Abstract
We recently proposed the use of engineered irregularly shaped zinc oxide nanoparticles (ZnO NPs) coated with oleylamine (OAm), as photosynthetic biostimulants, to enhance crop yield. In the current research, we tested newly engineered rod-shaped ZnO nanorods (NRs) coated with oleylamine (ZnO@OAm NRs) regarding their in vivo behavior related to photosynthetic function and reactive oxygen species (ROS) generation in tomato (Lycopersicon esculentum Mill.) plants. ZnO@OAm NRs were produced via solvothermal synthesis. Their physicochemical assessment revealed a crystallite size of 15 nm, an organic coating of 8.7% w/w, a hydrodynamic diameter of 122 nm, and a ζ-potential of −4.8 mV. The chlorophyll content of tomato leaflets after a foliar spray with 15 mg L−1 ZnO@OAm NRs presented a hormetic response, with an increased content 30 min after the spray, which dropped to control levels 90 min after the spray. Simultaneously, 90 min after the spray, the efficiency of the oxygen-evolving complex (OEC) decreased significantly (p < 0.05) compared to control values, with a concomitant increase in ROS generation, a decrease in the maximum efficiency of PSII photochemistry (Fv/Fm), a decrease in the electron transport rate (ETR), and a decrease in the effective quantum yield of PSII photochemistry (ΦPSII), indicating reduced PSII efficiency. The decreased ETR and ΦPSII were due to the reduced efficiency of PSII reaction centers (Fv’/Fm’). There were no alterations in the excess excitation energy at PSII or the fraction of open PSII reaction centers (qp). We discovered that rod-shaped ZnO@OAm NRs reduced PSII photochemistry, in contrast to irregularly shaped ZnO@OAm NPs, which enhanced PSII efficiency. Thus, the shape and organic coating of the nanoparticles play a critical role in the mechanism of their action and their impact on crop yield when they are used in agriculture.
1. Introduction
Nanoparticles (NPs) have been shown to have great potential to improve the agri-food sector and revolutionize agriculture []. They can act as fertilizers, improving plant performance and protecting crop plants from environmental stresses. They can also suppress plant disease and increase crop yield [,,]. NPs can be used to diminish the amounts of sprayed chemical products, reduce nutrient losses in fertilization, and drive precision and sustainability in agriculture [,]. However, some metal-based NPs have been reported to decrease plant growth and performance by causing phytotoxic effects in crops [,]. NPs cause oxidative stress that depends on the concentration, reactivity, surface area, and size used as well as the plant age and the treated plant species [,].
Zinc oxide (ZnO) is recognized for its biocompatibility, biodegradability, and safety in both medical and environmental contexts []. Engineered zinc oxide nanoparticles (ZnO NPs) have been successfully applied as novel nano-fertilizers for crop improvement and to enrich Zn-deficit soil with Zn []. The application of ZnO NPs can enhance crop yield, providing an effective solution for nutrient supplementation in farming systems, and these NPs were characterized as a novel alternative for sustainable agricultural development [,]. It has been proposed that ZnO NPs can be used as photosynthetic biostimulants for enhancing crop yields [], as they possess insecticidal [], antifungal [], and antibacterial properties []. The impact of ZnO NPs on metabolic attributes and the photosynthetic function of rice seedlings in hydroponic culture was found to be concentration-dependent when improving the qualitative and quantitative characteristics of the plants []. Foliar sprays with 20 and 100 mg L−1 ZnO NPs enhanced tomato growth by increasing the chlorophyll content and photosystem II (PSII) activity []. Similarly, ZnO NPs were found to amplify the enzymatic functions in Lycopersicon esculentum, triggering the defense mechanisms of the plant [,]. ZnO NPs have a favorable effect on plant growth and development in ordinary amounts []. Different concentrations of ZnO NPs showed various effects on the growth and development of Arabidopsis thaliana [,]. The response of Stevia rebaudiana to ZnO NPs also displayed a dual nature, exhibiting both favorable and adverse effects depending on the NP concentration []. Exposing Hordeum vulgare seedlings to ZnO NPs for 7 days reduced root growth in a dose-dependent manner []. A damaging impact is induced by replacing ions of other elements, causing the generation of ROS []. A negative impact of ZnO NPs through ethylene has been recorded in rice seedlings, causing ultrastructural and stomatal damage []. However, ZnO NPs have been reported to decrease the growth of spring barley through the modulation of oxidative stress and antioxidant enzyme metabolism [].
A positive impact of ZnO NPs on drought- and salt-stressed plants was recorded. ZnO NPs at 25 and 50 mg L−1 concentrations were proven to be the optimal treatments for alleviating drought-induced oxidative stress in tomato plants []. In salinity-stressed bean plants, a foliar spray of biosynthesized 50 mg L−1 ZnO NPs helped plants to overcome the negative effects of salinity by increasing enzymatic and non-enzymatic antioxidants that reduced oxidative stress and increased soluble sugars, glycine betaine, proline, and amino acids []. A foliar spray of 200 mg L−1 ZnO NPs decreased the adverse effects of salinity on both photosystems (PSI and PSII) of Pisum sativum as well as on stomatal closure, pigment content, and membrane integrity []. However, ZnO NPs could improve the chlorophyll content, wheat growth, and wheat grain yield under salt stress [] and mitigate salinity stress in rice seedlings by upregulating the antioxidative system and enhancing physiological and biochemical indices []. Significant improvements in wheat and rice growth and grain yield under salinity stress using ZnO NPs were also recorded by Mazhar et al. []. A foliar application of 16–35 nm ZnO NPs at 10 mg L−1 increased the activity of superoxide dismutase (SOD) and catalase (CAT) and the concentration of photosynthetic pigments in Abelmoschus esculentus L., while it reduced the total soluble sugars and proline [].
The early-stage recognition of any stress with imaging techniques permits the avoidance of plant injury and consequently important crop losses [,,]. Chlorophyll-a fluorescence imaging is among the best-qualified imaging techniques to estimate plant health. It also allows the pre-symptomatic monitoring of plant stress symptoms [,,]. Chlorophyll-a fluorescence analysis is a capable tool for evaluating NP effects on plant function and to monitor plant−NP relations [,,].
We recently proposed that engineered irregularly shaped ZnO NPs coated with oleylamine (ZnO@OAm NPs) can be effectively used to boost photosynthetic function and thus are appropriate for enhancing crop yield []. Nevertheless, the presented literature suggests that plant responses to zinc-based NPs are multifactorial and are influenced by factors such as the plant species, NP morphology and size, the applied dosage, and the application method. Thus, the purpose of the current research was to compare the newly engineered rod-shaped ZnO@OAm NRs with the previously examined irregularly shaped ZnO@OAm NPs for their behavior related to photosynthetic function and ROS generation. Since the impact of ZnO NPs on plants is influenced by their morphology and size, we hypothesized that the rod-shaped ZnO@OAm NRs would have a different mechanism of action than irregularly shaped ZnO@OAm NPs and therefore a different impact on crop yield; thus we decided to investigate it in the current study, as demonstrated below.
2. Results
2.1. Synthesis and Characterization of Zinc Oxide Nanorods
The crystal structure of the ZnO@OAm NRs was identified via XRD analysis. The XRD diffractogram displayed the primary diffraction peaks at 31.6° (100), 34° (002), and 36° (101) (Figure 1a), which were assigned to the wurtzite structure of zinc oxide (JCPDS card #89-0510) []. The crystallite size was calculated to be 15 nm. The percentage and profile of the organic coating on the ZnO@OAm NRs were determined through TGA, and the results illustrated the gradual decomposition of OAm (Figure 1b). In the initial stage, absorbed water molecules were removed (0.84% w/w) up to 150 °C, followed by a weight loss of 7.7% w/w in the subsequent stage due to OAm. The final stage evidenced a mass loss of 1% w/w, also linked to the removal of OAm from the metallic cores of the particles. The total weight loss attributed to OAm amounted to 8.7% w/w.
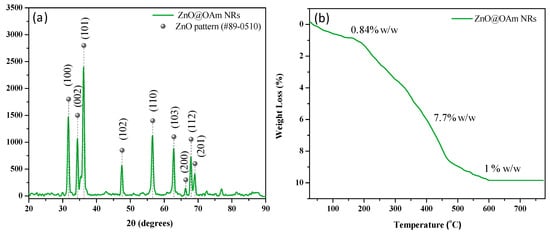
Figure 1.
X-ray diffraction (XRD) pattern (a) and thermogravimetric analysis (TGA) curve (b) of the ZnO@OAm NRs.
An analysis of the TEM images revealed that the particles possessed a distinctive rod-like structure (Figure 2a). To quantify the dimensions of these particles, we conducted a histogram analysis. The results illustrated that the ZnO@OAm NRs had an average width of 22 ± 2.1 nm (Figure 2b). Furthermore, the length of the NRs was measured, yielding an average value of 62 ± 1.3 nm.
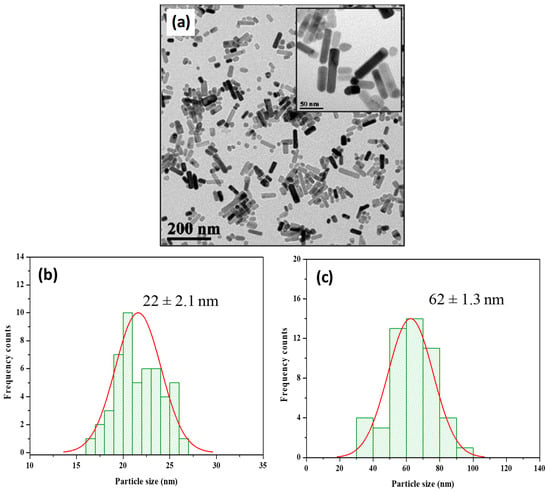
Figure 2.
Transmission electron microscopy (TEM) image of ZnO@OAm NRs (a), histogram of average width and Gaussian fit (22 ± 2.1 nm) (b), and average length histogram and Gaussian fit (62 ± 1.3 nm) (c). The average size of particles is expressed as mean size (±SD).
Optical characterization of the ZnO@OAm NRs, when dispersed in an ethanol/water mixture (1:3 ratio), was conducted using UV-Vis spectroscopy. The UV-Vis absorbance spectrum (Figure S1) prominently showcased an absorbance peak situated at 367 nm. Employing the Tauc plot, the band gap of the NRs was ascertained to be 3.16 eV.
ZnO@OAm NRs exhibiting hydrophobic characteristics were dispersed in an ethanol/water mixture (1:3 ratio) for DLS analysis. The hydrodynamic diameter, as discerned, was 122 ± 0.42 nm (Figure S2a), signifying monodispersity, with a PDI value of 0.17, which underscored the colloidal stability of the system. Furthermore, the ζ-potential measurements provided a surface charge for the nanoparticles at −4.8 ± 0.31 mV (Figure S2b).
2.2. Impact of Zinc Oxide Nanorods on Chlorophyll Content of Tomato Leaflets
The chlorophyll content increased significantly (p < 0.05) 30 min after spraying with ZnO@OAm NRs, but 90 min after the spray it returned to control values (Figure 3). Thus, the response of the chlorophyll content to ZnO@OAm NRs resembled a hormetic response.
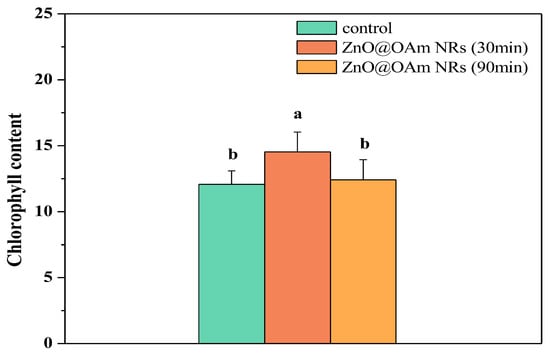
Figure 3.
The chlorophyll contents of control (sprayed with distilled water) tomato leaflets and tomato leaflets 30 and 90 min after spraying with 15 mg L−1 ZnO@OAm NRs. Different lowercase letters denote statistical difference (p < 0.05). Error bars in columns are SDs.
2.3. Changes in PSII Photochemistry due to Zinc Oxide Nanorods
2.3.1. Efficiency of the Oxygen-Evolving Complex and Maximum Efficiency of PSII Photochemistry
The efficiency of the oxygen-evolving complex (OEC) did not change 30 min after spraying with ZnO@OAm NRs, but 90 min after the spray it decreased significantly (p < 0.05) compared to the corresponding control values (Figure 4a). A similar response pattern was noticed in the maximum efficiency of PSII photochemistry (Fv/Fm) (Figure 4b).
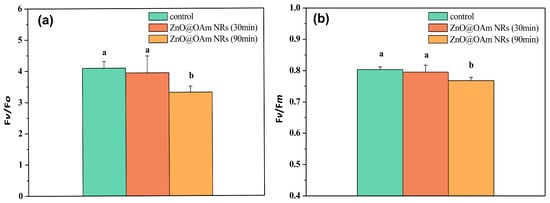
Figure 4.
The efficiency of the oxygen-evolving complex (OEC) (Fv/Fo) (a) and the maximum efficiency of PSII photochemistry (Fv/Fm) (b) of control (sprayed with distilled water) tomato leaflets and tomato leaflets 30 and 90 min after spraying with 15 mg L−1 ZnO@OAm NRs. Different lowercase letters denote statistical difference (p < 0.05). Error bars in columns are SDs.
2.3.2. Allocation of Absorbed Light Energy and Fraction of Open PSII Reaction Centers
Absorbed light energy is distributed to PSII photochemistry (ΦPSII), regulated non-photochemical energy loss in PSII (ΦNPQ), and non-regulated energy loss in PSII (ΦNO), with ΦPSII + ΦNPQ + ΦNO equal to 1 []. The effective quantum yield of PSII photochemistry (ΦPSII) did not change 30 min after spraying with ZnO@OAm NRs, but 90 min after the spray, it decreased significantly (p < 0.05) compared to the corresponding control values under both the growth-light (GL, 600 μmol photons m−2 s−1) and high-light (HL, 1000 μmol photons m−2 s−1) actinic light (AL) intensity conditions (Figure 5a). Under the GL intensity, all ΦPSII values were significantly (p < 0.05) higher compared to the corresponding HL intensity values (Figure 5a).
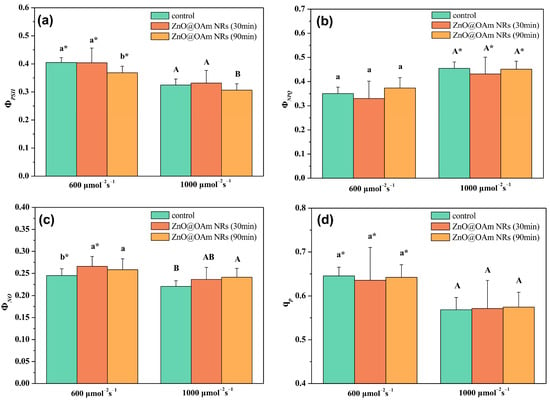
Figure 5.
The effective quantum yield of PSII photochemistry (ΦPSII) (a), the quantum yield of regulated non-photochemical energy loss in PSII (ΦNPQ) (b), the non-regulated energy loss in PSII (ΦNO) (c), and the fraction of open PSII reaction centers (d) of control (sprayed with distilled water) tomato leaflets and tomato leaflets 30 and 90 min after spraying with 15 mg L−1 ZnO@OAm NRs. Statistical differences (p < 0.05) at the growth light (600 μmol photons m−2 s−1) intensity are indicated by different lowercase letters, while statistical differences at the high light (1000 μmol photons m−2 s−1) intensity are indicated by capital letters. The asterisks (*) represent significant differences (p < 0.05) between the growth light intensity and the high light intensity for the same treatment. Error bars in columns are SDs.
The regulated non-photochemical energy loss as heat in PSII (ΦNPQ) did not change 30 or 90 min after spraying with ZnO@OAm NRs under both the GL and HL intensity conditions (Figure 5b). Under HL intensity, all ΦNPQ values were significantly (p < 0.05) higher compared to the corresponding GL intensity values (Figure 5b). The non-regulated energy loss in PSII (ΦNO) at the GL intensity increased significantly (p < 0.05) both 30 and 90 min after spraying with ZnO@OAm NRs, but at the HL intensity it only increased significantly (p < 0.05) 90 min after spraying with ZnO@OAm NRs (Figure 5c).
The redox state of quinone A (QA), or in other words the fraction of open PSII reaction centers, which indicates photochemical quenching (qp), did not change 30 or 90 min after spraying with ZnO@OAm NRs under both the GL and HL intensity conditions (Figure 5d).
2.3.3. Non-Photochemical Quenching and Electron Transport Rate
The parameter of non-photochemical quenching (NPQ), reflecting the dissipation of excess excitation energy as heat, did not change 30 or 90 min after spraying with ZnO@OAm NRs under both the GL and HL intensity conditions (Figure 6a). The electron transport rate (ETR) did not change 30 min after spraying with ZnO@OAm NRs, but 90 min after the spray, it decreased significantly (p < 0.05) compared to the corresponding control values under both the GL and HL intensity conditions (Figure 6b).
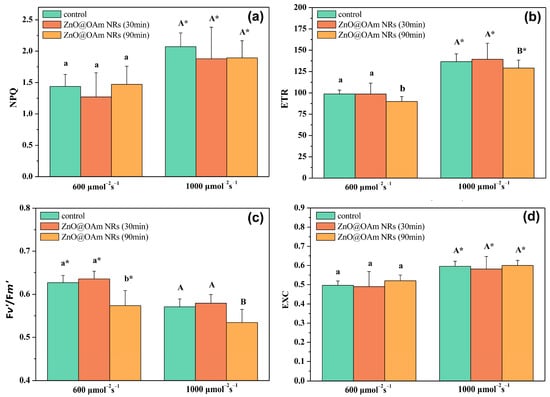
Figure 6.
The heat dissipation by non-photochemical quenching (NPQ) (a), the electron transport rate (ETR) (b), the efficiency of open PSII reaction centers (c), and the excess excitation energy at PSII (d) of control (sprayed with distilled water) tomato leaflets and tomato leaflets 30 and 90 min after spraying with 15 mg L−1 ZnO@OAm NRs. Statistical differences (p < 0.05) at the growth light (600 μmol photons m−2 s−1) intensity are indicated by different lowercase letters, while statistical differences at the high light (1000 μmol photons m−2 s−1) intensity are indicated by capital letters. The asterisks (*) represent significant differences (p < 0.05) between the growth light intensity and the high light intensity for the same treatment. Error bars in columns are SDs.
2.3.4. Efficiency of PSII Reaction Centers and Excess Excitation Energy
The efficiency of PSII reaction centers remained the same as in the controls 30 min after spraying with ZnO@OAm NRs, but 90 min after the spray, it decreased significantly (p < 0.05) compared to the corresponding control values under both the GL and HL intensity conditions (Figure 6c).
The excess excitation energy at PSII did not differ from controls 30 or 90 min after spraying with ZnO@OAm NRs under both the GL and HL intensity conditions (Figure 6d).
2.4. Impact of Zinc Oxide Nanoparticles on H2O2 Generation
90 minutes after spraying with 15 mg L−1 ZnO@OAm NRs, hydrogen peroxide (H2O2) formation was observed on leaf midribs and minor tomato lamina veins (Figure 7b). At the same time, in water-sprayed tomato leaflets, no H2O2 could be observed (Figure 7a).
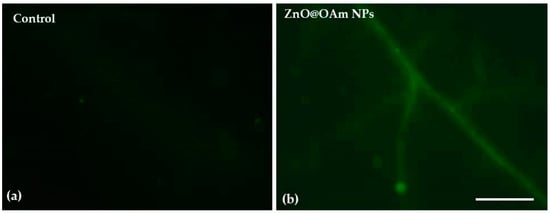
Figure 7.
Imaging of H2O2 production on tomato leaflets 90 min after spraying with distilled water (control) (a) or 15 mg L−1 ZnO@OAm NRs (b). The light green color denotes the H2O2 generation. Scale bar: 500 μm.
3. Discussion
ZnO NPs can be prepared in a very wide variety of sizes and shapes. Common ZnO NP morphologies like rods, spheres, flowers, and stars exhibit unique physicochemical properties influenced by their shapes and sizes []. Various synthetic approaches, including solvothermal and hydrothermal ones, produce ZnO NPs of distinct sizes and shapes []. Notably, rods and wires penetrate bacterial walls more effectively compared to spherical forms, while flower-shaped ZnO NPs show superior biocidal activity against E. coli and S. aureus [,]. Recent works, such as the study by Wang et al. [], have explored the interplay of ZnO NPs with plant photosynthesis, highlighting their promise for agricultural and environmental applications. In the present investigation, ZnO NRs that were coated with OAm were effectively produced using the polyol-solvothermal synthesis approach, which has gained prominence due to its simplicity and efficiency [].
Each polyol exhibits reductive capabilities that vary based on its molecular weight, leading to the creation of diverse intermediate complexes with the initial materials. This mechanism is influenced by selected reaction conditions, such as temperature, time, and pressure, as well as the inclusion of surfactants. Therefore, it results in NPs with varying sizes and shapes [,,]. Thus, in the present work, by using the lower-molecular-weight diethylene glycol in the presence of OAm and an 8 h reaction time, well-formed rods of ZnO@OAm NRs of 15 nm crystallite size were isolated (Figure 1a). Earlier findings [] showed that in the case of TrEG and an 8 h reaction time, marginally bigger crystallites of 18 nm, 38 nm width, and 83 nm length were formed, while in the sole presence of OAm irregularly shaped NPs of 19 nm were isolated. It has been noted that NPs with diameters below 50 nm, akin to our ZnO@OAm NRs, generally follow the plastid transport route within plants. In contrast, particles sized between 50 and 200 nm predominantly utilize the apoplast pathway [].
Additionally, based on the TGA results, the ZnO@OAm NRs (Figure 1b) were minimally coated with OAm (8.7% w/w), being hydrophobic. Nonpolar nanomaterials mainly exhibit hydrophobic characteristics due to the dominance of van der Waals forces []. Notably, the hydrophobicity of NPs significantly impacts their interactions within biological and environmental systems, influencing factors like protein and lipid corona formation [], immune responses [], cellular uptake [], bioaccumulation, and bioavailability [].
Considering the optical properties, the primary absorption peak (Figure S1) exhibited a noticeable blue shift that was predominantly ascribed to the Burstein–Moss effect [,]. This phenomenon is frequently seen in n-type semiconductors [] and is associated with reduced size, quantum confinement effects [,], impediments in low-energy transitions [], and alterations in surface morphology [,].
Rod-shaped nanostructures have certainly a higher aspect ratio compared to spherical and/or irregular NPs [,]. This difference in aspect ratio can influence their properties, including the way that they interact with cells and other biological systems []. For instance, rod-shaped gold NPs, despite being analogous in size to their spherical counterparts, exhibited enhanced absorption and integration []. In contrast, these rod-shaped particles exhibited reduced exocytosis compared to their spherical equivalents []. Elongated particles, due to their increased aspect ratios, demonstrate a more pronounced cellular uptake compared to spherical NPs of similar size [,,]. The distinct morphologies of NPs, coupled with their unique interfacial properties, lead to nuanced surface interactions that ultimately dictate their absorption kinetics [,].
ZnO NPs can provoke ROS generation, and thus they are considered to exhibit anticancer and antibacterial activities [,,]. In our work, the synthesized ZnO@OAm NRs decreased the efficiency of the OEC (Figure 4a) and increased ROS production (Figure 7b). Enhanced ROS production inhibits the repair of photodamaged PSII and especially the de novo synthesis of the D1 protein [,], thus reducing the efficiency of PSII reaction centers (Fv’/Fm’) (Figure 6c). A decreased ΦPSII can be assigned either to a decreased fraction of open PSII reaction centers (qp) or a decreased efficiency of reaction centers (Fv′/Fm′) []. In our case, the decreased ΦPSII was attributed to the reduced efficiency of the reaction centers (Fv′/Fm′) (Figure 6c) since the fraction of open PSII reaction centers (qp) remained unaltered (Figure 5d). Thus, the reduced efficiency of PSII reaction centers (Fv′/Fm′) resulted in a reduced electron transport rate (ETR) (Figure 6b) and a decreased effective quantum yield of PSII photochemistry (ΦPSII) (Figure 5a).
The decrease in the effective quantum yield of PSII photochemistry (ΦPSII) 90 min after spraying with ZnO@OAm NRs (Figure 5a) was accompanied by a significant (p < 0.05) increase in the non-regulated energy loss in PSII (ΦNO) at both GL and HL intensities (Figure 5c). The increase in ΦNO, which is a measure of the singlet oxygen (1O2) generation [,,], suggests an increase in ROS production. When the singlet excited chlorophyll state (1Chl*) is not quenched, the triplet chlorophyll state (3Chl*) is developed through intersystem crossing, which leads to singlet oxygen (1O2) formation [,,,]. ROS generation is prevented by downregulating 1Chl* through the mechanism of non-photochemical quenching (NPQ), quenching 3Chl*, or the antioxidant mechanisms that scavenge ROS and improve fitness [,,,]. However, in tomato leaves sprayed with ZnO@OAm NRs, the photoprotective mechanism of NPQ did not increase (Figure 6a) to prevent ROS production [,,,]. As a result, 1O2 (Figure 5c) and H2O2 (Figure 7b) were created, suggesting increased ROS generation.
ZnO@OAm NRs resulted in reduced efficiency in the OEC (Figure 4a), which caused photoinhibition, as judged by the decrease in the maximum photochemistry (Fv/Fm) (Figure 4b). A decline in OEC activity leads to a rapid decline in Fv/Fm, and the donor-side-induced photoinhibition further reduces Fv/Fm [,]. The production of H2O2 on leaf midribs and minor tomato lamina veins that were sprayed with ZnO@OAm NRs (Figure 7b) was probably due to donor-side photoinhibition as the result of the OEC malfunction []. The H2O2 production observed in the current research was in agreement with the increased ROS generation on the leaf midribs and minor lamina veins of Brassica juncea treated with ZnO NPs []. It has to be noted that the generation of H2O2 predominantly depends on the ZnO NP surface, leading to the production of more ROS [].
Upon exposure to light, ZnO NPs release oxygen molecules from their active sites, leading to the generation of various ROS on the surface of a ZnO nanocrystal []. ROS accumulation, due to the absence of the photoprotective mechanism of NPQ, is the result of the donor-side photoinhibition that is associated with H2O2 formation, which can be oxidized to the superoxide radical (O2•−) or reduced to the hydroxyl radical (HO•) []. When oxygen molecules bind to the rod-shaped ZnO NPs’ surfaces, they trap free electrons, as described by the equation O2(g) + e− → O2−(ad) []. Once the UV light source is discontinued, the concentration of holes becomes outnumbered by electrons within the ZnO NRs. As these holes recombine with the electrons, the oxygen molecules once again adhere to the surface, capturing the free electrons, which results in the conductance dropping [].
Despite their destructive action, ROS are described as second messengers in a plethora of cellular and developmental processes [,,,,]. ROS have been shown to diffuse through leaf veins and act as long-distance signaling molecules [,,,,,]. In fact, ROS primarily act by inhibiting the repair of photodamaged PSII reaction centers and not by damaging PSII reaction centers directly [].
ZnO NPs enter leaves through stomata. They are subsequently transported through the apoplast to mesophyll cells and are then translocated to chloroplasts [,,]. However, after leaf entry the long-distance transport of foliar-applied ZnO NPs is not well understood []. In chloroplasts, ZnO NPs alter PSII activity, measured as the maximum efficiency of PSII photochemistry (Fv/Fm) []. In mesophyll cells, ZnO NPs have been reported to decrease δ-aminolevulinic acid (ALA), indicating changes in the chlorophyll metabolic pathway []. Foliar sprays with 20 and 100 mg L−1 ZnO NPs enhanced the chlorophyll content in tomatoes [], while long-term foliar application of 3 mg L−1 ZnO NPs increased both chlorophyll a (Chl a), and chlorophyll b (Chl b) in tomatoes []. Faizan et al. [] reported that ZnO NPs increased the chlorophyll contents of tomato plants in a dose- and exposure-time-dependent way. In wheat plants under salt stress, the application of ZnO NPs increased the amounts of Chl a and Chl b by 24.6% and 10%, respectively []. Our data show that the response of the chlorophyll content to ZnO@OAm NRs resembled a hormetic response, with an increased content for a short period that decreased afterwards to the control level (Figure 3). A disturbance of homeostasis results in a hormetic stimulation as an overcompensation reaction [,]. Lately, hormesis has been revealed to occur in several organisms independent of the stressor or the biological function being examined [,,,,].
4. Materials and Methods
4.1. Synthesis of ZnO@OAm NRs
All the reagents used for the synthesis of ZnO@OAm NRs were of analytical grade. The synthesis of hydrophobic ZnO@OAm NRs was adapted from a previously reported polyol-solvothermal approach, with a modification in the glycol used. Specifically, DEG was employed as the solvent instead of the triethylene glycol (TrEG) used in an earlier study []. In the modified procedure, 1.06 mmol Zn(acac)2 was dissolved in a mixture of 4 mL of DEG and 4 mL of OAm. The solution was agitated at 30 °C for 15 min before being transferred to a Teflon-lined autoclave. The reaction was carried out under autogenous pressure at 200 °C for 8 h. Subsequently, the mixture was centrifuged at 5000 rpm for 20 min, followed by rinsing with ethyl alcohol four times to remove any untreated precursors.
4.2. Characterization of ZnO@OAm NRs
The crystal size and structure of the NRs were determined via X-ray diffraction (XRD) on a Philips PW 1820 diffractometer with monochromatized Cu Kα radiation (λ = 1.5406 Å) in the range of 2θ from 20 to 90°. The morphology and particle size were analyzed using a JEOL JEM 1200-EX (Tokyo, Japan) transmission electron microscope (TEM). The FT-IR spectra (ranging from 4000 to 450 cm−1) of the NRs were obtained using a Nicolet iS20 spectrometer equipped with a monolithic diamond ATR crystal (Thermo Fisher Scientific, Waltham, MA, USA). The thermal stability and the amount of the organic coating were evaluated via thermogravimetric analysis (TGA) using a SETA−RAM SetSys-1200 (KEP Technologies, Caluire, France) under a N2 atmosphere with a heating rate of 10 °C min−1. The optical properties of the NRs were investigated using a UV-Vis spectrophotometer (V-750, Jasco, Tokyo, Japan). The hydrodynamic size (nm), polydispersity (PDI), and ζ-potential (mV) of dispersed NRs in an ethanol/water solution (1:3 ratio) were determined via dynamic light scattering (DLS) at 25 °C using a Zetasizer (Nano ZS Malvern apparatus VASCO Flex™ Particle Size Analyzer NanoQ V2.5.4.0, Malvern, UK).
4.3. Plant Material and Growth Conditions
To test the impact of the synthesized ZnO@OAm NRs on photosynthetic function, we purchased tomato (Lycopersicon esculentum Mill. cv. Galli) plants from the market. The plants were left for three days to acclimate in a greenhouse at 25 ± 1/20 ± 1 °C day/night temperatures, with a 14 h day/night photoperiod provided by a photosynthetic photon flux density (PPFD) of 600 ± 10 μmol quanta m−2 s−1 and relative day/night humidities of 65 ± 5/75 ± 5%.
4.4. Exposure of Tomato Plants to ZnO@OAm NRs
After the three-day acclimation period, tomato plants were foliar-sprayed once with 15 mg L−1 ZnO@OAm NRs or double-distilled water (control). Each plant received 10 mL of 15 mg L−1 ZnO@OAm NRs or 10 mL of double-distilled water. Three to five plants were used in each treatment with three independent replicates.
4.5. Chlorophyll Content Measurements
The chlorophyll contents of the control plants and the ZnO@OAm NR-treated ones were estimated with a portable Chlorophyll Content Meter (Model Cl-01, Hansatech Instruments Ltd., Norfolk, UK) [,]. Six measurements were recorded around the middle leaf from each treatment, and the measurements were averaged from all biological replicates.
4.6. Chlorophyll Fluorescence Analysis
The impact of the synthesized ZnO@OAm NRs on PSII function was evaluated in dark-adapted (20 min) tomato leaflets, 30 and 90 min after spraying with 15 mg L−1 ZnO@OAm NRs or distilled water (control), using an Imaging PAM Fluorometer M-Series MINI-Version (Heinz Walz GmbH, Effeltrich, Germany), as described in detail previously []. Fluorescence was excited by a blue LED with a short-pass filter set to λ < 510 nm. Ten circular areas of interest (AOIs) were selected in each leaf to have the characteristic whole-leaf measurement. The actinic light (AL) used for the measurements was 600 μmol photons m−2 s−1, corresponding to the growth light (GL) of tomato plants, or 1000 μmol photons m−2 s−1, corresponding to high light (HL) intensity. The chlorophyll fluorescence parameters described in Table S1 were estimated using Win V2.41a software (Heinz Walz GmbH, Effeltrich, Germany).
4.7. Evaluation of Hydrogen Peroxide Generation
Hydrogen peroxide (H2O2) generation in tomato leaflets was evaluated 90 min after tomato plants were sprayed with 15 mg L−1 ZnO@OAm NRs or distilled water (control), as explained previously []. Leaves were incubated with 25 μM 2′, 7′-dichlorofluorescein diacetate (DCF-DA, Sigma Aldrich, Chemie GmbH, Schnelldorf, Germany) for 30 min in the dark and observed with a Zeiss AxioImager Z2 epi-fluorescence microscope equipped with an AxioCam MRc5 digital camera [].
4.8. Statistical Analysis
Significant differences were determined using an ANOVA, followed by Tukey’s post hoc tests for each parameter. Before the tests, we evaluated the assumptions for raw data normality and we used the variance homogeneity test to verify the parametric distribution of the data. Values were considered significantly different at p < 0.05.
5. Conclusions
Foliar spray with 15 mg L−1 ZnO@OAm NRs decreased the efficiency of the oxygen-evolving complex (OEC), with a concomitant increase in ROS generation, a decrease in the maximum efficiency of PSII photochemistry (Fv/Fm), a decrease in the electron transport rate (ETR), and a decrease in the effective quantum yield of PSII photochemistry (ΦPSII), indicating reduced PSII efficiency. We conclude that rod-shaped ZnO@OAm NRs reduced PSII photochemistry, in contrast to the irregularly shaped ZnO@OAm NPs, which enhanced PSII efficiency []. In the transport of NPs into plant mesophyll tissues, it is essential to recognize that the process does not depend solely on the particle size but also on its shape and electrostatic characteristics. Thus, the morphology of NPs, including their shape and organic coating, determines factors which are important for their mechanism of action and their impact on crop yield.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/plants12193502/s1, Figure S1: UV-Vis spectrum and optical band gap energy plot of ZnO@OAm NRs. Figure S2: Dynamic light scattering (DLS) spectra and the ζ-potential of NRs. Table S1: Definitions of the chlorophyll fluorescence parameters used in the experiments.
Author Contributions
Conceptualization, C.D.-S. and M.M.; methodology, P.T., I.S., I.-D.S.A. and M.M.; software, P.T., I.S., I.-D.S.A., S.M. and M.M.; validation, C.D.-S. and M.M.; formal analysis, P.T. and M.M.; resources, C.D.-S. and M.M.; data curation, P.T. and M.M.; writing—original draft preparation, P.T., C.D.-S. and M.M.; writing—review and editing, C.D.-S., M.M. and P.T.; supervision, C.D.-S. and M.M.; project administration, C.D.-S. and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-financed by Greece and the European Union (European Social Fund (ESF)) through the Operational Program “Human Resources Development, Education and Lifelong Learning” in the context of the Act “Enhancing Human Resources Research Potential by undertaking a Doctoral Research” Sub-action 2: IKY Scholarship Program for PhD candidates in Greek Universities (Tryfon Panagiota).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Silva, S.; Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Metabolomics as a tool to understand nano-plant interactions: The case study of metal-based nanoparticles. Plants 2023, 12, 491. [Google Scholar] [PubMed]
- Antonoglou, O.; Moustaka, J.; Adamakis, I.D.; Sperdouli, I.; Pantazaki, A.; Moustakas, M.; Dendrinou-Samara, C. Nanobrass CuZn nanoparticles as foliar spray non phytotoxic fungicides. ACS Appl. Mater. Interfaces 2018, 10, 4450–4461. [Google Scholar] [CrossRef] [PubMed]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-based sustainable agriculture and food science: Recent advances and future outlook. Front. Nanotechnol. 2020, 2, 579954. [Google Scholar]
- Singh, A.; Tiwari, S.; Pandey, J.; Lata, C.; Singh, I.K. Role of nanoparticles in crop improvement and abiotic stress management. J. Biotechnol. 2021, 337, 57–70. [Google Scholar]
- Dias, M.C.; Santos, C.; Pinto, G.; Silva, A.M.S.; Silva, S. Titanium dioxide nanoparticles impaired both photochemical and non-photochemical phases of photosynthesis in wheat. Protoplasma 2018, 256, 69–78. [Google Scholar] [CrossRef]
- Silva, S.; Ferreira de Oliveira, J.M.P.; Dias, M.C.; Silva, A.M.S.; Santos, C. Antioxidant mechanisms to counteract TiO2-nanoparticles toxicity in wheat leaves and roots are organ dependent. J. Hazard. Mater. 2019, 380, 120889. [Google Scholar] [CrossRef]
- Burman, U.; Saini, M.; Praveen, K. Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings. Toxicol. Environ. Chem. 2013, 95, 605–612. [Google Scholar] [CrossRef]
- Mogazy, A.M.; Hanafy, R.S. Foliar spray of biosynthesized zinc oxide nanoparticles alleviate salinity stress effect on Vicia faba plants. J. Soil Sci. Plant Nutr. 2022, 22, 2647–2662. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, N.; Wang, Z.L. Dissolving behavior and stability of ZnO wires in biofluids: A study on biodegradability and biocompatibility of ZnO nanostructures. Adv. Mater. 2006, 18, 2432–2435. [Google Scholar] [CrossRef]
- Yusefi-Tanha, E.; Fallah, S.; Rostamnejadi, A.; Pokhrel, L.R. Zinc oxide nanoparticles (ZnONPs) as a novel nanofertilizer: Influence on seed yield and antioxidant defense system in soil grown soybean (Glycine max cv. Kowsar). Sci. Total Environ. 2020, 738, 140240. [Google Scholar]
- Milani, N.; McLaughlin, M.J.; Stacey, S.P.; Kirby, J.K.; Hettiarachchi, G.M.; Beak, D.G.; Cornelis, G. Dissolution kinetics of macronutrient fertilizers coated with manufactured zinc oxide nanoparticles. J. Agric. Food Chem. 2012, 60, 3991–3998. [Google Scholar] [CrossRef]
- Ahmed, R.; Uddin, M.K.; Quddus, M.A.; Samad, M.Y.A.; Hossain, M.A.M.; Haque, A.N.A. Impact of foliar application of zinc and zinc oxide nanoparticles on growth, yield, nutrient uptake and quality of tomato. Horticulturae 2023, 9, 162. [Google Scholar] [CrossRef]
- Tryfon, P.; Sperdouli, I.; Adamakis, I.D.S.; Mourdikoudis, S.; Moustakas, M.; Dendrinou-Samara, C. Impact of coated zinc oxide nanoparticles on photosystem II of tomato plants. Materials 2023, 16, 5846. [Google Scholar] [CrossRef]
- Pittarate, S.; Rajula, J.; Rahman, A.; Vivekanandhan, P.; Thungrabeab, M.; Mekchay, S.; Krutmuang, P. Insecticidal effect of zinc oxide nanoparticles against Spodoptera frugiperda under laboratory conditions. Insects 2021, 12, 1017. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Luna, A.R.; Vásquez-López, A.; Rojas-Chávez, H.; Valdés-Madrigal, M.A.; Cruz-Martínez, H.; Medina, D.I. Engineered metal oxide nanoparticles as fungicides for plant disease control. Plants 2023, 12, 2461. [Google Scholar] [CrossRef] [PubMed]
- Kanakari, E.; Dendrinou-Samara, C. Fighting phytopathogens with engineered inorganic-based nanoparticles. Materials 2023, 16, 2388. [Google Scholar] [PubMed]
- Singh, A.; Prasad, S.M.; Singh, S. Impact of nano ZnO on metabolic attributes and fluorescence kinetics of rice seedlings. Environ. Nanotechnol. Monit. Manag. 2018, 9, 42–49. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Wang, R.; Wang, R.; Zhang, P.; Ju, Q.; Xu, J. Physiological, transcriptomic, and metabolomic analyses reveal zinc oxide nanoparticles modulate plant growth in tomato. Environ. Sci. Nano 2020, 7, 3587–3604. [Google Scholar] [CrossRef]
- Faizan, M.; Faraz, A.; Yusuf, M.; Khan, S.T.; Hayat, S. Zinc oxide nanoparticle-mediated changes in photosynthetic efficiency and antioxidant system of tomato plants. Photosynthetica 2018, 56, 678–686. [Google Scholar] [CrossRef]
- Faizan, M.; Faraz, A.; Mir, A.R.; Hayat, S. Role of zinc oxide nanoparticles in countering negative effects generated by cadmium in Lycopersicon esculentum. J. Plant Growth Regul. 2020, 40, 101–115. [Google Scholar] [CrossRef]
- Voloshina, M.; Rajput, V.D.; Minkina, T.; Vechkanov, E.; Mandzhieva, S.; Mazarji, M.; Churyukina, E.; Plotnikov, A.; Krepakova, M.; Wong, M.H. Zinc oxide nanoparticles: Physiological and biochemical responses in barley (Hordeum vulgare L.). Plants 2022, 11, 2759. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.M.G.; Chung, I.M. Regulation of morphological, molecular and nutrient status in Arabidopsis thaliana seedlings in response to ZnO nanoparticles and Zn ion exposure. Sci. Total Environ. 2017, 575, 187–198. [Google Scholar] [PubMed]
- Wan, J.; Wang, R.; Wang, R.; Ju, Q.; Wang, Y.; Xu, J. Comparative physiological and transcriptomic analyses reveal the toxic effects of ZnO nanoparticles on plant growth. Environ. Sci. Technol. 2019, 53, 4235–4244. [Google Scholar] [CrossRef]
- Javed, R.; Usman, M.; Yücesan, B.; Zia, M.; Gürel, E. Effect of zinc oxide (ZnO) nanoparticles on physiology and steviol glycosides production in micropropagated shoots of Stevia rebaudiana Bertoni. Plant Physiol. Biochem. 2017, 110, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Azarin, K.; Usatov, A.; Minkina, T.; Plotnikov, A.; Kasyanova, A.; Fedorenko, A.; Duplii, N.; Vechkanov, E.; Rajput, V.D.; Mandzhieva, S.; et al. Effects of ZnO nanoparticles and its bulk form on growth, antioxidant defense system and expression of oxidative stress related genes in Hordeum vulgare L. Chemosphere 2022, 287, 132167. [Google Scholar]
- Khan, A.R.; Azhar, W.; Wu, J.; Ulhassan, Z.; Salama, A.; Zaidi, S.H.R.; Yang, S.; Song, G.; Gan, Y. Ethylene participates in zinc oxide nanoparticles induced biochemical, molecular and ultrastructural changes in rice seedlings. Ecotoxicol. Environ. Saf. 2021, 226, 112844. [Google Scholar]
- El-Zohri, M.; Al-Wadaani, N.A.; Bafeel, S.O. Foliar sprayed green zinc oxide nanoparticles mitigate drought-induced oxidative stress in tomato. Plants 2021, 10, 2400. [Google Scholar] [CrossRef]
- Elshoky, H.A.; Yotsova, E.; Farghali, M.A.; Farroh, K.Y.; El-Sayed, K.; Elzorkany, H.E.; Rashkov, G.; Dobrikova, A.; Borisova, P.; Stefanov, M.; et al. Impact of foliar spray of zinc oxide nanoparticles on the photosynthesis of Pisum sativum L. under salt stress. Plant Physiol. Biochem. 2021, 167, 607–618. [Google Scholar]
- Adil, M.; Bashir, S.; Bashir, S.; Aslam, Z.; Ahmad, N.; Younas, T.; Asghar, R.M.A.; Alkahtani, J.; Dwiningsih, Y.; Elshikh, M.S. Zinc oxide nanoparticles improved chlorophyll contents, physical parameters, and wheat yield under salt stress. Front. Plant Sci. 2022, 13, 932861. [Google Scholar] [CrossRef]
- Singh, A.; Sengar, R.S.; Rajput, V.D.; Minkina, T.; Singh, R.K. Zinc oxide nanoparticles improve salt tolerance in rice seedlings by improving physiological and biochemical indices. Agriculture 2022, 12, 1014. [Google Scholar] [CrossRef]
- Mazhar, Z.; Akhtar, J.; Alhodaib, A.; Naz, T.; Zafar, M.I.; Iqbal, M.M.; Fatima, H.; Naz, I. Efficacy of ZnO nanoparticles in Zn fortification and partitioning of wheat and rice grains under salt stress. Sci. Rep. 2023, 13, 2022. [Google Scholar] [CrossRef]
- Alabdallah, N.M.; Alzahrani, H.S. The potential mitigation effect of ZnO nanoparticles on Abelmoschus esculentus L. Moench metabolism under salt stress conditions. Saudi. J. Biol. Sci. 2020, 27, 3132–3137. [Google Scholar] [CrossRef] [PubMed]
- Asfi, M.; Ouzounidou, G.; Panajiotidis, S.; Therios, I.; Moustakas, M. Toxicity effects of olive-mill wastewater on growth, photosynthesis and pollen morphology of spinach plants. Ecotoxicol. Environ. Saf. 2012, 80, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Sperdouli, I.; Mellidou, I.; Moustakas, M. Harnessing chlorophyll fluorescence for phenotyping analysis of wild and cultivated tomato for high photochemical efficiency under water deficit for climate change resilience. Climate 2021, 9, 154. [Google Scholar] [CrossRef]
- Ouzounidou, G.; Moustakas, M.; Lannoye, R. Chlorophyll fluorescence and photoacoustic characteristics in relationship to changes in chlorophyll and Ca2+ content of a Cu-tolerant Silene compacta ecotype under Cu treatment. Physiol. Plant. 1995, 93, 551–557. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Moustaka, J. Early drought stress warning in plants: Color pictures of photosystem II photochemistry. Climate 2022, 10, 179. [Google Scholar]
- Moustakas, M.; Guidi, L.; Calatayud, A. Editorial: Chlorophyll fluorescence analysis in biotic and abiotic stress, volume II. Front. Plant Sci. 2022, 13, 1066865. [Google Scholar] [CrossRef]
- Moustaka, J.; Moustakas, M. Early-stage detection by chlorophyll fluorescence imaging analysis of biotic and abiotic stress on plants. Biosensors 2023, 13, 796. [Google Scholar]
- Falco, W.F.; Queiroz, A.M.; Fernandes, J.; Botero, E.R.; Falcão, E.A.; Guimarães, F.E.G.; M’Peko, J.C.; Oliveira, S.L.; Colbeck, I.; Caires, A.R.L. Interaction between chlorophyll and silver nanoparticles: A close analysis of chlorophyll fluorescence quenching. J. Photochem. Photobiol. A 2015, 299, 203–209. [Google Scholar] [CrossRef]
- Tryfon, P.; Kamou, N.N.; Mourdikoudis, S.; Karamanoli, K.; Menkissoglu-Spiroudi, U.; Dendrinou-Samara, C. CuZn and ZnO nanoflowers as nano-fungicides against Botrytis cinerea and Sclerotinia sclerotiorum: Phytoprotection, translocation, and impact after foliar application. Materials 2021, 14, 7600. [Google Scholar] [CrossRef] [PubMed]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Aspoukeh, P.K.; Barzinjy, A.A.; Hamad, S.M. Synthesis, properties and uses of ZnO nanorods: A mini review. Int. Nano Lett. 2022, 12, 153–168. [Google Scholar]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A review of microwave synthesis of zinc oxide nanomaterials: Reactants, process parameters and morphologies. Nanomaterials 2020, 10, 1086. [Google Scholar]
- Yang, H.; Liu, C.; Yang, D.; Zhang, H.; Xi, Z. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: The role of particle size, shape and composition. J. Appl. Toxicol. 2009, 29, 69–78. [Google Scholar] [CrossRef]
- Ramani, M.; Ponnusamy, S.; Muthamizhchelvan, C.; Marsili, E. Amino acid-mediated synthesis of zinc oxide nanostructures and valuation of their facet-dependent antimicrobial activity. Colloid Surf. B 2014, 117, 233–239. [Google Scholar] [CrossRef]
- Wang, X.; Xie, H.; Wang, P.; Yin, H. Nanoparticles in plants: Uptake, transport and physiological activity in leaf and root. Materials 2023, 16, 3097. [Google Scholar]
- Khan, F.A. Synthesis of nanomaterials: Methods & Technology. In Applications of Nanomaterials in Human Health, 1st ed.; Khan, F.A., Ed.; Springer: Singapore, 2020; pp. 15–21. [Google Scholar] [CrossRef]
- Heinz, H.; Pramanik, C.; Heinz, O.; Ding, Y.; Mishra, R.K.; Marchon, D.; Flatt, R.J.; Estrela-Lopis, I.; Llop, J.; Moya, S.; et al. Nanoparticle decoration with surfactants: Molecular interactions, assembly, and applications. Surf. Sci. Rep. 2017, 72, 1–58. [Google Scholar]
- Ahammed, K.R.; Ashaduzzaman, M.; Paul, S.C.; Nath, M.R.; Bhowmik, S.; Saha, O.; Rahaman, M.M.; Bhowmik, S.; Aka, T.D. Microwave assisted synthesis of zinc oxide (ZnO) nanoparticles in a noble approach: Utilization for antibacterial and photocatalytic activity. SN Appl. Sci. 2020, 2, 955. [Google Scholar] [CrossRef]
- Harish, V.; Ansari, M.M.; Tewari, D.; Gaur, M.; Yadav, A.B.; García-Betancourt, M.-L.; Abdel-Haleem, F.M.; Bechelany, M.; Barhoum, A. Nanoparticle and nanostructure synthesis and controlled growth methods. Nanomaterials 2022, 12, 3226. [Google Scholar] [CrossRef] [PubMed]
- Tryfon, P.; Kamou, N.N.; Pavlou, A.; Mourdikoudis, S.; Menkissoglu-Spiroudi, U.; Dendrinou-Samara, C. Nanocapsules of ZnO nanorods and geraniol as a novel mean for the effective control of Botrytis cinerea in tomato and cucumber plants. Plants 2023, 12, 1074. [Google Scholar] [CrossRef] [PubMed]
- Raliya, R.; Tarafdar, J.C. ZnO Nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in clusterbean (Cyamopsis tetragonoloba L.). Agric. Res. 2013, 2, 48–57. [Google Scholar] [CrossRef]
- Feng, X.; Feng, L.; Jin, M.; Zhai, J.; Jiang, L.; Zhu, D. Reversible super-hydrophobicity to super-hydrophilicity transition of aligned ZnO nanorod films. J. Am. Chem. Soc. 2004, 126, 62–63. [Google Scholar] [CrossRef] [PubMed]
- Olenick, L.L.; Troiano, J.M.; Vartanian, A.; Melby, E.S.; Mensch, A.C.; Zhang, L.; Hong, J.; Mesele, O.; Qiu, T.; Bozich, J.; et al. Lipid corona formation from nanoparticle interactions with bilayers. Chem 2018, 4, 2709–2723. [Google Scholar] [CrossRef]
- Moyano, D.F.; Goldsmith, M.; Solfiell, D.J.; Landesman-Milo, D.; Miranda, O.R.; Peer, D.; Rotello, V.M. Nanoparticle hydrophobicity dictates immune response. J. Am. Chem. Soc. 2012, 134, 3965–3967. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.J.; Posati, T.; Moyano, D.F.; Tang, R.; Yan, B.; Vachet, R.W.; Rotello, V.M. The interplay of monolayer structure and serum protein interactions on the cellular uptake of gold nanoparticles. Small 2012, 8, 2659–2663. [Google Scholar] [CrossRef] [PubMed]
- Lasagna-Reeves, C.; Gonzalez-Romero, D.; Barria, M.A.; Olmedo, I.; Clos, A.; Sadagopa Ramanujam, V.M.; Urayama, A.; Vergara, L.; Kogan, M.J.; Soto, C. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochem. Biophys. Res. Commun. 2010, 393, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Chen, X.Y.; Feng, Y.; Yang, G.W. Physical mechanism of blue-shift of UV luminescence of a single pencil-like ZnO nanowire. Nano Lett. 2007, 7, 3879–3883. [Google Scholar] [CrossRef]
- Ghosh, A.; Choudhary, R.N.P. Optical emission and absorption spectra of Zn–ZnO core-shell nanostructures. J. Exp. Nanosci. 2010, 5, 134–142. [Google Scholar] [CrossRef]
- Neumark, Y.G.G.; Kuskovsky, I. Doping aspects of Zn-based wide-band-gap semiconductors. In Springer Handbook of Electronic and Photonic Materials; Kasap, P.C.S., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 843–854. [Google Scholar]
- Gu, Y.; Kuskovsky, I.L.; Yin, M.; O’Brien, S.; Neumark, G.F. Quantum confinement in ZnO nanorods. Appl. Phys. Lett. 2004, 85, 3833–3835. [Google Scholar] [CrossRef]
- Agarwal, S.; Jangir, L.K.; Rathore, K.S.; Kumar, M.; Awasthi, K. Morphology-dependent structural and optical properties of ZnO nanostructures. Appl. Phys. A 2019, 125, 553. [Google Scholar] [CrossRef]
- Ali, R.N.; Diao, K.; Naz, H.; Cui, X.; Xiang, B. Synthesis, characterization, and applications of zinc oxide nanoparticles and nanorods in acetone gas detection. Mater. Res. Express 2017, 4, 095015. [Google Scholar] [CrossRef]
- Köseoğlu, Y. A simple microwave-assisted combustion synthesis and structural, optical, and magnetic characterization of ZnO nanoplatelets. Ceram. Int. 2014, 40, 4673–4679. [Google Scholar] [CrossRef]
- Raji, R.; Gopchandran, K.G. ZnO nanostructures with tunable visible luminescence: Effects of kinetics of chemical reduction and annealing. J. Sci. Adv. Mater. Devices 2017, 2, 51–58. [Google Scholar] [CrossRef]
- Liu, B.; Zeng, H.C. Hydrothermal synthesis of ZnO nanorods in the diameter regime of 50 nm. J. Am. Chem. Soc. 2003, 125, 4430–4431. [Google Scholar] [CrossRef]
- Pariona, N.; Paraguay-Delgado, F.; Basurto-Cereceda, S.; Morales-Mendoza, J.E.; Hermida-Montero, L.A.; Mtz-Enriquez, A.I. Shape-dependent antifungal activity of ZnO particles against phytopathogenic fungi. Appl. Nanosci. 2019, 10, 435–443. [Google Scholar] [CrossRef]
- Higgins, S.G.; Becce, M.; Belessiotis-Richards, A.; Seong, H.; Sero, J.E.; Stevens, M.M. High-aspect-ratio nanostructured surfaces as biological metamaterials. Adv. Mater. 2020, 32, e1903862. [Google Scholar]
- Zhang, H.; Goh, N.S.; Wang, J.W.; Pinals, R.L.; González-Grandío, E.; Demirer, G.S.; Butrus, S.; Fakra, S.C.; Del Rio Flores, A.; Zhai, R.; et al. Nanoparticle cellular internalization is not required for RNA delivery to mature plant leaves. Nat. Nanotechnol. 2022, 17, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Zhang, S.; Zhang, B.; Zhang, C.; Fang, C.-Y.; Rehor, I.; Cigler, P.; Chang, H.-C.; Lin, G.; Liu, R. Unambiguous observation of shape effects on cellular fate of nanoparticles. Sci. Rep. 2014, 4, 4495. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Auth, T.; Gompper, G. Shape and orientation matter for the cellular uptake of nonspherical particles. Nano Lett. 2014, 14, 687–693. [Google Scholar] [CrossRef]
- Zubair, N.; Akhtar, K. Morphology controlled synthesis of ZnO nanoparticles for in-vitro evaluation of antibacterial activity. Trans. Nonferrous Met. Soc. China 2020, 30, 1605–1614. [Google Scholar] [CrossRef]
- Wu, H.; Li, Z. Nano-enabled agriculture: How do nanoparticles cross barriers in plants? Plant Commun. 2022, 3, 100346. [Google Scholar] [PubMed]
- Sun, H.; Lei, C.; Xu, J.; Li, R. Foliar uptake and leaf-to-root translocation of nanoplastics with different coating charge in maize plants. J. Hazard. Mater. 2021, 416, 125854. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, J.; Zhan, X.; Li, A.; White, J.C.; Gardea-Torresdey, J.L.; Xing, B. Role of charge and size in the translocation and distribution of zinc oxide particles in wheat cells. ACS Sustain. Chem. Eng. 2021, 9, 11556–11564. [Google Scholar] [CrossRef]
- Sivakumar, P.; Lee, M.; Kim, Y.S.; Shim, M.S. Phototriggered antibacterial and anticancer activities of zinc oxide nanoparticles. J. Mater. Chem. B 2018, 6, 4852–4871. [Google Scholar] [CrossRef] [PubMed]
- Donga, S.; Chanda, S. Caesalpinia crista seeds mediated green synthesis of zinc oxide nanoparticles for antibacterial, antioxidant, and anticancer activities. BioNanoScience 2022, 12, 451–462. [Google Scholar] [CrossRef]
- Yalcin, B.; Arda, L.; Yalcin, I.E.; Senturk, K.; Alphan, M.C.; Akcan, D.; Ozyigit, I.I. Exploration of the improving effect of Cd-doping on structural, photocatalytic, and biological properties of ZnO nanoparticles. J. Nanopart. Res. 2023, 25, 146. [Google Scholar]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta 2007, 1767, 414–421. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Murata, N. Revised scheme for the mechanism of photoinhibition and its application to enhance the abiotic stress tolerance of the photosynthetic machinery. Appl. Microbiol. Biotechnol. 2014, 98, 8777–8796. [Google Scholar]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Klughammer, C.; Schreiber, U. Complementary PSII quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl. Notes 2008, 1, 27–35. [Google Scholar]
- Kasajima, I.; Takahara, K.; Kawai-Yamada, M.; Uchimiya, H. Estimation of the relative sizes of rate constants for chlorophyll de-excitation processes through comparison of inverse fluorescence intensities. Plant Cell Physiol. 2009, 50, 1600–1616. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M.; Dobrikova, A.; Sperdouli, I.; Hanć, A.; Adamakis, I.-D.S.; Moustaka, J.; Apostolova, E.A. Hormetic spatiotemporal photosystem II response mechanism of salvia to excess zinc exposure. Int. J. Mol. Sci. 2022, 23, 11232. [Google Scholar] [CrossRef]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant. Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef]
- Kasajima, I.; Ebana, K.; Yamamoto, T.; Takahara, K.; Yano, M.; Kawai-Yamada, M.; Uchimiya, H. Molecular distinction in genetic regulation of nonphotochemical quenching in rice. Proc. Natl. Acad. Sci. USA 2011, 108, 13835–13840. [Google Scholar] [CrossRef]
- Gawrónski, P.; Witoón, D.; Vashutina, K.; Bederska, M.; Betlínski, B.; Rusaczonek, A.; Karpínski, S. Mitogen-activated protein kinase 4 is a salicylic acid-independent regulator of growth but not of photosynthesis in Arabidopsis. Mol. Plant 2014, 7, 1151–1166. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M. Plant photochemistry, reactive oxygen species, and photoprotection. Photochem 2022, 2, 5–8. [Google Scholar] [CrossRef]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, K.K. Safety valves for photosynthesis. Curr. Opin. Plant Biol. 2000, 3, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Adamakis, I.D.S. Editorial: Reactive oxygen species in chloroplasts and chloroplast antioxidants under abiotic stress. Front. Plant Sci. 2023, 14, 1208247. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Tóth, S.Z.; Nagy, V.; Puthur, J.T.; Kovács, L.; Garab, G. The physiological role of ascorbate as photosystem II electron donor: Protection against photoinactivation in heat-stressed leaves. Plant Physiol. 2011, 156, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Széles, E.; Kuntam, S.; Vidal-Meireles, A.; Nagy, V.; Nagy, K.; Ábrahám, Á.; Kovács, L.; Tóth, S.Z. Single-cell microfluidics in combination with chlorophyll a fluorescence measurements to assess the lifetime of the Chlamydomonas PSBO protein. Photosynthetica 2023, 61, 13–20. [Google Scholar] [CrossRef]
- Hamdani, S.; Khan, N.; Perveen, S.; Qu, M.; Jiang, J.; Govindjee; Zhu, X.G. Changes in the photosynthesis properties and photoprotection capacity in rice (Oryza sativa) grown under red, blue, or white light. Photosynth. Res. 2019, 139, 107–121. [Google Scholar] [PubMed]
- Geremew, A.; Carson, L.; Woldesenbet, S.; Wang, H.; Reeves, S.; Brooks, N., Jr.; Saganti, P.; Weerasooriya, A.; Peace, E. Effect of zinc oxide nanoparticles synthesized from Carya illinoinensis leaf extract on growth and antioxidant properties of mustard (Brassica juncea). Front. Plant Sci. 2023, 14, 1108186. [Google Scholar] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nanomicro Lett. 2015, 7, 219–242. [Google Scholar]
- Tiginyanu, I.M.; Lupan, O.; Ursaki, V.V.; Chow, L.; Enachi, M. Nanostructures of metal oxides. In Comprehensive Semiconductor Science and Technology, 1st ed.; Bhattacharya, P., Fornari, R., Kamimura, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 3, pp. 396–479. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Czarnocka, W.; Karpiński, S. Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radic. Biol. Med. 2018, 122, 4–20. [Google Scholar]
- Agrawal, V.; Singh, V.; Tripathi, B.N. Components and processes involved in retrograde signaling from chloroplast to nucleus. Physiol. Plant. 2023, 175, e13987. [Google Scholar] [CrossRef]
- Fichman, Y.; Rowland, L.; Oliver, M.J.; Mittler, R. ROS are evolutionary conserved cell-to-cell stress signals. Proc. Natl. Acad. Sci. USA 2023, 120, e2305496120. [Google Scholar] [CrossRef] [PubMed]
- Moustaka, J.; Tanou, G.; Adamakis, I.D.; Eleftheriou, E.P.; Moustakas, M. Leaf age dependent photoprotective and antioxidative mechanisms to paraquat-induced oxidative stress in Arabidopsis thaliana. Int. J. Mol. Sci. 2015, 16, 13989–14006. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.E.; Ivanov, A.G.; Öquist, G.; Grodzinski, B.; Sarhan, F.; Huner, N.P.A. Energy balance, organellar redox status, and acclimation to environmental stress. Can. J. Bot. 2006, 84, 1355–1370. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [PubMed]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signaling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fichman, Y.; Mittler, R. Vascular bundles mediate systemic reactive oxygen signaling during light stress. Plant Cell 2020, 32, 3425–3435. [Google Scholar]
- Sperdouli, I.; Andreadis, S.S.; Adamakis, I.S.; Moustaka, J.; Koutsogeorgiou, E.I.; Moustakas, M. Reactive oxygen species initiate defence responses of potato photosystem II to sap-sucking insect feeding. Insects 2022, 13, 409. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Allakhverdiev, S.I.; Murata, N. A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim. Biophys. Acta 2006, 1757, 742–749. [Google Scholar]
- Zhu, J.; Li, J.; Shen, Y.; Liu, S.; Zeng, N.; Zhan, X.; White, J.C.; Gardea-Torresdey, J.; Xing, B. Mechanism of zinc oxide nanoparticle entry into wheat seedling leaves. Environ. Sci. Nano 2020, 7, 3901–3913. [Google Scholar]
- Liu, L.; Nian, H.; Lian, T. Plants and rhizospheric environment: Affected by zinc oxide nanoparticles (ZnO NPs). A review. Plant Physiol. Biochem. 2022, 185, 91–100. [Google Scholar]
- Lv, W.; Geng, H.; Zhou, B.; Chen, H.; Yuan, R.; Ma, C.; Liu, R.; Xing, B.; Wang, F. The behavior, transport, and positive regulation mechanism of ZnO nanoparticles in a plant-soil-microbe environment. Environ. Pollut. 2022, 315, 120368. [Google Scholar]
- Malea, P.; Charitonidou, K.; Sperdouli, I.; Mylona, Z.; Moustakas, M. Zinc uptake, photosynthetic efficiency and oxidative stress in the seagrass Cymodocea nodosa exposed to ZnO nanoparticles. Materials 2019, 12, 2101. [Google Scholar] [CrossRef]
- Pejam, F.; Ardebili, Z.O.; Ladan-Moghadam, A.; Danaee, E. Zinc oxide nanoparticles mediated substantial physiological and molecular changes in tomato. PLoS ONE 2021, 16, e0248778. [Google Scholar]
- Calabrese, E.J. Evidence that hormesis represents an ‘‘overcompensation’’ response to a disruption in homeostasis. Ecotoxicol. Environ. Saf. 1999, 42, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M.; Moustaka, J.; Sperdouli, I. Hormesis in photosystem II: A mechanistic approach. Curr. Opin. Toxicol. 2022, 29, 57–64. [Google Scholar] [CrossRef]
- Agathokleous, E. The rise and fall of photosynthesis: Hormetic dose response in plants. J. For. Res. 2021, 32, 889–898. [Google Scholar]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Hormesis: Highly generalizable and beyond laboratory. Trends Plant Sci. 2020, 25, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Jiao, Q.; Agathokleous, E.; Liu, H.; Li, G.; Zhang, J.; Fahad, S.; Jiang, Y. Hormetic effects of zinc on growth and antioxidant defense system of wheat plants. Sci. Total Environ. 2022, 807, 150992. [Google Scholar] [CrossRef]
- Sperdouli, I.; Ouzounidou, G.; Moustakas, M. Hormesis responses of photosystem II in Arabidopsis thaliana under water deficit stress. Int. J. Mol. Sci. 2023, 24, 9573. [Google Scholar] [PubMed]
- Borek, M.; Bączek-Kwinta, R.; Rapacz, M. Photosynthetic activity of variegated leaves of Coleus × hybridus hort. cultivars characterised by chlorophyll fluorescence techniques. Photosynthetica 2016, 54, 331–339. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Adamakis, I.-D.S.; Moustaka, J.; İşgören, S.; Şaş, B. Harnessing the role of foliar applied salicylic acid in decreasing chlorophyll content to reassess photosystem II photoprotection in crop plants. Int. J. Mol. Sci. 2022, 23, 7038. [Google Scholar] [CrossRef] [PubMed]
- Moustaka, J.; Meyling, N.V.; Hauser, T.P. Induction of a compensatory photosynthetic response mechanism in tomato leaves upon short time feeding by the chewing insect Spodoptera Exigua. Insects 2021, 12, 562. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).