Zinc and Boron Soil Applications Affect Athelia rolfsii Stress Response in Sugar Beet (Beta vulgaris L.) Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Planting Material and Experimental Design
2.2. Pot Preparation, Fertilizer Application and Seed Sowing
2.3. Fungi Culture, Inoculation and Sample Collection
2.4. Determination of Soil-Plant-Analysis Development (SPAD) Value
2.5. Enzyme Extraction and Assay
2.6. Estimation of the Ascorbic Acid (AsA) Content
2.7. Determination of the Malondialdehyde (MDA) Content
2.8. Statistical Analysis
3. Results
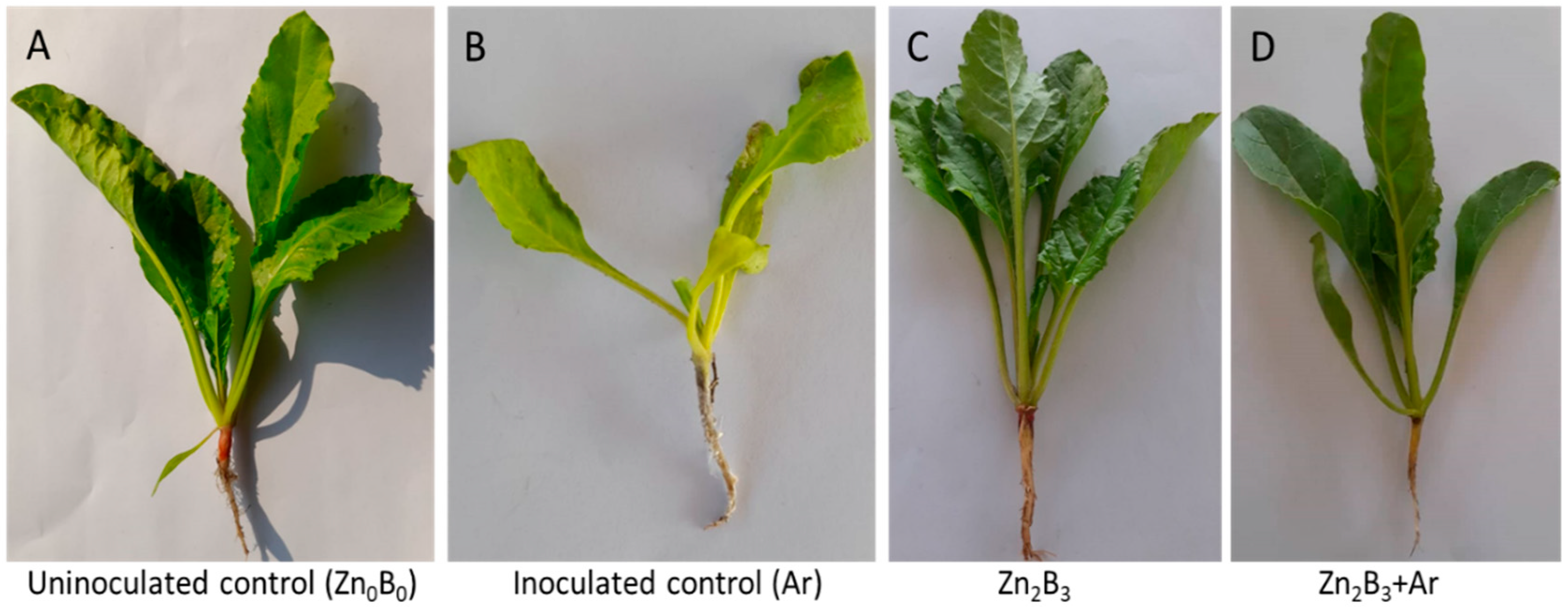
3.1. Effect of Zn and B on Plant Survival under A. rolfsii Stress
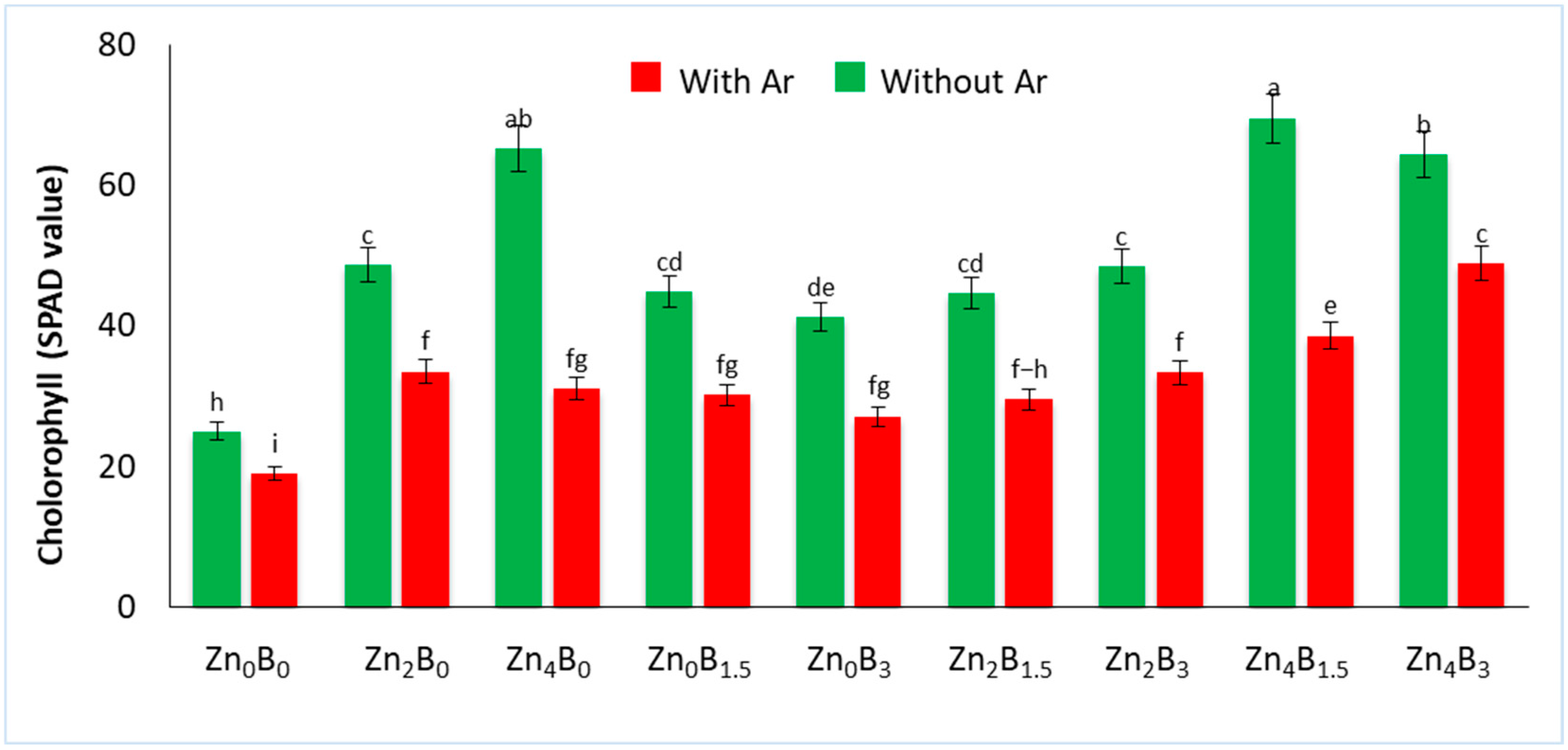
3.2. Effect of Zn and B on Chlorophyll Content (SPAD Value)
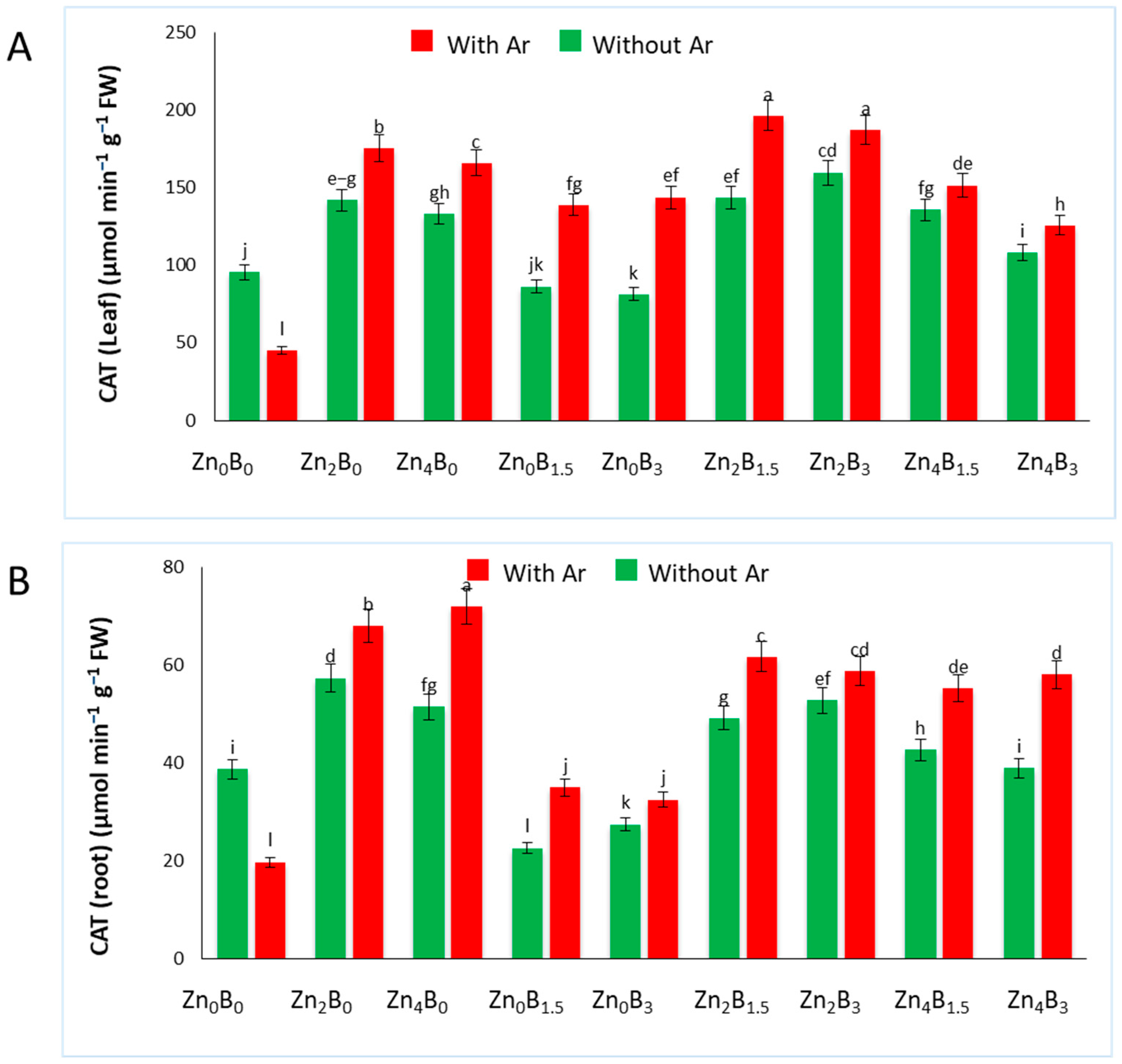
3.3. Effect of Zn and B on CAT Activity
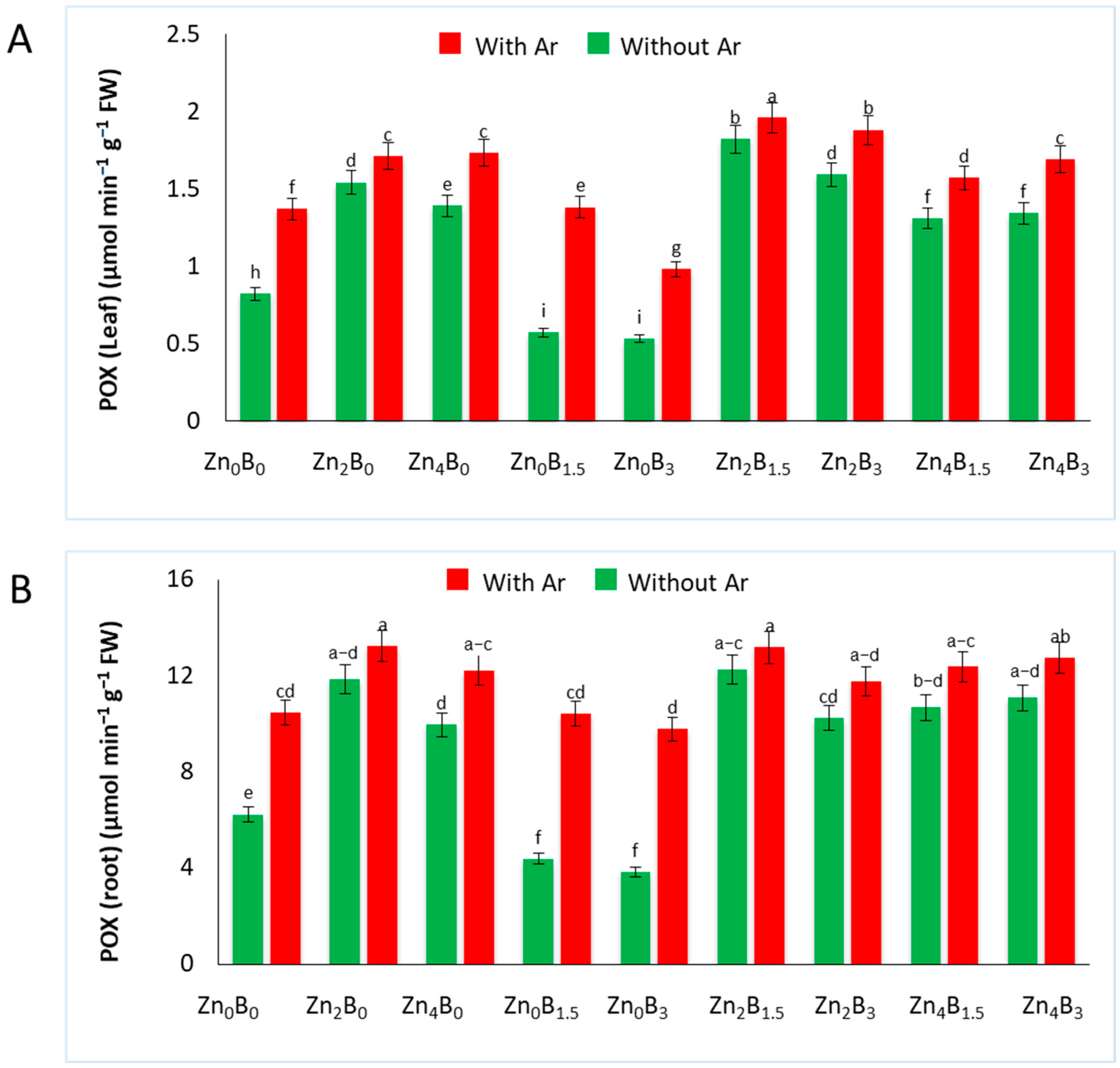
3.4. Effect of Zn and B on POX Activity
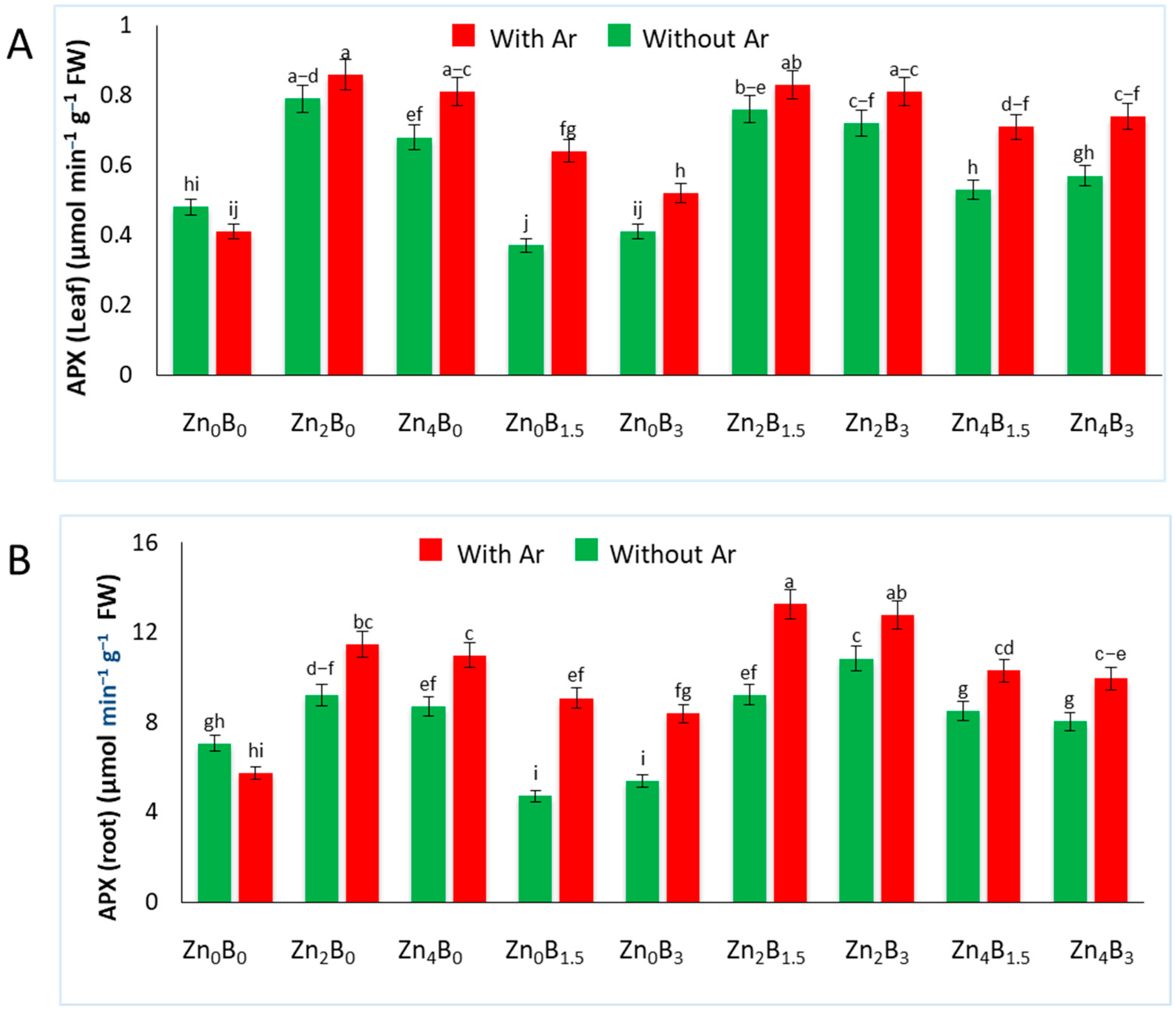
3.5. Effect of Zn and B on APX Activity
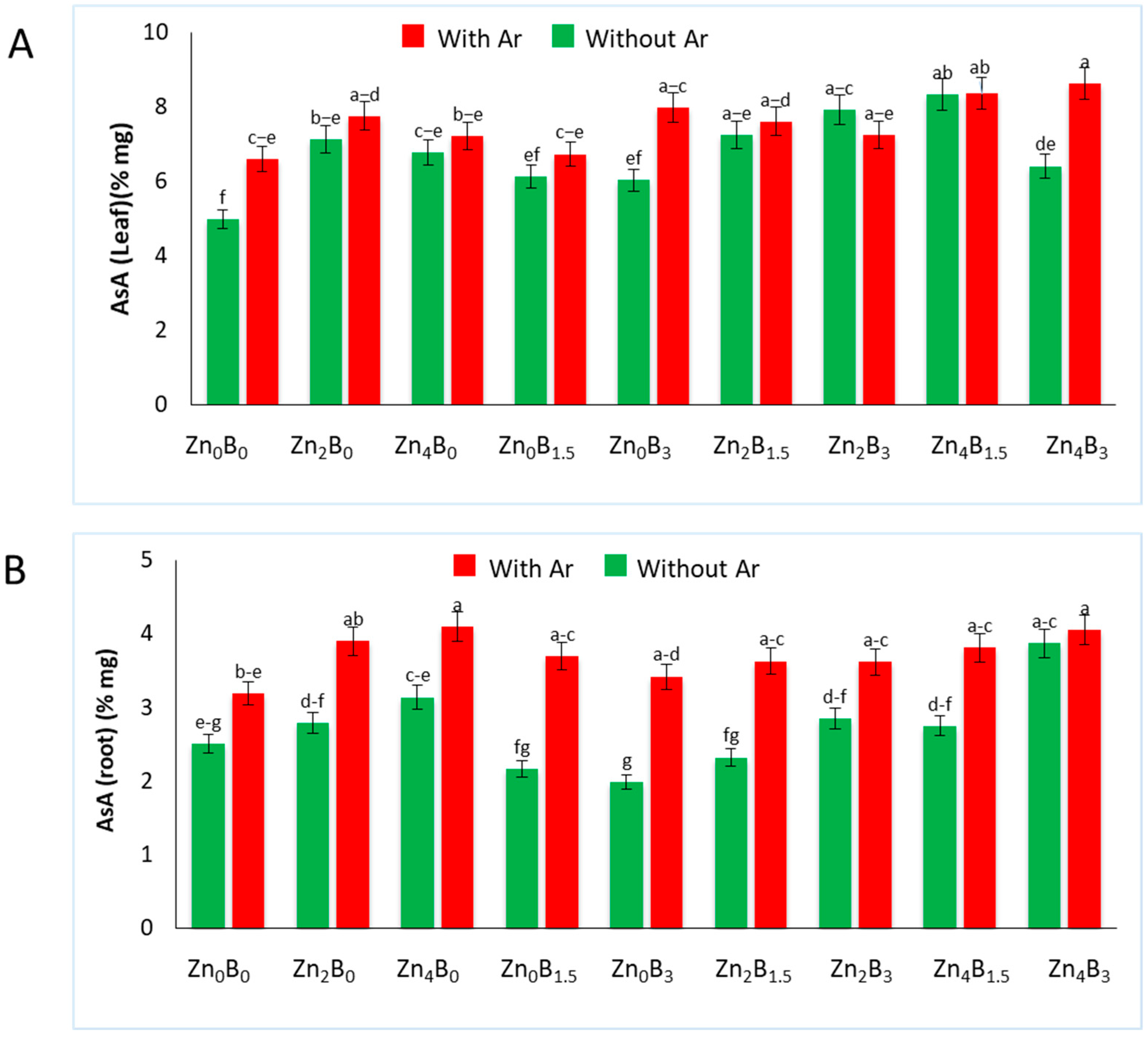
3.6. Effect of Zn and B on AsA Concentration
3.7. Effect of Zn and B on MDA Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brar, N.S.; Dhillon, B.S.; Saini, K.S.; Sharma, P.K. Agronomy of sugar beet cultivation—A review. Agric. Rev. 2015, 36, 184–197. [Google Scholar] [CrossRef]
- Bairagi, A.; Paul, S.K.; Kader, M.A.; Hossain, M.S. Yield of tropical sugarbeet as influenced by variety and rate of fertilizer application. Pak. Sugar J. 2013, 28, 13–20. [Google Scholar]
- Dohm, J.C.; Minochle, A.E.; Holtgrawe, D.; Capella-Gutiérrez, S.; Zakrzewski, F.; Tafer, H.; Rupp, O.; Sörensen, T.R.; Stracke, R.; Reinhardt, R.; et al. The genome of the recently domesticated crop plant sugar beet (Beta vulgaris). Nature 2014, 505, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Zubair, M.; Iqbal, N.; Cheema, N.M.; Mahmood, K. Evaluation of sugar beet hybrid varieties under Thal-Kumbi soil series of Pakistan. Int. J. Agric. Biol. 2012, 14, 605–608. [Google Scholar]
- Paul, S.K.; Paul, U.; Sarkar, M.A.R.; Hossain, M.S. Yield and quality of tropical sugarbeet as influenced by variety, spacing and fertilizer application. Sugar Tech 2018, 20, 175–181. [Google Scholar] [CrossRef]
- Mall, A.K.; Misra, V.; Santeshwari; Pathak, A.D.; Srivastava, S. Sugar beet cultivation in India: Prospects for bio-ethanol production and value-added co-products. Sugar Tech 2021, 23, 1218–1234. [Google Scholar] [CrossRef]
- Rinaldi, M.; Vonella, A.V. The response of autumn and spring sown sugarbeet (Beta vulgaris L.) to irrigation in southern Italy: Water and radiation use efficiency. Field Crops Res. 2006, 95, 103–114. [Google Scholar] [CrossRef]
- Paul, S.K.; Mahmud, N.U.; Gupta, D.R.; Surovy, M.Z.; Rahman, M.; Islam, M.T. Characterization of Sclerotium rolfsii causing root rot of sugar beet in Bangladesh. Sugar Tech 2021, 23, 1199–1205. [Google Scholar] [CrossRef]
- Errakhi, R.; Lebrihi, A.; Barakate, M. In vitro and in vivo antagonism of actinomycetes isolated from Moroccan rhizo-spherical soils against Sclerotium rolfsii: A causal agent of root rot on sugar beet (Beta vulgaris L.). J. Appl. Microbiol. 2009, 107, 672–681. [Google Scholar] [CrossRef]
- Rafi, S.; Shoaib, A.; Awan, Z.A.; Rizvi, N.B.; Shafiq, N.M. Chromium tolerance, oxidative stress response, morphological characteristics, and FTIR studies of phytopathogenic fungus Sclerotium rolfsii. Folia. Microbiol. 2017, 62, 207–219. [Google Scholar] [CrossRef]
- Liao, H.; Wen, X.; Deng, X.; Wu, Y.; Xu, J.; Li, X.; Zhou, S.; Li, X.; Zhu, C.; Luo, F.; et al. Integrated proteomic and metabolomic analyses reveal significant changes in chloroplasts and mitochondria of pepper (Capsicum annuum L.) during Sclerotium rolfsii infection. J. Microbiol. 2022, 60, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Shigeoka, S.; Ishikawa, T.; Tamoi, M.; Miyagawa, Y.; Takeda, T.; Yabuta, Y.; Yoshimura, K. Regulation and function of ascorbate peroxidase isoenzymes. J. Exp. Bot. 2002, 53, 1305–1319. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.S.; Zulfiqar, F.; Alam, M.M.; Fujita, M. Regulation of ROS metabolism in plants under environmental stress: A review of recent experimental evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef]
- Mansoor, S.; Ali Wani, O.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive oxygen species in plants: From source to sink. Antioxidants 2022, 11, 225. [Google Scholar] [CrossRef]
- Mathpal, B.; Srivastava, P.C.; Shankhdhar, D.; Shankhdhar, S.C. Improving key enzyme activities and quality of rice under various methods of zinc application. Physiol. Mol. Biol. Plants 2015, 21, 567–572. [Google Scholar] [CrossRef]
- Cabot, C.; Soledad, M.; Mercè, L.; Berta, G.; Roser, T.; Charlotte, P. A Role for Zinc in plant defense against pathogens and herbivores. Front. Plant Sci. 2019, 10, 1171. [Google Scholar] [CrossRef]
- Ilyas, A.; Ashraf, M.Y.; Hussain, M.; Ashraf, M.; Ahmed, R.; Kamal, A. Effect of micronutrients (Zn, Cu and B) on photosynthetic and fruit yield attributes of Citrus reticulata Blanco var. kinnow. Pak. J. Bot. 2015, 47, 1241–1247. [Google Scholar]
- Luo, Y.; Yao, A.; Tan, M.; Li, Z.; Qing, L.; Yang, S. Effects of manganese and zinc on the growth process of Phytophthora nicotianae and the possible inhibitory mechanisms. PeerJ 2020, 20, e8613. [Google Scholar] [CrossRef]
- Noreen, S.; Sultan, M.; Akhter, M.S.; Shah, K.H.; Ummara, U.; Manzoor, H.; Ulfat, M.; Alyemeni, M.N.; Ahmad, P. Foliar fertigation of ascorbic acid and zinc improves growth, antioxidant enzyme activity and harvest index in barley (Hordeum vulgare L.) grown under salt stress. Plant Physiol. Biochem. 2021, 158, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Umair Hassan, M.; Aamer, M.; Umer Chattha, M.; Haiying, T.; Shahzad, B.; Barbanti, L.; Nawaz, M.; Rasheed, A.; Afzal, A.; Liu, Y. The critical role of zinc in plants facing the drought stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- Aydin, M.; Tombuloglu, G.; Sakcali, M.S.; Hakeem, K.R.; Tombuloglu, H. Boron alleviates drought stress by enhancing gene expression and antioxidant enzyme activity. J. Soil Sci. Plant Nutr. 2019, 19, 545–555. [Google Scholar] [CrossRef]
- Kohli, S.K.; Kaur, H.; Khanna, K.; Handa, H.; Bhardwaj, R.; Rinklebe, J.; Ahmad, P. Boron in plants: Uptake, deficiency and biological potential. Plant Growth Regul. 2023, 100, 267–282. [Google Scholar] [CrossRef]
- Forand, A.D.; Finfrock, Y.Z.; Lavier, M.; Stobbs, J.; Qin, L.; Wang, S.; Karunakaran, C.; Wei, Y.; Ghosh, S.; Tanino, K.K. With a little help from my cell wall: Structural modifications in pectin may play a role to overcome both dehydration stress and fungal pathogens. Plants 2022, 11, 385. [Google Scholar] [CrossRef]
- Guo, H.; Bi, X.; Wang, Z.; Jiang, D.; Cai, M.; An, M.; Xia, Z.; Wu, Y. Reactive oxygen species-related genes participate in resistance to cucumber green mottle mosaic virus infection regulated by boron in Nicotiana benthamiana and watermelon. Front. Plant Sci. 2022, 13, 1027404. [Google Scholar] [CrossRef]
- Awan, Z.A.; Shoaib, A.; Khan, K.A. Crosstalk of Zn in combination with other fertilizers underpins interactive effects and induces resistance in tomato plant against early blight disease. Plant Pathol. J. 2019, 35, 330–340. [Google Scholar] [CrossRef]
- Rerhou, B.; Mosseddaq, F.; Ezzahiri, B.; Moughli, L.; Mokrini, F.; Bel-Lahbib, S.; Ibno Namr, K. Occurrence of Sclerotium rolfsii inducing sugar beet root rot and its sustainable management by acting on soil fertility in western Morocco. J. Ecol. Eng. 2023, 24, 54–70. [Google Scholar] [CrossRef]
- Figueredo, M.S.; Tonelli, M.L.; Ibáñez, F.; Morla, F.; Cerioni, G.; del Carmen Tordable, M.; Fabra, A. Induced systemic resistance and symbiotic performance of peanut plants challenged with fungal pathogens and co-inoculated with the biocontrol agent Bacillus sp. CHEP5 and Bradyrhizobium sp. SEMIA6144. Microbiol. Res. 2017, 197, 65–73. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Meth. Enzymol. 1981, 105, 121–126. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Rangana, S. Pectin analysis. In Handbook of Analysis and Quality Control for Fruit and Vegetable Products; Tata McGraw-Hill: New York, NY, USA, 1986; p. 342. [Google Scholar]
- Cakmak, L.; Horst, W.J. Effect of aluminum on lipid peroxidation, superoxide dismutase, catalase, and peroxide activity in root tip of soybean (Glycine max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Debona, D.; Rodrigues, F.Á.; Rios, J.A.; Nascimento, K.J.T. Biochemical changes in the leaves of wheat plants infected by Pyricularia oryzae. Phytopathology 2012, 102, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley & Sons: New Work, NY, USA, 1984; pp. 139–240. [Google Scholar]
- Slathia, S.; Sharma, Y.P.; Hakla, H.R.; Urfan, M.; Yadav, N.S.; Pal, S. Post-harvest management of Alternaria induced rot in tomato fruits with essential oil of Zanthoxylum armatum DC. Front. Sustain. Food Sys. 2021, 5, 225. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Lozowicka, B. Biochemical compounds and stress markers in lettuce upon exposure to pathogenic Botrytis cinerea and fungicides inhibiting oxidative phosphorylation. Planta 2022, 255, 61. [Google Scholar] [CrossRef]
- Zafar, S.; Ashraf, M.Y.; Ali, Q.; Ashraf, A.; Anwar, S.; Iqbal, N.; Kausar, A.; Nouman, A.; Ali, M.; Zafar, M.A. Antioxidant activity and secondary metabolites in selected vegetables irrigated with sewage water. Appl. Ecol. Environ. Res. 2016, 14, 35–48. [Google Scholar] [CrossRef]
- Singh, M.; Avtar, R.; Pal, A.; Punia, R.; Singh, V.K.; Bishnoi, M.; Singh, A.; Choudhary, R.R.; Mandhania, S. Genotype-specific antioxidant responses and assessment of resistance against Sclerotinia sclerotiorum causing Sclerotinia rot in Indian mustard. Pathogens 2020, 9, 892. [Google Scholar] [CrossRef]
- Ni, L.; Punja, Z.K. Effects of a foliar fertilizer containing boron on the development of Sclerotinia stem rot (Sclerotinia sclerotiorum) on canola (Brassica napus L.) leaves. J. Phytopathol. 2020, 168, 47–55. [Google Scholar] [CrossRef]
- Erper, I.; Yıldırım, E.; Türkkan, M. Antifungal effect of boron compounds against three Rhizoctonia solani AG-4 subgroups causing root and crown rot. Gesunde Pflanz. 2019, 71, 61–71. [Google Scholar] [CrossRef]
- Dong, X.; Wang, M.; Ling, N.; Shen, Q.; Guo, S. Effects of iron and boron combinations on the suppression of Fusarium wilt in banana. Sci. Rep. 2016, 201, 38944. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Łuniewski, S.; Kaczyński, P.; Łozowicka, B. The influence of humic acids and nitrophenols on metabolic compounds and pesticide behavior in wheat under biotic stress. Agronomy 2023, 13, 1378. [Google Scholar] [CrossRef]
- Noman, A.; Ali, Q.; Maqsood, J.; Iqbal, N.; Javed, M.T.; Rasool, N.; Naseem, J. Deciphering physio-biochemical, yield, and nutritional quality attributes of water-stressed radish (Raphanus sativus L.) plants grown from Zn-Lys primed seeds. Chemosphere 2018, 195, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Sarker, M.M.H.; Jahiruddin, M.; Moslehuddin, A.Z.M.; Islam, M.R. Optimization of zinc and boron doses for cauliflower-maize-rice pattern in floodplain soil. Commun. Soil Sci. Plant Anal. 2019, 50, 1425–1438. [Google Scholar] [CrossRef]
- Khattak, S.G.; Dominy, P.J.; Ahmad, W. Effect of Zn as soil addition and foliar application on yield and protein content of wheat in alkaline soil. J. Natl. Sci. Found. Sri Lanka 2016, 43, 303–312. [Google Scholar] [CrossRef]
- Khan, K.A.; Shoaib, A.; Awan, Z.A.; Basit, A.; Hussai, M. Macrophomina phaseolina alters the biochemical pathway in Vigna radiata chastened by Zn2+ and FYM to improve plant growth. J. Plant Interact. 2018, 13, 131–140. [Google Scholar] [CrossRef]
- Rehman, A.; Farooq, M.; Naveed, M.; Nawaz, A.; Shahzad, B. Seed priming of Zn with endophytic bacteria improves the productivity and grain biofortification of bread wheat. Eur. J. Agron. 2018, 94, 98–107. [Google Scholar] [CrossRef]
- Janků, M.; Luhová, L.; Petřivalský, M. On the origin and fate of reactive oxygen species in plant cell compartments. Antioxidants 2019, 8, 105. [Google Scholar] [CrossRef]
- Tufail, A.; Li, H.; Naeem, A.; Li, T.X. Leaf cell membrane stability-based mechanisms of zinc nutrition in mitigating salinity stress in rice. Plant Biol. 2018, 20, 338–345. [Google Scholar] [CrossRef]
- Yin, Q.; Kang, L.; Liu, Y.; Qaseem, M.F.; Qin, W.; Liu, T.; Li, H.; Deng, X.; Wu, A.-m. Boron deficiency disorders the cell wall in Neolamarckia cadamba. Ind. Crops Prod. 2022, 17, 114332. [Google Scholar] [CrossRef]
- Sharf, W.; Javaid, A.; Shoaib, A.; Khan, I.H. Induction of resistance in chili against Sclerotium rolfsii by plant-growth-promoting rhizobacteria and Anagallis arvensis. Egypt. J. Biol. Pest Control 2021, 31, 16. [Google Scholar] [CrossRef]
- Ma, J.; Liu, X.; Liu, J.; Wang, H. Changes of protective enzymes activities responding to Sclerotinia sclerotiorum inoculation in non-heading Chinese cabbages. Jiangsu J. Agric. Sci. 2012, 28, 1403–1408. [Google Scholar]
- Wen, L.; Tan, T.; Shu, J.; Chen, Y.; Liu, Y.; Yang, Z.; Zhang, Q.; Yin, M.; Tao, J.; Guan, C. Using proteomic analysis to find the proteins involved in resistance against Sclerotinia sclerotiorum in adult Brassica napus. Eur. J. Plant Pathol. 2013, 137, 505–523. [Google Scholar] [CrossRef]
- Na, R.; Luo, Y.; Bo, H.; Jia, R.; Meng, Q.; Zhou, H.; Hao, J.; Zhao, J. Responses of sunflower induced by Sclerotinia sclerotiorum infection. Physiol. Mol. Plant Pathol. 2018, 102, 113–121. [Google Scholar] [CrossRef]
- Liu, Y.; Khan, A.R.; Gan, Y. C2H2 zinc finger proteins response to abiotic stress in plants. Int. J. Mol. Sci. 2022, 23, 2730. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Hao, X.; Wang, X.; Yang, S.; Dong, Y.; Ding, Y.; Wang, Q.; Wang, X.; Zhou, J. Boron stress inhibits beet (Beta vulgaris L.) growth through influencing endogenous hormones and oxidative stress response. Soil Sci. Plant Nutr. 2019, 65, 346–352. [Google Scholar] [CrossRef]
- Wang, X.; Song, B.; Wu, Z.; Zhao, X.; Song, X.; Adil, F.M.; Riaz, M.; Lal, M.K.; Huang, W. Insights into physiological and molecular mechanisms underlying efficient utilization of boron in different boron efficient Beta vulgaris L. varieties. Plant Physiol. Biochem. 2023, 197, 107619. [Google Scholar] [CrossRef] [PubMed]
- Duran, P.; Barra, P.J.; Mora, M.d.l.L.; Nunes-Nesi, A.; Merino-Gergichevich, C. Boron and zinc diminish grey necrosis incidence by the promotion of desirable microorganisms on hazelnut orchards. Agronomy 2022, 12, 868. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhadra, T.; Mahapatra, C.K.; Hosenuzzaman, M.; Gupta, D.R.; Hashem, A.; Avila-Quezada, G.D.; Abd_Allah, E.F.; Hoque, M.A.; Paul, S.K. Zinc and Boron Soil Applications Affect Athelia rolfsii Stress Response in Sugar Beet (Beta vulgaris L.) Plants. Plants 2023, 12, 3509. https://doi.org/10.3390/plants12193509
Bhadra T, Mahapatra CK, Hosenuzzaman M, Gupta DR, Hashem A, Avila-Quezada GD, Abd_Allah EF, Hoque MA, Paul SK. Zinc and Boron Soil Applications Affect Athelia rolfsii Stress Response in Sugar Beet (Beta vulgaris L.) Plants. Plants. 2023; 12(19):3509. https://doi.org/10.3390/plants12193509
Chicago/Turabian StyleBhadra, Tamalika, Chandan Kumar Mahapatra, Md. Hosenuzzaman, Dipali Rani Gupta, Abeer Hashem, Graciela Dolores Avila-Quezada, Elsayed Fathi Abd_Allah, Md. Anamul Hoque, and Swapan Kumar Paul. 2023. "Zinc and Boron Soil Applications Affect Athelia rolfsii Stress Response in Sugar Beet (Beta vulgaris L.) Plants" Plants 12, no. 19: 3509. https://doi.org/10.3390/plants12193509
APA StyleBhadra, T., Mahapatra, C. K., Hosenuzzaman, M., Gupta, D. R., Hashem, A., Avila-Quezada, G. D., Abd_Allah, E. F., Hoque, M. A., & Paul, S. K. (2023). Zinc and Boron Soil Applications Affect Athelia rolfsii Stress Response in Sugar Beet (Beta vulgaris L.) Plants. Plants, 12(19), 3509. https://doi.org/10.3390/plants12193509







