Shoot Organogenesis and Regeneration from Leaf Seedlings of Diospyros oleifera Cheng
Abstract
:1. Introduction
2. Results
2.1. Explant Sterilization
2.2. Seed Germination
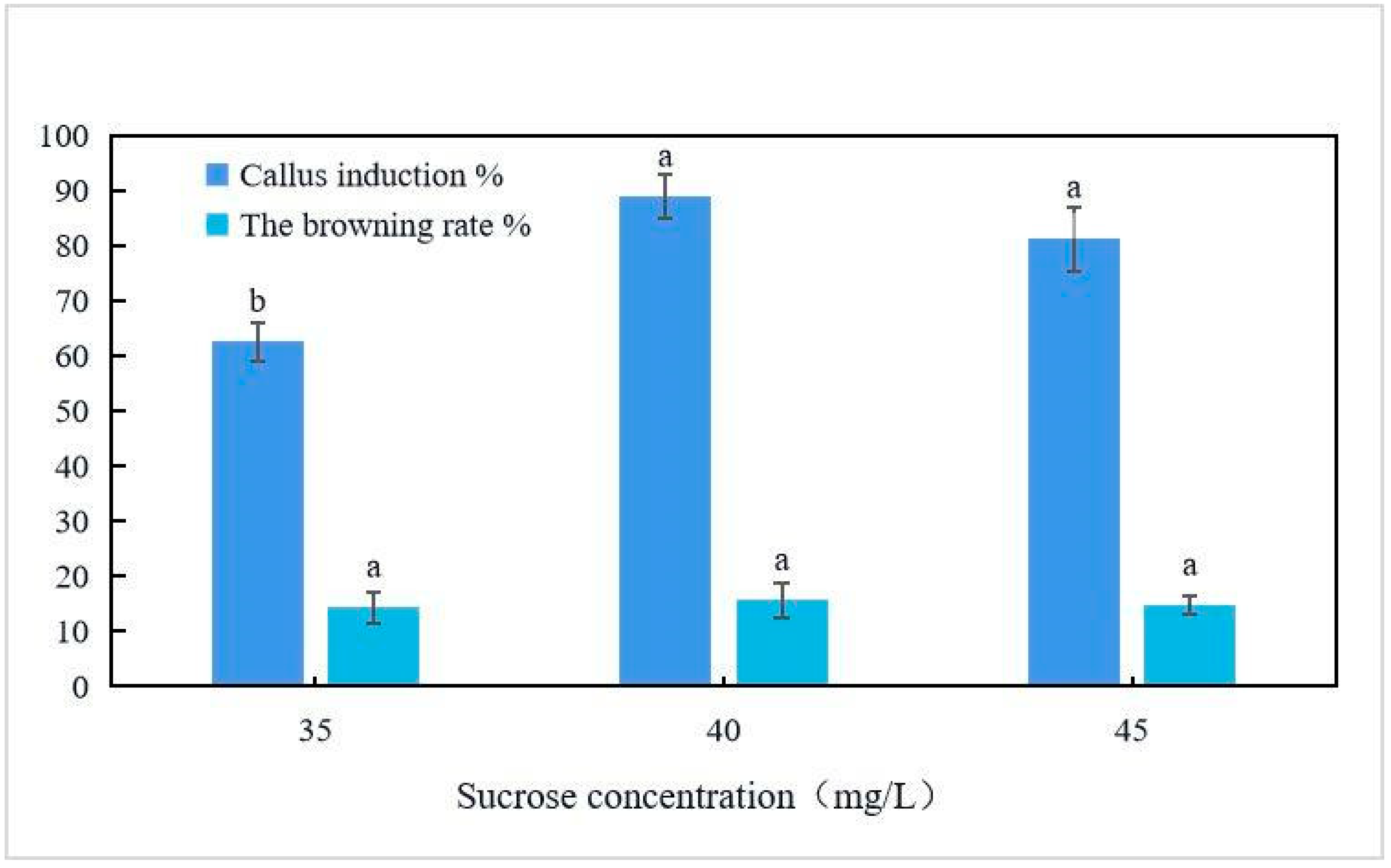
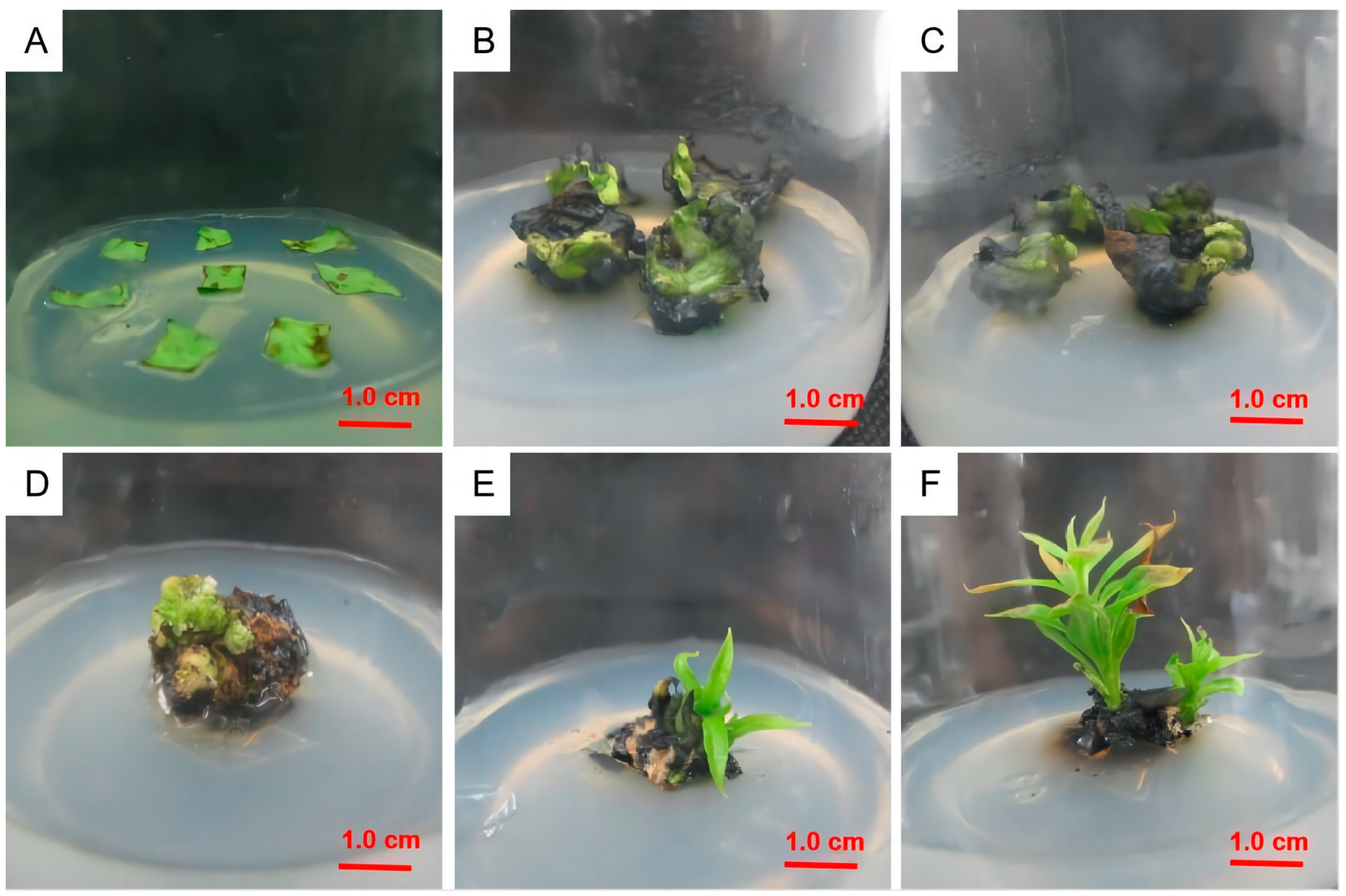
2.3. Leaf Disk Callus Induction
2.4. Induction of Adventitious Shoots from Callus
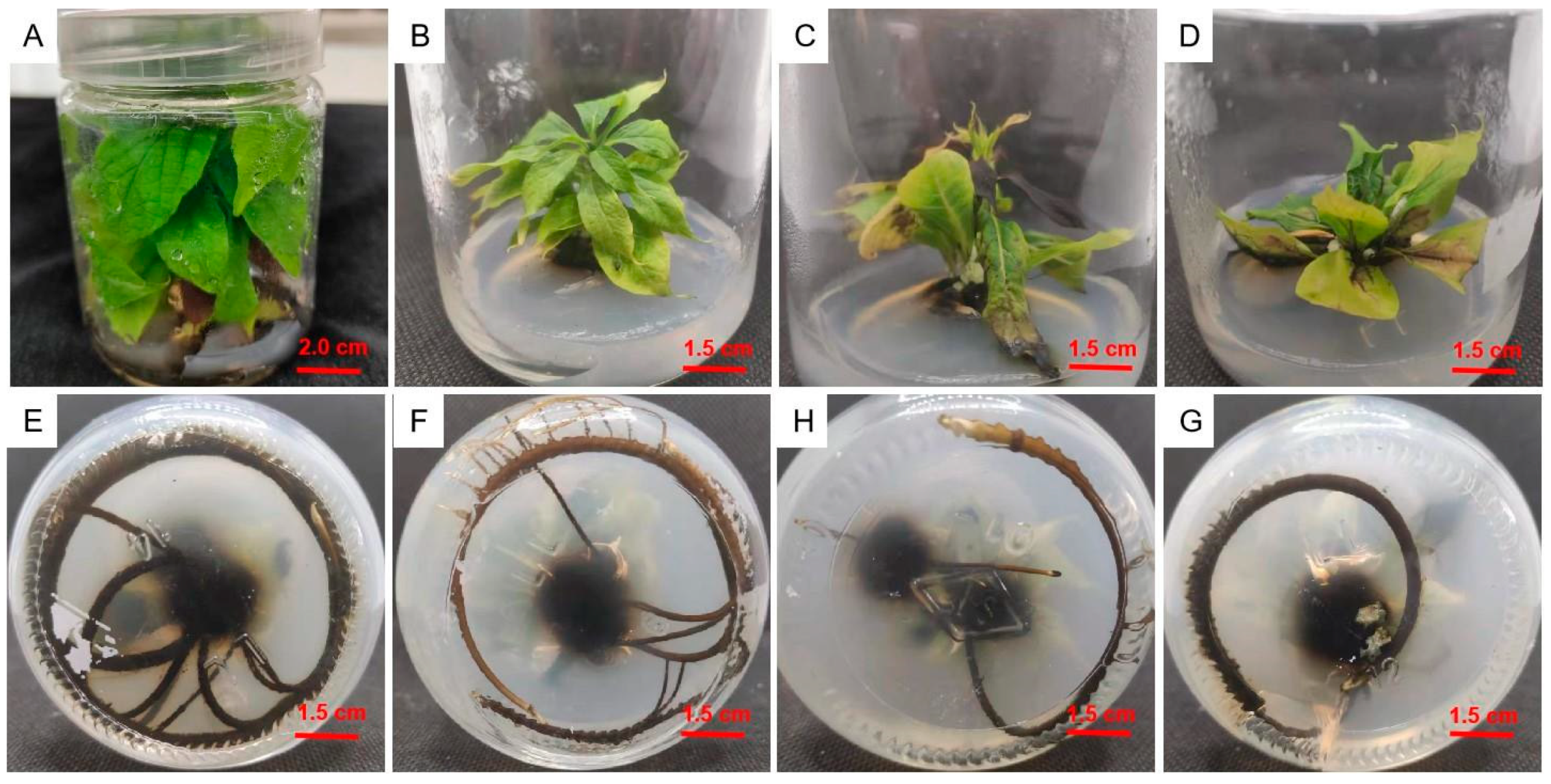
2.5. Adventitious Shoot Proliferation in Culture
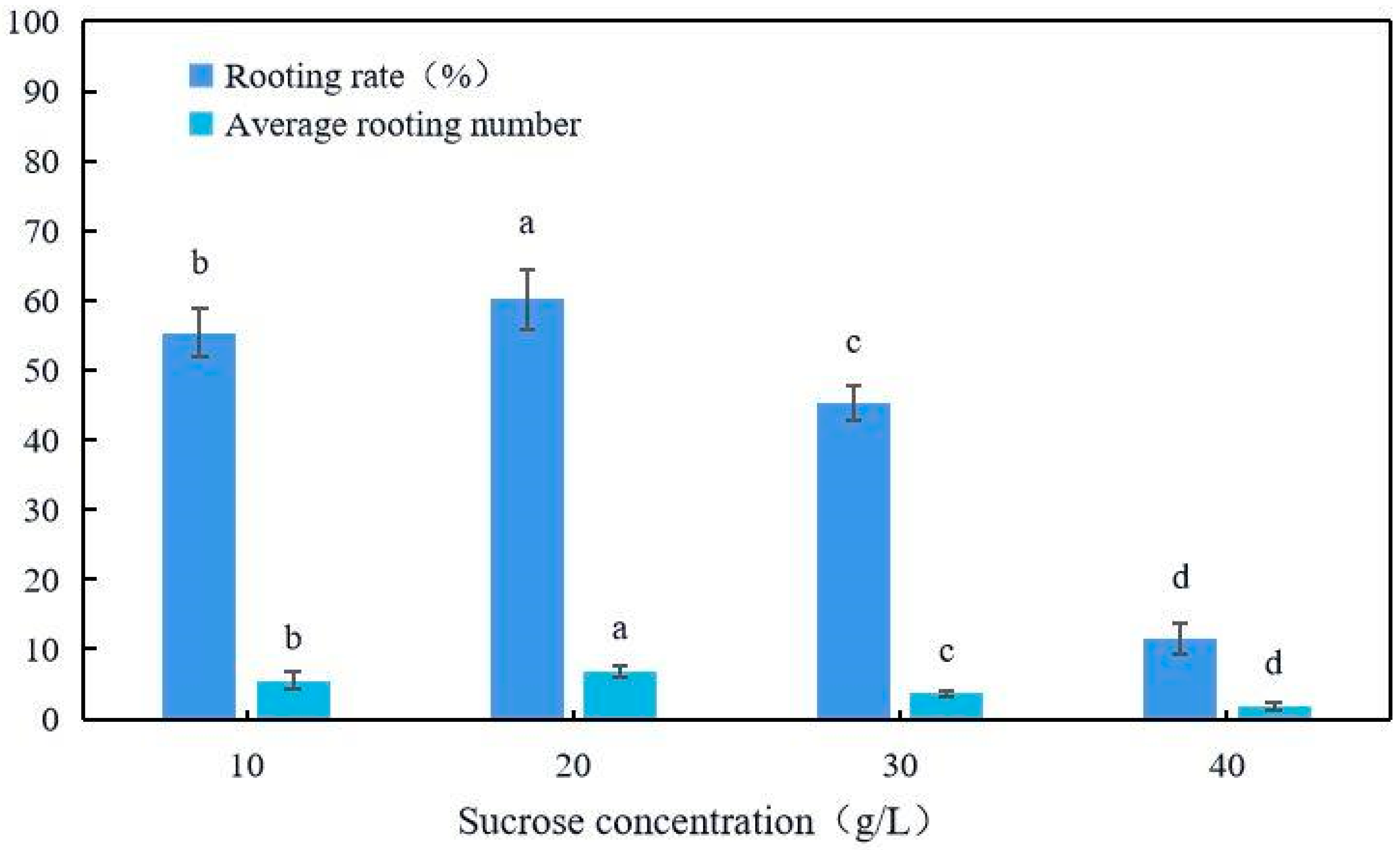
2.6. Rooting Culture
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Chemicals Used
4.3. Sterilization of Explants
4.4. Seed Germination
4.5. Leaf Disk Callus Induction
4.6. Induction of Adventitious Shoots from Callus
4.7. Adventitious Shoot Proliferation in Culture
4.8. Rooting Cultivation
4.9. Culture Conditions
4.10. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fu, J.; Liu, H.; Hu, J.; Liang, Y.; Liang, J.; Wuyun, T.; Tan, X. Five Complete Chloroplast Genome Sequences from Diospyros: Genome Organization and Comparative Analysis. PLoS ONE 2017, 11, e0159566. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Cheng, J.Y.; Zhong, X.H. Progress in persimmon Ti research. Mod. Gard. 2013, 7, 22–23. [Google Scholar] [CrossRef]
- Lin, J.F.; Lin, H.T.; Xie, L.H.; Lin, Q.Y.; Chen, S.H.; Zhao, Y.F. Chemical composition, pharmacology, clinical application and development and utilization of persimmon leaves. Food Ferment. Ind. 2005, 7, 90–96. [Google Scholar] [CrossRef]
- Xie, Q.X.; Zhuang, D.H.; Wu, Y.H. Culture of Diospyros oleifera Cheng hypocotyl regeneration plants. Fruit Tree J. 2007, 2, 157–161+255. [Google Scholar]
- Fu, J.; Liang, J.; Wuyun, T.N.; Liang, Y.; Sun, P.; Li, F. Sequence Analysis of ITS Regions and nd hA Gene for Determining Phylogenetic Relationship of Diospyros kaki (persimmon) With Other Related Wild Diospyros (Ebenaceae) Species. Plant Res. 2015, 35, 515–520. [Google Scholar]
- Li, W.Q.; Yang, Y.; Xie, X.M.; Qing, C.; Jin, X.B.; Suo, Z.L. Diospyros oleifera and D. deyange nsis Are Revealed as the Closest Relatives to D. kaki by E3 Ubiquitin-Protein Ligase UPL3 DNA Sequences. Agric. Sci. 2018, 8, 657–673. [Google Scholar]
- Han, W.J.; Fu, J.M.; Wang, L.Y.; Wang, Y.R.; Diao, S.F.; Li, H.W.; Sun, P.; Suo, Y.J. Research on physiological qualities during fruit ripening of Diospyros oleifera Cheng. J. Cent. South Univ. For. Technol. 2021, 41, 14–21. [Google Scholar] [CrossRef]
- Yonemori, K.; Sugiura, A.; Yamada, M. Persimmon Genetics and Breeding; John Wiley & Sons, Ltd.: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Ma, P.; Gong, B.C.; Jiang, X.B.; Wu, K.Y.; Xu, Y. Evaluation of growth physiology and affinity of grafted sweet persimmon with different rootstocks. For. Sci. Res. 2015, 28, 518–523. [Google Scholar]
- Mai, Y.; Liu, Y.; Yuan, J.; Ye, L.; Zhang, Y.; Diao, S.; Han, W.; Suo, Y.; Li, H.; Hu, R.; et al. Establishment of an efficient genetic transformation system: A case study of RNAi-induced silencing of the transcription factor MeGI in Diospyros oleifera Cheng seedlings. Sci. Hortic. 2023, 308, 111560. [Google Scholar] [CrossRef]
- Li, C.; Fu, J.M.; Wang, S.; Wen, Y.F.; Yan, C.; Sun, P.; Diao, S.F.; Han, W.J. Establishment of the tissue culture and regeneration system of Yangfeng sweet persimmon. J. Northwest For. Coll. 2016, 31, 159–164. [Google Scholar]
- Wei, W.H.; Yang, Y.T.; Bai, Y.J.; W, Y.J.; Yang, W.L.; Zhou, R.J. D. lotus stem segment initiation culture study. Mod. Gard. 2020, 43, 27–28. [Google Scholar]
- Li, S.H.; Gong, Q.T.; Jiang, E.S.; Li, Q.S.; Yu, L.L. Application of tissue culture in fruit trees. Shanxi Agric. Sci. 2013, 41, 1270–1273. [Google Scholar] [CrossRef]
- Zhou, R.J.; Zhang, X.N.; Huai, G.Z.; Zhou, R.J.; Li, G.R.; Hu, H.L. Studies on Factors Affecting Survival Rate of Micro-grafting in Vitro in Persimmon. Agric. Biotechnol. 2018, 7, 46–48. [Google Scholar] [CrossRef]
- Naval, M.M.; Llácer, G.; Badenes, M.L.; Giordani, E. Studies on the somaclonal variation of the persimmon (Diospyros Kaki Thunb) Cv. 'Rojo brillante' as a breeding tool. Acta Hortic. 2009, 814, e291294. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, S.; Chen, W.; Xu, L.; Guo, D.; Luo, Z.; Zhang, Q. An Efficient Agrobacterium-Mediated Genetic Transformation System for Persimmon (Diospyros kaki Thunb.). Horticulturae 2022, 8, 422. [Google Scholar]
- El-Hawary, S.S.E.-D.; Tadros, S.H.; Taha, H.; Abdelmohsen, M.; Nazif, N.M.; El Sheikh, I.; El-Nasr, M.S. Biotechnological and chemical analysis of Egyptian Diospyros kaki L. cv. Costata grown in Egypt. Bull. Natl. Res. Cent. 2020, 44, 2205–2212. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kobayashi, S.; Nakajima, I. Agrobacterium-mediated transformation and plant regeneration from hypocotyl segments of Japanese persimmon (Diospyros kaki Thunb). Plant Cell Rep. 1998, 17, 435–440. [Google Scholar] [CrossRef]
- Sun, Q.; Sun, H.; Liu, Q.; Shi, Y. Plant regeneration from hypocotyls of persimmon. Deciduous Fruits 2000, 5, 4–5. Available online: https://kns.cnki.net/kcms2/article/abstract?v=D8qIuhHbuGoueCEcJSMvJ1QswU-buD2bgxFoPDydYt_SwFqamiAkeLPLzZ1uWIONUqAbL31chE4RwIKGjmAO3J61fJpq5JMIN1W89s69tH2ZUAZNnKfxCX1zNkE0IUk7&uniplatform=NZKPT&flag=copy (accessed on 6 October 2023).
- Lv, Z.Y.; Guan, C.F.; Li, J.Y.; Ding, Y.; Fan, Z.R.; Yang, Y. Research progress in tissue culture techniques of persimmon plants. North. Hortic. 2023, 12, 129–137. [Google Scholar]
- Rine, I.A.; Alifi, N.D.; Samidjo, G.S. Utilization of Shoot Multiplication Medium for In-Vitro Conservation of Vanda tricolor. IOP Conf. Ser. Earth Environ. Sci. 2022, 985, 012009. [Google Scholar] [CrossRef]
- Larekeng, S.H.; Gusmiaty; Restu, M.; Tunggal, A.; Susilowati, A. Isolation and identification of rhizospheric fungus under Mahoni (Swietenia mahagoni) stands and its ability to produce IAA (Indole Acetid Acid) hormones. IOP Conf. Ser. Earth Environ. Sci. 2019, 343, 012051. [Google Scholar] [CrossRef]
- Yun, C.; Zhao, Z.; Gu, L.; Zhang, Z.; Wang, S.; Shi, Y.; Miao, N.; Ri, I.; Wang, W.; Wang, H. In vitro production of atractylon and β-eudesmol from Atractylodes chinensis by adventitious root culture. Appl. Microbiol. Biotechnol. 2022, 21, 106. [Google Scholar] [CrossRef] [PubMed]
- Aliabad, K.K.; Rezaee, A.; Homayoonfar, F.; Zamani, E. The Influence of Growth Regulators on In-Vitro Culture of Rosa Hybrida. J. Comput. Theor. Nanosci. Ence. 2019, 11, 2. [Google Scholar] [CrossRef]
- Khandaker, M.M.; Saidi, A.; Badaluddin, N.A.; Yusoff, N.; Majrashi, A.; Alenazi, M.M.; Saifuddin, M.; Alam, A.; Mohd, K.S. Effects of Indole-3-Butyric Acid (IBA) and rooting media on rooting and survival of air layered wax apple (Syzygium samarangense) CV Jambu Madu. Braz. J. Biol. Rev. Brasleira Biol. 2022, 82, e256277. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.J.; Yu, Y.F.; Huang, L.Q. Tissue Culture Analysis of Medicinal Plant Erigeron breviscapus. Mol. Plant Breed. 2023, 21, 5058–5065. [Google Scholar]
- Yin, M.H. Effects of KT and NAA on the Growth and Development of Anemarrhena asphodeloides Bunge. Plantlets Hubei Agric. Sci. 2014, 53, 607–608+612. [Google Scholar] [CrossRef]
- Ai, P.F.; Luo, Z.R. Study on rooting conditions of sweet persimmon tube seedlings. J. Huazhong Agric. Univ. 2002, 14, 154–157. [Google Scholar] [CrossRef]
- Manoj, K.; Yogesh, R.; Shardulya, S.; Ankita, S.; Ajay, Y.; Jagraj, S.; Deep, K. Hormonal Effect on Callus Induction and Shoot Multiplication on Leaf Explants in Gerbera (Gerbera jamesonii) Cultivar Grand. Int. J. Plant Soil Sci. 2022, 7, 538–544. [Google Scholar] [CrossRef]



| 75% Ethanol Min | 5% NaClO Min | 1% HgCl2 Min | Contamination Rate % | Germination Rate % |
|---|---|---|---|---|
| 1 | 0 | 5 | 55.7 ± 1.2 a | 27.6 ± 2.7 e |
| 1 | 0 | 6 | 47.3 ± 5.3 b | 28.5 ± 1.0 e |
| 1 | 0 | 7 | 42.5 ± 8.3 b | 30.1 ± 4.3 e |
| 2 | 0 | 5 | 43.3 ± 4.0 b | 38.6 ± 5.7 d |
| 2 | 0 | 6 | 39.7 ± 6.4 bc | 43.3 ± 6.3 c |
| 2 | 0 | 7 | 35.6 ± 1.3 c | 40.7 ± 7.7 cd |
| 3 | 0 | 5 | 36.0 ± 0.7 c | 42.3 ± 2.5 c |
| 3 | 0 | 6 | 33.6 ± 1.3 c | 37.3 ± 3.6 d |
| 3 | 0 | 7 | 30.3 ± 2.6 cd | 33.7 ± 4.6 d |
| 1 | 8 | 0 | 43.3 ± 3.6 b | 36.3 ± 5.7 d |
| 1 | 10 | 0 | 40.7 ± 1.3 bc | 38.3 ± 8.5 d |
| 1 | 12 | 0 | 36.7 ± 8.6 c | 40.7 ± 3.6 cd |
| 2 | 8 | 0 | 28.3 ± 9.6 cd | 53.7 ± 3.7 b |
| 2 | 10 | 0 | 25.0 ± 1.0 d | 63.5 ± 4.6 a |
| 2 | 12 | 0 | 21.7 ± 4.6 d | 54.3 ± 2.0 b |
| 3 | 8 | 0 | 22.0 ± 1.3 d | 39.3 ± 1.1 cd |
| 3 | 10 | 0 | 18.9 ± 5.7 e | 43.3 ± 1.2 c |
| 3 | 12 | 0 | 13.3 ± 4.6 e | 36.7 ± 3.7 d |
| Medium | Additives | Activated Carbon g/L | Germination Rate % |
|---|---|---|---|
| 1/2MS | 2.0 6-BA + 0.5 NAA | 1.0 | 51.2 ± 3.7 bc |
| 1/2MS | 0.5 GA3 | 1.0 | 67.3 ± 5.6 a |
| 1/2MS | 1.0 GA3 | 1.0 | 58.7 ± 8.0 b |
| (1/2N) MS | 2.0 6-BA + 0.5 NAA | 1.0 | 49.5 ± 6.6 c |
| (1/2N) MS | 0.5 GA3 | 1.0 | 58.3 ± 6.3 b |
| (1/2N) MS | 1.0 GA3 | 1.0 | 51.5 ± 7.5 bc |
| WPM | 2.0 6-BA + 0.5 NAA | 1.0 | 45.1 ± 8.7 c |
| WPM | 0.5 GA3 | 1.0 | 52.3 ± 9.8 b |
| WPM | 1.0 GA3 | 1.0 | 49.9 ± 6.4 bc |
| 1/2MS | 0.5 GA3 | 0 | 50.2 ± 3.3 bc |
| 1/2MS | 0.5 GA3 | 2.0 | 54.6 ± 4.3 b |
| Culture Medium | 6-BA mg/L | NAA mg/L | Callus Induction Rate % | Browning Rate % |
|---|---|---|---|---|
| 1/2MS | 1.0 | 0 | 3.8 ± 0.6 g | 8.0 ± 1.3 e |
| 1/2MS | 2.0 | 0 | 19.5 ± 2.7 f | 10.3 ± 1.6 e |
| 1/2MS | 3.0 | 0 | 24.5 ± 2.6 e | 21.3 ± 2.4 de |
| 1/2MS | 1.0 | 0.5 | 65.2 ± 3.7 b | 16.2 ± 1.7 f |
| 1/2MS | 2.0 | 0.5 | 88.9 ± 4.6 a | 15.7 ± 1.3 d |
| 1/2MS | 3.0 | 0.5 | 63.4 ± 2.6 b | 11.5 ± 3.4 e |
| 1/2MS | 1.0 | 1 | 51.5 ± 3.6 d | 25.1 ± 4.6 d |
| 1/2MS | 2.0 | 1 | 59.2 ± 2.7 c | 28.4 ± 1.5 c |
| 1/2MS | 3.0 | 1 | 41.7 ± 2.7 d | 35.3 ± 3.6 b |
| (1/2N) MS | 2.0 | 0.5 | 85.4 ± 5.9 a | 13.2 ± 2.1 d |
| MS | 2.0 | 0.5 | 68.6 ± 4.7 b | 9.9 ± 2.5 e |
| TDZ mg/L | ZT mg/L | NAA mg/L | Adventitious Shoot Induction Rate % | Adventitious Shoot Coefficient | Growth Status of the Adventitious Shoots |
|---|---|---|---|---|---|
| 1 | 1 | 0.5 | 20.4 ± 1.3 e | 2.2 ± 1.3 c | Yellow and short leaves |
| 1 | 2 | 0.5 | 32.4 ± 4.5 d | 2.7 ± 1.5 b | Yellow leaves with withered tips |
| 1 | 3 | 0.5 | 26.7 ± 3.3 e | 1.4 ± 0.3 e | Leaf tips rarely withered and soft leaves |
| 2 | 1 | 0.5 | 53.9 ± 1.5 b | 3.0 ± 1.3 b | Growth more vigorous, but leaf base softer |
| 2 | 2 | 0.5 | 83.3 ± 3.7 a | 5.4 ± 2.1 a | Large and green leaves |
| 2 | 3 | 0.5 | 47.2 ± 1.3 b | 1.5 ± 0.2 de | Growth more vigorous, leaves yellowed |
| 3 | 1 | 0.5 | 41.1 ± 2.3 bc | 1.6 ± 0.4 d | Growth relatively vigorous, but leaf tip withered |
| 3 | 2 | 0.5 | 34.6 ± 5.3 d | 1.2 ± 1.1 e | Test tube seedlings short, and leaves withered |
| 3 | 3 | 0.5 | 21.3 ± 3.2 e | 1.1 ± 0.7 e | Seedlings short and leaves yellow |
| 2 | 2 | 1 | 11.5 ± 2.2 f | 1.7 ± 0.8 d | Weak growth with curled, yellowed leaves |
| 2 | 2 | 1.5 | 10.4 ± 3.3 f | 1.0 ± 0.5 e | Vigorous growth, but very low value-added coefficient |
| 2 | 2 | 2 | 9.7 ± 2.3 f | 1.0 ± 0.3 e | Seedlings short with withered leaf tips |
| ZT mg/L | 2iP mg/L | IAA mg/L | Multiplication Coefficient | Material Growth Status |
|---|---|---|---|---|
| 0 | 0 | 0 | 1.0 | No proliferation |
| 1 | 1 | 0 | 1.9 ± 0.33 e | Test tube seedlings short; leaves with black spots |
| 1 | 2 | 0 | 2.4 ± 0.67 d | Seedlings taller, but leaves yellow and withered |
| 1 | 3 | 0 | 1.7 ± 0.25 e | Short with thick, green, large leaves |
| 2 | 1 | 0 | 3.1 ± 0.10 c | Tall but with withered leaf tips |
| 2 | 2 | 0 | 5.5 ± 1.33 b | Taller with large, emerald green leaves |
| 2 | 3 | 0 | 4.3 ± 0.33 c | Stem segments short; large yellow leaves |
| 3 | 1 | 0 | 4.0 ± 0.21 cd | Stem sections short; large yellow withered leaves |
| 3 | 2 | 0 | 4.2 ± 0.56 c | Seedlings taller with larger but yellow leaves |
| 3 | 3 | 0 | 4.1 ± 0.43 cd | Seedlings taller with large, emerald green leaves |
| 2 | 2 | 0.05 | 6.0 ± 0.52 b | Seedlings shorter with large, emerald green leaves |
| 2 | 2 | 0.1 | 7.5 ± 0.33 a | Seedlings taller with large, emerald green leaves |
| 2 | 3 | 0.15 | 2.5 ± 0.58 f | Seedlings shorter with large, emerald green leaves |
| Basal Culture Medium | IBA mg/L | NAA mg/L | KT mg/L | Rooting Rate % | Average Root Number |
|---|---|---|---|---|---|
| MS | 0 | 0 | 0 | 0 | 0 |
| 1/2MS | 0.5 | 0.5 | 1.0 | 33.4 ± 2.1 c | 2.1 ± 0.2 d |
| (1/2N) MS | 0.5 | 0.5 | 1.0 | 25.8 ± 3.9 d | 1.4 ± 0.3 e |
| MS | 0.5 | 0.5 | 1.0 | 17.5 ± 1.4 e | 1.3 ± 0.5 e |
| WPM | 0.5 | 0.5 | 1.0 | 16.2 ± 3.5 e | 1.4 ± 0.6 e |
| 1/2MS | 1.0 | 0.5 | 1.0 | 60.2 ± 4.2 a | 6.9 ± 2.8 a |
| (1/2N) MS | 1.0 | 0.5 | 1.0 | 44.5 ± 2.3 b | 4.4 ± 1.6 b |
| MS | 1.0 | 0.5 | 1.0 | 38.1 ± 6.4 c | 3.4 ± 1.4 c |
| WPM | 1.0 | 0.5 | 1.0 | 28.4 ± 4.1 d | 2.8 ± 1.5 d |
| 1/2MS | 1.5 | 0.5 | 1.0 | 44.6 ± 3.1 b | 4.3 ± 0.5 b |
| (1/2N) MS | 1.5 | 0.5 | 1.0 | 30.1 ± 2.6 c | 3.1 ± 1.6 c |
| MS | 1.5 | 0.5 | 1.0 | 23.2 ± 4.7 d | 2.0 ± 0.6 d |
| WPM | 1.5 | 0.5 | 1.0 | 22.1 ± 1.3 d | 1.5 ± 0.5 e |
| 1/2MS | 1.0 | 1.0 | 1.5 | 10.4 ± 3.5 e | 1.7 ± 1.1 e |
| 1/2MS | 1.0 | 0.5 | 2.0 | 21.0 ± 2.4 d | 4.3 ± 2.4 b |
| 1/2MS | 1.0 | 0.5 | 2.5 | 18.5 ± 3.4 de | 2.9 ± 1.2 d |
| 1/2MS | 1.0 | 0.5 | 0.5 | 41.4 ± 5.6 b | 4.5 ± 0.9 b |
| 1/2MS | 1.0 | 0.5 | 0 | 33.4 ± 2.1 c | 3.6 ± 1.2 c |
| 1/2MS | 1.0 | 0 | 1.0 | 22.5 ± 3.3 d | 1.1 ± 0.2 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhou, N.; Luo, C.; Zhang, Q.; Sun, P.; Fu, J.; Li, S.; Li, Z. Shoot Organogenesis and Regeneration from Leaf Seedlings of Diospyros oleifera Cheng. Plants 2023, 12, 3507. https://doi.org/10.3390/plants12193507
Liu Y, Zhou N, Luo C, Zhang Q, Sun P, Fu J, Li S, Li Z. Shoot Organogenesis and Regeneration from Leaf Seedlings of Diospyros oleifera Cheng. Plants. 2023; 12(19):3507. https://doi.org/10.3390/plants12193507
Chicago/Turabian StyleLiu, Yang, Naifu Zhou, Chengrui Luo, Qi Zhang, Peng Sun, Jianmin Fu, Shuzhan Li, and Ze Li. 2023. "Shoot Organogenesis and Regeneration from Leaf Seedlings of Diospyros oleifera Cheng" Plants 12, no. 19: 3507. https://doi.org/10.3390/plants12193507
APA StyleLiu, Y., Zhou, N., Luo, C., Zhang, Q., Sun, P., Fu, J., Li, S., & Li, Z. (2023). Shoot Organogenesis and Regeneration from Leaf Seedlings of Diospyros oleifera Cheng. Plants, 12(19), 3507. https://doi.org/10.3390/plants12193507






