Abstract
ATP-binding cassette transporters (ABC transporters) play crucial physiological roles in plants, such as being involved in the growth and development of organs, nutrient acquisition, response to biotic and abiotic stress, disease resistance, and the interaction of the plant with its environment. The ABCG subfamily of proteins are involved in the process of plant vegetative organ development. In contrast, the functions of the ABCG36 and ABCG40 transporters have received considerably less attention. Here, we investigated changes in the transcriptomic data of the stem tissue of transgenic tobacco (Nicotiana tabacum) with LkABCG36 and LkABCG40 (Larix kaempferi) overexpression, and compared them with those of the wild type (WT). Compared with the WT, we identified 1120 and 318 differentially expressed genes (DEGs) in the LkABCG36 and LkABCG40 groups, respectively. We then annotated the function of the DEGs against the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases. The results showed enrichment in cell wall biogenesis and hormone signal transduction functional classes in transgenic LkABCG36 tobacco. In transgenic LkABCG40 tobacco, the enrichment was involved in metabolic and biosynthetic processes, mainly those related to environmental adaptation. In addition, among these DEGs, many auxin-related genes were significantly upregulated in the LkABCG36 group, and we found key genes involved in environmental adaptation in the LkABCG40 group, including an encoding resistance protein and a WRKY transcription factor. These results suggest that LkABCG36 and LkABCG40 play important roles in plant development and environmental adaptation. LkABCG36 may promote plant organ growth and development by increasing auxin transport, whereas LkABCG40 may inhibit the expression of WRKY to improve the resistance of transgenic tobacco. Our results are beneficial to researchers pursuing further study of the functions of ABCG36 and ABCG40.
1. Introduction
ATP-binding cassette transporters (ABC transporters) are membrane-associated primary transporters forming a large and ubiquitous family that plays important roles in the physiological development of plants [1], including the growth and development of organs, nutrient acquisition, response to abiotic and biotic stress, disease resistance, and the interaction of the plant with its environment [2,3,4]. ABC transporters are mainly localized in the plasma membrane, tonoplast, chloroplast, mitochondria, and peroxisomes on the membrane structures of plants [5,6]. The spectrum of substrates that can be transported by ABC transporters ranges from small molecules such as heavy metals, inorganic acids, and peptides, to large molecules such as lipids, polysaccharides, steroids, and even intact proteins [7,8]. Compared with other eukaryotes, plants have more abundant ABC transporters, especially the ABCG subgroup [9,10].
ABCG transporters are involved in diverse processes in many plant species, including pathogen response, diffusion barrier formation, response to abiotic stress, and phytohormone transport [11,12]. As such, the dysfunction of some ABCG transporters profoundly affects plant growth, resulting in partial or complete sterility [13]. For example, AtABCG26 (Arabidopsis) mainly transports polyketides for antioxidant capacity; loss of function of AtABCG26 leads to sterility and reduced fertility [14]. Furthermore, ABCG transporters are involved in transporting lipid molecules, such as wax and keratin, to the epidermis to promote the stress resistance of the plant [15]. NpABCG5 (Nicotiana plumbaginifolia) is involved in facilitating a plant’s resistance to pathogens by transporting lipid molecules [16]. The ABCG transporters also play a crucial role in phytohormone transport over long distances in various directions [10]. For example, AtABCG1 and AtABCG16 can change the distribution and flow of auxin by transporting growth hormone that ensure the rapid growth of pollen tubes in pistils [17,18]. AtABCG37 transports substrates involved in auxin synthesis, such as endogenous auxin precursor indole-3-butyrate (IBA), which plays an important role in plant growth and development [19].
The AtABCG36 transporter plays a dual role in the transport of IBA [20,21], and AtABCG40 is a high-affinity abscisic acid (ABA) transporter [22]. However, no studies have been conducted on the function of LkABCG36 and LkABCG40 in Larix kaempferi. In addition, the mechanisms of the effects of LkABCG36 and LkABCG40 on plant organ growth, development, and environmental adaptation are still unclear. As such, to explore the function of LkABCG36 and LkABCG40, we overexpressed these genes in a model organism (Nicotiana tabacum).
Gene expression analysis is useful for studying the molecular mechanisms and physiology of plants. Many researchers have identified interesting marker genes with advantageous traits or the molecular mechanisms of target genes based on the differences in the mRNA expression profiles between wild type and transgenic groups.
In this study, we performed a transcriptomic analysis on the stem tissues from transgenic tobacco overexpressing LkABCG36 and LkABCG40, and we compared the results with those of wild type tobacco. We first searched for significant DEGs, and then performed GO and KEGG functional enrichment analysis on the DEGs. We screened a series of marker genes that may play crucial roles in the improvement of transgenic plant traits. Our findings provide insights into the functions of ABCG transporters in plant development and elucidate the molecular mechanisms of LkABCG36 and LkABCG40.
2. Results
2.1. Overexpression of LkABCG36 and LkABCG40 Promotes Plant Growth in Tobacco
To evaluate the effect of the overexpression of LkABCG36 and LkABCG40 on tobacco growth, we first examined the growth behavior and phenotype of these lines. Compared with the wild type (WT), the transgenic tobacco (LkABCG36 and LkABCG40) had larger leaves and longer stems under the same growth conditions (Figure 1). Next, we obtained transgenic plant stem tissues for use in transcriptome analysis to explore the functions of LkABCG36 and LkABCG40 and their effects on transgenic tobacco growth.

Figure 1.
Overexpression of LkABCG36 and LkABCG40 promoted tobacco plant growth. Morphology of wild type (WT) and transgenic (LkABCG36 and LkABCG40) tobacco plants at 11 weeks old.
2.2. Transcriptome Analysis in Stem of Transgenic Tobacco Plants Overexpressing LkABCG36 and LkABCG40
We first screened the DEGs according to the two classic criteria of |log2 (FC)| > 1 and significance level p < 0.05. The volcano plots of the significant DEGs between the LkABCG36 and WT groups are shown in Figure 2A,C. We identified 427 and 693 significantly up- and downregulated DEGs, respectively, in the LkABCG36 group compared with the WT group. The volcano plots of the significant DEGs between the LkABCG40 and WT groups are shown in Figure 2B,C. We identified 125 and 193 significantly up- and downregulated DEGs, respectively, in the LkABCG40 group compared with WT group. The expression levels of the DEGs are shown as heatmaps in Figure 2D,E.
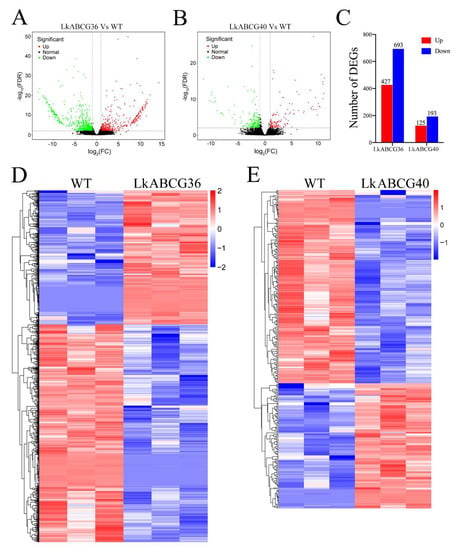
Figure 2.
Transcript level profiles analysis of significantly differentially expressed genes. (A,B) Volcano plots of significantly differentially expressed genes (DEGs) between LkABCG36 (A) or LkABCG40 (B) and WT groups. DEGs were identified by comparing gene expression values with |log2(fold change)| ≥ 1 and p < 0.05. (C) Number of DEGs was counted. We found 427 and 693 significantly up- and downregulated genes, respectively, in LkABCG36 group compared with WT group. We found 125 and 193 significantly up- and downregulated genes, respectively, in LkABCG40 group compared with WT group. (D,E) Heatmap of DEGs between ABCG36 and WT groups (D), and between LkABCG40 and WT groups (E). FPKM values (transcript levels) were transformed to log10 (FPKM) for color scaling.
In addition, to identify the highly correlated DEGs, we characterized the DEGs in the LkABCG36 and LkABCG40 groups by co-expression networks analysis. We found that most of the DEGs in the LkABCG36 group had high correlation coefficients (Figure 3A), whereas fewer DEGs had high correlation coefficients in the LkABCG40 group (Figure 3B).
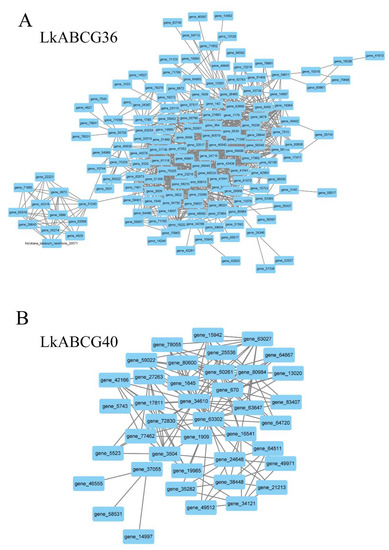
Figure 3.
Characterization of DEGs in LkABCG36 and LkABCG40 groups. (A) Co-expression network analysis of DEGs in LkABCG36 group; (B) Co-expression network analysis of DEGs in LkABCG40 group.
2.3. Functional Enrichment Analysis of DEGs in Transgenic Tobacco Overexpressing LkABCG36
To explore the functional significance of the genes that were differentially expressed between the LkABCG36 and WT groups, we first performed Gene Ontology (GO) enrichment analysis (Figure 4A). We found significant enrichment of genes relevant to cell wall biogenesis, including those involved in xyloglucan metabolism, xyloglucan and lignin biosynthetic processes, and cell wall modification. In addition, we found enrichment in energy metabolism processes such as the fructose 1,6-bisphosphate and glucose metabolic processes, the glycolytic process, and chlororespiration. Furthermore, we identified enrichment in the defense response processes, such as in the response to abscisic acid and salicylic acid, the wax biosynthetic process, and the cellular response to cytokinin stimulus. Moreover, certain transport and signal transduction terms were also enriched, such as in hormone transport and intracellular receptor signaling pathways.
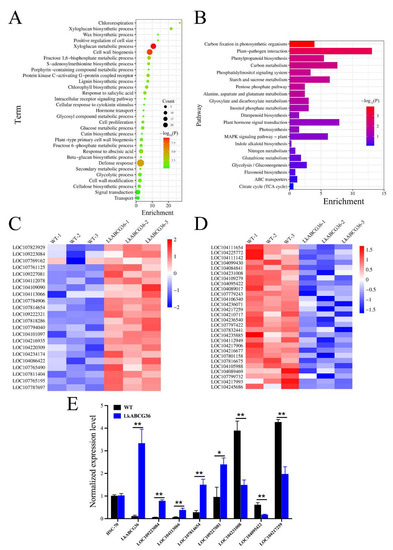
Figure 4.
Functional enrichment analysis of DEGs and expression of key genes in LkABCG36 group. (A) Gene Ontology (GO) enrichment analysis identifiers in cluster of overlapping DEGs in LkABCG36 group compared with WT group. (B) Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis identifiers in cluster of overlapping DEGs in LkABCG36 group compared with WT group. (C) Heatmap shows key upregulated DEGs involved in biosynthesis of some secondary metabolites, energy metabolism, and plant hormone signal transduction. Relative expression level of genes was calculated using log10 (FPKM). (D) Heatmap shows key downregulated DEGs involved in biosynthesis of signal transduction and energy metabolism. Relative expression level of genes was calculated using log10 (FPKM). (E) We used RT-PCR to detect changes in key up- and downregulated DEGs in stem tissue of transgenic tobacco. Data reported as mean ± SEM. Significance levels are noted as * p < 0.05; ** p < 0.01.
To further explore which signal pathways were directly affected by LkABCG36 overexpression, we analyzed DEG enrichment according to the KEGG pathway database. The results showed that the four main pathways involved were energy metabolism, biosynthesis of other secondary metabolites, environmental information processing, and carbohydrate metabolism (Figure 4B).
Various pathways, including carbon fixation in photosynthetic organisms, photosynthesis, and nitrogen metabolism, are involved in energy metabolism. In addition, pathways including phenylpropanoid biosynthesis, inositol phosphate metabolism, and flavonoid biosynthesis are involved in the biosynthesis of other secondary metabolites. Pathways including ABC transporters, MAPK signaling pathway—plant, plant hormone signal transduction, and phosphatidylinositol signaling systems are involved in environmental information processing. Finally, pathways including the citrate cycle (TCA cycle), glycolysis/gluconeogenesis, glyoxylate and dicarboxylate metabolism, and starch and sucrose metabolism are involved in carbohydrate metabolism.
To validate the signaling pathways identified in the aforementioned results, as well as to explore the key genes involved in transgenic tobacco overexpressing LkABCG36, we studied a number of key genes, and their expression levels are shown in Figure 4C,D. Of these DEGs, many auxin-related genes were significantly upregulated; examples include LOC109223084, encoding auxin-responsive protein; LOC104113066 and LOC107784906, encoding indole-3-acetic acid-amido synthetase; and LOC109227081, encoding auxin response factor. In addition, genes regulating hormone-related kinases were also significantly upregulated, including LOC107814654, LOC104086422, and LOC107761125 (Figure 4C). To further verify the reliability of sequencing, we detected the expression levels of the above genes in the stem of transgenic tobacco using qRT-PCR (Figure 4E). The significantly downregulated DEGs were mainly involved in carbohydrate metabolism; for example, LOC104231008, encoding glucose-6-phosphate 1-dehydrogenase; LOC104109279, encoding malate dehydrogenase; LOC104095422 and LOC104089017, encoding fructose-bisphosphate aldolase 1; LOC104217259 and LOC104112949, encoding glutamate dehydrogenase B; and LOC107801158, encoding glyceraldehyde-3-phosphate dehydrogenase B (Figure 4D). We also used qRT-PCR to detect the expression levels of the above downregulated genes in the stem of transgenic tobacco (Figure 4E). These findings suggested that LkABCG36 possibly has functions in processes involving both the transport of plant hormones and signal transduction.
2.4. Functional Enrichment Analysis of DEGs in Transgenic Tobacco Overexpressing LkABCG40
To explore the functional significance of the DEGs between the LkABCG40 and WT groups, we performed GO enrichment analysis (Figure 5A). We found that the genes are involved in the regulation of intracellular transport; we noted strong enrichment in protein transport, the regulation of ion transmembrane transport, and carbohydrate transport. In addition, we found gene enrichment in metabolic and biosynthetic processes, such as the salicylic acid and cellulose biosynthetic processes, and the carbohydrate and cellular aromatic compound metabolic processes. Furthermore, the terms including the response to hormones, the jasmonic-acid-mediated signaling pathway, and intracellular signal transduction were also enriched.
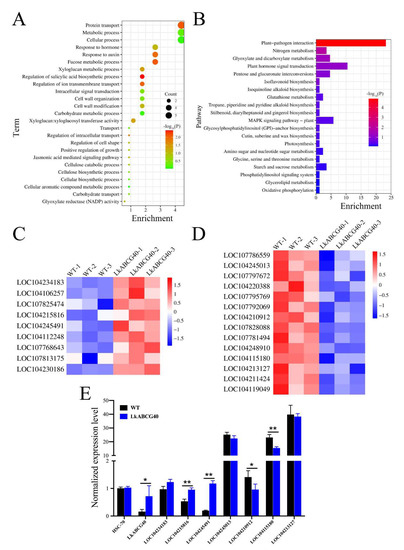
Figure 5.
Functional enrichment analysis of DEGs and expression of key genes in LkABCG40 group. (A) Gene Ontology (GO) enrichment analysis identifiers in cluster of overlapping DEGs in LkABCG40 group compared with WT group. (B) Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis identifiers in cluster of overlapping DEGs in LkABCG40 group compared with WT group. (C) Heatmap shows key upregulated DEGs involved in biosynthesis of environmental adaptation and energy metabolism. Relative expression level of genes was calculated using log10(FPKM). (D) Heatmap showing the key downregulated DEGs involved in biosynthesis of signal transduction and energy metabolism. Relative expression level of genes was calculated using log10 (FPKM). (E) RT-PCR was used to detect changes in key up- and downregulated DEGs in stem tissue of transgenic tobacco. Data shown as mean ± SEM. Significance levels are noted as * p < 0.05; ** p < 0.01.
To further explore which signal pathways were directly affected by the overexpression of LkABCG40, we analyzed DEG enrichment according the KEGG pathway database (Figure 5B). We found that the pathways were mainly involved in four areas: environmental adaptation, energy metabolism, biosynthesis of other secondary metabolites, and signal transduction.
Pathways including plant–pathogen interaction, and cutin, suberine, and wax biosyntheses are involved in environmental adaptation. In addition, pathways including oxidative phosphorylation, photosynthesis, and nitrogen metabolism are involved in energy metabolism. Furthermore, various pathways, including the biosynthesis of isoflavonoids; of tropane, piperidine, and pyridine alkaloids; and of stilbenoid, diarylheptanoid, and gingerol, are involved in the biosynthesis of other secondary metabolites. Moreover, certain pathways, including plant hormone signal transduction and the MAPK signaling pathway—plant, are involved in signal transduction.
To validate the signaling pathways identified in the aforementioned results, as well as to explore the key genes in transgenic tobacco overexpressing LkABCG40, we studied a number of key genes, and their expression levels are shown in Figure 5C,D. Of these DEGs, many key genes involved in environmental adaptation were significantly upregulated, such as LOC104234183, encoding probable disease resistance protein RF9; LOC104215816, encoding resistance protein homolog R1A-10; and LOC104245491, encoding resistance protein homolog R1B-16 (Figure 5C). To further verify the reliability of the sequencing, we detected the expression levels of the above genes in the stem of transgenic tobacco using qRT-PCR (Figure 5E). The significantly downregulated DEGs were mainly involved in plant–pathogen interactions, such as LOC104245013, encoding WRKY transcription factor 41; LOC104210912, encoding WRKY transcription factor 22; LOC104115180, encoding WRKY transcription factor 41-like; LOC104213127, encoding WRKY transcription factor 70; and LOC104119049, encoding probable WRKY transcription factor 41. We also used qRT-PCR to detect the expression levels of the above downregulated genes in the stem of transgenic tobacco (Figure 5E). These findings suggest that LkABCG40 is possibly involved in the environmental adaptation and resistance of transgenic tobacco.
3. Discussion
In our study, we investigated the transcriptomic changes in the stem tissues of transgenic tobacco plants overexpressing LkABCG36 and LkABCG40 and compared them with those in the wild type. Our findings showed that the functions of the DEGs in the stem tissues of transgenic tobacco overexpressing LkABCG36 were mainly enriched in plant hormone signal transduction, carbohydrate metabolism, and the biosynthesis of other secondary metabolites. The functions of the DEGs in the stem tissues of transgenic tobacco overexpressing LkABCG40 were mainly enriched in metabolism, biosynthesis, and environmental adaptation. Based on the above results, we speculated that LkABCG36 and LkABCG40 play crucial roles in plant development and environmental adaptation.
AtABCG36 possibly plays a dual role in IBA transport [20]. The phytohormone auxin IBA is involved in regulating plant growth and development, and it plays an important role in the responses to biotic and abiotic stresses, including drought, salinity, and cold [23]. In this study, we found that transgenic tobacco overexpressing LkABCG36 grew rapidly, which may have been related to LkABCG36 catalyzing the export of IBA [20,21]. In addition, we detected many significantly upregulated genes encoding auxin or auxin receptors in the stem tissue of transgenic tobacco overexpressing LkABCG36; for example, LOC109223084, encoding auxin-responsive protein and LOC104113066 and LOC107784906, encoding indole-3-acetic acid-amido (IAA) synthetase. This finding may be related to the dynamic spatiotemporal changes in the level of auxin that can accurately and rapidly trigger gene reprogramming [24]. Therefore, we speculated that the overexpression of LkABCG36 promotes the expression of auxin response factor (ARF) and increases the secretion of auxin in transgenic tobacco. Furthermore, Yu et al. [25] found that IAA20 (Eucalyptus grandis) is preferentially expressed in cambium and is involved in wood formation. Zhang et al. [26] found that maize (Zea mays L.) Aux/IAA protein RUM1 plays key roles in the regulation of the auxin signal transduction components and promotes vascular development in primary roots. This indicates that Aux/IAA not only plays an important role in regulating plant growth and development, but also provides protection for plants in adapting to environmental changes [24]. Therefore, we hypothesized that LkABCG36 promotes the expression of key auxin-related genes by transporting auxin, thereby accelerating plant growth and development, and ultimately enhancing the resistance of transgenic tobacco.
ABCG40 is also involved in hormone transport, but unlike AtABCG36 involvement in IBA transport, AtABCG40 is an exporter of abscisic acid (ABA) [10]. ABA is a stress hormone which plays key roles in regulating both biological and abiotic stress responses [27], and ABA improves drought tolerance and water-use efficiency by regulating stomatal conductance. Zhang et al. [28] found that AtABCG17 and AtABCG18 redundantly promote ABA import, restricting stomatal closure and thus allowing plants to respond to the changing environment. This suggests that ABCG40 may be involved in long-distance ABA transport, regulating the physiology and morphology of plants. In addition, we found that a number of the DEGs involved in environmental adaptation were significantly upregulated, such as LOC104234183, encoding probable disease resistance protein RF9; LOC104215816, encoding resistance protein homolog R1A-10; and LOC104245491, encoding resistance protein homolog R1B-16. These results indicated that the overexpression of LkABCG40 promotes resistance in transgenic tobacco. In addition, we found some DEGs were downregulated in encoding WRKY transcription factor; examples include LOC104245013, LOC104210912, LOC104115180, and LOC104213127. The WRKY gene family is one of the largest transcription factor families in higher plants [29]. WRKY proteins encoded by WRKY genes often act as repressors and activators [30]. These transcription factors play important roles in the response to biotic and abiotic stresses, mainly by regulating plant hormone signal transduction pathways in plants [31]. The expression of WRKY is significantly reduced by drought stress or treatment with mannitol and ABA [32,33]. Furthermore, it has been found that the concentration of ABA is lower than that of WT in Arabidopsis thaliana with ABCG40 knockout [34]. Therefore, we hypothesized that in overexpression of LkABCG40 the ABA concentration may be higher than that in WT, and the high concentration of ABA may inhibit the expression of WRKY.
However, some aspects need to be further explored, such as the difference in hormone transport between LkABCG36 and LkABCG40, and whether they differ in their specificities for transport substrates. In addition, auxin is a growth hormone, and ABA is often defined as a stress hormone. Both of them play important roles in plant development and enhancing plant traits, but the complex cross-talk relationships between different plant hormones are not well understood, so the LkABCG36 and LkABCG40 hormone transport mechanisms may provide insight into the environmental adaptation of plants.
In conclusion, we found that LkABCG36 and LkABCG40 play important roles in plant development and environmental adaptation. LkABCG36 may promote the growth of transgenic tobacco by promoting the transport of auxins such as IBA and IAA; LkABCG40 may inhibit the expression of WRKY by increasing the concentration of ABA to improve the stress resistance of transgenic tobacco. These results are beneficial information for future studies into the functions of LkABCG36 and LkABCG40.
4. Materials and Methods
4.1. Plant Materials
We sterilized tobacco (Nicotiana tabacum) seeds with 70% ethanol, then rinsed them three times with sterile water. After removing the disinfectant, we germinated the seeds on MS medium and placed them under a 16/8 h (light/dark) photoperiod at 25 °C for culture. To obtain LkABCG36 and LkABCG40 transgenic tobacco plants, we used Agrobacterium mediated transformation as previously described [35,36]. We collected leaves from 5-week-old tobacco plants, which we cleaned with reverse osmosis deionized (RODI) water, then disinfected with 4% NaClO. Following this, we washed the leaves three times with RODI water. Then, we cut the leaves into 1–2 cm2 explants, which we placed on a shoot-induction medium (SIM) for culture with the following supplements: 3% (w/v) sucrose, 1 mg/L 6-benzylaminopurine (BA), 0.1 mg/L 1-naphthaleneacetic acid (NAA), and 8% agar with pH adjusted to 5.7. In the next step, we immersed the explants in Agrobacterium (carrying a helper plasmid pBI121-GFP) suspension for 15–20 min. The sequences of LkABCG36 and LkABCG40 are shown in Supplementary Data S1.
We used sterile filter paper to blot the remaining fluid from the explants, which we then transferred to fresh SIM for co-culture over 24 h. Finally, we transferred the explants to a fresh SIM medium containing 30 mg/L meropenem and 200 mg/L kanamycin. When the roots of the explants reached 1–2 cm, we cut them into sections and transferred them into fresh RIM, which contained 3% (w/v) sucrose, 0.1 mg/L NAA, 30 mg/L meropenem, 200 mg/L kanamycin, and 8% agar with pH adjusted to 5.7, for further development. When the plantlets reached 5–8 cm in height, we transferred them to soil for acclimation and identification of transgenic positive plants. Ultimately, we obtained a total of 11 transgenic lines, and we selected a homozygous strain with high expressions of LkABCG36 or LkABCG40 for this study.
4.2. RNA-Seq and Data Processing
We derived the materials used for RNA-seq analysis from the stems of 11-week-old transgenic and wild-type plants, and we collected 3 samples from each group. We first extracted total RNA from the stem tissues using a Plant RN38 Kit (Aidlab, Beijing, China) according to the manufacturer’s instructions. Then, we prepared the RNA-seq library with a TruSeq RNA sample preparation kit (Illumina, San Diego, CA, USA), according to the manufacturer’s instructions; we sequenced the libraries on an Illumina HiSeq system. We used Skewer (v0.2.2) software (https://sourceforge.net) to dynamically remove joint sequence and low-quality fragments from the 3’ end of the sequencing data. We used FastQC (v0.11.5) software (http://www.bioinformatics) to conduct quality control analysis on the preprocessed data. We mapped the sequence data to the N. tabacum genome (https://solgenomics.net/organism/Nicotiana_tabacum/genome) using TopHat (2.0.12) software. We used StringTie (v1.3.1c) software (http://ccb.jhu.edu) to calculate the original sequence count of known genes for all samples, and we calculated the expression level of genes based on fragments per kilobase of transcript per million fragments mapped (FPKM) using Cufflinks software [37].
4.3. Differential Gene Expression Analysis
We analyzed the differentially expressed genes (DEGs) between WT and transgenic plants to identify significantly up- or downregulated genes. We assessed the DEGs using DESeq 2 (v1.16.1) software (https://bioconductor.org). We used the Benjamini–Hochberg multiple test correction method. We chose the DEGs according to |log2 fold change| ≥ 1 and adjusted p value < 0.05. We tested Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) (http://www.genome.jp) annotations for enrichment [38]. We constructed the gene co-expression network using weighted gene co-expression network analysis (WGCNA) methodology [39]. We plotted the heatmaps using Bioinformatics (https://www.bioinformatics.com.cn).
4.4. qRT-PCR Analysis
We selected a number of key genes for verification using qRT-PCR. We designed the primers with Primer Premier 5 software; all primers are listed in Table S1. We isolated the total RNA of the stems with a Plant RN38 Kit (Aidlab, Beijing, China), according to the manufacturer’s instructions. Then, we reverse-transcribed the total RNA into cDNA using a reverse-transcription kit (Aidlab, Beijing, China). We performed qRT-PCR using a 7500 Fast Real-Time PCR system (Applied Biosystems, Foster, CA, USA) with SYBR Premix Ex TaqTM (Aidlab, Beijing, China), and we used HSC70-1 as an internal reference gene. We ran the qRT-PCR program according to the manufacturer’s instructions, with three replicates for each sample. We used the 2−ΔΔCT approach to assess the relative expression levels of key genes; data are reported as mean ± SEM (n = 4); and we analyzed the significant differences with GraphPad Prism 8.0 software with a t-test (* p < 0.05, ** p < 0.01).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12020227/s1, Table S1: The list of primer sequence.
Author Contributions
All authors contributed to the study design. N.S. and C.L. analyzed the data; N.S. and X.J. performed the data collection; N.S. wrote the first draft of the manuscript; Y.G. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by National Key R&D Program of China (2022YFD2200100).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Theodoulou, F.L.; Kerr, I.D. ABC transporter research: Going strong 40 years on. Biochem. Soc. Trans. 2015, 43, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Do, T.H.T.; Martinoia, E.; Lee, Y. Functions of ABC transporters in plant growth and development. Curr. Opin. Plant Biol. 2018, 41, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Theodoulou, F.L. Plant ABC transporters. Biochim. Biophys. Acta 2000, 1465, 79–103. [Google Scholar] [CrossRef] [PubMed]
- Aryal, B.; Laurent, C.; Geisler, M. Learning from each other: ABC transporter regulation by protein phosphorylation in plant and mammalian systems. Biochem. Soc. Trans. 2015, 43, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Aller, S.G.; Beis, K.; Carpenter, E.P.; Chang, G.; Chen, L.; Dassa, E.; Dean, M.; Duong Van Hoa, F.; Ekiert, D.; et al. Structural and functional diversity calls for a new classification of ABC transporters. FEBS Lett. 2020, 594, 3767–3775. [Google Scholar] [CrossRef]
- Dahuja, A.; Kumar, R.R.; Sakhare, A.; Watts, A.; Singh, B.; Goswami, S.; Sachdev, A.; Praveen, S. Role of ATP-binding cassette transporters in maintaining plant homeostasis under abiotic and biotic stresses. Physiol. Plant 2021, 171, 785–801. [Google Scholar] [CrossRef]
- Crouzet, J.; Trombik, T.; Fraysse, A.S.; Boutry, M. Organization and function of the plant pleiotropic drug resistance ABC transporter family. FEBS Lett. 2006, 580, 1123–1130. [Google Scholar] [CrossRef]
- Lefèvre, F.; Baijot, A.; Boutry, M. Plant ABC transporters: Time for biochemistry? Biochem. Soc. Trans. 2015, 43, 931–936. [Google Scholar] [CrossRef]
- Dhara, A.; Raichaudhuri, A. ABCG transporter proteins with beneficial activity on plants. Phytochemistry 2021, 184, 112663. [Google Scholar] [CrossRef]
- Borghi, L.; Kang, J.; Ko, D.; Lee, Y.; Martinoia, E. The role of ABCG-type ABC transporters in phytohormone transport. Biochem. Soc. Trans. 2015, 43, 924–930. [Google Scholar] [CrossRef]
- Jarzyniak, K. Role of ABCG transporters in modulation of symbiotic interactions. Postepy. Biochem. 2020, 66, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Fang, C.; Li, Z.; Wang, Y.; Pan, S.; Wu, Y.; An, X.; Long, Y.; Wan, X. ATP-Binding Cassette G Transporters and Their Multiple Roles Especially for Male Fertility in Arabidopsis, Rice and Maize. Int. J. Mol. Sci. 2022, 23, 9304. [Google Scholar] [CrossRef] [PubMed]
- Ichino, T.; Yazaki, K. Modes of secretion of plant lipophilic metabolites via ABCG transporter-dependent transport and vesicle-mediated trafficking. Curr. Opin. Plant Biol. 2022, 66, 102184. [Google Scholar] [CrossRef] [PubMed]
- Quilichini, T.D.; Samuels, A.L.; Douglas, C.J. ABCG26-mediated polyketide trafficking and hydroxycinnamoyl spermidines contribute to pollen wall exine formation in Arabidopsis. Plant Cell 2014, 26, 4483–4498. [Google Scholar] [CrossRef]
- Zhao, G.; Shi, J.; Liang, W.; Zhang, D. ATP binding cassette G transporters and plant male reproduction. Plant Signal Behav. 2016, 11, e1136764. [Google Scholar] [CrossRef][Green Version]
- Toussaint, F.; Pierman, B.; Bertin, A.; Lévy, D.; Boutry, M. Purification and biochemical characterization of NpABCG5/NpPDR5, a plant pleiotropic drug resistance transporter expressed in Nicotiana tabacum BY-2 suspension cells. Biochem. J. 2017, 474, 1689–1703. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, L.; Chen, P.; Cai, H.; Hou, Z.; Jin, X.; Aslam, M.; Chai, M.; Lai, L.; He, Q.; et al. ATP binding cassette transporters ABCG1 and ABCG16 affect reproductive development via auxin signalling in Arabidopsis. Plant J. 2020, 102, 1172–1186. [Google Scholar] [CrossRef]
- Yim, S.; Khare, D.; Kang, J.; Hwang, J.U.; Liang, W.; Martinoia, E.; Zhang, D.; Kang, B.; Lee, Y. Postmeiotic development of pollen surface layers requires two Arabidopsis ABCG-type transporters. Plant Cell Rep. 2016, 35, 1863–1873. [Google Scholar] [CrossRef]
- Ruzicka, K.; Strader, L.C.; Bailly, A.; Yang, H.; Blakeslee, J.; Langowski, L.; Nejedlá, E.; Fujita, H.; Itoh, H.; Syono, K.; et al. Arabidopsis PIS1 encodes the ABCG37 transporter of auxinic compounds including the auxin precursor indole-3-butyric acid. Proc. Natl. Acad. Sci. USA 2010, 107, 10749–10753. [Google Scholar] [CrossRef]
- Aryal, B.; Huynh, J.; Schneuwly, J.; Siffert, A.; Liu, J.; Alejandro, S.; Ludwig-Müller, J.; Martinoia, E.; Geisler, M. ABCG36/PEN3/PDR8 Is an Exporter of the Auxin Precursor, Indole-3-Butyric Acid, and Involved in Auxin-Controlled Development. Front. Plant Sci. 2019, 10, 899. [Google Scholar] [CrossRef]
- Song, P.; Xu, H.; Zhang, J.; Chen, H.; Li, L.; Qu, Y.; Lin, F.; Zhang, Q. Functional analysis of indole 3-hexanoic acid as a novel auxin from Arabidopsis thaliana. Planta 2021, 254, 69. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.Q.; Yuan, J.J.; Xiao, C.C.; Li, G.X.; Yan, J.Y.; Zheng, S.J.; Ding, Z.J. RING-box proteins regulate leaf senescence and stomatal closure via repression of ABA transporter gene ABCG40. J. Integr. Plant Biol. 2022, 64, 979–994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Xu, C.; Liu, K.; Bi, H.; Ai, X. H2O2 Functions as a Downstream Signal of IAA to Mediate H2S-Induced Chilling Tolerance in Cucumber. Int. J. Mol. Sci. 2021, 22, 12910. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhou, J.J.; Zhang, J.Z. Aux/IAA Gene Family in Plants: Molecular Structure, Regulation, and Function. Int. J. Mol. Sci. 2018, 19, 259. [Google Scholar] [CrossRef]
- Yu, H.; Liu, M.; Zhu, Z.; Wu, A.; Mounet, F.; Pesquet, E.; Grima-Pettenati, J.; Cassan-Wang, H. Overexpression of EgrIAA20 from Eucalyptus grandis, a Non-Canonical Aux/IAA Gene, Specifically Decouples Lignification of the Different Cell-Types in Arabidopsis Secondary Xylem. Int. J. Mol. Sci. 2022, 23, 5068. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Paschold, A.; Marcon, C.; Liu, S.; Tai, H.; Nestler, J.; Yeh, C.T.; Opitz, N.; Lanz, C.; Schnable, P.S.; et al. The Aux/IAA gene rum1 involved in seminal and lateral root formation controls vascular patterning in maize (Zea mays L.) primary roots. J. Exp. Bot. 2014, 65, 4919–4930. [Google Scholar] [CrossRef]
- Emenecker, R.J.; Strader, L.C. Auxin-Abscisic Acid Interactions in Plant Growth and Development. Biomolecules 2020, 10, 281. [Google Scholar] [CrossRef]
- Zhang, Y.; Kilambi, H.V.; Liu, J.; Bar, H.; Lazary, S.; Egbaria, A.; Ripper, D.; Charrier, L.; Belew, Z.M.; Wulff, N.; et al. ABA homeostasis and long-distance translocation are redundantly regulated by ABCG ABA importers. Sci. Adv. 2021, 7, eabf6069. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, S.; Ye, N.; Jiang, M.; Cao, J.; Zhang, J. WRKY transcription factors in plant responses to stresses. J. Integr. Plant Biol. 2017, 59, 86–101. [Google Scholar] [CrossRef]
- Wani, S.H.; Anand, S.; Singh, B.; Bohra, A.; Joshi, R. WRKY transcription factors and plant defense responses: Latest discoveries and future prospects. Plant Cell Rep. 2021, 40, 1071–1085. [Google Scholar] [CrossRef]
- Mirza, Z.; Haque, M.M.; Gupta, M. WRKY transcription factors: A promising way to deal with arsenic stress in rice. Mol. Biol. Rep. 2022, 49, 10895–10904. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Kang, K.; Shim, Y.; Yoo, S.C.; Paek, N.C. Inactivating transcription factor OsWRKY5 enhances drought tolerance through abscisic acid signaling pathways. Plant Physiol. 2022, 188, 1900–1916. [Google Scholar] [CrossRef] [PubMed]
- Rushton, D.L.; Tripathi, P.; Rabara, R.C.; Lin, J.; Ringler, P.; Boken, A.K.; Langum, T.J.; Smidt, L.; Boomsma, D.D.; Emme, N.J.; et al. WRKY transcription factors: Key components in abscisic acid signalling. Plant Biotechnol. J. 2012, 10, 2–11. [Google Scholar] [CrossRef]
- Shimadzu, S.; Seo, M.; Terashima, I.; Yamori, W. Whole Irradiated Plant Leaves Showed Faster Photosynthetic Induction Than Individually Irradiated Leaves via Improved Stomatal Opening. Front. Plant Sci. 2019, 10, 1512. [Google Scholar] [CrossRef] [PubMed]
- Krenek, P.; Samajova, O.; Luptovciak, I.; Doskocilova, A.; Komis, G.; Samaj, J. Transient plant transformation mediated by Agrobacterium tumefaciens: Principles, methods and applications. Biotechnol. Adv. 2015, 33, 1024–1042. [Google Scholar] [CrossRef]
- Navet, N.; Tian, M. Agrobacterium-mediated Transformation of Sweet Basil (Ocimum basilicum). Bio. Protoc. 2020, 10, e3828. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).