Abstract
Leaf senescence is the final stage of leaf development and is essential for storage properties and crop productivity. WRKY transcription factors have been revealed to play crucial roles in several biological processes during plant growth and development, especially in leaf senescence. However, the functions of Brassica napus WRKY transcription factors in leaf senescence remain unclear. In the present study, Bna.A07.WRKY70, one paralogue of Brassica napus WRKY70, was cloned from the B. napus cultivar “Zhongshuang11 (ZS11)”. We found that Bna.A07.WRKY70 contains a highly conserved WRKY domain and is most closely related to Arabidopsis thaliana WRKY70. The subcellular localization and transcriptional self-activation assays indicated that Bna.A07.WRKY70 functions as a transcription factor. Meanwhile, RT-qPCR and promoter-GUS analysis showed that Bna.A07.WRKY70 is predominantly expressed in the leaves of B. napus and rosette leaves of A. thaliana. In addition, our results demonstrated that ectopic expression of Bna.A07.WRKY70 in A. thaliana wrky70 mutants could restore the senescence phenotypes to wild-type levels. Consistently, the expression levels of three senescence-related marker genes of wrky70 mutants were restored to wild-type levels by ectopic expression of Bna.A07.WRKY70. These findings improve our understanding of the function of Bna.A07.WRKY70 in B. napus and provide a novel strategy for breeding the new stay-green cultivars in rapeseed through genetic manipulation.
1. Introduction
Rapeseed (Brassica napus L., AACC, 2n = 38), an allotetraploid species, originated from spontaneous hybridization between two diploid Brassica species: Brassica rapa (AA, 2n = 20) and Brassica oleracea (CC, 2n = 18) [1]. It is a major oilseed crop grown worldwide for the production of edible oil in the human diet, livestock feed, and industrial materials [2]. Therefore, there is important social and economic significance for studying its associated biological processes, including leaf development. The leaf is the primary organ of photosynthesis and can produce nutrition and gather energy during plants’ growth and maturation stages. Leaf senescence, as a type of programmed cell death (PCD), is the terminal stage of leaf development. During leaf senescence, the chloroplast first starts disassembling, and is followed by a loss of chlorophyll together with the catabolism of macromolecules such as protein, lipids, nucleic acids, and RNA [3]. By general catabolism, cellular materials are converted into easily exportable nutrients, which from senescing leaves were subsequently transported to reproductive and developing structures [4]. Consequently, leaf senescence is a critical process for crop fitness and is particularly essential for the optimization of crop productivity. Generally, leaf senescence is influenced by various external environmental and endogenous factors. The environmental cues that affect leaf senescence include high temperature, light signals, drought, and biotic stress [5,6,7]. The endogenous factors include the accumulation of reactive oxygen species (ROS), variation of plant hormones, and, most importantly, regulation of multiple senescence-associated genes [8,9,10,11]. Therefore, mining the key genes regulating the leaf senescence process is of great importance in rapeseed.
The WRKY proteins are one of the largest and most important superfamilies of transcription factors (TFs) in plants. WRKY transcription factors encompass a core motif WRKYGQK (a highly conserved WRKY domain) at the N-terminus and an atypical Zinc finger motif at the C-terminus [12]. On the basis of both the number of WRKY domains and the features of the Zn-finger motif in their evolutionary history, the WRKY TFs can be divided into three different groups (I, II, and III). Group I contains two WRKY domains and a finger motif whose pattern is conserved zinc ligands (C–X4-5–C–X22-23–H–X1–H), which is the same as the Zinc finger motif of group II, but there is only one WRKY domain in group II. Instead of a C2–H2 pattern in group I and II, group III contains a pattern of C2–HC (C–X7–C–X23–H–X1–C) zinc finger-like motif and have one WRKY domain [13,14]. All three groups’ members of WRKY TFs have been demonstrated to interact with the specific DNA cis-acting element W-box (C/TTGACT/C) in the promoter regions of downstream genes and further regulate their expression [15]. In recent decades, experimental evidence has shown that WRKY proteins act as key regulators widely involved in various plant growth and development processes, such as leaf senescence, growth of roots [16], stem elongation [17], and multiple biotic and abiotic stressors [18,19]. In Arabidopsis thaliana, WRKY53 acts in a complex transcription factor signaling network regulating leaf senescence-specific gene expression [20], WRKY45 interacts with the DELLA protein RGL1 to positively regulate age-triggered leaf senescence [21], and WRKY71 mediates ethylene (ET) signaling and synthesis to hasten leaf senescence [22].
WRKY70 belongs to WRKY III TFs and has been reported in response to several developmental and physiological processes in diversified species [23]. In Arabidopsis, WRKY70 acts as a negative regulator of leaf senescence, with gradually increasing expression during leaf development [3], and WRKY70 is also crucial in plant defense against pathogens, controlling the cross-talk of salicylic acid (SA) and jasmonic acid (JA) signaling in plant defense [23,24]. Moreover, WRKY70 is an important signaling component that is positively involved in brassinolide (BR)-regulated growth and negatively involved in drought responses by inhibiting drought-responsive genes [25]. In chickpeas, WRKY70 was reported to regulate the expression of a chickpea HD-Zip transcription factor CaHDZ12, which improved tolerance to osmotic stresses under drought and salinity stress, and increased sensitivity to abscisic acid (ABA) in transgenic tobacco and chickpea [26]. In addition, it was suggested that GhWRKY70D13 negatively regulates cotton’s resistance to Verticillium dahliae mainly through its effect on ET and JA biosynthesis and signaling pathways [27]. A recent study demonstrated that TaWRKY70 positively regulates TaCAT5 by directly binding to the TaCAT5 promoter to enhance Cd tolerance in transgenic Arabidopsis [28]. In B. napus, the BnWRKY70 knockout plants by CRISPR/Cas9 system enhanced Sclerotinia sclerotiorum resistances, while overexpression of BnWRKY70 reduced resistance to S. sclerotiorum [29]. However, the roles of WRKY proteins in B. napus in the regulation of leaf senescence remain unclear.
In the current study, Bna.A07.WRKY70, one of the AtWRKY70 orthologues in B. napus, was isolated and functionally characterized. We found that Bna.A07.WRKY70 functioned as a TF and was specifically expressed in the leaves in A. thaliana and B. napus. We also demonstrated that ectopic expression of Bna.A07.WRKY70 in the A. thaliana wrky70 mutant restored the leaf senescence rate and chlorophyll content and greatly altered the expression of three senescence-related genes in this mutant. Our results may indicate that Bna.A07.WRKY70 functions as a negative regulator of leaf senescence in Arabidopsis, which might reveal a conserved role of WRKY70 proteins in regulating leaf senescence between A. thaliana and B. napus.
2. Results
2.1. Sequence Analysis of BnaWRKY70 Paralogs
In the B. napus cultivar “Zhongshuang11 (ZS11)”, six paralogs of BnaWRKY70 were predicted in BnPIR (http://cbi.hzau.edu.cn/bnapus/index.php, accessed on 9 September 2022) and were designated Bna.A07.WRKY70 (BnaA07G0195100ZS), Bna.C06.WRKY70 (BnaC06G0198900ZS), Bna.A04.WRKY70 (BnaA04G0035900ZS), Bna.C08.WRKY70 (BnaC08G0362900ZS), Bna.A09.WRKY70 (BnaA09G0519800ZS), and Bna.C04.WRKY70 (BnaC04G0308100ZS). With the multiple sequence alignment, we found that the WRKY70 protein from B. napus and A. thaliana possessed highly conserved WRKY domains, including WRKYGQ/KK core motif and a pattern of C2–HC zinc finger-like motif at the C-terminus (Figure 1A). Among them, Bna.A07.WRKY70 was predicted to share the highest identity in the amino acid sequence with the AtWRKY70 protein (66.01%) (Figure S1). A phylogenetic analysis was performed to investigate the evolutionary relationships between Bna.A07.WRKY70 and 20 WRKY70 proteins from seven plant species, including A. thaliana, B. napus, B. rapa, Glycine max, Zea mays, Oryza sativa, and Setaria italic. As illustrated in Figure 1B, Bna.A07.WRKY70 is most closely related to the WRKY70 protein from B. rapa (NP_001288847.1) and A. thaliana (AtWRKY70). These results suggested preliminarily that Bna.A07.WRKY70 might have similar functions as AtWRKY70.
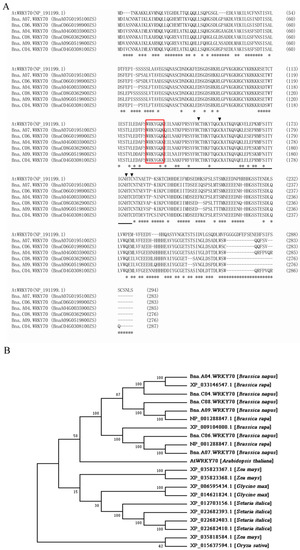
Figure 1.
Protein sequence and phylogenetic analyses of WRKY70 proteins. (A) Protein sequence alignment of WRKY70 from A. thaliana and B. napus was carried out using the MUSCLE program (http://www.ebi.ac.uk/Tools/msa/muscle/, accessed on 12 September 2022). Asterisks indicate non-conservative differences. The WRKY domain 125–185, as indicated by the Conserved Domain Search program (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi, accessed on 12 September 2022), is underlined. The highly conserved core sequence WRKYGQK in the WRKY domain is represented by a red box, together with the C and H residues in the CCHC zinc-finger-like motif indicated by a downward black triangle. (B) Phylogenetic analysis of Bna.A07.WRKY70 with 20 other WRKY70 proteins from seven plant species, including AtWRKY70 (Arabidopsis thaliana); Bna.A07.WRKY70 (BnaA07G0195100ZS), Bna.C06.WRKY70 (BnaC06G0198900ZS), Bna.A04.WRKY70 (BnaA04G0035900ZS), Bna.C08.WRKY70 (BnaC08G0362900ZS), Bna.A09.WRKY70 (BnaA09G0519800ZS), and Bna.C04.WRKY70 (BnaC04G0308100ZS (Brassica napus); NP_001288821.1, XP_033146547.1, XP_009104000.1, and NP_001288847.1 (Brassica rapa); XP_015637594.1 (Oryza sativa); XP_035823367.1, XP_035823368.1, and XP_035818584.1 (Zea mays); XP_012703156.1, XP_022682393.1, XP_022682403.1, and XP_022682410.1 (Setaria italica) and XP_006595434.1 and XP_014621824.1 (Glycine max). A neighbor-joining tree (Jones–Taylor–Thornton model) with 1000 replicates of bootstrap analysis was generated by MEGA7. Bootstrap values are indicated at the nodes, and the accession numbers of the species are labeled on the phylogenetic tree.
2.2. Subcellular Localization and Transcriptional Activity of Bna.A07.WRKY70
For subcellular localization, Bna.A07.WRKY70 was expressed in tobacco (Nicotiana benthamiana) leaf cells as a recombinant protein fused to a green fluorescent protein marker. The fluorescence signal was detected in the nucleus by laser scanning confocal microscopy (Figure 2A), suggesting that Bna.A07.WRKY70 might function as a transcription factor.
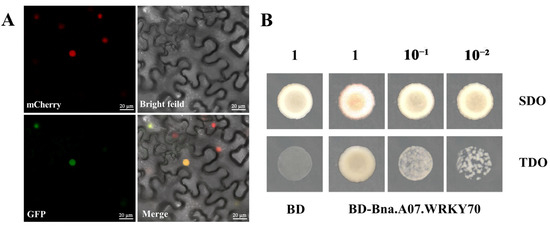
Figure 2.
Transcription factor characterization of Bna.A07.WRKY70. (A) Subcellular localization of Bna.A07.WRKY70 protein fused with GFP (35S:GFP-Bna.A07.WRKY70) in tobacco leaves (Nicotiana benthamiana). mCherry, a nuclear-localized protein fused with a red fluorescent protein; merge, merge of mCherry, GFP, and bright field images. (B) Transcriptional activation assays of Bna.A07.WRKY70 in yeast. BD: empty vector that contains GAL4 DNA-binding domain, BD-Bna.A07.WRKY70: cDNAs encoding of Bna.A07.WRKY70 transcripts were separately cloned into the pGBKT7/BD vector containing the GAL4 DNA binding domain, which transformed into the yeast strain Y2HGold, SDO: ability of yeast transformants to grow on medium lacking Trp, TDO: ability of yeast transformants to grow on medium lacking Trp, His and Ade indicates transcriptional activation. 1, 10−1, 10−2: the transformed strains were spotted on plates by diluting to different concentrations. The images show representative results from more than four independent yeast transformants.
To further characterize Bna.A07.WRKY70 function, we investigated whether Bna.A07.WRKY70 has transcription activation activity in yeast cells. The empty vector pGBKT7 as negative control and fusion construct (pBD-Bna.A07.WRKY70) were transformed separately into Y2HGold yeast cells, which were cultured on SDO (SD/-Trp) and TDO (SD/-Trp/-His/-Ade) medium. As shown in Figure 2B, on SDO (SD/-Trp) medium, all yeast transformants could grow normally, indicating that the constructs were transformed successfully into the Y2HGold yeast cells. Instead, on TDO (SD/-Trp/-His/-Ade) medium, the empty vector pGBKT7 did not grow, but yeast cells with Bna.A07.WRKY70 fusion constructs grew well, which demonstrated that Bna.A07.WRKY70 could activate the expression of the reporter genes. Given these findings, the Bna.A07.WRKY70 was testified to function as a transcription activator.
2.3. Analysis of Bna.A07.WRKY70 Expression Pattern
We further investigated the spatiotemporal expression pattern of Bna.A07.WRKY70 by analyzing the relative abundance of the mRNA in various tissues of B. napus cultivar “ZS11” using quantitative reverse transcription PCR (RT-qPCR). The results showed that Bna.A07.WRKY70 was widely expressed in different organs of B. napus, with higher expression in leaves, moderate in stems, flowers, and roots but low in developing seeds (Figure 3A). To comprehensively investigate the spatiotemporal expression pattern of Bna.A07.WRKY70 in A. thaliana, we obtained 16 pBna.A07.WRKY70:GUS in wild-type background independent lines of A. thaliana and the one representative line was used for promoter-GUS analysis because of similar GUS staining patterns in most lines. Consistent with the RT-qPCR data in B. napus, promoter-GUS activity staining was predominantly detected in rosette leaves of A. thaliana (Figure 3D) and then was also slightly detected in other organs of A. thaliana, including stems (Figure 3C), roots (Figure 3B), and flower abscission zones (Figure 3E). Conversely, it was not detected in the embryo (Figure 3G) and siliques (Figure 3F). In summary, these observations suggested that Bna.A07.WRKY70 might regulate a significant function during leaf development.
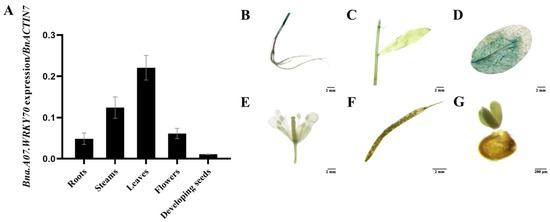
Figure 3.
Analysis of the Bna.A07.WRKY70 expression pattern. (A) RT-qPCR analysis of the Bna.A07.WRKY70 expression in various tissues of B. napus cultivar “ZS11”. The RT-qPCR result was normalized against the expression of BnACTIN7 as an internal control. Values are means ± SD (n = 3). Error bars denote standard deviations. (B) to (G), Histochemical GUS staining in 35-day-old ProBna.A07.WRKY70:GUS transgenic Arabidopsis plants. (B) Roots (bar = 2 mM); (C) Stems and leaves (bar = 2 mM); (D) rosette leaves (bar = 2 mM); (E) Flowers (bar = 2 mM); (F) siliques 12 days after pollination (bar = 2 mM); (G) Developing seeds 12 days after pollination (bar = 200 μM).
2.4. Bna.A07.WRKY70 Negatively Regulates Leaf Senescence in A. thaliana
To further explore the function of Bna.A07.WRKY70 on leaf development, we introduced the construct 35S:Bna.A07.WRKY70-GFP into A. thaliana wrky70 mutant (Figure 4A). Twelve independent T1 transgenic plants were generated using hygromycin selection, and five independent T3 homozygous transgenic lines wrky70 35S:Bna.A07.WRKY70-GFP were selected and confirmed by PCR amplification with the specific primers 35S-F/Bna.A07.WRKY70-GFP-BamHI-R (Figure 4B; Supplementary Table S1). Of these lines, three representative ones, wrky70 35S:Bna.A07.WRKY70-GFP #4, #6, and #12, with a relatively high expression level (Figure 4C), were selected for the follow-up experiment. As illustrated in Figure 5A,B, the loss-of-function mutants wrky70 exhibited markedly yellowing of leaves at 35 DAG (days after germination) and indicated earlier senescence compared to wild-type plants, which is in line with previous findings [3]. Interestingly, we found that the A. thaliana wrky70 mutant leaves were smaller than wild-type plants. Ectopic expression of Bna.A07.WRKY70 fully restored the rate of leaf senescence to wild-type levels in Arabidopsis wrky70 mutants (Figure 5A). Furthermore, by arranging the rosette leaves of 35-day-old Col-0, wrky70 mutant, and transgenic plants (#4, #6, #12) according to their age from older to younger, we found that three Bna.A07.WRKY70 transgenic lines in the wrky70 background delayed the premature senescence of leaves and rescued the phenotype of leaves relatively smaller in size compared to wild-type plants (Figure 5B). The results of the chlorophyll content indicated that the chlorophyll content of the wrky70 mutant intensified degradation from the fifth week, but the chlorophyll content of Bna.A07.WRKY70 transgenic lines were in keeping with that of Col-0 and clearly higher than that of the wrky70 mutant (Figure 5C).
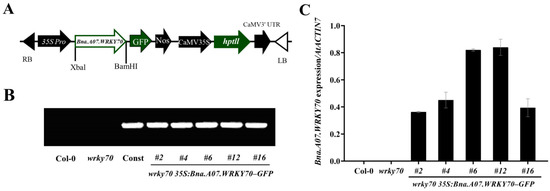
Figure 4.
Molecular characterization of wrky70 35S:Bna.A07.WRKY70-GFP transgenic plants. (A) Schematic diagram of constitutive expression cassette of the Bna.A07.WRKY70 gene in the binary vector pCAMBIA-1300 used for plant transformation. RB, right border; LB, left border; 35S Pro, CaMV 35S promoter; Nos, nopaline synthase terminator; CaMV35S, CaMV 35S promoter; hptII, hygromycin resistance gene. (B) PCR-based DNA genotyping of wrky70 35S:Bna.A07.WRKY70-GFP transgenic plants using specific primers of 35S_P/Bna.A07.WRKY70-GFP-BamHI-R. Const, 35S:Bna.A07.WRKY70-GFP construct. Col-0 and wrky70 indicate A. thaliana wild type and mutant plants, respectively. (C) Expression analysis of Bna.A07.WRKY70 in wrky70 35S:Bna.A07.WRKY70-GFP transgenic plants using RT-qPCR. The expression level was normalized against the expression of AtACTIN7, which was used as an internal control. Values are the means ± SD (n = 3). Error bars indicate standard deviation. # indicates the transgenic lines.
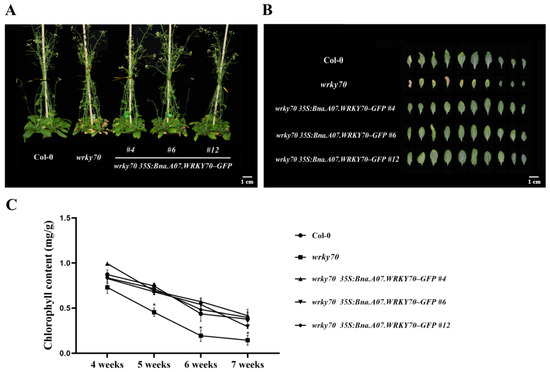
Figure 5.
Effects of Bna.A07.WRKY70 overexpression in the wrky70 mutant background on leaf senescence in A. thaliana. (A) The whole plant phenotypes of leaf senescence in the wild type (Col-0), wrky70 mutant, and wrky70 35S:Bna.A07.WRKY70-GFP transgenic plants. The images were taken 35 days after germination (DAG). Bar = 1 cM. (B) Phenotype of rosette leaves in 35-day-old plants, excised leaves are arranged according to age, from older to younger. Bar = 1 cM. (C) Comparisons of chlorophyll content of the fifth to sixth rosette leaves among wild-type (Col-0), wrky70 mutant, and wrky70 35S:Bna.A07.WRKY70 transgenic plants at the indicated ages. Values are means ± SD (n = 3). Asterisks indicate significant differences from wild-type (two-tailed paired Student’s t-test, p ≤ 0.05). Error bars indicate standard deviation. # indicates the transgenic lines.
In order to further confirm that the Bna.A07.WRKY70 regulates the progress of leaf senescence in A. thaliana, we assessed the transcript levels of representative genes relating to senescence in the fifth and sixth rosette leaves of A. thaliana wild-type, the wrky70 mutant, wrky70 35S:Bna.A07.WRKY70-GFP plants at 35 DAG. Compared to the wild type, the results showed that the expression of the senescence-related gene AtSAG13 (senescence-associated gene 13) and AtSEN1 (senescence-associated gene 1) were significantly increased, while the expression of photosynthesis-related AtCAB1 gene (chlorophyll a/b-binding protein) was significantly decreased in 35-day-old wrky70 mutant plants (Figure 6). However, when the 35S:Bna.A07.WRKY70-GFP was introduced into the wrky70 mutant, we found that the transcript abundance of these three senescence-related marker genes, including AtSEN1, AtCAB1, and AtSAG13, was restored to wild type levels. In brief, all results containing the premature senescence phenotype, chlorophyll content, and the expression of senescence-associated marker genes together revealed that Bna.A07.WRKY70 may negatively regulate the leaf senescence by adjusting the expression of senescence genes in A. thaliana and play a similar role with AtWRKY70 in A. thaliana.
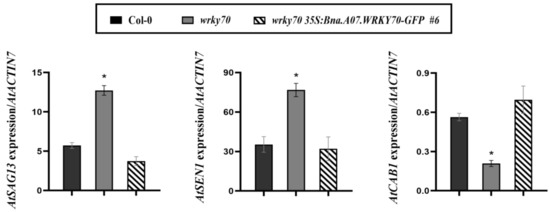
Figure 6.
Expression analysis of leaf senescence marker genes in the rosette leaves among wild-type (Col-0), wrky70 mutant, and wrky70 35S:Bna.A07.WRKY70-GFP transgenic plants at the 35 DAG, as measured by RT-qPCR. Expression levels were normalized to the expression of the internal reference gene, AtACTIN7. Values are means ± SD (n = 3). Error bars indicate standard deviations. Asterisks indicate statistically significant differences from wild type plants (two-tailed paired Student’s t-test, p ≤ 0.05). # indicates the transgenic lines.
3. Discussion
Leaf senescence is an indispensable portion and spans the latter half of leaf development. It is a highly intricate process regulated by multiple pathways [30]. As previously reported, the three largest groups of transcription factors, WRKY, NAC, and MYB superfamilies, are responsible for modulating transcriptional changes during leaf senescence [31], in which the AtWRKY70 has already been confirmed with a high level of expression in the late stage of leaf development and functions as an essential repressor during leaf senescence in A. thaliana [32]. However, the roles of WRKY70 transcription factors during leaf development in B. napus were lacking.
It has been widely known that B. napus was formed 7500 years ago by natural hybridization between B. rapa and B. oleracea [33]. B. napus, and diploid parental species B. rapa and B. oleracea, are believed to share a common ancestor with A. thaliana, a fact that has favored the transfer of knowledge from Arabidopsis to B. napus. As an allopolyploid plant, a large number and a high frequency of chromosome variation activities were identified, such as duplication, rearrangement, fusion, and deletion in the evolution processes of B. napus, which makes the genomics of B. napus more complicated. Generally, a single Arabidopsis gene is represented by two to eight paralogs in the B. napus genome [34,35]. Accordingly, six paralogues (Bna.A07.WRKY70, Bna.C06.WRKY70, Bna.A04.WRKY70, Bna.C08.WRKY70, Bna.A09.WRKY70, and Bna.C04.WRKY70) were found in the B. napus genome (Figure 1). In the WRKY transcription factor family, the WRKY domain is the major determinant of DNA-binding and specifically binds DNA cis-acting element W-box (C/TTGACT/C). Our results showed that all six Bna.WRKY70 had the WRKY protein domain containing the WRKYGQ/KK core motif and a pattern of C2–HC zinc finger-like motif at the C-terminus (Figure 1A). In the present study, among these BnaWRKY70 paralogues, Bna.A07.WRKY70, which was predicted to have the highest identity of protein sequence and the WRKY central conserved domains with AtWRKY70 (Figure 1), was cloned from the B. napus cultivar “ZS11” and functionally characterized. Bna.A07.WRKY70 was located in the nucleus of tobacco leaf cells, and we further demonstrated that Bna.A07.WRKY70 could activate the expression of the reporter genes in yeast cells (Figure 2). These results suggested that Bna.A07.WRKY70 functions as a transcription activator. Additionally, the Bna.A07.WRKY70 transcript was broadly present in different vegetative tissues, with the highest levels observed in leaves (Figure 3), suggesting that Bna.A07.WRKY70 might regulate a significant function during leaf development. Ectopic expression of Bna.A07.WRKY70 in the background of A. thaliana wrky70 mutants significantly delayed the senescence of leaves and restored the chlorophyll content to the wild type level (Figure 5). Moreover, the expression of senescence-associated genes (AtSEN1, AtSAG13, and AtCAB1) was clearly regulated by Bna.A07.WRKY70 during leaf senescence. Thus, these results may indicate that Bna.A07.WRKY70 functions as a negative factor in leaf senescence as the AtWRKY70.
During leaf senescence, the leaves turned yellow, resulting in photosynthesis deficiency and beginning with chloroplast dismantling, followed by degradation of chlorophyll and chlorophyll-protein complexes. Meanwhile, leaf senescence is accompanied by decreased expression of genes related to photosynthesis and protein synthesis and increased expression of senescence-associated genes (SAGs) [36]. Consistently, our results demonstrated that compared to the wild type, the expression of the photosynthesis-related AtCAB1 gene was decreased, and the expression of senescence-related gene AtSAG13 and AtSEN1 were increased in A. thaliana wrky70 mutant plants. The expression of these three marker genes was rescued to wild-type in wrky70 35S:Bna.A07.WRKY70-GFP transgenic plants, which proved that Bna.A07.WRKY70 indeed delayed the leaf senescence during plant senescence by affecting the expression of these three senescence genes in A. thaliana. Leaf senescence was widely influenced by a variety of external and internal factors, including environmental stresses and phytohormones. Recently, key gene regulatory networks comprising these TFs have been identified, indicating that leaf senescence is controlled by multiple cross-linking pathways, many of which are associated with stress response signaling [37,38,39]. Arabidopsis WRKY71 was reported that it is able to directly upregulate the ethylene signaling pathway genes EIN2 (ethylene insensitive2) and ORE1 (oresara1) and promote ethylene synthesis by directly activating the ACS2 gene to accelerate leaf senescence in Arabidopsis [22]. The cotton (Gossypium hirsutum L.) GhWRKY91 directly targets GhWRKY17, a gene associated with ABA signals and reactive oxygen species (ROS) production to negatively mediate leaf senescence and provide a foundation for further functional studies on natural and stress-induced leaf senescence [40]. OsWRKY53 of rice, as a positive regulator, repressed the transcript of ABA catabolic genes (OsABA8ox1 and OsABA8ox2) by directly binding to their promoters to promote ABA accumulation, and modulated ABA-induced leaf senescence [41]. In Arabidopsis, AtWRKY70 transcript levels were more strongly reduced in npr1 (non-expressor of PR 1) and pad4 (phytoalexin-deficient 4) and completely abolished in NahG (salicylate hydroxylase gene) plants compared to wild-type at 40 days post germination, among which, the NahG, pad4, and npr1 belonged to SA mutants and exhibited a delayed senescence phenotype [3]. These findings support the role of AtWRKY70 as a senescence-associated gene and indicate a functional requirement of SA for its normal expression. Besides, the preceding research illustrated that the pathway of plant hormones could respond to numerous abiotic stresses; for instance, GhWRKY17 from upland cotton modulated the increased sensitivity of plants to drought by reducing the level of ABA, and repressed transcript levels of ABA-inducible genes, including AREB (ABA-responsive element binding), DREB (dehydration-responsive element binding), NCED (9-cis-epoxycarotenoid dioxygenase), ERD (early responsive to dehydration) and LEA (late embryogenesis-abundant protein) under drought and salt stress conditions, indicating that GhWRKY17 responds to drought and salt stress through ABA signaling and the regulation of cellular ROS production in plants [42]. With the above findings in mind, whether Bna.A07.WRKY70 of B. napus adjusts the signaling pathways of phytohormone by combining with some key genes during the regulation of leaf senescence and responds to plant stress resistance mediated by the signaling pathways, can be explored further.
Interestingly, it has been reported that the Arabidopsis wrky70 knockout mutants were slightly reduced in size compared to wild-type plants during the entire period of development in A. thaliana [3]. However, from another report, no obvious growth phenotype was observed in a single knockout mutant of wrky70 compared with the wild-type A. thaliana [25]. In this study, our results found that the A. thaliana wrky70 mutant leaves were smaller than wild-type plants, and the leaf size of the wrky70 mutant was restored to wild type by the ectopic expression of Bna.A07.WRKY70. Whether or not Bna.A07.WRKY70 plays a role in controlling the size of leaves requires further investigation. Overall, based on the above, the multiple functions and regulation network of Bna.A07.WRKY70 still has great research potential.
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
The A. thaliana wild-type ecotype Columbia (Col-0), the T-DNA mutant wrky70 (SALK_025198) in the Col-0 background obtained from Arashare (https://www.arashare.cn/index/, accessed on 20 October 2020), and Brassica napus L. cultivar “Zhongshuang 11 (ZS11)”, were used in this study. The A. thaliana plants were grown in a growth chamber at 22 °C under a long day duration (LD, 16 h light/8 h dark) with moderate light intensity (160 μmol m−2 s−1). The B. napus cultivar “ZS11” was first grown in the greenhouse at 22 °C with a long day duration for six weeks. For vernalization, the plants were transferred to a cold chamber at 4 °C under LD conditions. After vernalization, the plants were returned to the initial greenhouse conditions for 10 weeks until harvest.
4.2. Protein Sequence and Phylogenetic Analysis
The protein sequences of WRKY70 were obtained from the National Center for Biotechnology Information (NCBI) database (https://www.ncbi.nlm.nih.gov/, accessed on 9 September 2022) and the B. napus pan-genome information resource (BnPIR) database (http://cbi.hzau.edu.cn/bnapus/index.php, accessed on 9 September 2022). Protein sequence alignment was carried out using MUSCLE (http://www.ebi.ac.uk/Tools/msa/muscle/, accessed on 12 September 2022). The conserved WRKY domain of Bna.A07.WRKY70 was indicated using the conserved domain search program in the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi, accessed on 12 September 2022). The phylogenetic tree was conducted using the neighbor-joining tree (Jones–Taylor–Thornton model) by MEGA7. Bootstrap analysis with 1000 replicates was performed to assess the statistical reliability of the tree topology.
4.3. Gene Cloning and Plasmid Construction
The full-length coding domain sequence (CDS) of Bna.A07.WRKY70 (XP_ 013648025.1) without stop codon was amplified by the specific primer designed in NCBI (https://www.ncbi.nlm.nih.gov/tools/primerblast/, accessed on 20 October 2020). The total RNA was extracted from young leaves of the B. napus cultivar “ZS11” by the SteadyPure Plant RNA Extraction Kit (Accurate Biology, Changsha, China), and the RNA concentration was determined by spectrometry (Nano Drop; Thermo Scientific, Wilmington, MA, USA) (Supplementary Table S3) and quality was checked by 1% agarose gel electrophoresis. For cloning, first-stand cDNA was synthesized from total RNA using EasyScript One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen, Beijing, China). The CDS of Bna.A07.WRKY70 was isolated from cDNA through PCR (Thermal Cycler Block, Thermo Fisher Scientific) using the high-fidelity thermostable DNA polymerase KOD-Plus-Neo (Toyobo Co., Ltd., Osaka, Japan). The PCR conditions were as follows: pre-denaturation at 94 °C for 2 min, followed by 35 cycles of 98 °C for 10 s, 55 °C for 30 s, and 68 °C for 1 min, and final extension at 68 °C for 7 min. Cloning primers are listed in Supplementary Table S1.
To construct the plasmid 35S:Bna.A07.WRKY70-GFP, the CDS of Bna.A07.WRKY70 without stop code was digested with the restriction endonucleases XbaI and BamHI and cloned into P1300-35S-green fluorescent protein (GFP) vector, which was driven by the CaMV35S (35S) promoter. Similarly, the digested PCR fragment of Bna.A07.WRKY70 was also cloned into pGreen-35S-eGFP to produce a fusion of GFP-Bna.A07.WRKY70 under the control of the 35S promoter. To obtain the construct of pBna.A07.WRKY70:GUS, the 2600 bp 5′ regulatory region upstream of the ATG start codon, as the Bna.A07.WRKY70 promoter region was amplified and cloned into pHY107 [43]. The CDS of Bna.A07.WRKY70 was cloned into the pGBKT7 vector containing the GAL4 DNA binding domain to form a construct of pBD-Bna.A07.WRKY70. Eight single colonies of each plasmid were selected randomly and sequenced by Sangon Biotechnology (Shanghai, China). Primers used for plasmid construction are listed in Supplementary Table S1.
4.4. Subcellular Localization of Bna.A07.WRKY70-GFP Protein
The 35S:GFP-Bna.A07.WRKY70 construct was transformed into Agrobacterium tumefaciens strain GV3101 and transiently expressed in the leaves of transgenic tobacco (Nicotiana benthamiana) carrying a nuclear localization signal as previously described [44]. Images of fluorescent signals were detected through a confocal laser scanning microscope (Leica TCS SP8 SR, Wetzlar, Germany) 72 h after agroinfiltration of the tobacco plants.
4.5. Transcriptional Activation Assays in Yeast
The construct of pBD-Bna.A07.WRKY70 and the negative control pGBKT7 vector were transformed separately into the yeast strain Y2HGold, including the HIS3 and ADE2 reporter genes. The transformed strains were cultured on synthetic dropout nutrient medium without tryptophan (SD/-Trp) plates and then were spotted on SDO (SD/-Trp) and TDO (SD/-Trp/-His/-Ade) plates by diluting to different concentrations. The transcription activation activity of each construct was observed according to the growth conditions of the corresponding yeast cells after incubating for 2–3 days in a 30 °C incubator.
4.6. Generation of A. thaliana Transgenic Plants
The construct of pBna.A07.WRKY70:GUS and 35S:Bna.A07.WRKY70-GFP was transformed into Agrobacterium tumefaciens strain GV3101, which was subsequently used to transform the A. thaliana wild type and wrky70 mutant plants using the floral dip method [45]. The transgenic lines of pBna.A07.WRKY70:GUS in wild type was selected on soil using Basta® and the transgenic lines of 35S:Bna.A07.WRKY70-GFP in wrky70 mutants were screened by hygromycin. All the transgenic plants were genotyped according to DNA and RNA analyses and selfed until T3 generation homozygous plants, which were generated and used for subsequent experiments.
4.7. RNA Extraction and RT-qPCR Analysis
The total RNA from various tissues of B. napus and leaves of A. thaliana were extracted using the SteadyPure Plant RNA Extraction Kit (Accurate Biology, Changsha, China). The quality of RNA was assessed using 1% agarose gel electrophoresis, and the concentration was determined by spectrometry (Nano Drop; Thermo Scientific, Wilmington, MA, USA) (Supplementary Table S3). RNA was reverse transcribed by EasyScript One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen, Beijing, China) according to the manufacturer’s instructions, and conditions were 37 °C for 15 min; 85 °C for 5 s, followed by maintaining at 4 °C. Quantitative real-time PCR (RT-qPCR) was utilized to evaluate gene expression with SYBR Green Master Mix (Cofitt, Hongkong, China) using the QuantStudioTM 7 Flex Real-Time PCR System (Thermo Scientific), which were performed by three independent biological replicates with two technical replicates for each biological replicate. Reactions were performed in a total volume of 20 μL containing 100 nM of each primer and 2 μL of diluted cDNA (50 ng/μL) templates and amplified using the following cycling conditions: 95 °C for 2 min, 40 cycles of 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 30 s. AtACTIN7 (amplified product with 161 bp) and BnACTIN7 (amplified product with 400 bp) were used as the internal control in Arabidopsis and rapeseed, respectively. For each reaction run, the specificity of the amplification was validated, and the threshold cycle (Ct) above the background was calculated using Bio-Rad iCycler (Bio-Rad, Hercules, CA, USA). The relative expression levels of the target genes were calculated using a modified double delta method [46]. Primers used for RT-qPCR analyses are listed in Supplementary Table S2.
4.8. Phenotypic Observation of A. thaliana Leaves
The seeds of the Col-0, the wrky70 mutant, and three independent lines—wrky70 35S:Bna.A07.WRKY70-GFP #4, #6, and #12—were germinated on 1/2 MS agar medium for one week. Subsequently, the seedlings were transplanted into 8 × 8 cm pots. When the A. thaliana plants grew 35 days after germination (DAG), the phenotype of leaf senescence was observed and photographed by a camera (D7500, Nikon, Tokyo, Japan).
4.9. Measurement of the Chlorophyll Content
The fifth and sixth rosette leaf samples of Col-0, wrky70 mutants, and Bna.A07.WRKY70 transgenic plants from the fourth, fifth, sixth, and seventh weeks were separately collected and weighed and then placed in a 1.5 mL centrifuge tube with 1 mL extraction solution (80% acetone), soaked the leaves in the dark for 24 h until they faded [47]. To calculate the chlorophyll content of leaves, the 0.2 mL supernatant was absorbed into Costar 96 Flat Transparent plate, and the absorbance values at 663 nm and 645 nm were measured using a microplate reader (Infinite M200pro, Tecan, Mannedorf, Switzerland). Each experiment was represented by three biological replicates.
5. Conclusions
As an indispensable portion, leaf senescence spans the latter half of leaf development, which is essential to guarantee better production and survival of the next generation. This study suggested that Bna.A07.WRKY70 may act as a negative regulator to share a conserved function with AtWRKY70 in controlling leaf senescence when it is expressed in A. thaliana. Thus, Bna.A07.WRKY70 can be utilized as a potential target to genetically manipulate leaf senescence and to create new stay-green materials to improve the rapeseed yield.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12020347/s1, Figure S1: Percent identity of full-length protein sequences of the WRKY70 protein from A. thaliana and B. napus; Table S1: Primers used for gene cloning and various constructs in the present study; Table S2: Primers used for RT-qPCR analysis in the present study; Table S3: The amount of RNA per sample in the Arabidopsis thaliana and Brassica napus.
Author Contributions
Y.G. and M.C. conceived and designed the experiments. T.L. conducted the experiments and analyzed the data. Y.L., C.W., D.Z., J.L. and M.H. conducted parts of the experiments. T.L. wrote the draft of the manuscript, and M.C. and Y.G. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by a grant from the Yang Ling Seed Industry Innovation Center (Grant no. K3031122024) and the National Natural Science Foundation of China (Grant no. 31801393).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data included in this study are available upon reasonable request by contact with the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chalhoub, B.; Denoeud, F.; Liu, S.; Parkin, I.A.; Tang, H.; Wang, X.; Chiquet, J.; Belcram, H.; Tong, C.; Samans, B.; et al. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 2014, 345, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Sashidhar, N.; Harloff, H.J.; Jung, C. Identification of phytic acid mutants in oilseed rape (Brassica napus) by large-scale screening of mutant populations through amplicon sequencing. New Phytol. 2020, 225, 2022–2034. [Google Scholar] [CrossRef]
- Ülker, B.; Shahid Mukhtar, M.; Somssich, I.E. The WRKY70 transcription factor of Arabidopsis influences both the plant senescence and defense signaling pathways. Planta 2007, 226, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.X.; Maodzeka, A.; Zhou, L.H.; Ali, E.; Wang, Z.; Jiang, L.X. Removal of DELLA repression promotes leaf senescence in Arabidopsis. Plant Sci. 2014, 219–220, 26–34. [Google Scholar] [CrossRef]
- Lim, P.O.; Kim, H.J.; Nam, H.G. Leaf Senescence. Annu. Rev. Plant Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef]
- Kim, C.; Kim, S.J.; Jeong, J.; Park, E.; Oh, E.; Park, Y.I.; Lim, P.O.; Choi, G. High ambient temperature accelerates leaf senescence via PHYTOCHROME-INTERACTING FACTOR 4 and 5 in Arabidopsis. Mol. Cells 2020, 43, 645–661. [Google Scholar] [PubMed]
- Lee, S.; Seo, P.J.; Lee, H.J.; Park, C.M. A NAC transcription factor NTL4 promotes reactive oxygen species production during drought-induced leaf senescence in Arabidopsis. Plant J. 2012, 70, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ye, C.F.; Zhao, Y.T.; Cheng, X.L.; Wang, Y.Q.; Jiang, Y.Q.; Yang, B. An oilseed rape WRKY-type transcription factor regulates ROS accumulation and leaf senescence in Nicotiana benthamiana and Arabidopsis through modulating transcription of RbohD and RbohF. Planta 2018, 247, 1323–1338. [Google Scholar] [CrossRef]
- Schippers, J.H. Transcriptional networks in leaf senescence. Curr. Opin. Plant Biol. 2015, 27, 77–83. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.H.; Lyu, J.I.; Woo, H.R.; Lim, P.O. New insights into the regulation of leaf senescence in Arabidopsis. J. Exp. Bot. 2018, 69, 787–799. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Guo, P.; Xia, X.; Guo, H.; Li, Z. Multiple layers of regulation on leaf senescence: New advances and perspectives. Front. Plant Sci. 2021, 12, 788996. [Google Scholar] [CrossRef]
- Liu, W.; Liang, X.; Cai, W.; Wang, H.; Liu, X.; Cheng, L.; Song, P.; Luo, G.; Han, D. Isolation and functional analysis of VvWRKY28, a Vitis Vinifera WRKY transcription factor gene, with functions in tolerance to cold and salt stress in transgenic Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 13418. [Google Scholar] [CrossRef] [PubMed]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY transcription factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef]
- Fan, Z.Q.; Tan, X.L.; Shan, W.; Kuang, J.F.; Lu, W.J.; Chen, J.Y. BrWRKY65, a WRKY transcription factor, is involved in regulating three leaf senescence-associated genes in Chinese flowering cabbage. Int. J. Mol. Sci. 2017, 18, 1228. [Google Scholar] [CrossRef]
- Devaiah, B.N.; Karthikeyan, A.S.; Raghothama, K.G. WRKY75 transcription factor is a modulator of phosphate acquisition and root development in Arabidopsis. Plant Physiol. 2007, 143, 1789–1801. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Xu, Y.; Lu, Y.; Yu, H.X.; Gu, M.H.; Liu, Q.Q. The WRKY transcription factor OsWRKY78 regulates stem elongation and seed development in rice. Planta 2011, 234, 541–554. [Google Scholar] [CrossRef]
- Pandey, S.P.; Somssich, I.E. The role of WRKY transcription factors in plant immunity. Plant Physiol. 2009, 150, 1648–1655. [Google Scholar] [CrossRef]
- Pan, L.J.; Jiang, L. Identification and expression of the WRKY transcription factors of Carica papaya in response to abiotic and biotic stresses. Mol. Biol. Rep. 2014, 41, 1215–1225. [Google Scholar] [CrossRef]
- Miao, Y.; Laun, T.; Zimmermann, P.; Zentgraf, U. Targets of the WRKY53 transcription factor and its role during leaf senescence in Arabidopsis. Plant Mol. Biol. 2004, 55, 853–867. [Google Scholar] [CrossRef]
- Chen, L.G.; Xiang, S.Y.; Chen, Y.L.; Li, D.B.; Yu, D.Q. Arabidopsis WRKY45 interacts with the DELLA protein RGL1 to positively regulate age-triggered leaf senescence. Mol. Plant 2017, 10, 1174–1189. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.C.; Qi, Y.A.; Xu, J.P.; Dai, X.H.; Chen, J.C.; Dong, C.H.; Xiang, F.N. Arabidopsis WRKY71 regulates ethylene-mediated leaf senescence by directly activating EIN2, ORE1 and ACS2 genes. Plant J. 2021, 107, 1819–1836. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.R.; Dong, Q.Y.; Yu, D.Q. Arabidopsis WRKY46 coordinates with WRKY70 and WRKY53 in basal resistance against pathogen Pseudomonas syringae. Plant Sci. 2012, 185–186, 288–297. [Google Scholar] [CrossRef]
- Li, J.; Brader, G.; Kariola, T.; Tapio Palva, E. WRKY70 modulates the selection of signaling pathways in plant defense. Plant J. 2006, 46, 477–491. [Google Scholar] [CrossRef]
- Chen, J.N.; Nolan, T.M.; Ye, H.X.; Zhang, M.C.; Tong, H.N.; Xin, P.Y.; Chu, J.F.; Chu, C.C.; Li, Z.H.; Yin, Y.H. Arabidopsis WRKY46, WRKY54 and WRKY70 transcription factors are involved in brassinosteroid-regulated plant growth and drought response. Plant Cell 2017, 29, 1425–1439. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Chakraborty, J.; Ghosh, P.; Basu, D.; Das, S. Chickpea WRKY70 regulates the expression of a homeodomain-leucine zipper (HD-Zip) I transcription factor CaHDZ12, which confers abiotic stress tolerance in transgenic tobacco and chickpea. Plant Cell Physiol. 2017, 58, 1934–1952. [Google Scholar] [CrossRef]
- Xiong, X.P.; Sun, S.C.; Zhang, X.Y.; Li, Y.J.; Liu, F.; Zhu, Q.H.; Xue, F.; Sun, J. GhWRKY70D13 regulates resistance to Verticillium dahliae in cotton through the ethylene and jasmonic acid signaling pathways. Front. Plant Sci. 2020, 11, 69. [Google Scholar] [CrossRef]
- Jia, Z.Z.; Li, M.Z.; Wang, H.C.; Zhu, B.; Gu, L.; Du, X.Y.; Ren, M.J. TaWRKY70 positively regulates TaCAT5 enhanced Cd tolerance in transgenic Arabidopsis. Environ. Exp. Bot. 2021, 190, 104591. [Google Scholar] [CrossRef]
- Sun, Q.F.; Lin, L.; Liu, D.X.; Wu, D.W.; Fang, Y.J.; Wu, J.; Wang, Y.P. CRISPR/Cas9-mediated multiplex genome editing of the BnWRKY11 and BnWRKY70 genes in Brassica napus L. Int. J. Mol. Sci. 2018, 19, 2716. [Google Scholar] [CrossRef]
- Woo, H.R.; Kim, H.J.; Lim, P.O.; Nam, H.G. Leaf senescence: Systems and dynamics aspects. Annu. Rev. Plant Biol. 2019, 70, 347–376. [Google Scholar] [CrossRef]
- Kim, H.J.; Nam, H.G.; Lim, P.O. Regulatory network of NAC transcription factors in leaf senescence. Curr. Opin. Plant Biol. 2016, 33, 48–56. [Google Scholar] [CrossRef]
- Besseau, S.; Li, J.; Palva, E.T. WRKY54 and WRKY70 co-operate as negative regulators of leaf senescence in Arabidopsis thaliana. J. Exp. Bot. 2012, 63, 2667–2679. [Google Scholar] [CrossRef]
- Osborn, T.C.; Kole, C.; Parkin, I.A.P.; Sharpe, A.G.; Kuiper, M.; Lydiatet, D.J.; Trick, M. Comparison of flowering time genes in Brassica rapa, B. napus and Arabidopsis thaliana. Genetics 1997, 146, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Cavell, A.C.; Lydiate, D.J.; Parkin, I.A.P.; Dean, C.; Trick, M. Collinearity between a 30-centimorgan segment of Arabidopsis thaliana chromosome 4 and duplicated regions within the Brassica napus genome. Genome 1998, 41, 62–69. [Google Scholar] [CrossRef]
- Scheffler, J.A.; Sharpe, A.G.; Schmidt, H.; Sperling, P.; Parkin, I.A.P.; Lühs, W.; Lydiate, D.J.; Heinz, E. Desaturase multigene families of Brassica napus arose through genome duplication. Theor. Appl. Genet. 1997, 94, 583–591. [Google Scholar] [CrossRef]
- Yoshida, S.; Ito, M.; Nishida, I.; Watanabe, A. Isolation and RNA gel blot analysis of genes that could serve as potential molecular markers for leaf senescence in Arabidopsis thaliana. Plant Cell Physiol. 2001, 42, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Breeze, E.; Harrison, E.; Mchattie, S.; Hughes, L.; Hickman, R.; Hill, C.; Kiddle, S.; Kim, Y.S.; Penfold, C.A.; Jenkins, D.; et al. High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell 2011, 23, 873–894. [Google Scholar] [CrossRef]
- Lee, H.N.; Lee, K.H.; Kim, C.S. Abscisic acid receptor PYRABACTIN RESISTANCE-LIKE 8, PYL8, is involved in glucose response and dark-induced leaf senescence in Arabidopsis. Biochem. Biophys. Res. Commun. 2015, 463, 24–28. [Google Scholar] [CrossRef]
- Penfold, C.A.; Buchanan-Wollaston, V. Modelling transcriptional networks in leaf senescence. J. Exp. Bot. 2014, 65, 3859–3873. [Google Scholar] [CrossRef]
- Gu, L.J.; Ma, Q.; Zhang, C.; Wang, C.C.; Wei, H.L.; Wang, H.T.; Yu, S.X. The cotton GhWRKY91 transcription factor mediates leaf senescence and responses to drought stress in transgenic Arabidopsis thaliana. Front. Plant Sci. 2019, 10, 1352. [Google Scholar] [CrossRef]
- Xie, W.Y.; Li, X.R.; Wang, S.P.; Yuan, M. OsWRKY53 promotes abscisic acid accumulation to accelerate leaf senescence and inhibit seed germination by downregulating abscisic acid catabolic genes in rice. Front. Plant Sci. 2022, 12, 816156. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.R.; Jia, H.H.; Chen, X.B.; Hao, L.L.; An, H.L.; Guo, X.Q. The cotton WRKY transcription factor GhWRKY17 functions in drought and salt stress in transgenic Nicotiana benthamiana through ABA signaling and the modulation of reactive oxygen species production. Plant Cell Physiol. 2014, 55, 2060–2076. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, J.; Bracha-Drori, K.; Yalovsky, S.; Ito, T.; Yu, H. Specification of Arabidopsis floral meristem identity by repression of flowering time genes. Development 2007, 134, 1901–1910. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.S.; Yang, B.; Li, J.Z.; Wang, Y.; Tao, R.Y.; Yang, F.; Wu, X.Y.; Yan, X.H.; Ahmad, M.; Shen, J.Q.; et al. ABA-responsive ABRE-BINDING FACTOR3 activates DAM3 expression to promote bud dormancy in Asian pear. Plant Cell Environ. 2020, 43, 1360–1375. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.L.; Ohlrogge, J.B. Turnover of fatty acids during natural senescence of Arabidopsis, Brachypodium, and Switchgrass and in Arabidopsis β-Oxidation mutants. Plant Physiol. 2009, 150, 1981–1989. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).