Sulla (Hedysarum coronarium L.) Response to Drought Stress during Early Vegetative Stage
Abstract
1. Introduction
2. Results
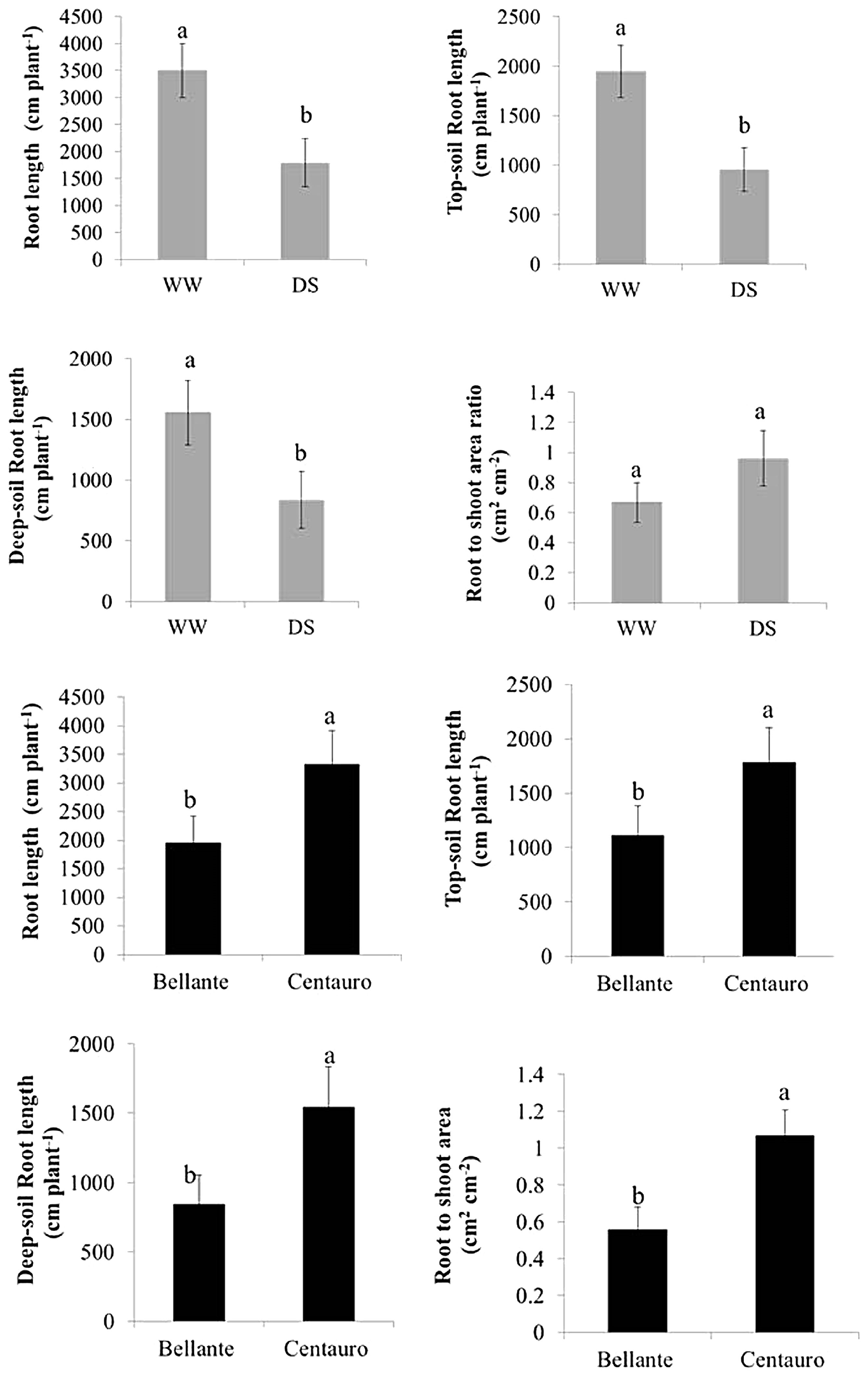
2.1. Shoot and Root Growth
2.2. Forage Quality
3. Discussion
3.1. Shoot and Root Growth
3.2. Forage Quality
4. Materials and Methods
4.1. Experimental Setup
- -
- Factor 1. Genotypes of sulla (Hedysarum coronarium L.) with two levels:
4.2. Shoot Biometry
4.3. Forage Quality
4.4. Root Traits
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flores, F.; Gutierrez, J.C.; Lopez, J.; Moreno, M.T.; Cubero, J.I. Multivariate analysis approach to evaluate a germplasm collection of Hedysarum coronarium L. Genet. Resour. Crops Evol. 1997, 44, 545–555. [Google Scholar] [CrossRef]
- Slim, S.; Louhaichi, M.; Gamoun, M.; Ates, S.; Hassan, S.; Romdhane, O.B.; Belgacem, A.O. Assessment of soil surface scarification and reseeding with sulla (Hedysarum coronarium L.) of degraded Mediterranean semi-arid rangelands. Afr. J. Range Forage Sci. 2021, 38 (Suppl. S1), S63–S72. [Google Scholar] [CrossRef]
- Slim, S.; Jeddi, F.B. Soil protection in mountainous areas of Tunisia with the northern sulla (Hedysarum coronarium L.). Sci. Chang. Planétaires/Sécheresse 2011, 22, 117–124. [Google Scholar]
- Leto, G.; Todaro, M.; Di Noto, A.M.; Alicata, M.L. Comparison of Sulla-hay and Sulla-silage in the lactating ewes and their effects on milk and cheese characteristics. Small Rumin. Res. 2002, 45, 301–306. [Google Scholar] [CrossRef]
- Dear, B.S.; Moore, G.A.; Hughes, S.J. Adaptation and potential contribution of temperate perennial legumes to the southern Australian wheatbelt: A review. Aust. J. Exp. Agric. 2003, 43, 1–18. [Google Scholar] [CrossRef]
- Watson, M.J. Hedysarum coronarium—A legume with potential for sil conservation and forage. N. Z. Agric. Sci. 1982. [Google Scholar]
- Amato, G.; Giambalvo, D.; Frenda, A.S.; Mazza, F.; Ruisi, P.; Saia, S.; Di Miceli, G. Sulla (Hedysarum coronarium L.) as potential feedstock for biofuel and protein. BioEnergy Res. 2016, 9, 711–719. [Google Scholar] [CrossRef]
- Borreani, G.; Roggero, P.P.; Sulas, L.; Valente, M.E. Quantifying Morphological Stage to Predict the Nutritive Value in Sulla (Hedysarum coronarium L.). Agron. J. 2003, 95, 1608–1617. [Google Scholar] [CrossRef]
- Molan, A.L.; Waghorn, G.C.; McNabb, W.C. Condensed Tannins and Gastro-Intestinal Parasites in Sheep; New Zealand Grassland Association: Dunedin, New Zealand, 2 January 1999; pp. 57–61. [Google Scholar]
- Niezen, J.H.; Waghorn, T.S.; Charleston, W.A.G.; Waghorn, G.C. Growth and gastrointestinal nematode parasitism in lambs grazing either lucerne (Medicago sativa) or sulla (Hedysarum coronarium) which contains condensed tannins. J. Agric. Sci. 1995, 125, 281–289. [Google Scholar] [CrossRef]
- Niezen, J.; Charleston, W.; Robertson, H.; Shelton, D.; Waghorn, G.; Green, R. The effect of feeding sulla (Hedysarum coronarium) or lucerne (Medicago sativa) on lamb parasite burdens and development of immunity to gastrointestinal nematodes. Vet. Parasitol. 2002, 105, 229–245. [Google Scholar] [CrossRef]
- Pomroy, W.; Adlington, B. Efficacy of short-term feeding of sulla (Hedysarum coronarium) to young goats against a mixed burden of gastrointestinal nematodes. Vet. Parasitol. 2006, 136, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Cabiddu, A.; Molle, G.; Decandia, M.; Spada, S.; Fiori, M.; Piredda, G.; Addis, M. Responses to condensed tannins of flowering sulla (Hedysarum coronarium L.) grazed by dairy sheep: Part 2: Effects on milk fatty acid profile. Livest. Sci. 2009, 123, 230–240. [Google Scholar] [CrossRef]
- Di Trana, A.; Bonanno, A.; Cecchini, S.; Giorgio, D.; Di Grigoli, A.; Claps, S. Effects of Sulla forage (Sulla coronarium L.) on the oxidative status and milk polyphenol content in goats. J. Dairy Sci. 2015, 98, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Rufino-Moya, P.J.; Bertolín, J.R.; Blanco, M.; Lobón, S.; Joy, M. Fatty acid profile, secondary compounds and antioxidant activities in the fresh forage, hay and silage of sainfoin (Onobrychis viciifolia) and sulla (Hedysarum coronarium). J. Sci. Food Agric. 2022, 102, 4736–4743. [Google Scholar] [CrossRef]
- Tava, A.; Biazzi, E.; Ronga, D.; Mella, M.; Doria, F.; D’Addabbo, T.; Candido, V.; Avato, P. Chemical identification of specialized metabolites from sulla (Hedysarum coronarium L.) collected in southern Italy. Molecules 2021, 26, 4606. [Google Scholar] [CrossRef]
- Akbarian, M.M.; Mojaradi, T.; Shirzadi, F. Effects of Hedysarum coronarium L. (sulla) as a Green Manure along with Nitrogen Fertilizer on Maize Production. Agritech 2021, 41, 95–106. [Google Scholar] [CrossRef]
- D’Addabbo, T.; Tava, A.; Argentieri, M.P.; Biazzi, E.; Candido, V.; Avato, P. Nematicidal potential of sulla (Hedysarum coronarium L.) against the root-knot nematode Meloidogyne incognita. Plants 2022, 11, 2550. [Google Scholar] [CrossRef]
- Gambacorta, E.; Simonetti, A.; Garrisi, N.; Intaglietta, I.; Perna, A. Antioxidant properties and phenolic content of sulla (H edysarum spp.) honeys from S outhern I taly. Int. J. Food Sci. Technol. 2014, 49, 2260–2268. [Google Scholar] [CrossRef]
- Yates, R.; Foster, K.; Nichols, P.; Ewing, M. Flamenco–a new variety of sulla for southern Australia. In Proceedings of the Australian Society of Agronomy Conference, Perth, Australia, 10–14 September 2006. [Google Scholar]
- Ruisi, P.; Siragusa, M.; Di Giorgio, G.; Graziano, D.; Amato, G.; Carimi, F.; Giambalvo, D. Pheno-morphological, agronomic and genetic diversity among natural populations of sulla (Hedysarum coronarium L.) collected in Sicily, Italy. Genet. Resour. Crops Evol. 2011, 58, 245–257. [Google Scholar] [CrossRef]
- Annicchiarico, P.; Ruisi, P.; Di Miceli, G.; Pecetti, L. Morpho-physiological and adaptive variation of Italian germplasm of sulla (Hedysarum coronarium L.). Crops Pasture Sci. 2014, 65, 206–213. [Google Scholar] [CrossRef]
- Fenta, B.A.; Beebe, S.E.; Kunert, K.J.; Burridge, J.D.; Barlow, K.M.; Lynch, J.P.; Foyer, C.H. Field phenotyping of soybean roots for drought stress tolerance. Agronomy 2014, 4, 418–435. [Google Scholar] [CrossRef]
- Kim, Y.; Chung, Y.S.; Lee, E.; Tripathi, P.; Heo, S.; Kim, K.-H. Root response to drought stress in rice (Oryza sativa L.). Int. J. Mol. Sci. 2020, 21, 1513. [Google Scholar] [CrossRef] [PubMed]
- Paez-Garcia, A.; Motes, C.M.; Scheible, W.-R.; Chen, R.; Blancaflor, E.B.; Monteros, M.J. Root traits and phenotyping strategies for plant improvement. Plants 2015, 4, 334–355. [Google Scholar] [CrossRef] [PubMed]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef]
- Kashiwagi, J.; Krishnamurthy, L.; Upadhyaya, H.D.; Krishna, H.; Chandra, S.; Vadez, V.; Serraj, R. Genetic variability of drought-avoidance root traits in the mini-core germplasm collection of chickpea (Cicer arietinum L.). Euphytica 2005, 146, 213–222. [Google Scholar] [CrossRef]
- Li, H.; Siri, M.; Wang, B.; He, Y.; Liu, C.; Feng, C.; Liu, K. Global root traits research during 2000–2021: A bibliometric analysis. Agronomy 2022, 12, 2471. [Google Scholar] [CrossRef]
- Hofer, D.; Suter, M.; Haughey, E.; Finn, J.A.; Hoekstra, N.J.; Buchmann, N.; Lüscher, A. Yield of temperate forage grassland species is either largely resistant or resilient to experimental summer drought. J. Appl. Ecol. 2016, 53, 1023–1034. [Google Scholar] [CrossRef]
- Komainda, M.; Küchenmeister, K.; Küchenmeister, F.; Breitsameter, L.; Wrage-Mönnig, N.; Kayser, M.; Isselstein, J. Forage legumes for future dry climates: Lower relative biomass losses of minor forage legumes compared to Trifolium repens under conditions of periodic drought stress. J. Agron. Crop Sci. 2019, 205, 460–469. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Q.; Ge, G.; Han, G.; Jia, Y. Influence of drought stress on afalfa yields and nutritional composition. BMC Plant Biol. 2018, 18, 13. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Bennett, J.M.; Muchow, R.C. Relative sensitivity of grain yield and biomass accumulation to drought in field-grown maize. Crops Sci. 1990, 30, 690–693. [Google Scholar] [CrossRef]
- Malisch, C.S.; Salminen, J.P.; Kölliker, R.; Engström, M.T.; Suter, D.; Studer, B.; Lüscher, A. Drought effects on proanthocyanidins in sainfoin (Onobrychis viciifolia Scop.) are dependent on the plant’s ontogenetic stage. J. Agric. Food Chem. 2016, 64, 9307–9316. [Google Scholar] [CrossRef]
- Sebastian, J.; Yee, M.-C.; Viana, W.G.; Rellán-Álvarez, R.; Feldman, M.; Priest, H.D.; Trontin, C.; Lee, T.; Jiang, H.; Baxter, I.; et al. Grasses suppress shoot-borne roots to conserve water during drought. Proc. Natl. Acad. Sci. USA 2016, 113, 8861–8866. [Google Scholar] [CrossRef]
- Zhan, A.; Schneider, H.; Lynch, J.P. Reduced lateral root branching density improves drought tolerance in maize. Plant Physiol. 2015, 168, 1603–1615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shi, S.; Wang, B.; Zhao, J. Physiological and biochemical changes in different drought-tolerant alfalfa (Medicago sativa L.) varieties under PEG-induced drought stress. Acta Physiol. Plant. 2018, 40, 25. [Google Scholar] [CrossRef]
- Palta, J.A.; Chen, X.; Milroy, S.P.; Rebetzke, G.J.; Dreccer, M.F.; Watt, M. Large root systems: Are they useful in adapting wheat to dry environments? Funct. Plant Biol. 2011, 38, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Seminario, A.; Udvardi, M.; Annicchiarico, P. Physiological and biochemical adaptive traits support the specific breeding of alfalfa (Medicago sativa) for severely drought-stressed or moisture-favourable environments. J. Agron. Crops Sci. 2023, 209, 132–143. [Google Scholar] [CrossRef]
- Richards, R.A.; Passioura, J. A breeding program to reduce the diameter of the major xylem vessel in the seminal roots of wheat and its effect on grain yield in rain-fed environments. Aust. J. Agric. Res. 1989, 40, 943–950. [Google Scholar] [CrossRef]
- Lynch, J.P. Rightsizing root phenotypes for drought resistance. J. Exp. Bot. 2018, 69, 3279–3292. [Google Scholar] [CrossRef]
- Tron, S.; Bodner, G.; Laio, F.; Ridolfi, L.; Leitner, D. Can diversity in root architecture explain plant water use efficiency? A modeling study. Ecol. Model. 2015, 312, 200–210. [Google Scholar] [CrossRef]
- Wasson, A.P.; Richards, R.A.; Chatrath, R.; Misra, S.C.; Prasad, S.V.S.; Rebetzke, G.J.; Kirkegaard, J.A.; Christopher, J.; Watt, M. Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J. Exp. Bot. 2012, 63, 3485–3498. [Google Scholar] [CrossRef]
- Tola, E.; Henriquez-Sabà, J.L.; Polone, E.; Dazzo, F.B.; Concheri, G.; Casella, S.; Squartini, A. Shovel roots: A unique stress-avoiding developmental strategy of the legume plant Hedysarum coronarium L. Plant Soil 2009, 322, 25–37. [Google Scholar] [CrossRef]
- Rossi, R.; Picuno, P.; Fagnano, M.; Amato, M. Soil reinforcement potential of cultivated cardoon (Cynara cardunculus L.): First data of root tensile strength and density. CATENA 2022, 211, 106016. [Google Scholar] [CrossRef]
- Rasse, D.P.; Rumpel, C.; Dignac, M.-F. Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 2005, 269, 341–356. [Google Scholar] [CrossRef]
- Chen, Y.; Ghanem, M.E.; Siddique, K.H.M. Characterising root trait variability in chickpea (Cicer arietinum L.) germplasm. J. Exp. Bot. 2017, 68, 1987–1999. [Google Scholar] [PubMed]
- Amato, M.; Pardo, A. Root length and biomass losses during sample preparation with different screen mesh sizes. Plant Soil 1994, 161, 299–303. [Google Scholar] [CrossRef]
- Norris, I.B.; Thomas, H. Recovery of ryegrass species from drought. J. Agric. Sci. 1982, 98, 623–628. [Google Scholar] [CrossRef]
- Leafe, E.L.; Jones, M.B.; Stiles, W. The physiological effects of water stress on perennial ryegrass in the field. In Proceedings of the 13th International Grassland Congress, Lexington, KY, USA, 15–24 June 1977; pp. 253–260. [Google Scholar]
- Jones, M.B.; Leafe, E.L.; Stiles, W. Water stress in field-grown perennial ryegrass.II. Its effect on leaf water status, stomatal resistance and leaf morphology. Ann. Appl. Biol. 1980, 96, 103–110. [Google Scholar] [CrossRef]
- Hoogenboom, G.; Peterson, C.M.; Huck, M.G. Shoot Growth Rate of Soybean as Affected by Drought Stress 1. Agron. J. 1987, 79, 598–607. [Google Scholar] [CrossRef]
- Barry, T.N.; McNabb, W.C. The implications of condensed tannins on the nutritive value of temperate forages fed to ruminants. Br. J. Nutr. 1999, 81, 263–272. [Google Scholar] [CrossRef]
- Gourlay, G.; Constabel, C.P. Condensed tannins are inducible antioxidants and protect hybrid poplar against oxidative stress. Tree Physiol. 2019, 39, 345–355. [Google Scholar] [CrossRef]
- Waghorn, G.C.; Shelton, I.D. Effect of condensed tannins in Lotus corniculatus on the nutritive value of pasture for sheep. J. Agric. Sci. 1997, 128, 365–372. [Google Scholar] [CrossRef]
- Terrill, T.H.; Rowan, A.M.; Douglas, G.B.; Barry, T.N. Determination of extractable and bound condensed tannin concentrations in forage plants, protein concentrate meals and cereal grains. J. Sci. Food Agric. 1992, 58, 321–329. [Google Scholar] [CrossRef]
- Terrill, T.H.; Douglas, G.B.; Foote, A.G.; Purchas, R.W.; Wilson, G.F.; Barry, T.N. Effect of condensed tannins upon body growth, wool growth and rumen metabolism in sheep grazing sulla (Hedysarum coronarium) and perennial pasture. J. Agric. Sci. 1992, 119, 265–273. [Google Scholar] [CrossRef]
- Stienezen, M.; Waghorn, G.C.; Douglas, G.B. Digestibility and effects of condensed tannins on digestion of sulla (Hedysarum coronarium) when fed to sheep. N. Z. J. Agric. Res. 1996, 39, 215–221. [Google Scholar] [CrossRef]
- Molan, A.L.; Waghorn, G.C.; Min, B.R.; McNabb, W.C. The effect of condensed tannins from seven herbages on Trichostrongylus colubriformis larval migration in vitro. Folia Parasitol. 2000, 47, 39–44. [Google Scholar] [CrossRef]
- Bonanno, A.; Di Miceli, G.; Di Grigoli, A.; Frenda, A.S.; Tornambè, G.; Giambalvo, D.; Amato, G. Effects of feeding green forage of sulla (Hedysarum coronarium L.) on lamb growth and carcass and meat quality. Animal 2011, 5, 148–154. [Google Scholar] [CrossRef]
- Tzamaloukas, O.; Athanasiadou, S.; Kyriazakis, I.; Jackson, F.; Coop, R.L. The consequences of short-term grazing of bioactive forages on established adult and incoming larvae populations of Teladorsagia circumcincta in lambs. Int. J. Parasitol. 2005, 35, 329–335. [Google Scholar] [CrossRef]
- Zhang, L.H.; Shao, H.B.; Ye, G.F.; Lin, Y.M. Effects of fertilization and drought stress on tannin biosynthesis of Casuarina equisetifolia seedlings branchlets. Acta Physiol. Plant. 2012, 34, 1639–1649. [Google Scholar] [CrossRef][Green Version]
- Popović, B.M.; Štajner, D.; Ždero-Pavlović, R.; Tumbas-Šaponjac, V.; Čanadanović-Brunet, J.; Orlović, S. Water stress induces changes in polyphenol profile and antioxidant capacity in poplar plants (Populus spp.). Plant Physiol. Biochem. 2016, 105, 242–250. [Google Scholar] [CrossRef]
- McKiernan, A.B. The Effects of Soil Water Deficit on Physiological, Morphological and Chemical Traits of Eucalyptus. Doctoral Dissertation, University of Tasmania, Hobart, TAS, Australia, 2015. [Google Scholar]
- Anuraga, M.; Duarsa, P.; Hill, M.; Lovett, J. Soil moisture and temperature affect condensed tannin concentrations and growth in Lotus corniculatus and Lotus pedunculatus. Aust. J. Agric. Res. 1993, 44, 1667–1681. [Google Scholar] [CrossRef]
- Malisch, C.S.; Lewandowski, L.; Salminen, J.P.; Taube, F.; Lüscher, A. Low Concentrations of Protein-and Fiber-Bound Proanthocyanidins in Sainfoin (Onobrychis viciifolia) Are Stable across Accessions, Growth Stages, and Drought Conditions. J. Agric. Food Chem. 2020, 68, 7369–7377. [Google Scholar] [CrossRef] [PubMed]
- Oukaltouma, K.; El Moukhtari, A.; Lahrizi, Y.; Mouradi, M.; Farissi, M.; Willems, A.; Qaddoury, A.; Bekkaoui, F.; Ghoulam, C. Phosphorus deficiency enhances water deficit impact on some morphological and physiological traits in four faba bean (Vicia faba L.) varieties. Ital. J. Agron. 2020, 16, 1662. [Google Scholar] [CrossRef]
- Van Soest, P.J.; McQueen, R.W. The chemistry and estimation of fibre. Proc. Nutr. Soc. 1973, 32, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Ainwsorth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; Volume 574, p. 574. [Google Scholar]
- Wood, S. Package ‘mgcv’. R Package Version. 2015. 1, 729. Available online: https://cran.uib.no/web/packages/mgcv/mgcv.pdf (accessed on 19 July 2023).



| Mean | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Irrigation | Variety | Fresh Biomass Plant−1 (g) | Dry Biomass Plant−1 (g) | Dry Matter % | N° of Shoots | N° of Leaves | Max Shoot Length (cm) | Leaf Area (cm2) | Root Length (cm) Plant−1 | Top- Root Length (cm) Plant−1 | Deep- Root Length (cm) Plant−1 | Root Area (cm2) Plant−1 | Top- Root Area (cm2) Plant−1 | Deep- Root Area (cm2) Plant−1 | N° Shovel Roots Plant−1 | Root-to-Shoot-Area Ratio |
| WW | Bellante | 41.5 | 3.49 | 8.38 | 27 | 139 | 16.3 | 510 | 2645 | 1542 | 1103 | 217 | 126 | 90.8 | 61.3 | 0.43 |
| Centauro | 38.2 | 3.35 | 8.77 | 40.7 | 183 | 13.8 | 468 | 4354 | 2345 | 2009 | 403 | 219 | 184 | 50.3 | 0.907 | |
| DS | Bellante | 10.6 | 1.18 | 11 | 15 | 49.7 | 10.8 | 147 | 1272 | 681 | 592 | 95.4 | 56.4 | 39 | 34.7 | 0.691 |
| Centauro | 14.4 | 1.74 | 11.7 | 19.3 | 53 | 12.3 | 159 | 2310 | 1229 | 1081 | 200 | 114 | 85.3 | 52.7 | 1.23 | |
| Standard Deviation | ||||||||||||||||
| WW | Bellante | 12.2 | 1.07 | 0.206 | 4.58 | 34.8 | 3.4 | 137 | 1206 | 722 | 539 | 96.7 | 52.6 | 49.3 | 7.77 | 0.18 |
| Centauro | 5.15 | 0.53 | 0.41 | 5.51 | 21.4 | 3.62 | 140 | 216 | 191 | 376 | 31.5 | 12.1 | 36.5 | 39.7 | 0.238 | |
| DS | Bellante | 0.678 | 0.155 | 0.839 | 5.29 | 6.51 | 1.04 | 28 | 479 | 142 | 393 | 36.5 | 12.8 | 25.7 | 29.7 | 0.364 |
| Centauro | 4.38 | 0.814 | 2.06 | 1.53 | 7 | 3.01 | 33.4 | 1394 | 701 | 698 | 91.1 | 52 | 40.8 | 33.5 | 0.39 | |
| Coefficient of variation % | ||||||||||||||||
| WW | Bellante | 29.4 | 30.66 | 2.46 | 16.96 | 25.04 | 20.86 | 26.86 | 45.6 | 46.82 | 48.87 | 44.56 | 41.75 | 54.3 | 12.68 | 41.86 |
| Centauro | 13.48 | 15.82 | 4.68 | 13.54 | 11.69 | 26.23 | 29.91 | 4.96 | 8.14 | 18.72 | 7.82 | 5.53 | 19.84 | 78.93 | 26.24 | |
| DS | Bellante | 6.4 | 13.14 | 7.63 | 35.27 | 13.1 | 9.63 | 19.05 | 37.66 | 20.85 | 66.39 | 38.26 | 22.7 | 65.9 | 85.59 | 52.68 |
| Centauro | 30.42 | 46.78 | 17.61 | 7.93 | 13.21 | 24.47 | 21.01 | 60.35 | 57.04 | 64.57 | 45.55 | 45.61 | 47.83 | 63.57 | 31.71 | |
| Drought Stress | Variety | Drought Stress × Variety | |
|---|---|---|---|
| N° of leaves | P = 1.7 × 10−5 | P = 0.08 ns | ns |
| N° of shoots | P = 0.008696 | P = 0.000212 | ns |
| Fresh Biomass plant−1 (g) | P = 0.000142 | ns | ns |
| Dry Biomass plant−1 (g) | P = 0.00156 | ns | ns |
| Max shoot length (cm) | P = 0.074 ns | ns | ns |
| Leaf area (cm2) | P = 0.000408 | ns | ns |
| Root Length (cm) plant−1 | P = 0.0149 | P = 0.038 | ns |
| Top-Root Length (cm) plant−1 | P = 0.0106 | P = 0.0533 | ns |
| Deep-Root Length (cm) plant−1 | P = 0.0427 | P = 0.048 | ns |
| Root Area (cm2) plant−1 | P = 0.00408 | P = 0.00739 | ns |
| Top-Root Area (cm2) plant−1 | P = 0.0042 | P = 0.00889 | ns |
| Deep-Root Area (cm2) plant−1 | P = 0.0101 | P = 0.0145 | ns |
| Root-to-Shoot-Area Ratio | ns | P = 0.0205 | ns |
| N° Shovel root plant−1 | ns | ns | ns |
| Family: Gaussian-Link function: identity | ||||
| Leaf Area~treatment + s (days, k = 6, by = treatment) | ||||
| Parametric coefficients: | ||||
| Estimate | Std.Error | t value | Pr(>|t|) | |
| (Intercept) | 85.664 | 4.662 | 18.37 | 3.33 × 10−13 *** |
| treatmentWW | 121.307 | 6.593 | 18.40 | 3.25 × 10−13 *** |
| Approximate significance of smooth terms: | ||||
| edf | Ref.df | F | p-value | |
| s (days): treatmentDS | 1.00 | 1.000 | 83.3 | <2 × 10−16 *** |
| s (days): treatmentWW | 2.79 | 3.397 | 319.6 | <2 × 10−16 *** |
| Signif. codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘ ’ 1 | ||||
| R-sq.(adj) = 0.985; Deviance explained = 98.8% | ||||
| GCV = 343.78; Scale est. = 260.84; n = 24 | ||||
| Leaf Number~treatment + varieties + s (days, k = 6, by = treatment) | ||||
| Parametric coefficients: | ||||
| Estimate | Std. Error | t value | Pr(>|t|) | |
| (Intercept) | 19.417 | 2.537 | 7.652 | 7.15 × 10−7 *** |
| treatmentWW | 36.167 | 2.930 | 12.344 | 7.50 × 10−10*** |
| varietiesCentauro | 8.000 | 2.930 | 2.730 | 0.0143 * |
| Approximate significance of smooth terms: | ||||
| edf | Ref.df | F | p-value | |
| s (days): treatmentDS | 1.452 | 1.763 | 35.75 | 2.66 × 10−6 *** |
| s (days): treatmentWW | 2.740 | 3.339 | 198.50 | <2 × 10−16 *** |
| --- | ||||
| Signif. codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘ ’ 1 | ||||
| R-sq.(adj) = 0.975; Deviance explained = 98.1% | ||||
| GCV = 73.541; Scale est. = 51.506; n = 24 | ||||
| Number of shoots~treatment + varieties + s (days, k = 6, by = treatment) | ||||
| Parametric coefficients: | ||||
| Estimate | Std. Error | t value | Pr(>|t|) | |
| (Intercept) | 8.5208 | 0.7611 | 11.195 | 2.01 × 10−9 *** |
| treatmentWW | 6.2083 | 0.8789 | 7.064 | 1.58 × 10−6 *** |
| varieties Centauro | 2.7083 | 0.8789 | 3.082 | 0.00657 ** |
| Approximate significance of smooth terms: | ||||
| edf | Ref.df | F | p-value | |
| s (days): treatmentDS | 1.126 | 1.240 | 36.16 | 6.51 × 10−6 *** |
| s (days): treatmentWW | 2.307 | 2.828 | 88.20 | <2 × 10−16 *** |
| --- | ||||
| Signif. codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘ ’ 1 | ||||
| R-sq.(adj) = 0.938; Deviance explained = 95.3% | ||||
| GCV = 6.3317; Scale est. = 4.6344; n = 24 | ||||
| Extractable CT (mg CE g−1 DW) | Protein-Bound CT (mg g−1 DW) | Fiber-Bound CT (mg g−1 DW) | Total CT (mg g−1 DW) | |||||||||||||
| Bellante | Centauro | Bellante | Centauro | Bellante | Centauro | Bellante | Centauro | |||||||||
| mean | sd | mean | sd | mean | sd | mean | sd | mean | sd | mean | sd | mean | sd | mean | sd | |
| DS | 3.24 ± 0.16 | 4.37 ± 0.28 | 3.32 ± 1.55 | 3.15 ± 0.27 | 0.49 ± 0.26 | 0.52 ± 0.05 | 7.05 ± 1.17 | 8.04 ± 0.03 | ||||||||
| WW | 4.13 ± 0.10 | 2.68 ± 0.91 | 2.16 ± 0.57 | 2.49 ± 0.81 | 0.43 ± 0.14 | 0.38 ± 0.28 | 6.71 ± 0.60 | 5.55 ± 0.35 | ||||||||
| Crude protein | NDF g 100 g−1 DW | Total Polyphenol (mg GAE g−1 DW) | ||||||||||||||
| Bellante | Centauro | Bellante | Centauro | Bellante | Centauro | |||||||||||
| mean | sd | mean | sd | mean | sd | mean | sd | mean | sd | mean | sd | |||||
| DS | 24.79 ± 3.95 | 24.93 ± 1.55 | 24.19 ± 2.44 | 25.23 ± 3.15 | 7.46 ± 1.2 | 6.67 ± 0.39 | ||||||||||
| WW | 27.12 ± 1.52 | 29.75 ± 0.87 | 26.90 ± 1.25 | 25.83 ± 4.92 | 7.62 ± 2.93 | 6.31 ± 0.69 | ||||||||||
| Condensed Tannins | Drought Stress | Variety | Drought Stress × Variety |
|---|---|---|---|
| Extractable tannins | P = 0.05 | ns | P = 0.000577 |
| Protein bound tannins | ns | ns | ns |
| Fiber bound tannins | ns | ns | ns |
| Total tannins | P = 0.00304 | ns | P = 0.01029 |
| Total N | P = 0.0272 | ns | ns |
| NDF | ns | ns | ns |
| Total Polyphenols | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, R.; Amato, M.; Claps, S. Sulla (Hedysarum coronarium L.) Response to Drought Stress during Early Vegetative Stage. Plants 2023, 12, 3396. https://doi.org/10.3390/plants12193396
Rossi R, Amato M, Claps S. Sulla (Hedysarum coronarium L.) Response to Drought Stress during Early Vegetative Stage. Plants. 2023; 12(19):3396. https://doi.org/10.3390/plants12193396
Chicago/Turabian StyleRossi, Roberta, Mariana Amato, and Salvatore Claps. 2023. "Sulla (Hedysarum coronarium L.) Response to Drought Stress during Early Vegetative Stage" Plants 12, no. 19: 3396. https://doi.org/10.3390/plants12193396
APA StyleRossi, R., Amato, M., & Claps, S. (2023). Sulla (Hedysarum coronarium L.) Response to Drought Stress during Early Vegetative Stage. Plants, 12(19), 3396. https://doi.org/10.3390/plants12193396






