Diversity, Ecology and Phytogeography of Bryophytes across Temperate Forest Communities—Insight from Mt. Papuk (Croatia, SE Europe)
Abstract
1. Introduction
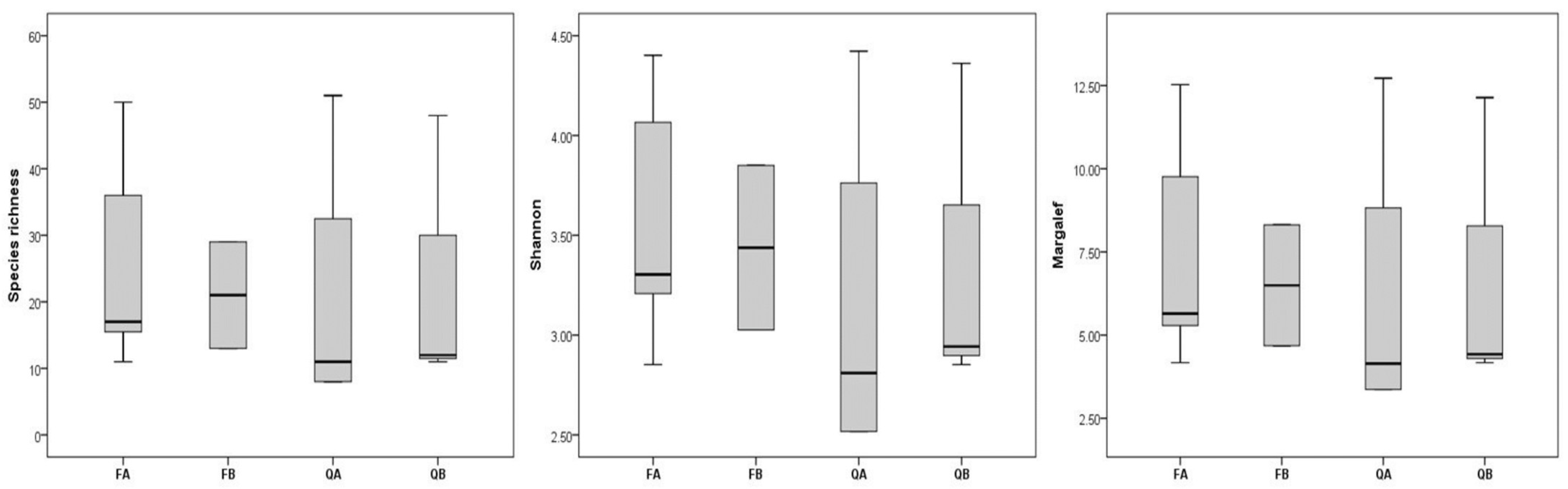
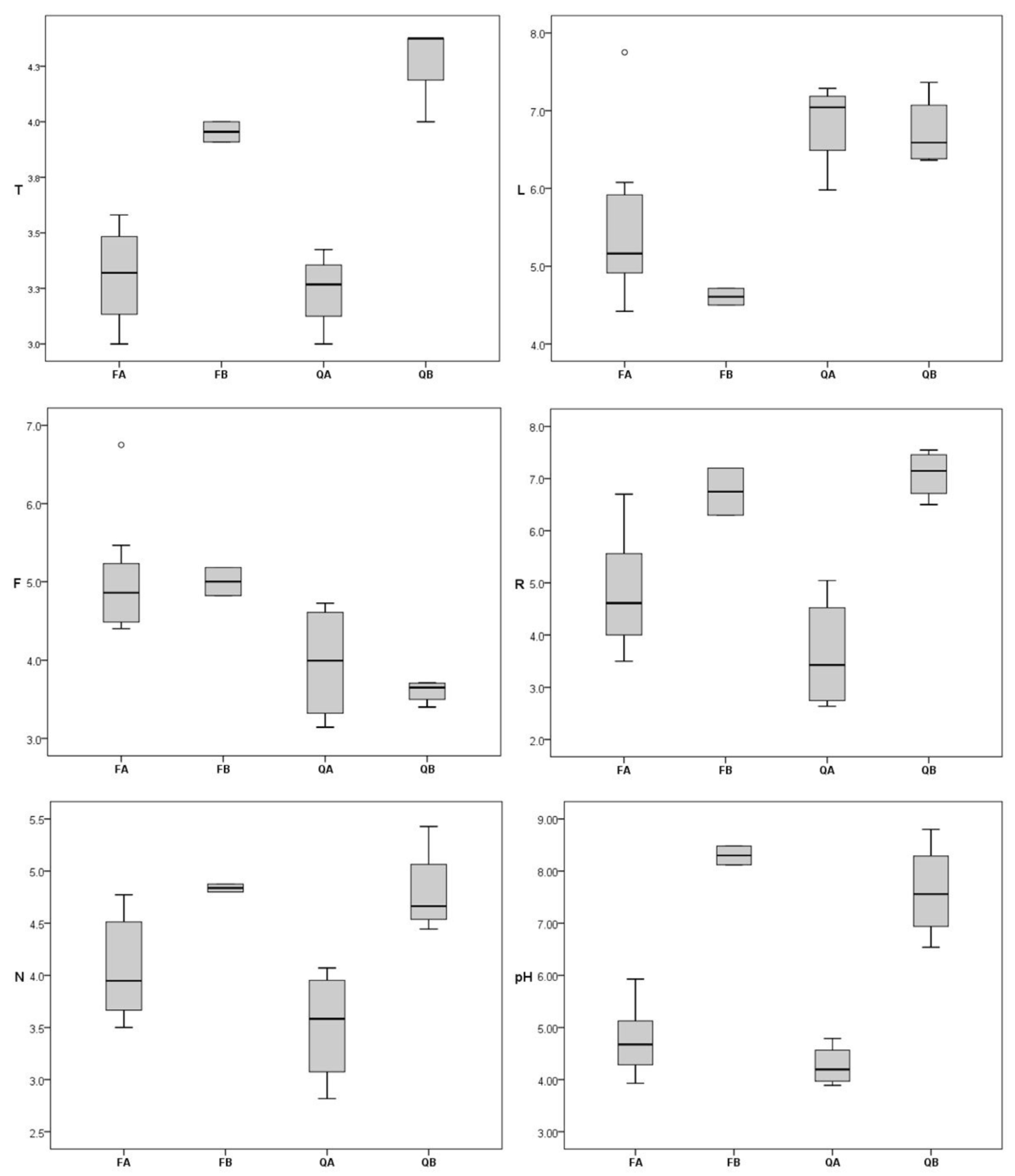
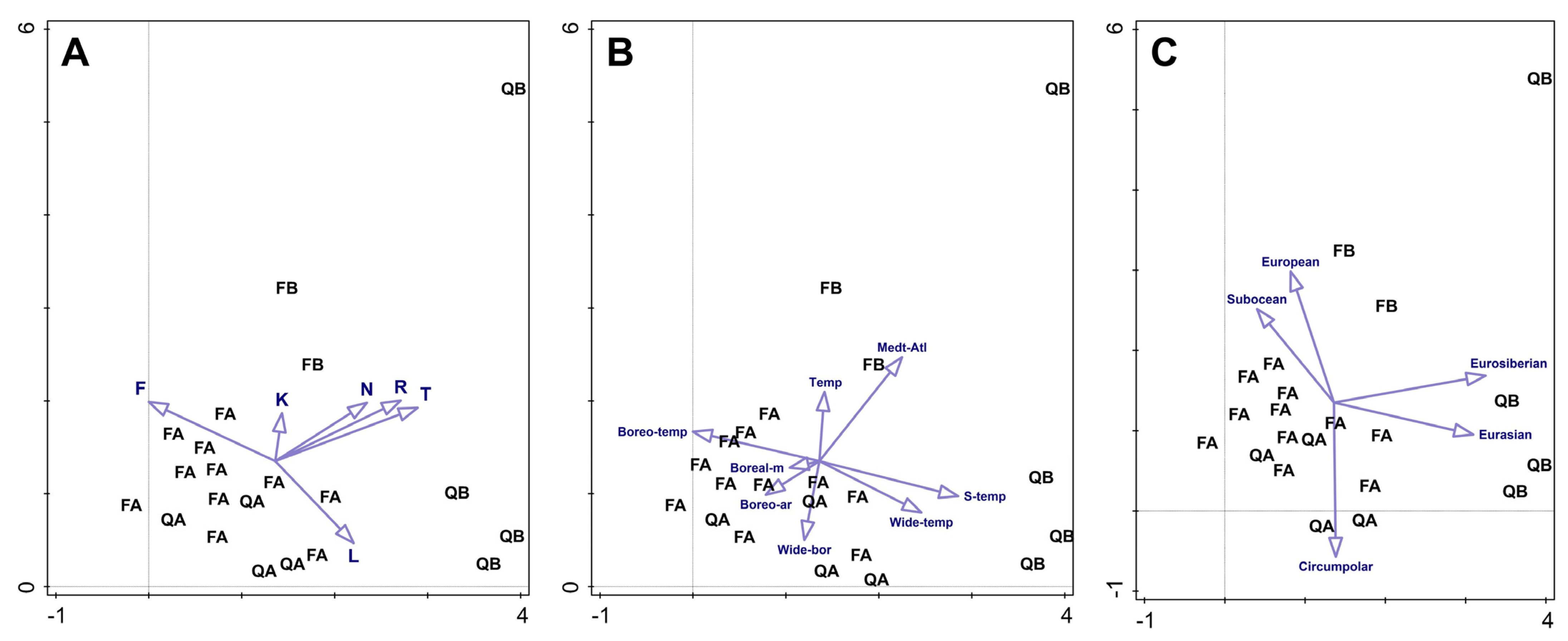
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Area
4.2. Methods
- FA—acidophilous beech forests (suballiance Luzulo luzuloidis-Fagenion (Lohm. et Tx. 1954) Oberd. 1957 and ass. Festuco drymeiae-Abietetum Vukelić et Baričević 2007);
- FB—basiphilous beech forests (ass. Vicio oroboidi-Fagetum sylvaticae (Horvat 1938) Pocs et Borhidi in Borhidi 1960 from the suballiance Epimedio-fagenion (Borhidi 1963) Martinček et al. 1993);
- QA—acidophilous oak forests (ass. Festuco drymeiae-Quercetum petrae (Janković 1968) Hruška-Dell’Uomo 1975, ass. Molinio arundinaceae-Quercetum petraeae Šugar 1972 and Quercus petraea-Calluna vulgaris community, all three belonging to the alliance Quercion robori-petraeae Tx. (1931) 1937);
- QB—basiphilous oak forests (Lathyro nigri-Quercetum petraeae Horvat (1938) 1958, Fraxino orni-Quercetum pubescentis Klika 1938, both from the alliance Quercion pubescenti-petraeae Br.-Bl. 1932).
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Synoptic Table with Percentage Frequency and Fidelity Index ϕ-Coefficients
| Forest Type | FA | FB | QA | QB | ||||
|---|---|---|---|---|---|---|---|---|
| Number of Relevés | 11 | 2 | 4 | 4 | ||||
| Hypnum cupressiforme Hedw. | 100 | 44.7 | . | 100 | 50 | |||
| Polytrichum formosum Hedw. | 64 | . | 100 | . | ||||
| Isothecium alopecuroides (Lam. ex Dubois) | 73 | 52.6 | . | 25 | 25 | |||
| Dicranella heteromalla (Hedw.) Schimp. | 64 | . | 75 | . | ||||
| Brachythecium rutabulum (Hedw.) Schimp. | 55 | 100 | 25 | 25 | ||||
| Plagiochila porelloides (Torr. ex Nees) | 73 | 42.8 | 50 | 25 | . | |||
| Metzgeria furcata (L.) Corda | 73 | 64.9 | . | 25 | . | |||
| Dicranum scoparium Hedw. | 45 | . | 50 | 25 | ||||
| Hedwigia ciliata (Hedw.) P.Beauv. | 36 | 50 | 50 | . | ||||
| Pseudanomodon attenuatus (Hedw.) Ignatov | 45 | 50 | 25 | . | ||||
| Sciuro-hypnum populeum (Hedw.) Ignatov & | 45 | 50 | 25 | |||||
| Oxyrrhynchium hians (Hedw.) Loeske | 27 | 100 | 50 | |||||
| Alleniella complanata (Hedw.) S.Olsson, | 36 | 50 | 25 | . | ||||
| Rhizomnium punctatum (Hedw.) T.J.Kop. | 55 | 68.8 | . | . | ||||
| Thamnobryum alopecurum (Hedw.) Gangulee | 36 | 100 | . | |||||
| Ctenidium molluscum (Hedw.) Mitt. | 27 | 100 | 25 | |||||
| Plagiothecium cavifolium (Brid.) Z.Iwats | 45 | . | 25 | . | ||||
| Lophocolea heterophylla (Schrad.) Dumort | 45 | 62 | . | . | ||||
| Anomodon viticulosus (Hedw.) Hook. & Tay | 27 | 50 | 25 | |||||
| Pseudotaxiphyllum elegans (Brid.) Z.Iwat | 36 | . | 25 | . | ||||
| Frullania dilatata (L.) Dumort. | 27 | . | 50 | . | ||||
| Radula complanata (L.) Dumort. | 36 | . | 25 | . | ||||
| Atrichum undulatum (Hedw.) P.Beauv. | 27 | . | 50 | . | ||||
| Polytrichum piliferum Hedw. | 9 | . | 100 | 94.3 | . | |||
| Atrichum angustatum (Brid.) Bruch & Schi | 27 | . | 25 | . | ||||
| Plagiomnium cuspidatum (Hedw.) T.J.Kop. | 36 | . | . | |||||
| Metzgeria conjugata Lindb. | 27 | . | 25 | . | ||||
| Mnium stellare Hedw. | 18 | 100 | 89.2 | . | ||||
| Plagiomnium rostratum (Schrad.) T.J.Kop. | 18 | 100 | 89.2 | . | ||||
| Pogonatum urnigerum (Hedw.) P.Beauv. | 27 | . | 25 | . | ||||
| Frullania tamarisci (L.) Dumort. | 27 | . | 25 | . | ||||
| Eurhynchium angustirete (Broth.) T.J.Kop | 27 | . | 25 | . | ||||
| Thuidium delicatulum (Hedw.) Schimp. | 18 | . | 50 | |||||
| Brachytheciastrum velutinum (Hedw.) Igna | 36 | . | . | |||||
| Diplophyllum albicans (L.) Dumort. | 27 | 50 | . | |||||
| Plagiomnium undulatum (Hedw.) T.J.Kop. | 18 | 100 | 89.2 | . | ||||
| Brachythecium tommasinii (Sendtn. ex Bou) | 9 | 100 | 81.3 | 25 | . | |||
| Ptychostomum capillare (Hedw.) Holyoak & | 9 | 50 | 25 | 25 | ||||
| Didymodon fallax (Hedw.) R.H.Zander | . | 50 | 25 | 50 | ||||
| Ceratodon purpureus (Hedw.) Brid. | . | . | 75 | 66.7 | 25 | |||
| Leucodon sciuroides (Hedw.) Schwägr. | . | . | 25 | 75 | 66.7 | |||
| Hylocomium splendens (Hedw.) Schimp. | 27 | . | . | |||||
| Pogonatum aloides (Hedw.) P.Beauv. | 27 | . | . | |||||
| Solenostoma gracillimum (Sm.) R.M.Schust | 18 | . | 25 | . | ||||
| Plagiothecium nemorale (Mitt.) A.Jaeger | 27 | . | . | |||||
| Pseudotaxiphyllum elegans (Brid.) Z.Iwat | 18 | . | 25 | . | ||||
| Pleurozium schreberi (Willd. ex Brid.) M | 18 | . | 25 | . | ||||
| Dicranum fulvum Hook. | 27 | . | . | |||||
| Conocephalum salebrosum Szweyk., Buczk. | 9 | 100 | 94.3 | . | ||||
| Pedinophyllum interruptum(Nees) Kaal. | 18 | . | 25 | . | ||||
| Plagiomnium affine (Blandow ex Funck) T. | 9 | 50 | 25 | . | ||||
| Pterigynandrum filiforme Hedw. | 27 | . | . | . | ||||
| Paraleucobryum longifolium (Hedw.) Loesk | 18 | . | . | 25 | ||||
| Scapania nemorea (L.) Grolle | 27 | . | . | . | ||||
| Leucobryum juniperoideum (Brid.) Müll.Ha | 9 | . | 50 | . | ||||
| Blepharostoma trichophyllum (L.) Dumort. | 18 | . | 25 | . | ||||
| Lepidozia reptans (L.) Dumort. | 18 | . | 25 | . | ||||
| Lophozia ventricosa (Dicks.) Dumort. | 18 | . | 25 | . | ||||
| Brachythecium salebrosum (Hoffm. ex F.We | 18 | . | . | 25 | ||||
| Exsertotheca crispa (Hedw.) S.Olsson, En | 9 | 50 | . | 25 | ||||
| Homalothecium philippeanum (Spruce) Schi | 9 | 100 | 94.3 | . | . | |||
| Orthotrichum cupulatum Brid. | 9 | . | 25 | 25 | ||||
| Barbula unguiculata Hedw. | . | . | . | 75 | 83.2 | |||
| Tortula muralis Hedw. | . | . | . | 75 | 83.2 | |||
| Encalypta streptocarpa Hedw. | . | . | . | 75 | 83.2 | |||
| Diplophyllum obtusifolium (Hook.) Dumort | 18 | . | . | . | ||||
| Mnium marginatum (Dicks.) P.Beauv. | 18 | . | . | . | ||||
| Chiloscyphus polyanthos (L.) Corda | 18 | . | . | . | ||||
| Plagiochila asplenioides (L.) Dumort | 18 | . | . | . | ||||
| Dichodontium pellucidum (Hedw.) Schimp. | 18 | . | . | . | ||||
| Cirriphyllum crassinervium (Taylor) Loes | 18 | . | . | . | ||||
| Bartramia pomiformis Hedw. | 18 | . | . | . | ||||
| Pohlia nutans (Hedw.) Lindb. | 9 | . | 25 | . | ||||
| Dicranella varia (Hedw.) Schimp. | 9 | . | . | 25 | ||||
| Antitrichia curtipendula (Hedw.) Brid. | 9 | . | 25 | . | ||||
| Plagiothecium denticulatum (Hedw.) Schim | 18 | . | . | . | ||||
| Calypogeia fissa (L.) Raddi | 18 | . | . | . | ||||
| Pellia neesiana (Gottsche) Limpr. | 9 | 50 | . | . | ||||
| Solenostoma hyalinum (Lyell) Mitt. | 18 | . | . | . | ||||
| Cephaloziella divaricata (Sm.) Schiffn. | 9 | . | 25 | . | ||||
| Homalia trichomanioides (Hedw.) Brid. | 18 | . | . | . | ||||
| Mnium hornum Hedw. | 18 | . | . | . | ||||
| Heterocladium heteropterum (Brid.) Schim | 18 | . | . | . | ||||
| Polytrichastrum alpinum (Hedw.) G.L.Sm. | 9 | . | 25 | . | ||||
| Conocephalum conicum (L.) Dumort. | 18 | . | . | . | ||||
| Polytrichum juniperinum Hedw. | 9 | . | 25 | . | ||||
| Hylocomiadelphus triquetrus (Hedw.) Ochy | 9 | . | . | 25 | ||||
| Fissidens dubius P.Beauv. | 9 | . | . | 25 | ||||
| Dicranoweisia cirrata (Hedw.) Lindb. | 9 | . | 25 | . | ||||
| Orthotrichum anomalum Hedw. | 9 | . | 25 | . | ||||
| Syzygiella autumnalis (DC.) K.Feldberg, | 9 | . | 25 | . | ||||
| Grimmia trichophylla Grev. | 9 | . | 25 | . | ||||
| Pseudoleskeella catenulata (Brid. ex Sch | 9 | . | 25 | . | ||||
| Lewinskya striata (Hedw.) F.Lara, Garill | 9 | . | 25 | . | ||||
| Rhabdoweisia fugax (Hedw.) Bruch. & Schi | 9 | . | 25 | . | ||||
| Platygyrium repens (Brid.) Schimp. | 9 | . | 25 | . | ||||
| Orthotrichum stramineum Hornsch. ex Brid | 9 | . | 25 | . | ||||
| Lewinskya speciosa (Nees) F.Lara, Garill | 9 | . | 25 | . | ||||
| Cynodontium polycarpon (Hedw.) Schimp. | 9 | . | 25 | . | ||||
| Schistidium crassipilum H.H.Blom | 9 | . | . | 25 | ||||
| Cephalozia bicuspidata (L.) Dumort. | . | 50 | 25 | . | ||||
| Fissidens taxifolius Hedw. | . | 50 | . | 25 | ||||
| Thuidium recognitum (Hedw.) Lindb. | . | . | 25 | 25 | ||||
| Didymodon rigidulus Hedw. | . | . | 25 | 25 | ||||
| Hypnum cupressiforme var. lacunosum Brid. | . | . | 25 | 25 | ||||
| Homalothecium lutescens (Hedw.) H.Rob. | . | . | . | 50 | 65.5 | |||
| Didymodon acutus (Brid.) K.Saito | . | . | . | 50 | 65.5 | |||
| Homalothecium sericeum (Hedw.) Schimp. | . | . | . | 50 | 65.5 | |||
| Grimmia pulvinata (Hedw.) Sm. | . | . | . | 50 | 65.5 | |||
| Tortella tortuosa (Hedw.) Limpr. | . | . | . | 50 | 65.5 | |||
| Flexitrichum flexicaule (Schwägr.) Ignat | . | . | . | 50 | 65.5 | |||
| Plagiothecium platyphyllum Mönk. | 9 | . | . | . | ||||
| Leskea polycarpa Hedw. | 9 | . | . | . | ||||
| Grimmia hartmanii Schimp. | 9 | . | . | . | ||||
| Porella arboris-vitae (With.) Grolle | 9 | . | . | . | ||||
| Dicranum spurium Hedw. | 9 | . | . | . | ||||
| Diphyscium foliosum (Hedw.) D.Mohr | 9 | . | . | . | ||||
| Leucobryum glaucum (Hedw.) Ĺngstr. | 9 | . | . | . | ||||
| Jungermannia pumila With. | 9 | . | . | . | ||||
| Polytrichum commune Hedw. | 9 | . | . | . | ||||
| Sphagnum quinquefarium (Braithw.) Warnst | 9 | . | . | . | ||||
| Porella platyphylla (L.) Pfeiff. | 9 | . | . | . | ||||
| Pseudoscleropodium purum (Hedw.) M.Fleis | 9 | . | . | . | ||||
| Dicranum polysetum Sw. ex anon. | 9 | . | . | . | ||||
| Bazzania trilobata (L.) Gray | 9 | . | . | . | ||||
| Porella baueri (Schiffn.) C.E.O.Jensen | 9 | . | . | . | ||||
| Lewinskya affinis (Schrad. ex Brid.) F.L | 9 | . | . | . | ||||
| Callicladium imponens (Hedw.) Hedenäs, S | 9 | . | . | . | ||||
| Ulota bruchii Hornsch. ex Brid. | 9 | . | . | . | ||||
| Campylopus pyriformis (Schultz) Brid. | 9 | . | . | . | ||||
| Plagiothecium succulentum (Wilson) Lindb | 9 | . | . | . | ||||
| Plagiothecium laetum Schimp. | 9 | . | . | . | ||||
| Microlejeunea ulicina (Taylor) Steph. | 9 | . | . | . | ||||
| Amblystegium serpens (Hedw.) Schimp. | 9 | . | . | . | ||||
| Tritomaria exsectiformis (Breidl.) Schif | 9 | . | . | . | ||||
| Isothecium myosuroides Brid. | 9 | . | . | . | ||||
| Sciuro-hipnum flotowianum (Sendtn.) Ignatov | 9 | . | . | . | ||||
| Sciuro-hypnum plumosum (Hedw.) Ignatov & | 9 | . | . | . | ||||
| Taxiphyllum wissgrillii (Garov.) Wijk & | 9 | . | . | . | ||||
| Pellia epiphylla (L.) Corda | . | 50 | . | . | ||||
| Plasteurhynchium striatulum (Spruce) M.F | . | 50 | . | . | ||||
| Rhynchostegiella teneriffae (Mont.) Dirk | . | 50 | . | . | ||||
| Gymnostomum calcareum Nees & Hornsch. | . | 50 | . | . | ||||
| Liochlaena lanceolata Nees | . | 50 | . | . | ||||
| Conardia compacta (Drumm. ex Müll.Hal.) H | . | 50 | . | . | ||||
| Seligeria pusilla (Hedw.) Bruch & Schimp | . | 50 | . | . | ||||
| Plagiomnium ellipticum (Brid.) T.J.Kop. | . | 50 | . | . | ||||
| Barbilophozia barbata (Schmidel ex Schre | . | . | 25 | . | ||||
| Funaria hygrometrica Hedw. | . | . | 25 | . | ||||
| Orthotrichum pallens Bruch ex Brid. | . | . | 25 | . | ||||
| Amphidium mougeotii (Schimp.) Schimp. | . | . | 25 | . | ||||
| Grimmia muehlenbeckii Schimp. | . | . | 25 | . | ||||
| Ulota crispula Bruch | . | . | 25 | . | ||||
| Abietinella abietina (Hedw.) M.Fleisch. | . | . | . | 25 | ||||
| Ptychostomum rubens (Mitt.) Holyoak & N | . | . | . | 25 | ||||
| Ptychostomum moravicum (Podp.) Ros & Maz | . | . | . | 25 | ||||
| Syntrichia montana Nees | . | . | . | 25 | ||||
| Trichostomum brachydontium Bruch | . | . | . | 25 | ||||
| Tortella inclinata (R.Hedw.) Limpr. | . | . | . | 25 | ||||
| Thuidium assimile (Mitt.) A.Jaeger | . | . | . | 25 | ||||
| Encalypta vulgaris Hedw. | . | . | . | 25 | ||||
| Didymodon vinealis (Brid.) R.H.Zander | . | . | . | 25 | ||||
| Tortula lindbergii Broth. | . | . | . | 25 | ||||
| Weisia longifolia Mitt. | . | . | . | 25 | ||||
| Homomallium incurvatum (Schrad. ex Brid. | . | . | . | 25 | ||||
| Bryum argenteum Hedw. | . | . | . | 25 | ||||
| Brachythecium glaerosum (Bruch ex Spruce | . | . | . | 25 | ||||
| Campyliadelphus chrysophyllus (Brid.) R. | . | . | . | 25 | ||||
| Bryum ruderale Crundw. & Nyholm | . | . | . | 25 | ||||
| Weisia controversa Hedw. | . | . | . | 25 | ||||
| Didymodon spadiceus (Mitt.) Limpr. | . | . | . | 25 | ||||
| Syntrichia ruralis (Hedw.) F.Weber & D.M | . | . | . | 25 | ||||
| Tortella fasciculata (Culm.) Culm. | . | . | . | 25 | ||||
| Syntrichia calcicola J.J.Amann | . | . | . | 25 | ||||
| Tortella squarrosa (Brid.) Limpr. | . | . | . | 25 | ||||
| Bryoerythrophyllum recurvirostrum (Hedw.) | . | . | . | 25 | ||||
| Didymodon sinuosus (Mitt.) Delonge | . | . | . | 25 | ||||
| Pulvigera lyellii (Hook. & Taylor) Pláše | . | . | . | 25 | ||||
| Dicranella howei Renauld & Cardot | . | . | . | 25 | ||||
| Trichostomum crispulum Bruch | . | . | . | 25 | ||||
| Campylophyllopsis calcarea (Crundw. & Ny) | . | . | . | 25 | ||||
| Streblotrichum convolutum (Hedw.) P.Beau | . | . | . | 25 | ||||
| Didymodon cordatus Jur. | . | . | . | 25 | ||||
| Weissia brachycarpa (Nees et Hornsch.) J | . | . | . | 25 | ||||
Appendix B. Investigated Localities
| Locality | Coordinates WGS84 | Habitat Characteristics | ||||||
|---|---|---|---|---|---|---|---|---|
| No. | Name | y | x | Geological Bedrock | Forest Type | Vegetation | pH (H2O) | pH (KCl) |
| 1. | Šimića put—Molišće | 45.48704 | 17.64151 | quartzite | QA | Festuco drymeiae-Quercetum petrae | 4.05 | 3.46 |
| 2. | Vrhovački mlin | 45.46325 | 17.57752 | sandstone interlayed with algal limestone | QB | Fraxino orni-Quercetum pubescentis | 8.80 | 7.97 |
| 3. | Vranovo | 45.48917 | 17.56697 | phyllite | FA | Luzulo luzuloidis-Fagenion | 5.32 | 3.95 |
| 4. | Čarugin kamen | 45.51306 | 17.53556 | migmatite | FA | Luzulo luzuloidis-Fagenion | 4.67 | 4.13 |
| 5. | Točak (below peak) | 45.57584 | 17.53478 | amphibolite | FA | Luzulo luzuloidis-Fagenion, with several Qurecus petrea trees | 4.30 | 3.94 |
| 6. | Dva hrasta | 45.56671 | 17.50290 | migmatite | FA | Festuco drymeiae-Abietetum, with only scattered low Abies alba trees | 4.93 | 4.02 |
| 7. | Rupnica | 45.60513 | 17.53190 | albitic rhyolite | QB | Lathyro nigri-Quercetum petraeae | 6.54 | 5.91 |
| 8. | Gudnoga | 45.57435 | 17.63108 | granite | FA | Luzulo luzuloidis-Fagenion | 4.42 | 3.95 |
| 9. | Kovačica brook, near Jankovac | 45.53149 | 17.69876 | gneiss | FA | Luzulo luzuloidis-Fagenion | 5.36 | 5.07 |
| 10. | Mala Rijeka | 45.46467 | 17.86223 | phyllite | FA | Luzulo luzuloidis-Fagenion | 4.17 | 3.82 |
| 11. | Remetska Rijeka | 45.47461 | 17.86411 | chlorite schists | FA | Luzulo luzuloidis-Fagenion | 4.68 | 3.79 |
| 12. | Djedov nos | 45.49369 | 17.86581 | dolomitic breccia-conglomerates | QB | Lathyro nigri-Quercetum petraeae | 7.78 | 7.40 |
| 13. | Šaševa—Rastova kosa | 45.49537 | 17.81560 | slate, quartzite | QA | Qurecus petraea-Calluna vulgaris community | 3.89 | 3.13 |
| 14. | Viljevačka kosa | 45.51000 | 17.80319 | schist | QA | Festuco drymeiae-Quercetum petrae | 4.34 | 3.47 |
| 15. | Pištanska kosa | 45.51340 | 17.76675 | sand with grains of schits and phyllites | QA | Molinio arundinaceae-Quercetum petraeae | 4.79 | 3.98 |
| 16. | Jankovac, near brook spring | 45.51867 | 17.68675 | limestone, tuffa | FB | Vicio oroboidi-Fagetum sylvaticae | 8.48 | 8.01 |
| 17. | Jankovac | 45.52304 | 17.68300 | limestone, tuffa | FB | Vicio oroboidi-Fagetum sylvaticae | 8.12 | 8.04 |
| 18. | Svinjarevac | 45.50098 | 17.52846 | quartzite, gneiss | FA | 4.27 | 3.32 | |
| 19. | Radovanovačke Sokoline | 45.47723 | 17.62205 | quartzite | FA | Luzulo luzuloidis-Fagenion, with Quercus cerris and Q. petera | 3.93 | 3.49 |
| 20. | Velika Pliš | 45.46956 | 17.64227 | dolomites | QB | Lathyro nigri-Quercetum petraeae with patches of Fraxino orni-Quercetum pubescentis | 7.34 | 7.23 |
| 21. | Velika Raduča, Crni virovi | 45.53005 | 17.72536 | slate, quartzite | FA | Luzulo luzuloidis-Fagenion | 4.70 | 4.13 |
References
- Steel, J.B.; Wilson, B.; Anderson, B.J.; Lodge, R.H.E.; Tangney, R.S. Are bryophyte communities different from higher-plant communities? Abundance relations. Oikos 2004, 104, 479–486. [Google Scholar] [CrossRef]
- Grytnes, J.A.; Heegaard, E.; Ihlan, P.G. Species richness of vascular plants, bryophytes and lichens along an altitudinal gradient in western Norway. Acta Oecol. 2006, 29, 241–246. [Google Scholar] [CrossRef]
- Hofmeister, J.; Hošek, J.; Brabec, M.; Dvořák, D.; Beran, M.; Deckerová, H.; Burel, J.; Kříž, M.; Borovička, J.; Běťák, J.; et al. Value of old forest attributes related to cryptogam species richness in temperate forests: A quantitative assessment. Ecol. Ind. 2015, 57, 497–504. [Google Scholar] [CrossRef]
- Spitale, D. Forest and substrate type drive bryophyte distribution in the Alps. J. Bryol. 2017, 39, 128–140. [Google Scholar] [CrossRef]
- Wierzcholska, S.; Dyderski, M.K.; Pielech, R.; Gazda, A.; Smoczyk, M.; Malicki, M.; Horodecki, P.; Kamczyc, J.; Skorupski, M.; Hachułka, M.; et al. Natural forest remnants as refugia for bryophyte diversity in a transformed mountain river valley landscape. Sci. Total Environ. 2018, 640, 954–964. [Google Scholar] [CrossRef]
- Müller, J.; Boch, S.; Prati, D.; Socher, S.A.; Pommer, U.; Hessenmöller, D.; Schall, P.; Schulze, E.D.; Fischer, M. Effects of forest management on bryophyte species richness in Central European forests. For. Ecol. Manag. 2019, 432, 850–859. [Google Scholar] [CrossRef]
- Porley, R.; Hodgetts, N. Mosses and Liverworts; Collins: London, UK, 2005. [Google Scholar]
- Frahm, J.-P. Biologie der Moose; Spectrum Akademischer Verlag: Heidelberg/Berlin, Germany, 2001. [Google Scholar]
- Saxena, D.K.; Harinder. Uses of bryophytes. Resonance 2004, 9, 56–65. [Google Scholar] [CrossRef]
- Glime, J. The role of bryophytes in temperate forest ecosystems. Hikobia 2001, 13, 267–289. [Google Scholar]
- Sun, S.; Wu, Y.; Wang, G.; Zhou, J.; Yu, D.; Bing, H.; Luo, J. Bryophyte species richness and composition along an altitudinal gradient in Gongga Mountain. China. PLoS ONE 2013, 8, e58131. [Google Scholar] [CrossRef]
- Sveinbjörnsson, B.; Oechel, W.C. Controls on growth and productivity of bryophytes: Environmental limitations under current and anticipated conditions. In Bryophytes and lichens in a Changing Environment; Bates, J.W., Farmer, A.M., Eds.; Clarendon Press: Oxford, UK, 1992; pp. 77–102. [Google Scholar]
- Heilmann-Clausen, J.; Ande, E.; van Dort, K.; Christensen, M.; Piltaver, A.; Veerkamp, M.; Walleyn, R.; Siller, I.; Standovár, T.; Ódor, P. Communities of wood-inhabiting bryophytes and fungi on dead beech logs in Europe—Reflecting substrate quality or shaped by climate and forest conditions? J. Biogeogr. 2014, 41, 2269–2282. [Google Scholar] [CrossRef]
- Bruun, H.H.; Moen, J.; Virtanen, R.; Grytnes, J.A.; Oksanen, L.; Angerbjörn, A. Effects of altitude and topography on species richness of vascular plants, bryophytes and lichens in alpine communities. J. Veg. Sci. 2006, 17, 37–46. [Google Scholar] [CrossRef]
- Frego, K.A. Bryophytes as potential indicators of forest integrity. For. Ecol. Manag. 2007, 242, 65–75. [Google Scholar] [CrossRef]
- Bengtsson, J.; Nilsson, S.G.; Franc, A.; Menozzi, P. Biodiversity, disturbances, ecosystem function and management of European forests. For. Ecol. Manag. 2000, 132, 39–50. [Google Scholar] [CrossRef]
- Paillet, Y.; Bergés, L.; Hjältén, J.; Ódor, P.; Avon, C.; Bernhardt-Römermann, M.; Bijlsma, R.J.; de Bruyn, L.; Fuhr, M.; Grandin, U.; et al. Biodiversity differences between managed and unmanaged forests: Meta-analysis of species richness in Europe. Conserv. Biol. 2010, 24, 101–112. [Google Scholar] [CrossRef]
- Kantvilas, G.; Jarman, S.J.; Minchin, P.R. Early impacts of disturbance on lichens, mosses and liverworts in Tasmania’s wet eucalypt production forests. Aust. Forestry 2015, 78, 92–107. [Google Scholar] [CrossRef]
- Czerepko, J.; Gawryś, R.; Szymczyk, R.; Pisarek, W.; Janek, M.; Haidt, A.; Kowalewska, A.; Piegdoń, A.; Stebel, A.; Kukwa, M.; et al. How sensitive are epiphytic and epixylic cryptogams as indicators of forest naturalness? Testing bryophyte and lichen predictive power in stands under different management regimes in the Białowieża forest. Ecol. Indic. 2021, 125, 107532. [Google Scholar] [CrossRef]
- Márialigeti, S.; Németh, B.; Tinya, F.; Ódor, P. The effects of standstructure on ground-floor bryophyte assemblages in temperate mixed forests. Biodivers. Conserv. 2009, 18, 2223–2241. [Google Scholar] [CrossRef]
- Tinya, F.; Márialigeti, S.; Király, I.; Németh, B.; Ódor, P. The effect of light conditions on herbs, bryophytes and seedlings of temperate mixed forests in Őrség, Western Hungary. Plant Ecol. 2009, 204, 69–81. [Google Scholar] [CrossRef]
- Tinya, F.; Kovács, B.; Bidló, A.; Dima, B.; Király, I.; Kutszegi, G.; Lakatos, F.; Mag, Z.; Márialigeti, S.; Nascimbene, J.; et al. Environmental drivers of forest biodiversity in temperate mixed forests—A multi-taxon approach. Sci. Total Environ. 2021, 795, 148720. [Google Scholar] [CrossRef]
- Király, I.; Ódor, P. The effect of stand structure and tree species composition on epiphytic bryophytes in mixed deciduous–coniferous forests of Western Hungary. Biol. Conserv. 2010, 143, 2063–2069. [Google Scholar] [CrossRef]
- Tinya, F.; Ódor, P. Congruence of the spatial pattern of light and understory vegetation in an old-growth, temperate mixed forest. For. Ecol. Manag. 2016, 381, 84–92. [Google Scholar] [CrossRef]
- Vitt, D.H.; Li, Y.; Belland, R. Patterns of bryophyte diversity in peatlands of continental western Canada. Bryologist 1995, 98, 218–227. [Google Scholar] [CrossRef]
- Martinez, M.L.; Maun, M.A. Responses of dune mosses to experimental burial by sand under natural and greenhouse conditions. Plant Ecol. 1999, 145, 209–219. [Google Scholar] [CrossRef]
- Zamfir, M.; Dai, X.; van der Maarel, E. Bryophytes, lichens and phanerogams in an alvar grassland: Relationships at different scales and contributions to plant community pattern. Ecography 1999, 22, 40–52. [Google Scholar] [CrossRef]
- Erfanzadeh, R. Impact of phanerogam and soil characteristics on bryophyte assemblages with respect to restoration practices (case study: IJzermonding, Belgium). Ecopersia 2013, 1, 41–51. [Google Scholar]
- Nelson, C.R.; Halpern, C.B. Short-term effects of timber harvest and forest edges on ground-layer mosses and liverworts. Can. J. Bot. 2005, 83, 610–620. [Google Scholar] [CrossRef]
- Caners, R.T.; Macdonald, S.E.; Belland, R.J. Linking the biological traits of boreal bryophytes to forest habitat change after partial harvesting. For. Ecol. Manag. 2013, 303, 184–194. [Google Scholar] [CrossRef]
- Ewald, J. Epigeic bryophytes do not improve bioindication by Ellenberg values in mountain forests. Basic Appl. Ecol. 2009, 10, 420–426. [Google Scholar] [CrossRef]
- Berg, C.; Dengler, J. Moose und Flechten als diagnostische Arten von Pflanzengesellschaften—Eine Übersicht aus Mecklenburg-Vorpommern. Herzogia 2005, 18, 145–161. [Google Scholar]
- Diekmann, M. Use and improvement of Ellenberg’s indicator values in deciduous forests of the Boreo-nemoral zone in Sweden. Ecography 1995, 18, 178–189. [Google Scholar] [CrossRef]
- Düll, R. Zeigwerte von Laub- und Lebermoosen. In Zeigwerte von Pflanzen in Mitteleuropa, 3rd ed.; Ellenberg, H., Weber, H.E., Düll, R., Wirth, V., Werner, W., Eds.; Scripta geobotanica 18; Verlag Erich Goltze KG: Göttigen, Germany, 2001; pp. 9–166. [Google Scholar]
- Dierßen, K. Distribution, Ecological Amplitude and Phytosociological Characterization of European Bryophytes; Bryophytorum Bibliotheca; J. Cramer: Berlin, Germany, 2001. [Google Scholar]
- Sabovljević, M.; Vujičić, M.; Sabovljević, A. Diversity of saproxylic bryophytes in old-growth and managed beech forests in the Central Balkans. Plant Biosyst. 2010, 144, 234–240. [Google Scholar] [CrossRef]
- Ewald, J. Comparing indicator values of bryophyte and vascular understorey plants in mountain forests. Mitteilungen Der Arbeitsgemeinschaft Geobot. Schleswig-Holst. Und Hambg. 2008, 65, 117–126. [Google Scholar]
- Gabriel, R.; Bates, J.W. Bryophyte community composition and habitat specificity in the natural forests of Terceira, Azores. Plant Ecol. 2005, 177, 125–144. [Google Scholar] [CrossRef]
- Perhans, K.; Gustafsson, L.; Jonsson, F.; Nordin, U.; Weibull, H. 2007—Bryophytes and lichens in different types of forests set-asides in boreal Sweden. For. Ecol. Manag. 2007, 242, 347–390. [Google Scholar] [CrossRef]
- Baldwin, L.K.; Bradfield, G.E. Bryophyte responses to fragmentation in temperate coastal rainforests: A functional group approach. Biol. Conserv. 2007, 136, 408–422. [Google Scholar] [CrossRef]
- Stefańska-Krzaczek, E.; Swacha, G.; Żarnowiec, J.; Raduła, M.W.; Kącki, Z.; Staniaszek-Kik, M. Central European forest floor bryophytes: Richness, species composition, coexistence and diagnostic significance across environmental gradients of forest habitats. Ecol. Indic. 2022, 139, 108954. [Google Scholar] [CrossRef]
- Kutnar, L.; Kermavnar, J.; Sabovljević, M.S. Bryophyte diversity, composition and functional traits in relation to bedrock and tree species composition in close-to-nature managed forests. Eur. J. For. Res. 2023, 142, 1–18. [Google Scholar] [CrossRef]
- Rola, K.; Plášek, V.; Rożek, K.; Zubek, S. Effect of tree species identity and related habitat parameters on understorey bryophytes—interrelationships between bryophyte, soil and tree factors in a 50-year-old experimental forest. Plant Soil 2021, 466, 613–630. [Google Scholar] [CrossRef]
- Ilić, M.; Igić, R.; Ćuk, M.; Veljić, M.; Radulović, S.; Orlović, S.; Vukov, D. Environmental drivers of ground-floor bryophytes diversity in temperate forests. Oecologia 2023, 202, 275–285. [Google Scholar] [CrossRef]
- EU Habitats Directive (Council Directive 92/43/ECC). 1992. Available online: https://environment.ec.europa.eu/topics/nature-and-biodiversity/habitats-directive_en (accessed on 5 September 2023).
- Hokkanen, P.J. Bryophyte communities in herb-rich forests in Koli, eastern Finland: Comparison of forest classifications based on bryophytes and vascular plants. Ann. Bot. Fenn. 2004, 41, 331–365. [Google Scholar]
- Wolski, G.J.; Kruk, A. Determination of plant communities based on bryophytes: The combined use of Kohonen artificial neural network and indicator species analysis. Ecol. Ind. 2020, 113, 106160. [Google Scholar] [CrossRef]
- Staniaszek-Kik, M.; Żarnowiec, J.; Stefańska-Krzaczek, E. Diversity and composition of moss guilds on uprooted trees in Central European mountain forests: Effects of uprooting components and environmental variables. Ann. For. Sci. 2021, 78, 45. [Google Scholar] [CrossRef]
- Vukelić, J. Šumska Vegetacija Hrvatske. Forest Vegetation in Croatia; Šumarski Fakultet Sveučilišta u Zagrebu, Državni Zavod za Zaštitu Prirode: Zagreb, Croatia, 2012. [Google Scholar]
- Ellenberg, H. Vegetation Mitteleuropas mit den Alpen, 5th ed.; E. Ulmer Verlag: Stuttgart, Germany, 1996. [Google Scholar]
- Rapson, G.L. At what scales and in what vegetation types should we sample nonvascular plants? In Vegetation Structure and Function at Multiple Spatial, Temporal and Conceptual Scales; Box, E., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 389–403. [Google Scholar]
- Bagella, S. Does cross-taxon analysis show similarity in diversity patterns between vascular plants and bryophytes? Some answers from a literature review. C. R. Biol. 2014, 337, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Möls, T.; Vellak, K.; Vellak, A.; Ingerpuu, N. Global gradients in moss and vascular plant diversity. Biodivers. Conserv. 2013, 22, 1537–1551. [Google Scholar] [CrossRef]
- Dynesius, M.; Zinko, U. Species richness correlations omong primary producers in boreal forests. Divers. Distrib. 2006, 12, 703–713. [Google Scholar] [CrossRef]
- Hájková, P.; Hájek, M. Bryophyte and vascular plant responses to base-richness and water level gradients in Western Carpathian Sphagnum-rich mires. Folia Geobot. 2004, 39, 335–351. [Google Scholar] [CrossRef]
- Ugarković, D.; Tikvić, I.; Mikac, S.; Stankić, I.; Balta, D. The influence of changing climate extremes on the ecological niche of pedunculated oak in Croatia. South-East Eur. For. 2016, 7, 143–148. [Google Scholar] [CrossRef][Green Version]
- Škvorc, Ž.; Franjić, J.; Krstonošić, D.; Sever, K.; Alešković, I. Vegetacijska obilježja bukovih šuma Psunja, Papuka i Krndije—Vegetation Fetures of Beech Forests of Psunj, Papuk and Krndija Mountains. Croat. J. For. Eng. 2011, 32, 157–176. [Google Scholar]
- Government of the Republic of Croatia. Uredba o ekološkoj mreži [Regulation on Ecological Network]. Off. Gaz. 2013, 124. [Google Scholar]
- Mühlenberg, M. Freilandökologie, 3rd ed.; Quelle & Meyer: Heidelberg-Wiesbaden, Germany, 1993; pp. 45–47. [Google Scholar]
- Hodgetts, N.G.; Söderström, L.; Blockeel, L.; Caspari, S.; Ignatov, M.S.; Konstantinova, N.A.; Lockhart, N.; Papp, B.; Schröck, C.; Sim-Sim, M.; et al. An annotated checklist of bryophytes of Europe, Macaronesia and Cyprus. J. Bryol. 2020, 42, 1–116. [Google Scholar] [CrossRef]
- Tichý, L.; Holt, J.; Nejezchlebová, M. JUICE Program for Management, Analysis and Classification of Ecological Data. Program Manual; Masaryk University Brno: Brno, Czech Republic, 2006. [Google Scholar]
- Van Zuijlen, K.; Nobis, M.P.; Hedenäs, L.; Hodgetts, N.; Calleja Alarcón, J.A.; Albertos, B.; Bernhardt-Römermann, M.; Gabriel, R.; Garilleti, R.; Lara, F.; et al. Bryophytes of Europe Traits (BET) data set: A fundamental tool for ecological studies. J. Veg. Sci. 2023, 34, e13179. [Google Scholar] [CrossRef]
- Hill, M.O.; Preston, C.D. The geographical relationships of British and Irish bryophytes. J. Bryol. 1998, 20, 127–226. [Google Scholar] [CrossRef]
- Hill, M.O.; Preston, C.D.; Bossanquet, S.D.S.; Roy, D.B. Bryoatt—Attributes of British and Irish Mosses, Liverworts and Hornworts; NERC Centre for Ecology and Hydrology and Countryside Council for Wales: Huntigdon, UK, 2007. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Paleontol. Electron. 2001, 4, 9. [Google Scholar]
- ter Braak, C.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using Canoco 5.0, 2nd ed.; Cambridge University Press: New York, NY, USA, 2014. [Google Scholar]
- McCune, B.; Mefford, M.J. PC-ORD. Multivariate Analysis of Ecological Data, Version 7.09; Wild Blueberry Media: Corvallis, OR, USA, 2018. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alegro, A.; Šegota, V.; Rimac, A.; Papp, B. Diversity, Ecology and Phytogeography of Bryophytes across Temperate Forest Communities—Insight from Mt. Papuk (Croatia, SE Europe). Plants 2023, 12, 3346. https://doi.org/10.3390/plants12193346
Alegro A, Šegota V, Rimac A, Papp B. Diversity, Ecology and Phytogeography of Bryophytes across Temperate Forest Communities—Insight from Mt. Papuk (Croatia, SE Europe). Plants. 2023; 12(19):3346. https://doi.org/10.3390/plants12193346
Chicago/Turabian StyleAlegro, Antun, Vedran Šegota, Anja Rimac, and Beáta Papp. 2023. "Diversity, Ecology and Phytogeography of Bryophytes across Temperate Forest Communities—Insight from Mt. Papuk (Croatia, SE Europe)" Plants 12, no. 19: 3346. https://doi.org/10.3390/plants12193346
APA StyleAlegro, A., Šegota, V., Rimac, A., & Papp, B. (2023). Diversity, Ecology and Phytogeography of Bryophytes across Temperate Forest Communities—Insight from Mt. Papuk (Croatia, SE Europe). Plants, 12(19), 3346. https://doi.org/10.3390/plants12193346







