with sterile water, and the OD600nm was adjusted to the required inoculation titer with sterile water. Root exudates (100 µL) of each condition were aliquoted on six or more wells of a 96-well microtiter plate and inoculated with Ps WCS417r. Plates were incubated under constant agitation at 28 °C, and the culture density was monitored over time by reading OD600nm in a microtiter plate reader (SpectraMax® i3x, Molecular Devices, San Jose, CA, USA).
5.7. Root Colonization
Seedings were grown on vertical plates, as described above. Two weeks after sowing, six (6) uniformly growing seedlings were transferred to each of 5 plates containing 3 MM paper wetted with 5 mL half-strength MS without sucrose. Plates were horizontally incubated for one day to allow seedlings to attach to the paper surface. The next day, the edge of the paper opposite to the seedling’s-shoot side was flooded with 0.5X MS alone (contamination control) or inoculated with freshly harvested Ps WCS417r diluted to OD600nm = 0.0002. Bacteria were allowed to colonize the roots for 72 h. The capacity of roots to support bacterial growth was analyzed in 6 to 30 individual seedlings per condition. To assess colony forming units (CFU) per mg of root, the root mass was recorded right after harvesting. Immediately after weighing, the root tissue was ground with metal beads in a TissueLyser (QIAGEN, Hilden, Germany). Aliquots of the ground roots were base-ten serial diluted in sterile water and plated on OmniTray NuncTM plates (Thermo Fisher Scientific, Waltham, MA, USA) containing LB agar medium supplemented with 50 µg/mL of rifampicin. Plates were incubated at 28 °C overnight, and colonies were counted under a microscope. Only replicates with no colonies in the “contamination control” were included in the analysis.
5.8. Competitive Chemotaxis Assay
Competitive chemotaxis assay was designed by modifying capillary assays previously described [34,38] using syringes (Figure S3). Briefly, Ps WCS417r cultures grown and harvested, as described above, were resuspended at OD600 = 0.002 in chemotaxis buffer (10 mM potassium phosphate pH 7.2, 1 mM MgCl2, and 0.1 mM EDTA). Bacterial suspensions (40 mL) were pipetted into empty sterile Petri dishes. Then, 200 µL of the test samples (e.g., unplanted MS, wild-type-root exudates, or lht1-root exudates) were loaded into each of two 1 mL sterile syringes (without needles) and the tips of the syringes were dipped below the liquid surface in the Petri dish containing the Ps WCS417r in chemotaxis buffer suspension. The syringes loaded with exudates were allowed to attract bacteria from the suspension for 30 min. The syringes were removed from the bacterial suspension, and colony-forming units in the exudates contained in the syringes were assessed via dilution and plating, as described above (see root colonization assay for details).
5.9. Biofilm Formation Assays
Unplanted medium, wild-type root exudates, and lht1 root exudates were tested for their capacity to promote biofilm formation upon Ps WCS417r inoculation as follows. Each well of a 96-well round-bottom plate (Millipore-Sigma, Darmstadt, Germany; Cat#CLS2797,) was first loaded with 200 µL of root-exudate sample and then inoculated with 2 µL of freshly harvested Ps WCS417r (see details above) resuspended at OD600 = 0.02. Biofilms were allowed to grow statically for 48 h at 28 °C. Subsequently, the medium and non-adherent bacterial cells were carefully removed by pipetting, and wells were washed with sterile water without disturbing the biofilms attached to the bottom of the plate. The plates were air-dried for 5–10 min. Next, the wells were stained with 125 µL of a 0.1% (w/v) solution of crystal violet in water for 15 min. The stain was gently rinsed with water, without disturbing the stained biofilm. One hundred and fifty (150) µL of a 6:3:1 sterile water:methanol:acetic acid solution was added to each well to solubilize the crystal violet for 15 min. To quantify biofilm biomass, 125 µL of the 150 µL solution of stained biofilm were transferred into a well of a polyvinyl chloride 96-well flat-bottom plate (Millipore-Sigma, Darmstadt, Germany; Cat#CLS2595) for absorbance assessment at 550 nm in a microplate reader (SpectraMax® i3x, Molecular Devices, San Jose, CA, USA). Biofilm formation was tested on 3 to 6 independent samples per condition.
5.10. Plant Growth on Exudates ± Ps WCS417r
The capacity of Ps WCS417r to promote the growth of Arabidopsis seedlings when combined with WT exudates, lht1 exudates, or WT exudates supplemented with Gln or Ser was assessed as follows. Seedings were grown on 1X MS PhytoAgar with 0.5% sucrose. Plates were incubated in Conviron Adaptis 1000 reach-in plant growth incubators at 25 ± 0.2 °C, 75% RH, 16 h Light/8 h Dark, and 100 µmoles/m2/s light intensity. After two weeks, six (6) uniformly growing seedlings were transferred to each of 3 to 7 plates containing 3 MM paper wetted with 5 mL half-strength MS without sucrose. Plates were horizontally incubated for one day to allow seedlings to stabilize. The next day, the seedlings in each plate were root flooded (liquid added to the edge of the paper opposite to the seedlings’ shoot side) with half-strength MS alone (contamination control) or with freshly harvested Ps WCS417r diluted to OD600nm = 0.2 in the following carriers: Plate (1) WT exudate, and Plate (2) lht-1 exudate. In independent experiments we tested half-strength MS alone as contamination control or Ps WCS417r diluted to OD600nm = 0.2 in the following carriers: Plate (1) WT exudate, Plate (2) WT exudate supplemented with 1 mM Gln, Plate (3) WT exudate supplemented with 10 mM Gln, Plate (4) WT exudate supplemented with 1 mM Ser, and Plate (5) WT exudate supplemented with 10 mM Ser. We also included an “overgrowth control” in which we root-flooded the seedlings with Ps WCS417r diluted in WT exudate at final OD600nm = 1.6 (instead of 0.2); this control was included to test whether overgrowth of Ps WCS417r would be sufficient to inhibit plant growth.
5.11. Ps WCS417r Excreted Ammonium Assessment
The capacity of Ps WCS417r to produce ammonia through metabolizing exudates of interest was measured using a modified version of the Berthelot colorimetric method (Figure S4). Briefly, 24 wells of a 96-well plate were filled with 90 µL of WT or lht1 exudate, as well as 0.5X MS media as control. Twelve wells of each condition and of each independent source of exudate (e.g., 12 wells of WT exudate obtained in biological replicate 1, 12 wells of WT exudate obtained from biological replicate 2, etc.) were inoculated with freshly harvested and three-times-washed Ps WCS417r to reach a final OD600nm = 0.5, while the remaining 12 wells of each condition and biological replicate were left bacteria-free to measure the basal levels of ammonia in each exudate (negative controls). Plates were incubated at 28 °C with constant shaking. After 5, 10, 20, and 30 min, the content of 3 wells per condition was transferred to 1.5 mL tubes and centrifuged for 15 min at 15,000× g. The 3 supernatants from each treatment and control condition were pooled and filtered sterilized. Two microliter aliquots of the supernatants were transferred to wells of a 96-well plate prefilled with 98 µL of assay buffer of the ammonia assay kit (AbCam, Cambridge, UK; Cat#ab102509), and ammonia levels were quantified according to the manufacturer’s guidelines. Each supernatant was tested in triplicate, and, as described above, three independent exudates per condition were tested in parallel.
5.12. Plant Tissue Gene Expression Analysis
Root/leaf tissues were harvested one at a time into previously weighed tubes, and the weight of the tissues was measured. Immediately after weighing a sample, it was flash-frozen in liquid nitrogen and kept at −80 °C until processing. RNA was isolated using TRIzol® Reagent (Invitrogen, Waltham, MA, USA; Cat#15596018) and DNAse-I treated (Promega, Madison, WI, USA; Cat# M6101) and quantified in a NanoDrop-ND1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Two (2) µg of total RNA were used for cDNA synthesis with MMLV (Promega, Madison, WI, USA; Cat# M1701) and random hexamers (Invitrogen, Waltham, MA, USA; Cat# N8080127). The qPCR was performed using real-time SYBR-Green quantification (BioRad, Hercules, CA, USA; Cat# #1725150. There were two technical replicates per condition per biological replicate. The reactions were performed using an ABI 7500 Fast Real-Time PCR system (Applied Biosystems, Waltham, MA, USA). Data were analyzed using the ∆∆Ct method [61]. Gene expression was measured on ≥3 independent samples per condition.
5.13. Statistical Analysis
Data analyses and graphs were generated using Excel and GraphPad. A two-sided Student’s t-test was performed for statistical comparison of two means or a Welch’s t-test for two means with unequal variances when relevant. For comparison of more than two means, a one-way ANOVA followed by Tukey’s post hoc test, or a Kruskal–Wallis test for unequal variances followed by Dunn’s post hoc test was performed, as indicated in the relevant figure legends. For statistical analysis of bacterial growth curves, the CGGC (comparison of groups of growth curves) permutation test [62] was used to compare pairs of samples (i.e., unplanted vs. wild type; unplanted vs. lht1; wild type vs. lht1) over the course of growth (24 h). The test statistic (mean t) is the two-sample t-statistic to compare the OD600 values between the two groups at each hour, averaged over the course of growth (24 h). A p-value was obtained for the test statistic by simulation. Samples were randomly allocated to each of the two groups and the mean, t, was recalculated for 10,000 data sets generated through this permutation. The p-value is the proportion of permutations where the mean, t, is greater in absolute value than the mean, t, for the original data set.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12020371/s1.
Author Contributions
I.D.K.A. and C.H.D. conceived and designed the experiments; I.D.K.A. and C.H.D. performed experiments with help from B.T.K.; I.D.K.A. analyzed the data with input from C.H.D.; I.D.K.A. and C.H.D. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the University of Virginia 4-VA grant SG00409 (to C. H. Danna) and by the National Science Foundation CAREER Award IOS-1943120 grant (to C. H. Danna).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data and materials are available upon request.
Acknowledgments
We thank Nishikant Wase of the W. M. Keck Biomedical Mass Spectrometry Laboratory at the University of Virginia, School of Medicine, for LC-MS quantification of AA in root exudates. This study was partially supported by funds from the University of Virginia through a 4-VA grant awarded to C.H.D.
Conflicts of Interest
The authors have declared that no competing interest exist.
References
- Pieterse, C.M.J.; Berendsen, R.L.; de Jonge, R.; Stringlis, I.A.; Van Dijken, A.J.H.; Van Pelt, J.A.; Van Wees, S.C.M.; Yu, K.; Zamioudis, C.; Bakker, P.A.H.M. Pseudomonas Simiae WCS417: Star Track of a Model Beneficial Rhizobacterium. Plant Soil 2021, 461, 245–263. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Badri, D.V.; Bakker, M.G.; Sugiyama, A.; Manter, D.K.; Vivanco, J.M. Root Exudation of Phytochemicals in Arabidopsis Follows Specific Patterns That Are Developmentally Programmed and Correlate with Soil Microbial Functions. PLoS ONE 2013, 8, e55731. [Google Scholar] [CrossRef]
- Jaeger, C.H., III; Lindow, S.E.; Miller, W.; Clark, E. Mapping of Sugar and Amino Acid Availability in Soil around Roots with Bacterial Sensors of Sucrose and Tryptophan. Appl. Environ. Microbiol. 1999, 65, 2685. [Google Scholar] [CrossRef] [PubMed]
- Strehmel, N.; Böttcher, C.; Schmidt, S.; Scheel, D. Profiling of Secondary Metabolites in Root Exudates of Arabidopsis Thaliana. Phytochemistry 2014, 108, 35–46. [Google Scholar] [CrossRef]
- Defoirdt, T. Amino Acid–Derived Quorum Sensing Molecules Controlling the Virulence of Vibrios (and Beyond). PLoS Pathog. 2019, 15, e1007815. [Google Scholar] [CrossRef]
- Champalal, L.; Kumar, U.S.; Krishnan, N.; Vaseeharan, B.; Mariappanadar, V.; Raman, P. Modulation of Quorum Sensing-Controlled Virulence Factors in Chromobacterium violaceum by Selective Amino Acids. FEMS Microbiol. Lett. 2018, 365, fny252. [Google Scholar] [CrossRef]
- Wen, J.; Yu, Y.; Chen, M.; Cui, L.; Xia, Q.; Zeng, X.; Guo, Y.; Pan, D.; Wu, Z. Amino Acid-Derived Quorum Sensing Molecule Alanine on the Gastrointestinal Tract Tolerance of the Lactobacillus Strains in the Cocultured Fermentation Model. Microbiol. Spectr. 2022, 10, e00832-21. [Google Scholar] [CrossRef]
- Oku, S.; Komatsu, A.; Tajima, T.; Nakashimada, Y.; Kato, J. Identification of Chemotaxis Sensory Proteins for Amino Acids in Pseudomonas fluorescens Pf0-1 and Their Involvement in Chemotaxis to Tomato Root Exudate and Root Colonization. Microbes Environ. 2012, 27, 462–469. [Google Scholar] [CrossRef]
- Acosta Aragón, Y.; Jatkauskas, J.; Vrotniakienė, V. The Effect of a Silage Inoculant on Silage Quality, Aerobic Stability, and Meat Production on Farm Scale. ISRN Vet. Sci. 2012, 2012, 345927. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Zhang, N.; Li, Z.; Zhang, G.; Xu, Y.; Shen, Q.; Zhang, R. Plant-Microbe Communication Enhances Auxin Biosynthesis by a Root-Associated Bacterium, Bacillus amyloliquefaciens SQR9. Mol. Plant-Microbe Interact. 2016, 29, 324–330. [Google Scholar] [CrossRef]
- Liu, Y.; Von Wirén, N. Ammonium as a Signal for Physiological and Morphological Responses in Plants. J. Exp. Bot. 2017, 68, 2581–2592. [Google Scholar] [CrossRef]
- Britto, D.T.; Siddiqi, M.Y.; Glass, A.D.M.; Kronzucker, H.J. Futile Transmembrane NH4+ Cycling: A Cellular Hypothesis to Explain Ammonium Toxicity in Plants. Proc. Natl. Acad. Sci. USA 2001, 98, 4255–4258. [Google Scholar] [CrossRef] [PubMed]
- Näsholm, T.; Ekblad, A.; Nordin, A.; Giesler, R.; Högberg, M.; Högberg, P. Boreal Forest Plants Take up Organic Nitrogen. Nature 1998, 392, 914–917. [Google Scholar] [CrossRef]
- Melin, E.; Nilsson, H. Transfer of Labelled Nitrogen from Glutamic Acid to Pine Seedlings through the Mycelium of Boletus variegatus (Sw.) Fr. Nature 1953, 171, 134. [Google Scholar] [CrossRef] [PubMed]
- Lipson, D.; Näsholm, T. The Unexpected Versatility of Plants: Organic Nitrogen Use and Availability in Terrestrial Ecosystems. Oecologia 2001, 128, 305–316. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, Q.; Kraus, T.E.C.; Dahlgren, R.A.; Anastasio, C.; Zasoski, R.J. Contribution of Amino Compounds to Dissolved Organic Nitrogen in Forest Soils. Biogeochemistry 2002, 61, 173–198. [Google Scholar] [CrossRef]
- Senwo, Z.N.; Tabatabai, M.A. Amino Acid Composition of Soil Organic Matter. Biol. Fertil. Soils 1998, 26, 235–242. [Google Scholar] [CrossRef]
- Wipf, D.; Ludewig, U.; Tegeder, M.; Rentsch, D.; Koch, W.; Frommer, W.B. Conservation of Amino Acid Transporters in Fungi, Plants and Animals. Trends Biochem. Sci. 2002, 27, 139–147. [Google Scholar] [CrossRef]
- Lalonde, S.; Wipf, D.; Frommer, W.B. Transport Mechanisms For Organic Forms Of Carbon And Nitrogen Between Source And Sink. Annu. Rev. Plant Biol. 2004, 55, 341–372. [Google Scholar] [CrossRef]
- Okumoto, S.; Pilot, G. Amino Acid Export in Plants: A Missing Link in Nitrogen Cycling. Mol. Plant 2011, 4, 453–463. [Google Scholar] [CrossRef]
- Hirner, A.; Ladwig, F.; Stransky, H.; Okumoto, S.; Keinath, M.; Harms, A.; Frommer, W.B.; Koch, W. Arabidopsis LHT1 Is a High-Affinity Transporter for Cellular Amino Acid Uptake in Both Root Epidermis and Leaf Mesophyll. Plant Cell 2006, 18, 1931–1946. [Google Scholar] [CrossRef]
- Svennerstam, H.; Ganeteg, U.; Bellini, C.; Näsholm, T. Comprehensive Screening of Arabidopsis Mutants Suggests the Lysine Histidine Transporter 1 to Be Involved in Plant Uptake of Amino Acids. Plant Physiol. 2007, 143, 1853–1860. [Google Scholar] [CrossRef]
- Svennerstam, H.; Ganeteg, U.; Näsholm, T. Root Uptake of Cationic Amino Acids by Arabidopsis Depends on Functional Expression of Amino Acid Permease 5. New Phytol. 2008, 180, 620–630. [Google Scholar] [CrossRef]
- Svennerstam, H.; Jämtgård, S.; Ahmad, I.; Huss-Danell, K.; Näsholm, T.; Ganeteg, U. Transporters in Arabidopsis Roots Mediating Uptake of Amino Acids at Naturally Occurring Concentrations. New Phytol. 2011, 191, 459–467. [Google Scholar] [CrossRef]
- Lehmann, S.; Gumy, C.; Blatter, E.; Boeffel, S.; Fricke, W.; Rentsch, D. In Planta Function of Compatible Solute Transporters of the AtProT Family. J. Exp. Bot. 2011, 62, 787–796. [Google Scholar] [CrossRef]
- Fischer, W.-N.; Kwart, M.; Hummel, S.; Frommer, W.B. Substrate Specificity and Expression Profile of Amino Acid Transporters (AAPs) in Arabidopsis. J. Biol. Chem. 1995, 270, 16315–16320. [Google Scholar] [CrossRef]
- Grallath, S.; Weimar, T.; Meyer, A.; Gumy, C.; Suter-Grotemeyer, M.; Neuhaus, J.-M.; Rentsch, D. The AtProT Family. Compatible Solute Transporters with Similar Substrate Specificity But Differential Expression Patterns. Plant Physiol. 2005, 137, 117–126. [Google Scholar] [CrossRef]
- Hida, A.; Oku, S.; Kawasaki, T.; Nakashimada, Y.; Tajima, T.; Kato, J. Identification of the McpA and McpM Genes, Encoding Methyl-Accepting Proteins Involved in Amino Acid and l-Malate Chemotaxis, and Involvement of McpM-Mediated Chemotaxis in Plant Infection by Ralstonia pseudosolanacearum (Formerly Ralstonia solanacearum Phylotypes I and III). Appl. Environ. Microbiol. 2015, 81, 7420–7430. [Google Scholar] [CrossRef]
- Webb, B.A.; Helm, R.F.; Scharf, B.E. Contribution of Individual Chemoreceptors to Sinorhizobium meliloti Chemotaxis Towards Amino Acids of Host and Nonhost Seed Exudates. Mol. Plant. Microbe Interact. 2016, 29, 231–239. [Google Scholar] [CrossRef]
- Moe, L.A. Amino Acids in the Rhizosphere: From Plants to Microbes. Am. J. Bot. 2013, 100, 1692–1705. [Google Scholar] [CrossRef]
- Haney, C.H.; Samuel, B.S.; Bush, J.; Ausubel, F.M. Associations with Rhizosphere Bacteria Can Confer an Adaptive Advantage to Plants. Nat. Plants 2015, 1, 15051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Khadka, P.; Puchalski, P.; Leehan, J.D.; Rossi, F.R.; Okumoto, S.; Pilot, G.; Danna, C.H. MAMP-Elicited Changes in Amino Acid Transport Activity Contribute to Restricting Bacterial Growth. Plant Physiol. 2022, 189, 2315–2331. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Nie, J.; Bai, R.; Sui, X. Amino Acid Transporters in Plants: Identification and Function. Plants 2020, 9, 972. [Google Scholar] [CrossRef] [PubMed]
- Allard-Massicotte, R.; Tessier, L.; Lécuyer, F.; Lakshmanan, V.; Lucier, J.; Garneau, D.; Caudwell, L.; Vlamakis, H.; Bais, H.P.; Beauregard, P.B. Bacillus Subtilis Early Colonization of Arabidopsis Thaliana Roots Involves Multiple Chemotaxis Receptors. MBio 2016, 7, e01664-16. [Google Scholar] [CrossRef]
- De Weert, S.; Vermeiren, H.; Mulders, I.H.M.; Kuiper, I.; Hendrickx, N.; Bloemberg, G.V.; Vanderleyden, J.; De Mot, R.; Lugtenberg, B.J.J. Flagella-Driven Chemotaxis towards Exudate Components Is an Important Trait for Tomato Root Colonization by Pseudomonas fluorescens. Mol. Plant-Microbe Interact. 2002, 15, 1173–1180. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, D.; Liu, Y.; Li, S.; Shen, Q.; Zhang, R. Effects of Different Plant Root Exudates and Their Organic Acid Components on Chemotaxis, Biofilm Formation and Colonization by Beneficial Rhizosphere-Associated Bacterial Strains. Plant Soil 2014, 374, 689–700. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, N.; Huang, Q.; Raza, W.; Li, R.; Vivanco, J.M.; Shen, Q. Organic Acids from Root Exudates of Banana Help Root Colonization of PGPR Strain Bacillus amyloliquefaciens NJN-6. Sci. Rep. 2015, 5, 13438. [Google Scholar] [CrossRef]
- Liu, X.; Parales, R.E. Bacterial Chemotaxis to Atrazine and Related S-Triazines. Appl. Environ. Microbiol. 2009, 75, 5481–5488. [Google Scholar] [CrossRef]
- Heil, M.; Baldwin, I.T. Fitness Costs of Induced Resistance: Emerging Experimental Support for a Slippery Concept. Trends Plant Sci. 2002, 7, 61–67. [Google Scholar] [CrossRef]
- Bohnert, H.J.; Nelson, D.E.; Jensen, R.G. Adaptations to Environmental Stresses. Plant Cell 1995, 7, 1099–1111. [Google Scholar] [CrossRef]
- Burdon, J.J.; Thrall, P.H. The Fitness Costs to Plants of Resistance to Pathogens. Genome Biol. 2003, 4, 227. [Google Scholar] [CrossRef] [PubMed]
- Herms, D.A.; Mattson, W.J. The Dilemma of Plants: To Grow or Defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef]
- Coley, P.D.; Bryant, J.P.; Chapin, F.S. Resource Availability and Plant Antiherbivore Defense. Science 1985, 230, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth–Defense Tradeoffs in Plants: A Balancing Act to Optimize Fitness. Mol. Plant 2014, 7, 1267–1287. [Google Scholar] [CrossRef] [PubMed]
- Maleck, K.; Levine, A.; Eulgem, T.; Morgan, A.; Schmid, J.; Lawton, K.A.; Dangl, J.L.; Dietrich, R.A. The Transcriptome of Arabidopsis Thaliana during Systemic Acquired Resistance. Nat. Genet. 2000, 26, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Tena, G.; Plotnikova, J.; Willmann, M.R.; Chiu, W.L.; Gomez-Gomez, L.; Boller, T.; Ausubel, F.M.; Sheen, J. Map Kinase Signalling Cascade in Arabidopsis Innate Immunity. Nature 2002, 415, 977–983. [Google Scholar] [CrossRef]
- Park, J.E.; Park, J.Y.; Kim, Y.S.; Staswick, P.E.; Jeon, J.; Yun, J.; Kim, S.Y.; Kim, J.; Lee, Y.H.; Park, C.M. GH3-Mediated Auxin Homeostasis Links Growth Regulation with Stress Adaptation Response in Arabidopsis. J. Biol. Chem. 2007, 282, 10036–10046. [Google Scholar] [CrossRef]
- Korver, R.A.; Koevoets, I.T.; Testerink, C. Out of Shape During Stress: A Key Role for Auxin. Trends Plant Sci. 2018, 23, 783–793. [Google Scholar] [CrossRef]
- Di, D.-W.; Li, G.; Sun, L.; Wu, J.; Wang, M.; Kronzucker, H.J.; Fang, S.; Chu, J.; Shi, W. High Ammonium Inhibits Root Growth in Arabidopsis Thaliana by Promoting Auxin Conjugation Rather than Inhibiting Auxin Biosynthesis. J. Plant Physiol. 2021, 261, 153415. [Google Scholar] [CrossRef]
- Savage, J.A.; Clearwater, M.J.; Haines, D.F.; Klein, T.; Mencuccini, M.; Sevanto, S.; Turgeon, R.; Zhang, C. Allocation, Stress Tolerance and Carbon Transport in Plants: How Does Phloem Physiology Affect Plant Ecology? Plant Cell Environ. 2016, 39, 709–725. [Google Scholar] [CrossRef]
- Jones, D.L.; Nguyen, C.; Finlay, R.D. Carbon Flow in the Rhizosphere: Carbon Trading at the Soil-Root Interface. Plant Soil 2009, 321, 5–33. [Google Scholar] [CrossRef]
- Stringlis, I.A.; Proietti, S.; Hickman, R.; Van Verk, M.C.; Zamioudis, C.; Pieterse, C.M.J. Root Transcriptional Dynamics Induced by Beneficial Rhizobacteria and Microbial Immune Elicitors Reveal Signatures of Adaptation to Mutualists. Plant J. 2018, 417, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.R.; James, E.K.; Poole, P.S. The Plant Microbiome. Genome Biol. 2013, 14, 209. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of Action of Plant Growth Promoting Bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Leon-Reyes, A.; Van der Ent, S.; Van Wees, S.C.M. Networking by Small-Molecule Hormones in Plant Immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef]
- Sharma, S.; Lin, W.; Villamor, J.G.; Verslues, P.E. Divergent Low Water Potential Response in Arabidopsis Thaliana Accessions Landsberg Erecta and Shahdara. Plant. Cell Environ. 2013, 36, 994–1008. [Google Scholar] [CrossRef]
- Fomsgaard, I.S.; Mortensen, A.G.; Carlsen, S.C.K. Microbial Transformation Products of Benzoxazolinone and Benzoxazinone Allelochemicals—A Review. Chemosphere 2004, 54, 1025–1038. [Google Scholar] [CrossRef]
- Soulas, G.; Lagacherie, B. Modelling of Microbial Degradation of Pesticides in Soils. Biol. Fertil. Soils 2001, 33, 551–557. [Google Scholar] [CrossRef]
- Alonso, J.M.; Stepanova, A.N.; Leisse, T.J.; Kim, C.J.; Chen, H.; Shinn, P.; Stevenson, D.K.; Zimmerman, J.; Barajas, P.; Cheuk, R.; et al. Genome-Wide Insertional Mutagenesis of Arabidopsis thaliana. Science 2003, 301, 653–657. [Google Scholar] [CrossRef]
- Nemkov, T.; D’Alessandro, A.; Hansen, K.C. Three-Minute Method for Amino Acid Analysis by UHPLC and High-Resolution Quadrupole Orbitrap Mass Spectrometry. Amino Acids 2015, 47, 2345–2357. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zerbe, G.O. Randomization Analysis of the Completely Randomized Design Extended to Growth and Response Curves. J. Am. Stat. Assoc. 1979, 74, 215–221. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).