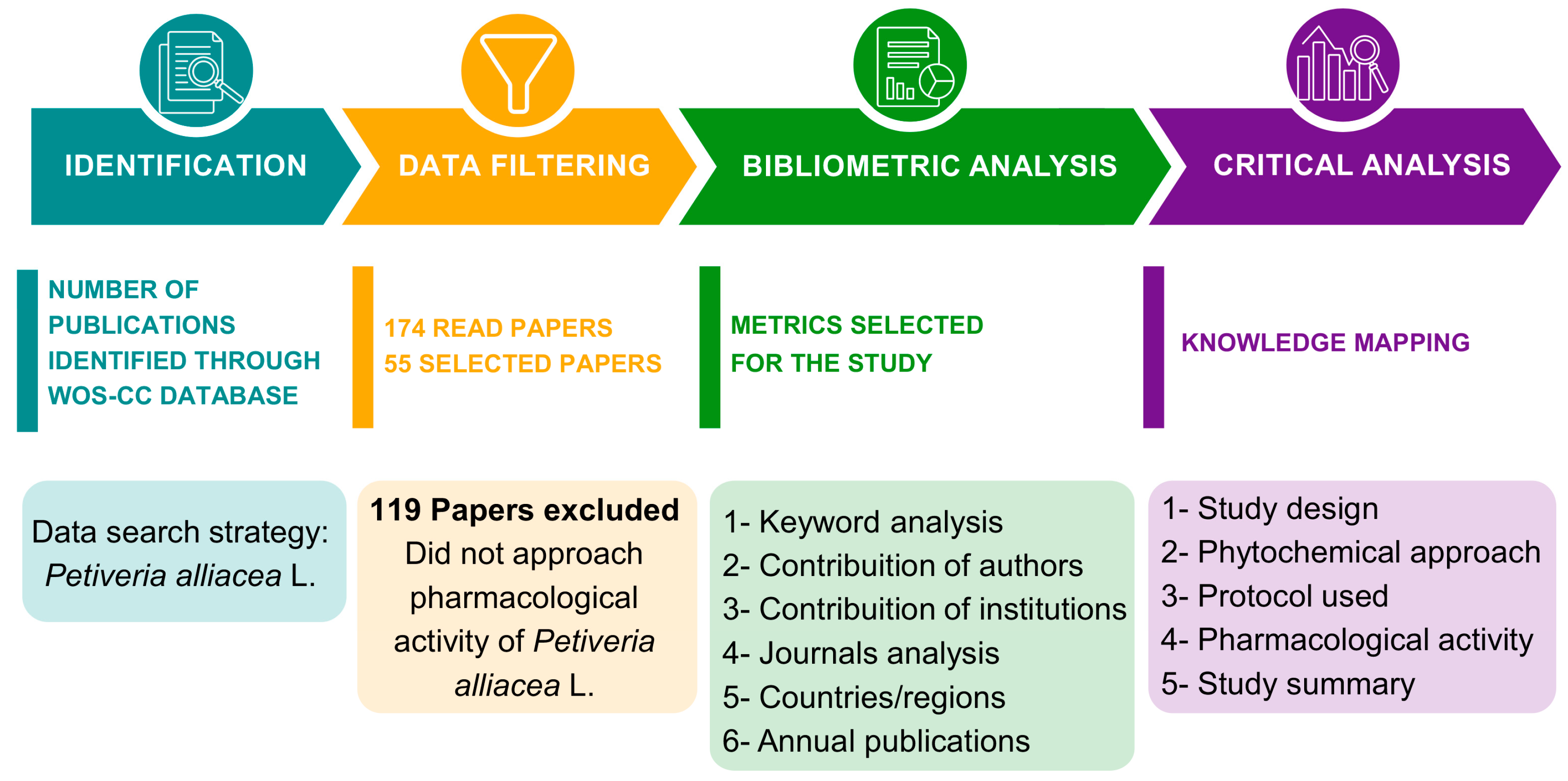
Amazonian Plants: A Global Bibliometric Approach to Petiveria alliacea L. Pharmacological and Toxicological Properties
Abstract
1. Introduction
2. Results
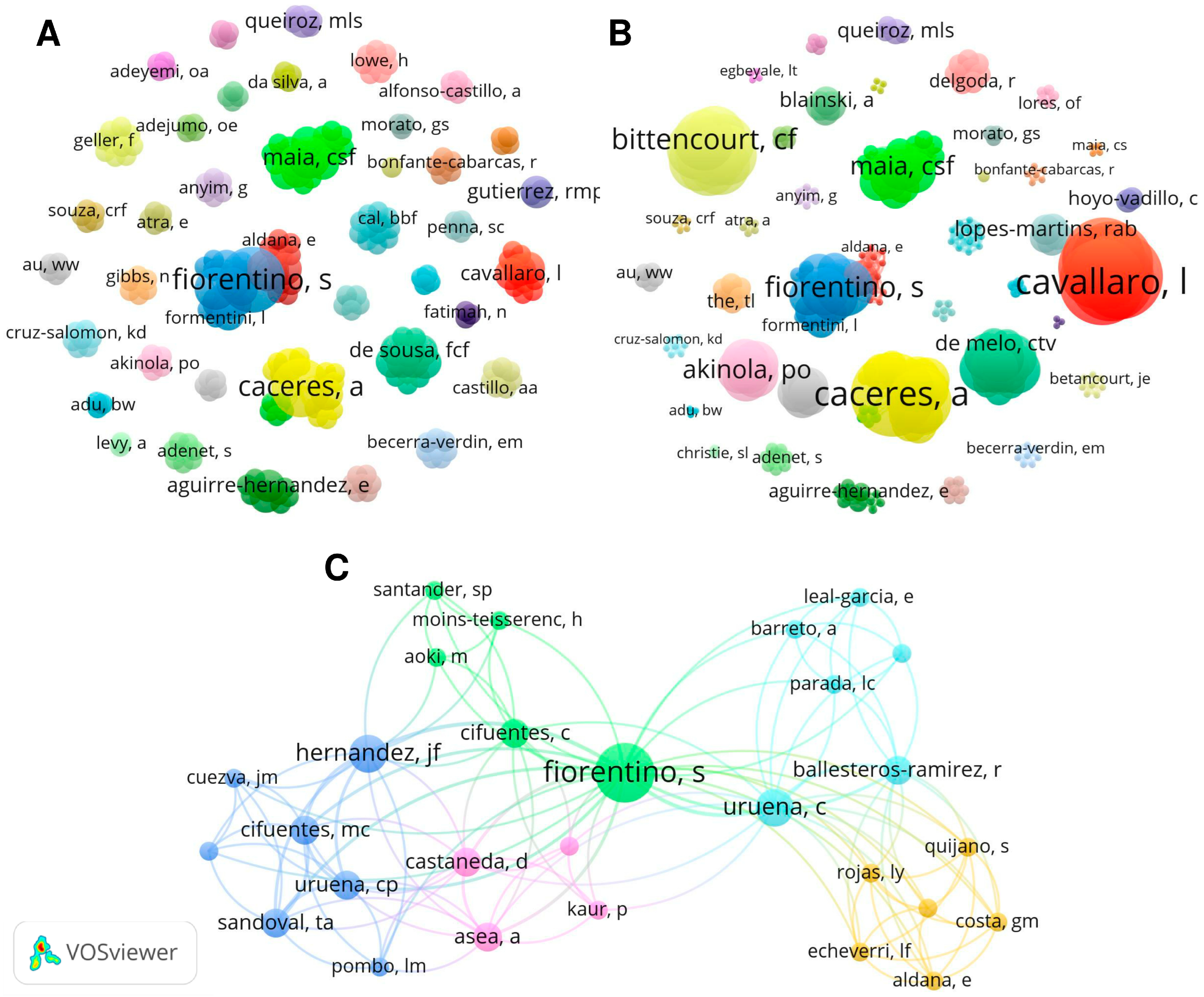
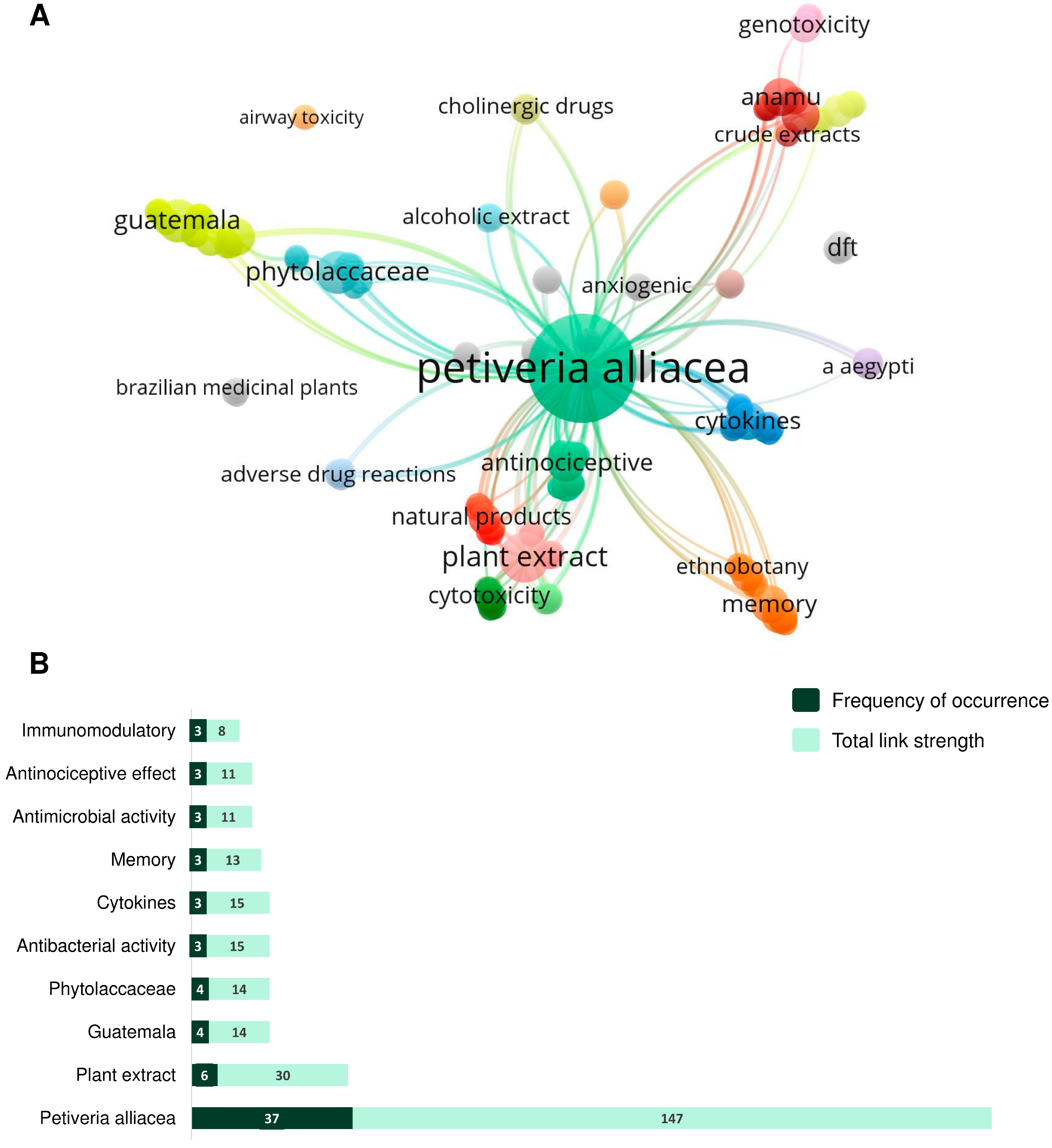
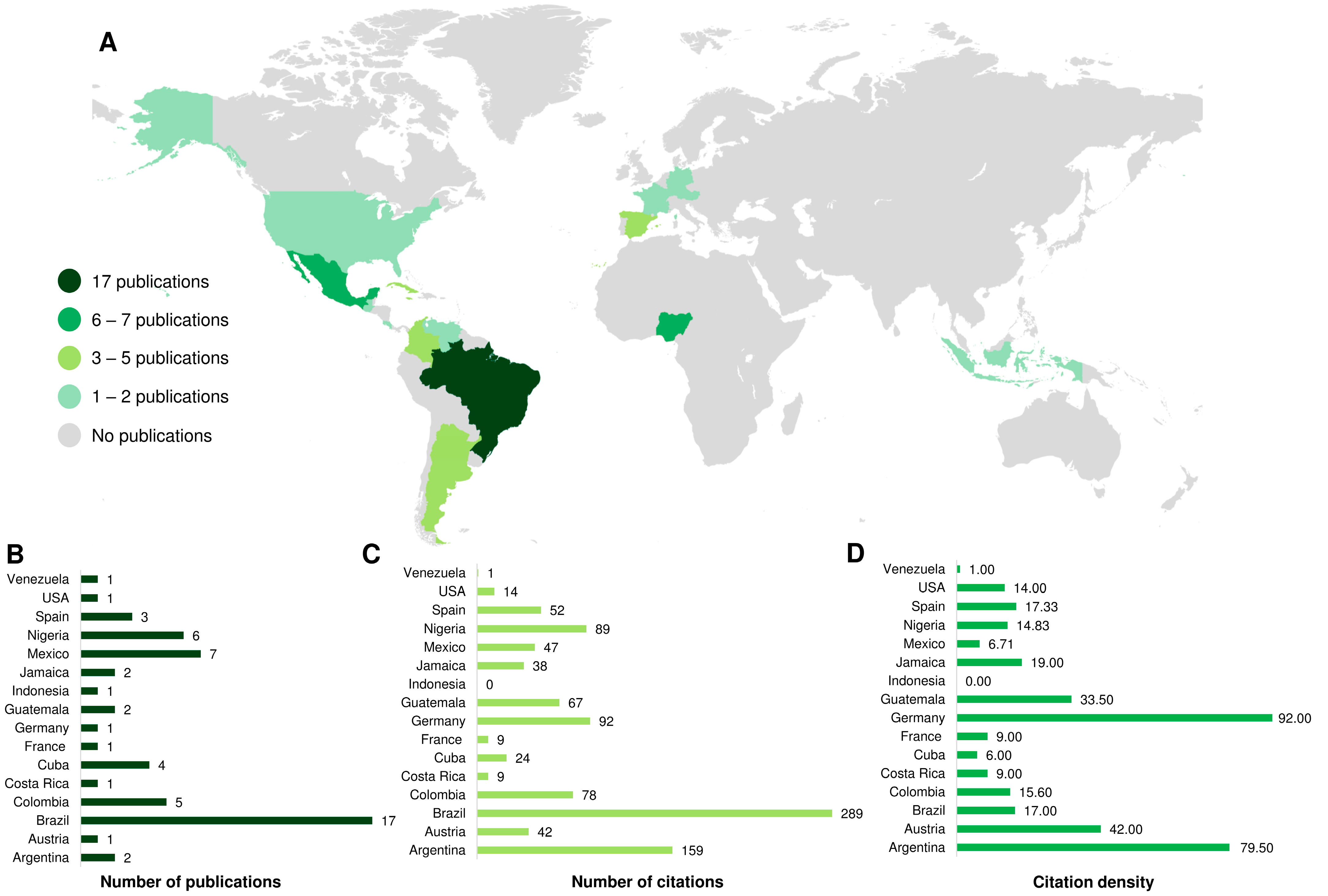
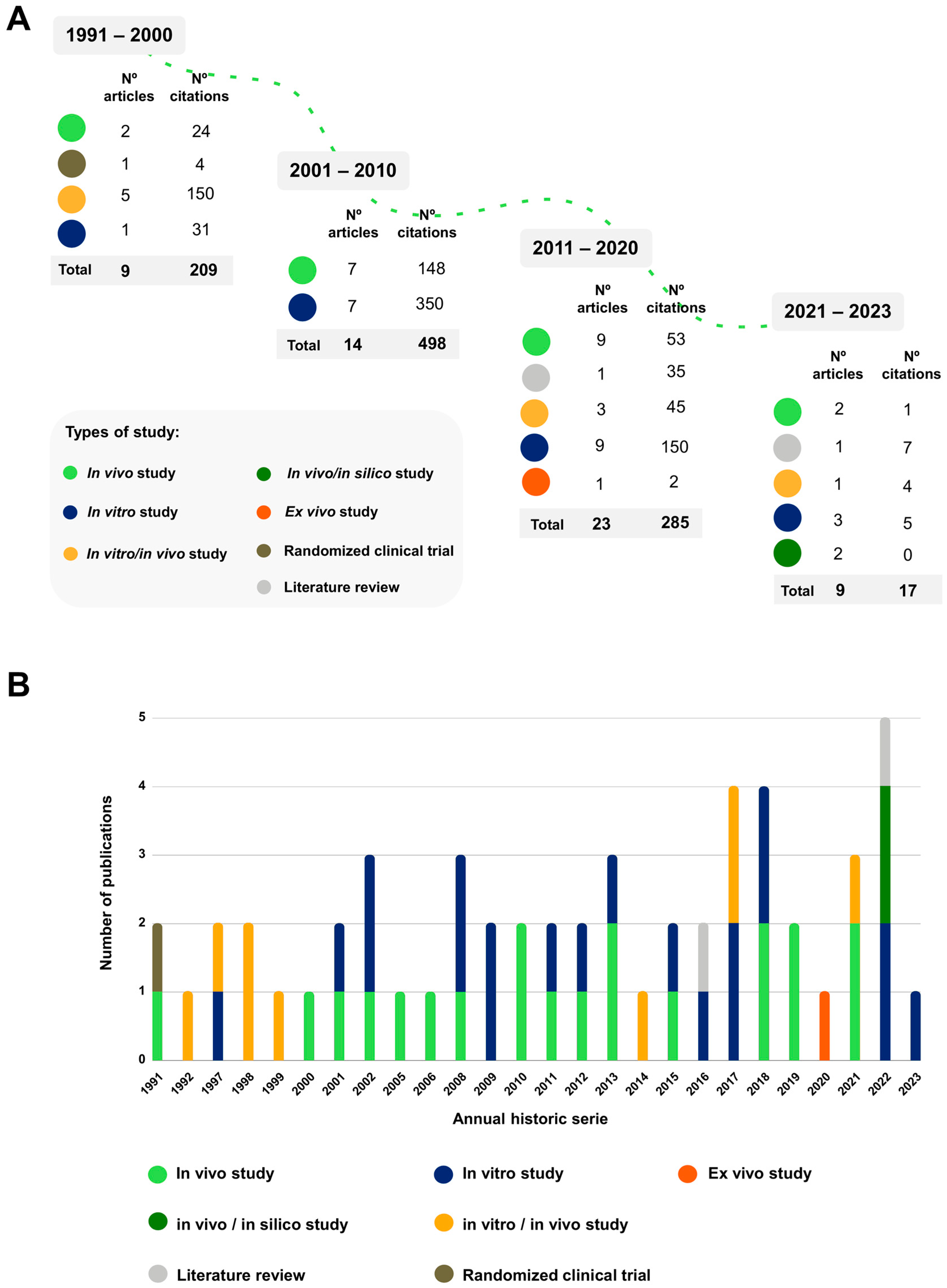
2.1. Bibliometric Analysis
| Authors/Year | Article Title | DOI/URL (Accessed on 3 August 2023) | Number of Citations | ||
|---|---|---|---|---|---|
| WoS-CC | Scopus | Google Scholar | |||
| Prada et al., 2023 [17] | Doxorubicin Activity Is Modulated by Traditional Herbal Extracts in a 2D and 3D Multicellular Sphere Model of Leukemia | https://doi.org//10.3390/pharmaceutics15061690 | 0 | 0 | 0 |
| Cal et al., 2022 [6] | Cytotoxicity of Extracts from Petiveria alliacea Leaves on Yeast | https://doi.org//10.3390/plants11233263 | 0 | 0 | 1 |
| Cruz-Salomon et al., 2022 [8] | In vivo and In Silico Study of the Antinociceptive and Toxicological Effect of the Extracts of Petiveria alliacea L. Leaves | https://doi.org//10.3390/ph15080943 | 0 | 0 | 1 |
| Olajubutu et al., 2022 [19] | Topical Anti-Inflammatory Activity of Petiveria alliacea, Chemical Profiling and Computational Investigation of Phytoconstituents Identified from its Active Fraction | https://doi.org//10.1007/s42250-022-00363-y | 4 | 0 | 0 |
| Zavala-Ocampo et al., 2022 [2] | Acetylcholinesterase inhibition and antioxidant activity properties of Petiveria alliacea L. | https://doi.org//10.1016/j.jep.2022.115239 | 5 | 6 | 7 |
| Castaneda et al., 2022 [12] | Medicinal plants used in traditional Mayan medicine for the treatment of central nervous system disorders: An overview | https://doi.org//10.1016/j.jep.2021.114746 | 7 | 7 | 8 |
| Mustika et al., 2021 [4] | The self-nanoemulsifying drug delivery system of Petiveria alliacea extract reduced the homeostatic model assessment-insulin resistance value, interleukin-6, and tumor necrosis factor-alpha level in diabetic rat models | https://doi.org//10.14202/vetworld.2021.3229-3234 | 0 | 0 | 1 |
| Oyeleke et al., 2021 [7] | Antimicrobial, immunomodulatory and hepatomodulatory effects of aqueous extracts of Petiveria alliacea root and leaf on growing pullets | https://doi.org//10.5424/sjar/2021191-17300 | 1 | 1 | 2 |
| Ileke et al., 2021 [9] | Bioefficacy of two indigenous Nigerian botanicals on the developmental stages of malaria vector, Anopheles gambiaegiles [Diptera: Culicidae] | https://doi.org//10.1007/s42690-020-00281-x | 1 | 1 | 2 |
| Ballesteros-Ramirez et al., 2020 [10] | Preferential Activity of Petiveria alliacea Extract on Primary Myeloid Leukemic Blast | https://doi.org//10.1155/2020/4736206 | 2 | 2 | 6 |
| Caicedo-Pinto et al., 2019 [11] | Effects of Petiveria alliacea and cholinergic drugs on the habituation cognitive behavior | https://doi.org//10.35588/blacpma.19.18.6.42 | 1 | 1 | 1 |
| Alves et al., 2019 [13] | Petiveria alliacea, a plant used in Afro-Brazilian smoke rituals, triggers pulmonary inflammation in rats | https://doi.org//10.1016/j.bjp.2019.06.005 | 4 | 5 | 9 |
| Garcia-Perez et al., 2018 [20] | Toxicological evaluation of an aqueous suspension from leaves and stems of Petiveria alliacea L. (Phytolaccaceae) | https://doi.org//10.1016/j.jep.2017.09.022 | 7 | 9 | 18 |
| Gutierrez and Flores, 2018 [14] | Petiveria alliacea Suppresses Airway Inflammation and Allergen-Specific Th2 Responses in Ovalbumin-Sensitized Murine Model of Asthma | https://doi.org//10.1007/s11655-018-2566-5 | 2 | 4 | 6 |
| Lateef et al., 2018 [21] | Characterization, antimicrobial, antioxidant, and anticoagulant activities of silver nanoparticles synthesized from Petiveria alliacea L. Leaf extract | https://doi.org//10.1080/10826068.2018.1479864 | 63 | 75 | 100 |
| Hartmann et al., 2018 [22] | Investigation of the Larvicidal Effect of Guinea (Petiveria alliacea) on Larvae of Mosquitoes of the Species A. aegypti | https://doi.org//10.21577/1984-6835.20180040 | 1 | 2 | 3 |
| Flota-Burgos et al., 2017 [23] | Anthelminthic activity of methanol extracts of Diospyros anisandra and Petiveria alliacea on cyathostomin (Nematoda: Cyathostominae) larval development and egg hatching | https://doi.org/10.1016/j.vetpar.2017.10.016 | 7 | 9 | 16 |
| Zavala-Ocampo et al., 2017 [24] | Antiamoebic Activity of Petiveria alliacea Leaves and Their Main Component, Isoarborinol | https://doi.org//10.4014/jmb.1705.05003 | 10 | 15 | 19 |
| Hernandez et al., 2017 [25] | A cytotoxic Petiveria alliacea dry extract induces ATP depletion and decreases beta-F1-ATPase expression in breast cancer cells and promotes survival in tumor-bearing mice | https://doi.org//10.1016/j.bjp.2016.09.008 | 9 | 11 | 20 |
| Gutierrez and Vadillo, 2017 [26] | Anti-inflammatory Potential of Petiveria alliacea on Activated RAW264.7 Murine Macrophages | https://doi.org//10.4103/pm.pm_479_16 | 20 | 22 | 28 |
| Luz et al., 2016 [27] | Ethnobotany, phytochemistry and neuropharmacological effects of Petiveria alliacea L. (Phytolaccaceae): A review | https://doi.org//10.1016/j.jep.2016.02.053 | 35 | 45 | 70 |
| Murray et al., 2016 [28] | Significant inhibitory impact of dibenzyl trisulfide and extracts of Petiveria alliacea on the activities of major drug-metabolizing enzymes in vitro: An assessment of the potential for medicinal plant-drug interactions | https://doi.org//10.1016/j.fitote.2016.04.011 | 19 | 19 | 25 |
| Silva et al., 2015 [29] | Petiveria alliacea exerts mnemonic and learning effects on rats | https://doi.org//10.1016/j.jep.2015.04.005 | 11 | 12 | 21 |
| Kerdudo et al., 2015 [30] | Essential oil composition and biological activities of Petiveria alliacea L. From Martinique | https://doi.org//10.1080/10412905.2015.1014118 | 9 | 11 | 13 |
| Hernandez et al., 2014 [31] | A Petiveria alliacea standardized fraction induces breast adenocarcinoma cell death by modulating glycolytic metabolism | https://doi.org//10.1016/j.jep.2014.03.013 | 26 | 33 | 59 |
| Christie and Levy, 2013 [32] | Evaluation of the Hypoglycaemic Activity of Petiveria alliacea (Guinea Hen Weed) Extracts in Normoglycaemic and Diabetic Rat Models | https://www.mona.uwi.edu/fms/wimj/system/files/article_pdfs/dr_christie_wimj_november.qxd_.pdf | 6 | 7 | 13 |
| Fletes-Arjona et al., 2013 [33] | Morphologic Alterations in the Respiratory Tract of Wistar Rats Induced by Steams of the Root of Hierba del Zorrillo (Petiveria alliacea) from Southwest Mexico | https://doi.org//10.4067/S0717-95022013000100019 | 3 | 3 | 1 |
| Pacheco et al., 2013 [34] | In vitro antimicrobial activity of total extracts of the leaves of Petiveria alliacea L. (Anamu) | https://doi.org//10.1590/S1984-82502013000200006 | 8 | 9 | 19 |
| de Andrade et al., 2012 [35] | Potential behavioral and pro-oxidant effects of Petiveria alliacea L. Extract in adult rats | https://doi.org//10.1016/j.jep.2012.07.020 | 16 | 20 | 33 |
| Santander et al., 2012 [36] | Immunomodulatory Effects of Aqueous and Organic Fractions from Petiveria alliacea on Human Dendritic Cells | https://doi.org//10.1142/S0192415X12500620 | 11 | 13 | 23 |
| Adejumo et al., 2011 [37] | Phytochemical and antisickling activities of Entandrophragma utile, Chenopodium ambrosioides and Petiveria alliacea | https://academicjournals.org/journal/JMPR | 12 | 13 | 33 |
| Duharte et al., 2011 [38] | Protecting effect of Petiveria alliacea (Anamu) on the immunosuppression induced by 5-fluorouracil in Balb/c mice | https://www.redalyc.org/pdf/856/85618379009.pdf | 3 | 4 | 3 |
| Maia et al., 2010 [39] | Analysis of Fetal and Placental Development in Rats after Administration of Hydroalcoholic Extract from the Root of Petiveria alliacea L. (Phytolaccaceae) | https://doi.org//10.4067/S0717-95022010000100023 | 2 | 2 | 5 |
| Blainski et al., 2010 [40] | Dual effects of crude extracts obtained from Petiveria alliacea L. (Phytolaccaceae) on experimental anxiety in mice | https://doi.org/10.1016/j.jep.2010.01.012 | 30 | 32 | 56 |
| Guedes et al., 2009 [41] | Antimicrobial activity of crude extracts of Petiveria alliacea L. | http://www.latamjpharm.org/trabajos/28/4/LAJOP_28_4_1_7_AIT6W1N9TT.pdf | 3 | 8 | 2 |
| Schmidt et al., 2009 [42] | Biological studies on Brazilian plants used in wound healing | https://doi.org//10.1016/j.jep.2009.01.022 | 92 | 101 | 194 |
| Gomes et al., 2008 [43] | Central effects of isolated fractions from the root of Petiveria alliacea L. (tipi) in mice | https://doi.org//10.1016/j.jep.2008.08.012 | 35 | 39 | 59 |
| Urueña et al., 2008 [44] | Petiveria alliacea extracts use multiple mechanisms to inhibit growth of human and mouse tumoral cells | https://doi.org//10.1186/1472-6882-8-60 | 38 | 46 | 92 |
| Adomi, 2008 [45] | Screening of the leaves of three Nigerian medicinal plants for antibacterial activity | https://www.ajol.info/index.php/ajb/article/view/59084 | 8 | 9 | 33 |
| Garcia-Gonzalez et al., 2006 [46] | Subchronic and acute preclinic toxicity and some pharmacological effects of the water extract from leaves of Petiveria alliacea (Phytolaccaceae) | https://doi.org//10.15517/rbt.v54i4.3108 | 9 | 11 | 18 |
| Gomes et al., 2005 [47] | Study of antinociceptive effect of isolated fractions from Petiveria alliacea L. (tipi) in mice | https://doi.org//10.1248/bpb.28.42 | 29 | 34 | 64 |
| Ruffa et al., 2002 [48] | Antiviral activity of Petiveria alliacea against the bovine viral diarrhea virus | https://doi.org//10.1159/000064920 | 10 | 12 | 28 |
| Lopes-Martins et al., 2002 [49] | The anti-inflammatory and analgesic effects of a crude extract of Petiveria alliacea L. (Phytolaccaceae) | https://doi.org//10.1078/0944-7113-00118 | 32 | 46 | 94 |
| Ruffa et al., 2002 [18] | Cytotoxic effect of Argentine medicinal plant extracts on human hepatocellular carcinoma cell line | https://doi.org//10.1016/S0378-8741(01)00400-7 | 149 | 171 | 302 |
| Benevides et al., 2001 [50] | Antifungal polysulphides from Petiveria alliacea L. | https://doi.org//10.1016/S0031-9422(01)00079-6 | 50 | 62 | 114 |
| Morales et al., 2001 [51] | Preliminary screening of five ethnomedicinal plants of Guatemala | https://doi.org//10.1016/S0014-827X(01)01107-7 | 11 | 13 | 21 |
| Queiroz et al., 2000 [52] | Cytokine profile and natural killer cell activity in Listeria monocytogenes infected mice treated orally with Petiveria alliacea extract | https://doi.org//10.3109/08923970009026008 | 10 | 11 | 24 |
| Quadros et al., 1999 [53] | Petiveria alliacea L. extract protects mice against Listeria monocytogenes infection-Effects on bone marrow progenitor cells | https://doi.org//10.3109/08923979909016397 | 15 | 16 | 35 |
| Caceres et al., 1998 [54] | Plants used in Guatemala for the treatment of protozoal infections. I. Screening of activity to bacteria, fungi and American trypanosomes of 13 native plants | https://doi.org//10.1016/S0378-8741(98)00140-8 | 60 | 67 | 187 |
| Berger et al., 1998 [55] | Plants used in Guatemala for the treatment of protozoal infections-II. Activity of extracts and fractions of five Guatemalan plants against Trypanosoma cruzi | https://doi.org//10.1016/S0378-8741(98)00011-7 | 42 | 48 | 100 |
| Villar et al., 1997 [56] | Screening of 17 Guatemalan medicinal plants for platelet antiaggregant activity | https://doi.org//10.1002/(SICI)1099-1573(199709)11:6<441::AID-PTR126>3.0.CO;2-T | 31 | 35 | 82 |
| Williams et al., 1997 [57] | Immunomodulatory activities of Petiveria alliacea L. | https://doi.org//10.1002/(SICI)1099-1573(199705)11:3<251::AID-PTR75>3.0.CO;2-B | 19 | 24 | 45 |
| Hoyos et al., 1992 [58] | Evaluation of the genotoxic effects of a folk medicine, Petiveria alliacea (Anamu) | https://doi.org//10.1016/0165-1218(92)90015-r | 14 | 15 | 48 |
| de Lima et al., 1991 [16] | Evaluation of antinociceptive effect of Petiveria alliacea (guine) in animals | https://doi.org//10.1590/S0074-02761991000600035 | 12 | 16 | 30 |
| Ferraz et al., 1991 [15] | The effectiveness of tipi in the treatment of hip and knee osteoarthritis-a preliminary-report | https://doi.org//10.1590/S0074-02761991000600054 | 4 | 5 | 11 |
2.2. Content Analysis
3. Discussion
4. Materials and Methods
4.1. Data Source and Collection
4.2. Establishment of Inclusion Criteria
4.3. Articles Selection
4.4. Bibliometric Analysis
4.5. Content Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Taylor, J.L.S.; Rabe, T.; McGaw, L.J.; Jäger, A.K.; Van Staden, J. Towards the Scientific Validation of Traditional Medicinal Plants. Plant Growth Regul. 2001, 34, 23–37. [Google Scholar] [CrossRef]
- Zavala-Ocampo, L.M.; Aguirre-Hernández, E.; López-Camacho, P.Y.; Cárdenas-Vázquez, R.; Dorazco-González, A.; Basurto-Islas, G. Acetylcholinesterase Inhibition and Antioxidant Activity Properties of Petiveria alliacea L. J. Ethnopharmacol. 2022, 292, 115239. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Xiao, P. Pharmaceutical Resource Discovery from Traditional Medicinal Plants: Pharmacophylogeny and Pharmacophylogenomics. Chin. Herb. Med. 2020, 12, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Mustika, A.; Fatimah, N.; Sari, G.M. The Self-Nanoemulsifying Drug Delivery System of Petiveria alliacea Extract Reduced the Homeostatic Model Assessment-Insulin Resistance Value, Interleukin-6, and Tumor Necrosis Factor-α Level in Diabetic Rat Models. Vet. World 2021, 14, 3229–3234. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Serrano, V.; McLaren, B.; Carrasco, J.C.; Alday, J.G.; Fiallos, L.; Amigo, J.; Onaindia, M. Traditional Ecological Knowledge and Medicinal Plant Diversity in Ecuadorian Amazon Home Gardens. Glob. Ecol. Conserv. 2019, 17, e00524. [Google Scholar] [CrossRef]
- Cal, B.B.F.; Araújo, L.B.N.; Nunes, B.M.; da Silva, C.R.; Oliveira, M.B.N.; Soares, B.O.; Leitão, A.A.C.; de Pádula, M.; Nascimento, D.; Chaves, D.S.A.; et al. Cytotoxicity of Extracts from Petiveria alliacea Leaves on Yeast. Plants 2022, 11, 3263. [Google Scholar] [CrossRef]
- Oyeleke, A.M.; Adeyemi, O.A.; Egbeyale, L.T.; Sobayo, R.A. Antimicrobial, Immunomodulatory and Hepatomodulatory Effects of Aqueous Extracts of Petiveria alliacea Root and Leaf on Growing Pullets. Span. J. Agric. Res. 2021, 19, e0502. [Google Scholar] [CrossRef]
- Cruz-Salomón, K.d.C.; Cruz-Rodríguez, R.I.; Espinosa-Juárez, J.V.; Cruz-Salomón, A.; Briones-Aranda, A.; Ruiz-Lau, N.; Ruíz-Valdiviezo, V.M. In Vivo and in Silico Study of the Antinociceptive and Toxicological Effect of the Extracts of Petiveria alliacea L. Leaves. Pharmaceuticals 2022, 15, 943. [Google Scholar] [CrossRef]
- Ileke, K.D.; Adu, B.; Olabimi, I.O. Bioefficacy of Two Indigenous Nigerian Botanicals on the Developmental Stages of Malaria Vector, Anopheles Gambiae Giles [Diptera: Culicidae]. Int. J. Trop. Insect Sci. 2021, 41, 999–1010. [Google Scholar] [CrossRef]
- Ballesteros-Ramírez, R.; Aldana, E.; Herrera, M.V.; Urueña, C.; Rojas, L.Y.; Echeverri, L.F.; Costa, G.M.; Quijano, S.; Fiorentino, S. Preferential Activity of Petiveria alliacea Extract on Primary Myeloid Leukemic Blast. Evid-Based Complement. Altern. Med. 2020, 2020, e4736206. [Google Scholar] [CrossRef]
- Caicedo-Pinto, P.; Lucena-Gallardo, D.F.; Correa-Rivera, M.A.; Yang-Yeung, K.; Terán, O.; Bonfante-Cabarcas, R. Efecto de Petiveria alliacea Y Drogas Colinérgicas Sobre La Habituación. Boletín Latinoam. Caribe Plantas Med. Y Aromát. 2019, 18, 595–606. [Google Scholar]
- Castañeda, R.; Cáceres, A.; Velásquez, D.; Rodríguez, C.; Morales, D.; Castillo, A. Medicinal Plants Used in Traditional Mayan Medicine for the Treatment of Central Nervous System Disorders: An Overview. J. Ethnopharmacol. 2022, 283, 114746. [Google Scholar] [CrossRef]
- Alves, T.C.; Rodrigues, E.; Lago, J.H.G.; Prado, C.M.; Girardi, C.E.N.; Hipólide, D.C. Petiveria alliacea, a Plant Used in Afro-Brazilian Smoke Rituals, Triggers Pulmonary Inflammation in Rats. Rev. Bras. Farmacogn. 2019, 29, 656–664. [Google Scholar] [CrossRef]
- Gutierrez, R.M.P.; Flores, J.M.M. Petiveria alliacea Suppresses Airway Inflammation and Allergen-Specific Th2 Responses in Ovalbumin-Sensitized Murine Model of Asthma. Chin. J. Integr. Med. 2018, 24, 912–919. [Google Scholar] [CrossRef]
- Ferraz, M.B.; Pereira, R.B.; Andrade, L.E.C.; Atra, E. The Effectiveness of Tipi in the Treatment of Hip and Knee Osteoarthritis: A Preliminary Report. Memórias Inst. Oswaldo Cruz 1991, 86 (Suppl. S2), 241–243. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Lima, T.C.M.; Morato, G.S.; Takahashi, R.N. Evaluation of Antinociceptive Effect of Petiveria alliacea (Guiné) in Animals. Memórias Inst. Oswaldo Cruz 1991, 86 (Suppl. S2), 153–158. [Google Scholar] [CrossRef]
- Prada, L.F.; Urueña, C.; Leal-Garcia, E.; Barreto, A.; Ballesteros-Ramírez, R.; Rodríguez-Pardo, V.M.; Fiorentino, S. Doxorubicin Activity Is Modulated by Traditional Herbal Extracts in a 2D and 3D Multicellular Sphere Model of Leukemia. Pharmaceutics 2023, 15, 1690. [Google Scholar] [CrossRef] [PubMed]
- Ruffa, M.J.; Ferraro, G.; Wagner, M.L.; Calcagno, M.L.; Campos, R.H.; Cavallaro, L. Cytotoxic Effect of Argentine Medicinal Plant Extracts on Human Hepatocellular Carcinoma Cell Line. J. Ethnopharmacol. 2002, 79, 335–339. [Google Scholar] [CrossRef]
- Olajubutu, O.G.; Ogunremi, B.I.; Adewole, A.H.; Awotuya, O.I.; Fakola, E.G.; Anyim, G.; Faloye, K.O. Correction To: Topical Anti-Inflammatory Activity of Petiveria alliacea, Chemical Profiling and Computational Investigation of Phytoconstituents Identified from Its Active Fraction. Chem. Afr. 2022, 5, 567. [Google Scholar] [CrossRef]
- García-Pérez, M.-E.; Alfonso-Castillo, A.; Lores, O.F.; Batista-Duharte, A.; Lemus-Rodríguez, Z. Toxicological Evaluation of an Aqueous Suspension from Leaves and Stems of Petiveria alliacea L. (Phytolaccaceae). J. Ethnopharmacol. 2018, 211, 29–37. [Google Scholar] [CrossRef]
- Lateef, A.; Folarin, B.I.; Oladejo, S.M.; Akinola, P.O.; Beukes, L.S.; Gueguim-Kana, E.B. Characterization, Antimicrobial, Antioxidant, and Anticoagulant Activities of Silver Nanoparticles Synthesized from Petiveria alliacea L. Leaf Extract. Prep. Biochem. Biotechnol. 2018, 48, 646–652. [Google Scholar] [CrossRef]
- Hartmann, I.; Da Silva, A.; Walter, M.E.; Jeremias, W.D.J. Investigation of the Larvicidal Effect of Guinea (Petiveria alliacea) on Larvae of Mosquitoes of the Species A. aegypti. Rev. Virtual Química 2018, 10, 529–541. [Google Scholar] [CrossRef]
- Flota-Burgos, G.J.; Rosado-Aguilar, J.A.; Rodríguez-Vivas, R.I.; Arjona-Cambranes, K.A. Anthelminthic Activity of Methanol Extracts of Diospyros Anisandra and Petiveria alliacea on Cyathostomin (Nematoda: Cyathostominae) Larval Development and Egg Hatching. Vet. Parasitol. 2017, 248, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Zavala-Ocampo, L.M.; Aguirre-Hernández, E.; Pérez-Hernández, N.; Rivera, G.; Marchat, L.A.; Ramírez-Moreno, E. Antiamoebic Activity of Petiveria alliacea Leaves and Their Main Component, Isoarborinol. J. Microbiol. Biotechnol. 2017, 27, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.F.; Urueña, C.P.; Sandoval, T.A.; Cifuentes, M.C.; Formentini, L.; Cuezva, J.M.; Fiorentino, S. A Cytotoxic Petiveria alliacea Dry Extract Induces ATP Depletion and Decreases β-F1-ATPase Expression in Breast Cancer Cells and Promotes Survival in Tumor-Bearing Mice. Rev. Bras. Farmacogn. 2017, 27, 306–314. [Google Scholar] [CrossRef]
- Gutierrez, R.M.P.; Vadillo, C.H. Anti-Inflammatory Potential of Petiveria alliacea on Activated RAW264.7 Murine Macrophages. Pharmacogn. Mag. 2017, 13, 174. [Google Scholar] [CrossRef]
- Luz, D.A.; Pinheiro, A.M.; Silva, M.L.; Monteiro, M.C.; Prediger, R.D.; Ferraz Maia, C.S.; Fontes-Júnior, E.A. Ethnobotany, Phytochemistry and Neuropharmacological Effects of Petiveria alliacea L. (Phytolaccaceae): A Review. J. Ethnopharmacol. 2016, 185, 182–201. [Google Scholar] [CrossRef]
- Murray, J.; Picking, D.; Lamm, A.; McKenzie, J.; Hartley, S.; Watson, C.; Williams, L.; Lowe, H.; Delgoda, R. Significant Inhibitory Impact of Dibenzyl Trisulfide and Extracts of Petiveria alliacea on the Activities of Major Drug-Metabolizing Enzymes in Vitro: An Assessment of the Potential for Medicinal Plant-Drug Interactions. Fitoterapia 2016, 111, 138–146. [Google Scholar] [CrossRef]
- Silva, M.L.; Luz, D.A.; da Paixão, T.P.; Silva, J.P.B.; Belém-Filho, I.J.A.; Fernandes, L.M.P.; Gonçalves, A.C.B.; Fontes-Júnior, E.A.; de Andrade, M.A.; Maia, C.S.F. Petiveria alliacea Exerts Mnemonic and Learning Effects on Rats. J. Ethnopharmacol. 2015, 169, 124–129. [Google Scholar] [CrossRef]
- Kerdudo, A.; Gonnot, V.; Ellong, E.N.; Boyer, L.; Michel, T.; Adenet, S.; Rochefort, K.; Fernandez, X. Essential Oil Composition and Biological Activities of Petiveria alliacea L. from Martinique. J. Essent. Oil Res. 2015, 27, 186–196. [Google Scholar] [CrossRef]
- Hernandez, J.F.; Urueña, C.; Cifuentes, M.C.; Sandoval, T.A.; Pombo, L.L.; Castañeda, D.; Asea, A.; Fiorentino, S. A Petiveria alliacea Standardized Fraction Induces Breast Adenocarcinoma Cell Death by Modulating Glycolytic Metabolism. J. Ethnopharmacol. 2014, 153, 641–649. [Google Scholar] [CrossRef]
- Christie, S.L.; Levy, A. Evaluation of the Hypoglycaemic Activity of Petiveria alliacea (Guinea Hen Weed) Extracts in Normoglycaemic and Diabetic Rat Models. West Indian Med. J. 2013, 62, 685–691. [Google Scholar] [PubMed]
- Fletes-Arjona, V.M.; Soto-Domínguez, A.; García-Garza, R.; Morán-Martínez, J.; Benítez-Valle, C.; Castañeda-Martínez, A.; Montalvo-González, R.; Becerra-Verdín, E.M. Alteraciones Morfológicas En El Tracto Respiratorio de Ratas Wistar Inducidas Por Vapores de La Raíz de Hierba Del Zorrillo (Petiveria alliacea) Del Suroeste de México. Int. J. Morphol. 2013, 31, 121–127. [Google Scholar] [CrossRef]
- Pacheco, A.O.; Moran, J.M.; Giro, Z.G.; Rodríguez, A.R.; Mujawimana, R.J.; González, K.T.; Frómeta, S.S. In Vitro Antimicrobial Activity of Total Extracts of the Leaves of Petiveria alliacea L. (Anamu). Braz. J. Pharm. Sci. 2013, 49, 241–250. [Google Scholar] [CrossRef]
- De Andrade, T.M.; de Melo, A.S.; Dias, R.G.C.; Varela, E.L.P.; de Oliveira, F.R.; Vieira, J.L.F.; de Andrade, M.A.; Baetas, A.C.; Monteiro, M.C.; Maia, C.D.S.F. Potential Behavioral and Pro-Oxidant Effects of Petiveria alliacea L. Extract in Adult Rats. J. Ethnopharmacol. 2012, 143, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Santander, S.P.; Hernández, J.F.; Barreto, C.C.; Masayuki, A.; Moins-Teisserenc, H.; Fiorentino, S. Immunomodulatory Effects of Aqueous and Organic Fractions from Petiveria alliacea on Human Dendritic Cells. Am. J. Chin. Med. 2012, 40, 833–844. [Google Scholar] [CrossRef]
- Adejumo, O.E.; Owa-Agbanah, I.S.; Kolapo, A.L.; Ayoola, M.D. Phytochemical and Antisickling Activities of Entandrophragma Utile, Chenopodium Ambrosioides and Petiveria alliacea. J. Med. Plants Res. 2011, 5, 1531–1535. [Google Scholar]
- Duharte, A.B.; Laffita, I.U.; Suárez, M.C.; Betancourt, J.F.; Zapata, E.; Castillo, A.; Martínez, H.S.; Soledad, M. Efecto Protector de Petiveria alliacea L. (Anamú) Sobre La Inmunosupresión Inducida Por 5-Fluoruracilo En Ratones Balb/C. Bol. Latinoam. Caribe Plantas Med. Aromat. 2011, 10, 256–264. [Google Scholar]
- Maia, C.S.; Teixeira, V.L.; Aguiar, Á.; Teles-Filho, N. Analysis of Fetal and Placental Development in Rats after Administration of Hydroalcoholic Extract from the Root of Petiveria alliacea L. (Phytolaccaceae). Int. J. Morphol. 2010, 28, 165–169. [Google Scholar] [CrossRef]
- Blainski, A.; Piccolo, V.K.; Mello, J.C.P.; de Oliveira, R.M.W. Dual Effects of Crude Extracts Obtained from Petiveria alliacea L. (Phytolaccaceae) on Experimental Anxiety in Mice. J. Ethnopharmacol. 2010, 128, 541–544. [Google Scholar] [CrossRef]
- Guedes, R.C.; Nogueira, N.G.; Fusco-Almeida, A.M.; Souza, C.R.; Oliveira, W.P. Antimicrobial Activity of Crude Extratcs of Petiveria alliacea L. Lat. Am. J. Pharm. 2009, 28, 520–524. [Google Scholar]
- Schmidt, C.; Fronza, M.; Goettert, M.; Geller, F.; Luik, S.; Flores, E.M.M.; Bittencourt, C.F.; Zanetti, G.D.; Heinzmann, B.M.; Laufer, S.; et al. Biological Studies on Brazilian Plants Used in Wound Healing. J. Ethnopharmacol. 2009, 122, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.B.; Noronha, E.C.; de Melo, C.T.V.; Bezerra, J.N.S.; Neto, M.A.; Lino, C.S.; Vasconcelos, S.M.M.; Viana, G.S.B.; de Sousa, F.C.F. Central Effects of Isolated Fractions from the Root of Petiveria alliacea L. (Tipi) in Mice. J. Ethnopharmacol. 2008, 120, 209–214. [Google Scholar] [CrossRef]
- Urueña, C.; Cifuentes, C.; Castañeda, D.; Arango, A.; Kaur, P.; Asea, A.; Fiorentino, S. Petiveria alliacea Extracts Uses Multiple Mechanisms to Inhibit Growth of Human and Mouse Tumoral Cells. BMC Complement. Altern. Med. 2008, 8, 60. [Google Scholar] [CrossRef]
- Adomi, P. Screening of the Leaves of Three Nigerian Medicinal Plants for Antibacterial Activity. Afr. J. Biotechnol. 2008, 7, 15. [Google Scholar]
- García-González, M.; Coto-Morales, T.; Ocampo, R.; Pazos, L. Subchronic and Acute Preclinic Toxicity and Some Pharmacological Effects of the Water Extract from Leaves of Petiveria alliacea (Phytolaccaceae). Rev. Biol. Trop. 2006, 54, 1323–1326. [Google Scholar] [CrossRef]
- Gomes, P.B.; Oliveira, M.M.d.S.; Nogueira, C.R.A.; Noronha, E.C.; Carneiro, L.M.V.; Bezerra, J.N.S.; Neto, M.A.; Vasconcelos, S.M.M.; Fonteles, M.M.F.; Viana, G.S.B.; et al. Study of Antinociceptive Effect of Isolated Fractions from Petiveria alliacea L. (Tipi) in Mice. Biol. Pharm. Bull. 2005, 28, 42–46. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruffa, M.; Perusina, M.; Alfonso, V.; Wagner, M.; Suriano, M.; Vicente, C.; Campos, R.; Cavallaro, L. Antiviral Activity of Petiveria alliacea against the Bovine Viral Diarrhea Virus. Chemoterapy 2002, 48, 144–147. [Google Scholar] [CrossRef]
- Lopes-Martins, R.; Pegoraro, D.; Woisky, R.; Penna, S.; Sertié, J. The Anti-Inflammatory and Analgesic Effects of a Crude Extract of Petiveria alliacea L. (Phytolaccaceae). Phytomedicine 2002, 9, 245–248. [Google Scholar] [CrossRef]
- Benevides, P.J.C.; Young, M.C.M.; Giesbrecht, A.M.; Roque, N.F.; Bolzani, V.d.S. Antifungal Polysulphides from Petiveria alliacea L. Phytochemistry 2001, 57, 743–747. [Google Scholar] [CrossRef]
- Morales, C.; Gómez-Serranillos, M.P.; Iglesias, I.; Villar, A.M.; Cáceres, A. Preliminary Screening of Five Ethnomedicinal Plants of Guatemala. Il Farm. 2001, 56, 523–526. [Google Scholar] [CrossRef]
- Queiroz, M.S.; Quadros, M.R.; Santos, L.M.B. Cytokine Profile and Natural Killer Cell Activity in Listeria Monocytogenes Infected Mice Treated Orally with Petiveria alliacea Extract. Immunopharmacol. Immunotoxicol. 2000, 22, 501–518. [Google Scholar] [CrossRef]
- Quadros, M.; Brito, A.R.M.S.; Queiroz, M.L.S. Petiveria alliacea I. Extract Protects Mice against Listeria Monocytogenes Infection-Effects on Bone Marrow Progenitor Cells. Immunopharmacol. Immunotoxicol. 1999, 21, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, A.; López, B.; González, S.; Berger, I.; Tada, I.; Maki, J. Plants Used in Guatemala for the Treatment of Protozoal Infections. I. Screening of Activity to Bacteria, Fungi and American Trypanosomes of 13 Native Plants. J. Ethnopharmacol. 1998, 62, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Berger, I.; Barrientos, A.C.; Cáceres, A.; Hernández, M.; Rastrelli, L.; Passreiter, C.M.; Kubelka, W. Plants Used in Guatemala for the Treatment of Protozoal Infections: II. Activity of Extracts and Fractions of Five Guatemalan Plants against Trypanosoma Cruzi. J. Ethnopharmacol. 1998, 62, 107–115. [Google Scholar] [CrossRef]
- Villar, R.M.; Calleja, J.L.; Morales, C.; Cáceres, A. Screening of 17 Guatemalan Medicinal Plants for Platelet Antiaggregant Activity. Phytoterapy Res. 1997, 11, 441–445. [Google Scholar] [CrossRef]
- Williams, L.A.D.; The, T.L.; Gardner, M.T.; Fletcher, C.K.; Naravane, A.; Gibbs, N.; Fleishacker, R. Immunomodulatory Activities of Petiveria alliacea L. Phytother. Res. 1997, 11, 251–253. [Google Scholar] [CrossRef]
- Hoyos, L.S.; Au, W.W.; Heo, M.Y.; Morris, D.L.; Legator, M.S. Evaluation of the Genotoxic Effects of a Folk Medicine, Petiveria alliacea (Anamu). Mutat. Res./Genet. Toxicol. 1992, 280, 29–34. [Google Scholar] [CrossRef]
- Sahoo, N.; Manchikanti, P.; Dey, S. Herbal Drugs: Standards and Regulation. Fitoterapia 2010, 81, 462–471. [Google Scholar] [CrossRef]
- Donthu, N.; Kumar, S.; Mukherjee, D.; Pandey, N.; Lim, W.M. How to Conduct a Bibliometric Analysis: An Overview and Guidelines. J. Bus. Res. 2021, 133, 285–296. [Google Scholar] [CrossRef]
- Figueiredo, R.; Luiz, O.; Vieira-Neto, J.; Ferreira, J.J. The Role of Knowledge Intensive Business Services in Economic Development: A Bibliometric Analysis from Bradford, Lotka and Zipf Laws. Gestão Produção 2019, 26. [Google Scholar] [CrossRef]
- Egghe, L. The Exact Place of Zipf’s and Pareto’s Law amongst the Classical Informetric Laws. Scientometrics 1991, 20, 93–106. [Google Scholar] [CrossRef]
- Lotka, A.J. The Frequency Distribution of Scientific Productivity. J. Frankl. Inst. 1926, 202, 271. [Google Scholar] [CrossRef]
- Kawamura, M.; Thomas, C.D.L.; Tsurumoto, A.; Sasahara, H.; Kawaguchi, Y. Lotka’s Law and Productivity Index of Authors in a Scientific Journal. J. Oral Sci. 2000, 42, 75–78. [Google Scholar] [CrossRef]
- Brookes, B.C. Bradford’s Law and the Bibliography of Science. Nature 1969, 224, 953–956. [Google Scholar] [CrossRef] [PubMed]
- Nongrang, K.; Laloo, B. Bibliometric Study of Biochemistry Literature in North Eastern Hill University during 2000 to 2010. COLLNET J. Scientometr. Inf. Manag. 2016, 10, 197–207. [Google Scholar] [CrossRef]
- Belter, C.W. Bibliometric Indicators: Opportunities and Limits. J. Med. Libr. Assoc. JMLA 2015, 103, 219–221. [Google Scholar] [CrossRef]
- Palhares, R.M.; Baratto, L.C.; Scopel, M.; Mügge, F.L.B.; Brandão, M.G.L. Medicinal Plants and Herbal Products from Brazil: How Can We Improve Quality? Front. Pharmacol. 2021, 11, 606623. [Google Scholar] [CrossRef]
- Howes, M.R.; Quave, C.L.; Collemare, J.; Tatsis, E.C.; Twilley, D.; Lulekal, E.; Farlow, A.; Li, L.; Cazar, M.; Leaman, D.J.; et al. Molecules from Nature: Reconciling Biodiversity Conservation and Global Healthcare Imperatives for Sustainable Use of Medicinal Plants and Fungi. Plants People Planet 2020, 2, 463–481. [Google Scholar] [CrossRef]
- Carmona, F.; Pereira, A.M.S. Herbal Medicines: Old and New Concepts, Truths and Misunderstandings. Rev. Bras. Farmacogn. 2013, 23, 379–385. [Google Scholar] [CrossRef]
- Braga, F.C. Brazilian Traditional Medicine: Historical Basis, Features and Potentialities for Pharmaceutical Development. J. Tradit. Chin. Med. Sci. 2020, 8, S44–S50. [Google Scholar] [CrossRef]
- Maia, M.L.F.; Pantoja, L.V.P.S.; Da Conceição, B.C.; Machado-Ferraro, K.M.; Gonçalves, J.K.M.; Dos Santos-Filho, P.M.; Lima, R.R.; Fontes-Junior, E.A.; Maia, C.S.F. Ketamine Clinical Use on the Pediatric Critically Ill Infant: A Global Bibliometric and Critical Review of Literature. J. Clin. Med. 2023, 12, 4643. [Google Scholar] [CrossRef] [PubMed]

| Journals | IF a | Number of Articles | Number of Citations | Citation (%) b |
|---|---|---|---|---|
| African Journal of Biotechnology | * | 1 | 8 | 0.79 |
| American Journal of Chinese Medicine | 5.7 | 1 | 11 | 1.09 |
| Biological & Pharmaceutical Bulletin | 2 | 1 | 29 | 2.87 |
| Bmc Complementary and Alternative Medicine | * | 1 | 39 | 3.86 |
| Boletin Latinoamericano y del Caribe de Plantas Medicinales y Aromaticas | 0.7 | 2 | 4 | 0.40 |
| Brazilian Journal of Pharmaceutical Sciences | 1.3 | 1 | 8 | 0.79 |
| Brazilian Journal of Pharmacognosy | 1.6 | 2 | 14 | 1.39 |
| Chemistry Africa | 2.6 | 1 | 4 | 0.40 |
| Chemotherapy | 3.3 | 1 | 10 | 0.99 |
| Chinese Journal of Integrative Medicine | 2.9 | 1 | 2 | 0.20 |
| Evidence-Based Complementary and Alternative Medicine | * | 1 | 2 | 0.20 |
| Farmaco | * | 1 | 11 | 1.09 |
| Fitoterapia | 3.4 | 1 | 19 | 1.88 |
| Immunopharmacology and Immunotoxicology | 3.3 | 2 | 25 | 2.48 |
| International Journal of Morphology | 0.5 | 2 | 5 | 0.50 |
| International Journal of Tropical Insect Science | 1.2 | 1 | 1 | 0.10 |
| Journal of Essential Oil Research | 3 | 1 | 9 | 0.89 |
| Journal of Ethnopharmacology | 5.4 | 13 | 515 | 50.99 |
| Journal of Medicinal Plants Research | * | 1 | 12 | 1.19 |
| Journal of Microbiology and Biotechnology | 2.8 | 1 | 10 | 0.99 |
| Latin American Journal of Pharmacy | 0.2 | 1 | 3 | 0.30 |
| Memorias do Instituto Oswaldo Cruz | 2.8 | 2 | 16 | 1.58 |
| Mutation Research | * | 1 | 14 | 1.39 |
| Pharmaceuticals | 4.6 | 1 | 0 | 0.00 |
| Pharmaceutics | 5.4 | 1 | 0 | 0.00 |
| Pharmacognosy Magazine | 0.7 | 1 | 20 | 1.98 |
| Phytochemistry | 3.8 | 1 | 50 | 4.95 |
| Phytomedicine | 7.9 | 1 | 32 | 3.17 |
| Phytotherapy Research | 7.2 | 2 | 50 | 4,95 |
| Plants-Basel | 4.5 | 1 | 0 | 0.00 |
| Preparative Biochemistry & Biotechnology | 2.9 | 1 | 63 | 6.24 |
| Revista de Biologia Tropical | 0.6 | 1 | 9 | 0.89 |
| Revista Virtual de Quimica | 0.5 | 1 | 1 | 0.10 |
| Spanish Journal of Agricultural Research | 0.9 | 1 | 1 | 0.10 |
| Veterinary Parasitology | 2.6 | 1 | 7 | 0.69 |
| Veterinary World | 1.6 | 1 | 0 | 0.00 |
| West Indian Medical Journal | 0.1 | 1 | 6 | 0.59 |
| Authors/Year | Design | Phytochemical Approach | Protocol Used | Pharmacological Activity | Study Summary | |||
|---|---|---|---|---|---|---|---|---|
| Plant Parts Used | Type of Extraction | Phytochemical Constitution | Dose/ Concentration | Route and Period of Administration | ||||
| Clinical study | ||||||||
| Ferraz et al., 1991 [15] | Randomized clinical trial | Leaves and stems | Infusion method; Solvent: water | Not investigated | 200 mL of 15 mg/mL solution | Oral administration; 3 times a day, for 3 weeks | Antinociceptive study | In this study, the authors investigated the antinociceptive potential of P. alliacea in 14 patients with osteoarthritis. The authors reported no statistically significant evidence that P. alliacea was superior to placebo in reducing the severity of pain. However, the authors emphasized the possibility that the small sample of patients compromised the statistical analysis performed. |
| In vivo study | ||||||||
| de Lima et al., 1991 [16] | Pre-clinical in vivo study (male and female mice) | Roots | Infusion method; Solvent: water | Not investigated | 125–2000 mg/kg | Oral administration (gavage) or parenteral administration (intraperitoneal); Single dose | Antinociceptive study | In this study, the authors evaluated the effects of aqueous crude extract of P. alliacea on sedative and analgesic properties in mice and rats. The authors stated that the antinociceptive effect in acetic acid, acetylcholine, and hypertonic saline induced animal constrictions but not in hot-plate and tail flick tests. The authors also reported that P. alliacea did not produce any CNS depressor effect. |
| Queiroz et al., 2000 [52] | Pre-clinical in vivo study (male mice) | Roots | Maceration method; Solvents: ethanol and water | Not investigated | 1000 mg/kg | Oral administration (gavage); Daily for 5 days, once a day | Immunomodulatory study | In this study, the authors investigated the effects of P. alliacea extract on the production of Th1-type and Th2-type cytokines and on NK cell activity in normal and infected mice. The authors suggest that P. alliacea administration up-regulates antibacterial immune response by enhancing both Th1 function and the activity of NK cells. |
| Morales et al., 2001 [51] | Pre-clinical in vivo study (male and female mice) | Roots | Maceration and percolation method; Solvents: hexane and chloroform, methanol and ethanol, water | Not investigated | 1.25 g/kg | Parenteral administration (Intraperitoneal); Single dose | Neuropharmacological study | In this study, the authors screened four different South American plants to investigate neuropharmacological activities through the Irwin test method. Regarding P. alliacea, the authors stated that the extract from roots only lightly decreased spontaneous motor activity, and the leaf extract showed hyperexcitability. The authors emphasize that P. alliacea has little effect on behavioral, neurologic, and autonomic signs compared to other plants studied. |
| Lopes-Martins et al., 2002 [49] | Pre-clinical in vivo study (male rats) | Roots | Percolation method; Solvents: ethanol (66.7%) | Not investigated | 31.4 and 43.9 mg/kg | Oral administration (gavage), single dose | Anti-inflammatory and analgesic study | In this study, the authors investigated the anti-inflammatory properties of P. alliacea crude extract administered to rats with pleurisy. The authors reported that the oral administration did not reduce the total number of leukocytes at the doses tested. However, the highest dose (43.9 mg/kg) reduced mononuclear cell migration, in addition to having an analgesic effect. The authors emphasized that the results provide a basis for folk medicine use, but further studies are necessary to elucidate the mechanism of anti-inflammatory and analgesic actions. |
| Gomes et al., 2005 [47] | Pre-clinical in vivo study (female mice) | Roots | Maceration method; Solvents: ethanol and water; Fractionation process with hexane and ethyl acetate | Not investigated | 100 and 200 mg/kg | Parenteral administration (Intraperitoneal), single dose | Antinociceptive study | In this study, the authors evaluated four fractions (acetate, hexanic, hydroalcoholic, and precipitated hydroalcoholic) from P. alliacea roots for antinociceptive effects. The authors also evaluated psychomotor and myorelaxant activities. It was reported that all fractions showed reduced locomotor activity on the open field test; however, different fractions had different antinociceptive potentials. |
| García-Gonzáles et al., 2006 [46] | Pre-clinical in vivo study (male mice) | Leaves | Infusion method; Solvents: water | Not investigated | 1000 and 2000 mg/kg (both acute toxicity and sub-chronic toxicity) | Oral administration (gavage); 5 consecutive days for 3 weeks (acute toxicity) and 5 consecutive days for 8 weeks | Toxicological study (acute and sub-chronic) | In this study, the authors tested the effects of an aqueous extract of P. alliacea on acute and sub-chronic toxicity in male mice. The authors reported that no mortality nor any toxicity signs could be observed. The authors also stated that no significant differences in intestinal motility or blood glucose levels could be found. |
| Gomes et al., 2008 [43] | Pre-clinical in vivo study (female mice) | Roots | Maceration method; Solvents: ethanol and water; Fractionation process with hexane and ethyl acetate | Not investigated | 100 and 200 mg/kg | Parenteral administration (Intraperitoneal) and oral administration (gavage); Single dose for each route of administration | Neuropharmacological study | In this study, the authors investigated the neuropharmacological properties of fractions from P. alliacea hydroalcoholic extract. The authors reported that all fractions suggested central depressant activity on the open field test and indicated an absence of anxiolytic-like effect on the elevated plus-maze test. The authors conclude that P. alliacea contains biological substances that have significant depressant and anticonvulsant potentials, supporting folk medicine use. |
| Blainski et al., 2010 [40] | Pre-clinical in vivo study (male mice) | Whole plant, aerial parts, and roots | Turbo-extraction method; Solvent: ethanol (50%) | Not investigated (only total flavonoid content) | 300, 600, and 900 mg/kg (All extracts) | Oral administration (gavage); Single dose | Behavioral study-anxiolytic activity | In this study, the authors investigated the behavioral effects of three different types of extracts from P. alliacea in mice using the open field and elevated plus maze apparatus. The authors described that the whole plant extract caused anxiolytic-like effects, and the aerial parts extract induced anxiogenic-like effects. The authors also suggested that the total flavonoid content may have a pivotal role in the obtained results, but further study is required. |
| Maia et al., 2010 [39] | Pre-clinical in vivo study (female rats) | Roots | Hydroalcoholic extract (preparation not described) | Not investigated | 18 mg/kg | Oral administration (gavage), single dose, 5th day of pregnancy | Toxicological study | In this study, the authors investigated the effect of a hydroalcoholic extract of P. alliacea roots on fetal and placental development in female rats. The extract caused a significant reduction in the number of implantation sites but no histological alterations in these sites and placenta. The authors also observed no significant alterations in the number, length, or weight of the offspring. |
| Duharte et al., 2011 [38] | Pre-clinical in vivo study (female mice) | Leaves and stems | There was no extraction process. The plant drug (P. alliacea) was dissolved in a solution of carboxymethylcellulose and water | Not investigated | 400 and 1200 mg/kg | Oral administration (gavage); Daily administration for 8 days, once a day | Immunomodulatory study | In this study, the authors determined the protective properties of P. alliacea on 5-FU immunosuppressed animals. The authors found that the group treated with the highest dose of P. alliacea was less affected by 5-FU-induced immunosuppression compared with the other treated groups. These results suggest that the plant could be used in patients under antineoplastic regimens to avoid the deleterious adverse effects of immunosuppressive drugs. |
| de Andrade et al., 2012 [35] | Pre-clinical in vivo study (female and male rats) | Leaves, roots, and stems | Maceration method; Solvent: ethanol (70%) | Not investigated | 2000 mg/kg or 5000 mg/kg (Oral acute toxicity); 900 mg/kg (behavioral assays) | Oral administration (gavage) for both acute toxicity and behavioral assays; Single dose for both assays | Behavioral plus oxidative study | In this study, the authors evaluated the behavioral and oxidative stress effects of hydroalcoholic extracts of P. alliacea in rats. The authors reported increased locomotor activity, as well as antidepressant and anxiolytic, in behavioral tests. The oxidative stress assessment showed pro-oxidative effects in vivo and in vitro. The authors also suggest that the polyphenols found in P. alliacea may have neuronal mechanisms that potentially regulate anxiety as well as oxidative stress. |
| Fletes-Arjona et al., 2013 [33] | Pre-clinical in vivo study (male rats) | Roots | There was no extraction process. The roots were used fresh | Not investigated | 150 mg | Inhalation route; Single exposure for 3 min | Toxicological study (acute) | In this study, the authors evaluated the morphologic alterations in the respiratory tract of female rats induced by the inhalation of P. alliacea steams. The authors reported that the main histological and morphological alterations were hyperplasia on the trachea epithelium, signs of elevated secretion from goblet cells, and vascular congestion on the bronchiole. The authors also suggest that the morphologic alterations happened due to coumarins present in the chemical composition of P. alliacea. |
| Christie and Levy, 2013 [34] | Pre-clinical in vivo study (male rats) | Leaves | Decoction method; Solvent: water | Not investigated | 200 and 400 mg/kg | Oral administration (gavage); Single dose | Antidiabetic study | In this study, the authors evaluated the hypoglycemic effect of P. alliacea on a model of diabetes induced with streptozotocin, as well as the effect of P. alliacea in normoglycemic female rats. The authors report that they did not observe a hypoglycemic effect in the model used. The authors state that the results are contrary to the medicinal use of the species, which does not support the use of P. alliacea for hypoglycemic purposes. |
| Silva et al., 2015 [29] | Pre-clinical in vivo study (female and male rats) | Leaves | Maceration method; Solvent: ethanol (70%) | Organosulfur compounds | 900 mg/kg | Oral administration (gavage); Single dose | Behavioral study—procognitive activity | In this study, the authors investigated the activities of P. alliacea on cognition (learning and memory). The authors reported that there were effects on the memory process and improved learning in the tests performed in the study. They also emphasized the importance of studies that investigate the pharmacological mechanisms involved in these observed activities. |
| Gutierrez and Flores, 2018 [14] | Pre-clinical in vivo study (male mice) | Leaves | Soxhlet extraction (SE); Solvent: methanol | Not investigated | 100, 200, and 400 mg/kg | Oral administration (gavage); Daily administration for 6 days, once a day | Anti-inflammatory and immunomodulatory study | In this study, the authors proposed to investigate the effect of P. alliaceae in a murine model of chronic asthma to assess the impacts on airway inflammation. The authors report that P. alliaceae can inhibit airway inflammation and regulate chemokines and cytokines, improving lung conditions in the asthma model used. |
| Garcia-Perez et al., 2018 [20] | Pre-clinical in vivo study (female and male rats) | Leaves and stems | Soxhlet extraction (SE); Solvent: ethanol (96%). Fractionation process with hexane, ethyl acetate, and water | Hexanic fraction: Phytol, (R)-(-)-(Z)-14-Methyl-8-hexadecen-1-ol, 3-Eicosyne, (3,7,11,15-Tetramethyl-2-hexadecen-1-ol), 1R,2c,3t,4t-Tetramethyl-cyclohexaxe; Ethyl acetate fraction: Phytol, (R)-(-)-(Z)-14-Methyl-8-hexadecen-1-ol, 3-Eicosyne, (3,7,11,15-Tetramethyl-2-hexadecen-1-ol), 2-Fluorobenzoic acid, 2-formyl-4,6- dichlorophenyl ester; Aqueous fraction: Methyl β-dimethylaminoisobutyrate, 1-(2-Hydrohyethyl)-1,2,4-triazole, 2-butanone,4-(dimethylamino)-3-methyl, Cis-3-Hydroxy-2-propylpiperidine | For the acute toxicity test, P. alliacea powder was administered at 2000 mg/kg. For the repeated oral toxicity test, the dose of 1000 mg/kg was chosen | Oral administration (gavage); Acute toxicity: single dose; Repeated toxicity: daily administration for 28 days, once a day | Toxicological study (acute and subchronic) | In this study, the authors investigated the acute and repeated toxicity of a powdered suspension of leaves and stems of P. alliacea. The authors reported that there was no death of the animals and no adverse effects that impacted the weight, general condition, and histopathological characteristics of the animals were observed. |
| Alves et al., 2019 [13] | Pre-clinical in vivo study (male rats) | Whole plant | No extraction method was adopted. Petiveria alliacea powder (vegetable drug) was used | For the phytochemical characterization, the authors used fresh leaves of P. alliacea, in which the following were identified: Dimethylsulfide, Diethylsulfide, α-Pinene, ß-Pinene, Di-n-propylsulfide and Nerolidol | 25 or 50 mg of the dried plant were burned to charcoal in a specific inhalation device | Inhalation route: Single exposure (each animal remained for 60 s in the inhalation chamber) | Behavioral study (anxiolytic activity) plus toxicological study (evaluation of lung inflammation) | In this study, the authors investigated the composition and exposure of animals to P. alliacea smoke. The objective of the study was to evaluate the potential anxiolytic and toxic effects of this exposure. The authors reported that there was no anxiolytic effect in the animals, but after histological analysis, there was possible pulmonary inflammation. |
| Caicedo-Pinto et al., 2019 [11] | Pre-clinical in vivo study (male rats) | Leaves | Infusion method; Solvent: water | Not investigated | The average consumption of P. alliacea infusion per animal was 7.59 mL | Oral administration (via drinking water); Administration period not specified | Behavioral study—anxiolytic activity | In this study, the authors evaluated the potential effect of P. alliacea and cholinergic drugs on cognitive behavior. The authors described that P. alliacea had an anxiolytic and antidepressant effect, and when combined with nicotine, it demonstrated potentiation of the anxiogenic effect. |
| Oyeleke et al., 2021 [7] | Pre-clinical in vivo study (female chickens) | Roots and leaves | Maceration method; Solvent: water | Not investigated | 15, 30, and 45 g/L (both root and leaf extract) | Oral administration (via drinking water); Twice per week for 10 weeks | Antimicrobial, immunomodulatory, and hepatomodulatory study | In this study, the authors investigated the antimicrobial, immunomodulatory, and hepatomodulatory activities of P. alliacea. Based on tests, the authors demonstrated that P. alliacea has the activities they set out to investigate. |
| Mustika et al., 2022 [4] | Pre-clinical in vivo study (male rats) | Leaves | Maceration method; Solvent: ethanol (70%) | Not investigated | 50, 100, and 200 mg/kg | Oral administration; 14 days (once a day) | Anti-inflammatory study | In this study, the authors investigated the extract of P. alliacea using a self-nanoemulsifying drug release model in an animal model with insulin resistance. The study evaluates the levels of interleukin (IL)-6 and TNF-α with the aim of evaluating the effects of P. alliacea on inflammation associated with diabetes. The authors reported that the proposed model enabled the reduction in insulin resistance, as well as suppressed the levels of (IL)-6 and TNF-α. |
| In vitro study | ||||||||
| Villar et al., 1997 [56] | Pre-clinical in vitro study (human platelets) | Leaves | Decoction method; Solvents: water | Oxidable compounds | 4 μL | Not applicable | Hematological study | In this study, the authors assayed 17 different aqueous plant extracts used in Guatemala for platelet antiaggregant activity. Regarding P. alliacea, the authors stated that the aqueous extract produced an inhibitory effect on the aggregation of washed human platelets induced by thrombin in a concentration-dependent form. |
| Benevides et al., 2001 [50] | Pre-clinical in vitro study (Phytochemical isolation) | Leaves, stems, and roots | Maceration method; Solvents: dichloromethane, methanol; Fractionation process with methanol, water, chloroform, ethyl acetate, and hexane | Dipropyl disulfide, Dibenzyl sulfide, Dibenzyl disulfide, Dibenzyl trisulfide, Dibenzyl tetrasulfide, enzyl hydroxymethyl sulfide, Di(benzyltrithio) methane | 20, 10, 1, and 0.1 μg for pure compounds and 100, 50, 30, and 20 μg for the crude extracts or fractions, respectively | Not applicable | Antimicrobial and phytochemical study | In this study, the authors conducted a phytochemical investigation on the extract of P. alliacea roots, isolating five substances known as polysulfides. The authors also tested different fractions for antifungal activity. The authors reported that the fractionation led to the isolation of dipropyl disulfide, dibenzyl disulfide, dibenzyl trisulfide, benzylhydroxymethyl sulfide, and di(benzyltrithio) methane, the last three substances being new compounds. |
| Ruffa et al., 2002 [17] | Pre-clinical in vitro study (liver cells) | Leaves | Maceration method; Solvents: methanol | Not investigated | 15.5–1000 μg/mL | Not applicable | Cytotoxicity study | In this study, the authors evaluated eight different methanolic extracts on cytotoxic effects on hepatocellular carcinoma cell lines. Regarding P. alliacea, the authors stated that the extract did not show any inhibitory effect on Hep G2 in the range of doses assayed (15.5–1000 μg/mL). |
| Ruffa et al., 2002 [48] | Pre-clinical in vitro study (bovine cells) | Leaves and stems | Maceration method; Solvents: methanol; Fractionation process with ether oil | Not investigated | 10 mg/mL | Not applicable | Antiviral study | In this study, the authors assayed five different medicinal plants used in Argentina to detect the inhibition of viral growth. Regarding P. alliacea, the authors stated that the extract inhibited bovine viral diarrhea virus replication but showed no activity against herpes simplex virus type 1, poliovirus type 1, adenovirus serotype 7, and vesicular stomatitis virus type 1. The authors conclude that, among the five different plants studied, P. alliacea was worth studying in the future. |
| Adomi, 2008 [45] | Pre-clinical in vitro study (bacteria) | Leaves | Maceration method; Solvents: water and absolute ethanol | Thiobenzaldehyde s-oxide, senfol, coumarin, pinitol, dibenzyl sulfide, s-(2-hydroxyethy)phenylmethaenethiosulfinate), 3,5-diphenyltritiolan, dibenzyl trisulfide, leridol 5-methyl ether, 4-ethylpetiveral, glutamyl-S-benzyl cysteine, lignoceric acid, myricitrin | 250–1000 mg/mL | Not applicable | Antibacterial study | In this study, the author screened three plants for antibacterial activity. Regarding P. alliacea, the author reported that both aqueous and ethanolic extracts did not show antibacterial activity. |
| Urueña et al., 2008 [44] | Pre-clinical in vitro study (tumoral cells) | Leaves and stems | Reflux method; Solvents: ethanol (96%); Fractionation process with ethyl acetate | Not investigated | 125 μg/mL | Not applicable | Anticancer study | In this study, the authors assessed multiple in vitro biological activities of an ethyl acetate fraction of P. alliacea over tumoral cell lines. The authors reported that the fraction altered actin cytoskeleton organization, inducing G2 cell cycle arrest and causing apoptotic cell death. The authors also suggested that P. alliacea exerts multiple biological activities and has potential as an antitumor agent. |
| Schimdt et al., 2009 [42] | Pre-clinical in vitro study (T cell lineage and fibroblasts; bacteria) | Leaves, aerial parts, and flowers | Soxhlet and ultra-sound method; Solvents: n-hexane, ethanol | Not investigated | 100 μg/mL | Not applicable | Immunodulatory and antimicrobial study | In this study, the authors investigated twelve plants used in Brazilian traditional medicine for various biological activities. Regarding P. alliacea, the authors reported that ethanolic extract showed promising wound-healing properties related to TNF-α suppression and Nf-κB binding. |
| Guedes et al., 2009 [41] | Pre-clinical in vitro study (microorganisms) | Aerial parts | Dynamic maceration method; Solvents: hexane, methylic alcohol, ethylic alcohol, ethanol (70%), purified water | Not investigated (total flavonoid and polyphenol) | 240–3960 μg/mL | Not applicable | Antimicrobial study | In this study, the authors evaluated the antifungal and antibacterial activity of several crude leaf extracts of P. alliacea against different strains of bacteria and yeasts. The authors reported that promising results were shown for the 70% ethanolic extract, which presented a minimum inhibitory concentration from 250 to 760 μg/mL for yeast. |
| Adejumo et al., 2011 [37] | Pre-clinical in vitro study (sickle cell) | Roots | Maceration method; Solvent: methanol. Fractionation process with methanol-water mixture and chloroform | Alkaloids, saponins, and tannins | 10, 1.0, and 0.1 mg/mL (both methanolic extract and aqueous fraction) | Not applicable | Hematological study | In this study, the authors evaluated the anticycling activities of aqueous fractions of P. alliacea on hematological cells. The authors reported that three fractions showed significant anticycling activity. The phytochemical screening revealed the presence of saponins, tannins, and alkaloids. The authors also emphasize that the popular use of P. alliacea for sickle cell disease is justified. |
| Santander et al., 2012 [36] | Pre-clinical in vitro study (monocytic cells) | Leaves and stems | Soxhlet extraction (SE); Solvent: ethanol (96%). Fractionation process with ethyl acetate (organic fraction) and water (aqueous fraction) | Not investigated | 31.2, 62.5, 125, 250 and 500 μg/mL (both aqueous and organic fractions) | Not applicable | Immunomodulatory study | In this study, the authors evaluated the immunomodulatory activity of aqueous and organic plant fractions from P. alliacea using human monocyte-derived dendritic cells. The authors found that the aqueous fraction induced morphological changes in dendritic cells and pro-inflammatory cytokines secretion. The fraction also increased NF-kB gene expression while down-regulating TGF β gene expression. The authors also emphasize that the organic fraction by itself showed no immunomodulatory activity. |
| Pacheco et al., 2013 [34] | Pre-clinical in vitro study (microorganisms) | Leaves | In this study, soft extracts and blended extracts were prepared; Solvents: hydroalcoholic solution and isopropyl alcohol (soft extracts); hydroalcoholic solution (blended extracts) | Not investigated | 10 μL of each extract | Not applicable | Antimicrobial study | In this study, the authors evaluated the antimicrobial activity of 13 different extracts from P. alliacea. The authors reported that nine extracts presented antibacterial activity, with blended extracts more potent than soft extracts. The authors also reported that B8 and E3, the most concentrated extracts, had the greatest spectrum of antibacterial action, being related to apolar substances. |
| Kerdudo et al., 2015 [30] | Pre-clinical in vitro study (microorganisms) | Aerial parts | Hydrodistillation method (Clevenger apparatus). Solvent: water | Benzaldehyde, toluenethiol, 1,2,5-trithiepane, dibenzyl disulfide, phytol, ethyl linolenate, dibenzyl trisulfide | 0.5% and 0.05% w/v of essential oil | Not applicable | Antimicrobial study | In this study, the authors investigated the potential antimicrobial activity of P. alliacea from different regions of Martinique. The authors reported that the essential oil extracted from one of the selected sites inhibited the growth of several microorganisms, including some multiresistant strains. The authors also commented on the importance of studies with fractionated oil to identify the antimicrobial molecules involved in the observed activity. |
| Murray et al., 2016 [28] | Pre-clinical in vitro study (enzymatic assay) | Whole plant | Three types of extract were prepared: aqueous (whole plant, roots), 65% ethanolic (whole plant, leaves and trunk, roots), and 96.5% ethanolic (roots only). A tincture was also prepared using rum as solvent (alc/vol = 63%). In total, seven extracts were prepared; Aqueous type extracts were prepared by the infusion method; the ethanolic ones, by maceration | Not investigated | In this paper, the adopted concentrations were not clearly presented | Not applicable | Pharmacokinetic study | In this study, the authors carried out an analysis of the effects of dibenzyl trisulfide (DTS) on the activities of the cytochrome P450 enzyme, an important enzyme for the process of drug metabolism. The authors demonstrate in their study that DTS acts as a cyp450 inhibitor and consider P. alliacea a valuable species for many studies that seek to investigate the impact of high DTS content on drug interactions important for clinical therapy. |
| Gutierrez and Hoyo-Vadillo, 2017 [26] | Pre-clinical in vitro study (RAW 264.7 murine macrophage cells) | Leaves | Soxhlet extraction (SE); Solvent: ethanol | Not investigated | 50, 100 and 200 μg/mL | Not applicable | Anti-inflammatory study | In this study, the authors evaluated P. alliacea in a murine model of RAW264 macrophages to analyze whether there is inflammation attenuation. The authors report that P. alliacea suppressed the secretion of prostaglandin and other inflammatory mediators. With the results obtained in this work, the authors state that P. alliacea has antioxidant activity and great anti-inflammatory potential. |
| Flota-Burgos et al., 2017 [23] | Pre-clinical in vitro study (nematode larvae) | Leaves and stems | Maceration method; Solvent: methanol | Not investigated | 600, 300, 150, 75, 37.5, 18.7, and 9.3 μg/ml | Not applicable | Anthelmintic study | In this study, the authors evaluated the ability of P. alliacea to control nematodes. The authors reported that in their study, they observed a strong effect of preventing the hatching of eggs when treated with P. alliacea, which is a response to the hypothesis proposed in the study, the use of plants as an alternative for anthelmintic treatment. |
| Hartmann et al., 2018 [22] | Pre-clinical in vitro study (mosquito larvae) | Leaves | Percolation method; Solvents: water and ethanol (hydroalcoholic solution) | Not investigated | 1%, 3%, 5%, 10%, 25%, and 50% v/v. | Not applicable | Anti-dengue study | In this study, the authors evaluated the larvicidal capacity of P. alliacea. The authors report that in this study, the cytotoxic effect of P. alliacea on Aedes aegypti larvae was confirmed, which proves its larvicidal activity. |
| Lateef et al., 2018 [21] | Pre-clinical in vitro study (microorganisms and enzymatic assays) | Leaves | Infusion method; Solvent: water | Not investigated | 5, 8, 10, 12, and 15 μg/mL (Antibacterial assay); 100 and 150 μg/mL (Antifungal assay); 100 μg/mL (Anticoagulant assay); 5, 10, 20 and 40 μg/mL (DPPH assay); 1, 2, 5, 10, and 15 μg/mL (Hydrogen peroxide scavenging activity) | Not applicable | Antimicrobial and anticoagulant study | In this study, the authors investigated the synthesis of silver nanoparticles using P. alliacea. The authors report that the described model showed 100% inhibition against Klebsiella pneumoniae, Escherichia coli, and Staphylococcus aureus, as well as antifungal activity and prevented human blood clotting, which confirms the antimicrobial and anticoagulant activities of P. alliacea. |
| Zavala-Ocampo et al., 2022 [2] | Pre-clinical in vitro study (enzymatic assay and human neuroblastoma cell line SH-SY5Y) | Leaves | Maceration method; Solvent: methanol. Fractionation process with methanol, hexane, and ethyl acetate | Methanolic extract: Not investigated; Hexanic fraction: Not investigated; Ethyl acetate fraction: Not investigated; Methanolic extract: Capreoside, Narcissin, Indane, (-)Isocaryophyllene, (-)β -Pinene, €-Tagetone, Peonidin 3-O-sambu -, bioside 5-O-glucoside | Concentrations including 1, 25, 50, 100, 250, 500, 1000, and 2000 μg/mL for the methanolic extract and fractions (Acetylcholinesterase activity); Concentrations including 25, 50, 125, 250, and 500 or the methanolic extract and fractions; Concentrations including 500, 1000, and 2000 μg/mL (Cellular Viability assay) and 1000 μg/mL (Antioxidant activity) | Not applicable | Antioxidant and cognitive study | In this study, the authors investigated extracts, fractions, subfractions, and isolated compounds from P. alliacea to study the antioxidant activity and the inhibition of acetylcholinesterase. The authors report in this work that P. alliacea has antioxidant properties and demonstrated activity in inhibiting acetylcholinesterase. They also emphasize the importance of investigations in animal models that further explore the therapeutic potential of the species. |
| Cal et al., 2022 [6] | Pre-clinical in vitro study (cytotoxicity) | Leaves | Maceration method; Solvent: ethanol. Fractionation process with n-hexane, dichloromethane, ethyl acetate, and n-butanol | Protocatechuic acid, cinnamic or benzoic acids, catechin, and epicatechin | 5, 10, 15, 20, 25, 50, and 100 μg/mL | Not applicable | Cytotoxicity study | In this study, the authors assessed the potential cytotoxic, genotoxic, and mutagenic effects of ethanolic extract of P. alliacea on Saccharomyces cerevisiae strains. The results indicate that fractions of mid-polarity of the ethanolic extract at the studied concentrations can induce mutagenicity mediated by oxidative lesions in the mitochondrial and genomic genomes. The authors also state that the lesions caused by the fractions of P. alliacea ethanolic extract can be mediated by reactive oxygen species and can reach multiple molecular targets to exert their toxicity. |
| Prada et al., 2023 [17] | Pre-clinical in vitro study (cytotoxicity) | Leaves | Percolation method; Solvent: ethanol | Myricetin and dibenzyl disulfide | 0–500 µg/mL | Not applicable | Cytotoxicity study | In this study, the authors investigated the cytotoxic action of the ethanolic extract of Petiveria alliacea against Leukemic cell lines and normal cells originating from the medulla. The results showed that the extract exerted a selective cytotoxic action on leukemic cells, not harming normal marrow cells. |
| Ex vivo study | ||||||||
| Ballesteros-Ramirez et al., 2020 [10] | Pre-clinical ex vivo study (human leukemic cell line K562) | Leaves | Supercritical fluid extraction (SFE); Solvents: Ethyl acetate, hexane, and methanol. Fractionation process with hexane, chloroform, and methanol solution. According to the authors, three fractions were obtained (FA, FB, and FC). Only FB was used for phytochemical characterization and biological assays | Myricetin and Dibenzyl disulfide | Antioxidant activity tests (ORAC, FRAP, and ABTS assays) and cytotoxicity were performed using a FB stock solution at 250 μg/L | Not applicable | Cytotoxicity study | In this study, the authors evaluated blasts from patients with acute leukemia to analyze the sensitivity of these cells to induction chemotherapy in an ex vivo model. In the work, P. alliacea is used to verify if there is a modulation of the sensitivity of these cells to death. The authors state that P. alliacea demonstrated positive results in inducing cell death and reinforcing the importance of tests in an ex vivo model to predict treatments, including herbal medicines. |
| In vitro/in vivo study | ||||||||
| Hoyos et al., 1992 [58] | Pre-clinical in vitro (human lymphocytes) and in vivo study (male mice) | Not applicable (capsules were purchased) | Capsules of P. alliacea were mixed with ethanol. After evaporation, the extract was redissolved with dimethyl sulfoxide | Not investigated | 1–1000 μg/mL (in vitro assay); 0.51, 102, 153, and 204 mg/kg (in vivo assay) | Not applicable | Toxicological study (genotoxicity) | In this study, the authors investigated the genotoxic effects of P. alliacea extract by conducting a sister chromatid exchange method in vitro and in vivo. The authors stated that the plant contains mutagenic and potentially carcinogenic agents. The authors also emphasized that further studies with cells from exposed individuals and experimental animals should be conducted to provide a better evaluation of health risks from the use of the drug. |
| Williams et al., 1999 [57] | Pre-clinical in vitro (microorganisms) and in vivo study (male mice) | Leaves and stems | Maceration method; Solvents: hexane; Fractionation process with cyclohexane and chloroform | Not investigated | 1 mg/mL (in vitro assay); 11 and 23 mg/kg (in vivo assay) | Parenteral administration (Intraperitoneal); Twice weekly for 3 weeks | Immunomodulatory study | In this study, the authors aimed to isolate and characterize the molecules responsible for the immunomodulatory properties of P. alliacea. The authors assayed in vitro phagocytosis, in vivo organ assessment, and isolation of dibenzyltrisulfide. The authors stated that P. alliacea extract and its isolated compound caused an alteration in blood cell count and an increase in the weight of the thymus and Peyer’s patches, suggesting an increase in the cellular and endocrine process responsible for T-cell differentiation. The authors emphasized that further studies were necessary to elucidate the appropriate mechanisms. |
| Berger et al., 1998 [55] | Pre-clinical in vitro (bacteria and T. cruzi) and in vivo study (mice) | Roots | Percolation method; Solvents: ethanol 95%, n-hexane and methanol. Infusion method; Solvent: water | Not investigated | 0.5% (in vitro assays); 100, 10, and 1 mg/kg (in vivo assay) | Oral administration (gavage); Intermittent regimen (every 48 h) for 3 weeks | Antiprotozoal study | In this study, the authors investigated the antiprotozoal activity of extracts from five different plants against Trypanosoma cruzi in vitro. Regarding P. alliacea, the authors state that the hexane and ethanol extracts of P. alliacea leaves and hexane and methanol extracts of the root showed activity against trypomastigotes. However, both plant parts were not effective against epimastigotes. The authors also reported that leaf extracts showed no toxicity in vitro, whereas hexane and methanol extracts were toxic. The authors conclude that the chloroform fraction was very active against trypomastigotes and showed little activity against epimastigotes but showed in vitro toxicity. |
| Cáceres et al., 1998 [54] | Pre-clinical in vitro (microorganisms) and in vivo study (male mice) | Leaves | Maceration method; Solvents: dichloromethane, ethanol, and water | Not investigated | 10 mg/mL (antibacterial and antifungal activity); 1 mg/mL (antitrypanosomal); 10–1000 ppm (cytotoxicity); 500 mg/kg (oral toxicity) | Oral administration (gavage); Intermittent regimen (every 48 h) for 3 weeks | Antimicrobial study | In this study, the authors screened 13 different native plants used in Guatemala for the treatment of protozoal infections, assessing antibacterial and antifungal activities. Regarding P. alliacea, the authors state that the extract showed antitrypanosomal activity in vitro but not in vivo. In the toxicity assay, no toxic effects were present in the studied dose (500 mg/kg). |
| Quadros et al., 1999 [53] | Pre-clinical in vitro study (microorganisms) and in vivo study (male mice) | Roots | Maceration method; Solvents: ethanol and water | Not investigated | 1000 mg/kg | Oral administration (gavage); Daily for 5 days, once a day | Immunomodulatory study | In this study, the authors investigated the effects of Petiveria alliacea on the hematopoietic response of mice infected with Listeria monocytogenes. The authors state that there was a protective effect of the crude extract of P. alliacea since the survival of the treated infected was higher than that in the infected group. The authors also suggest an immunomodulation effect of Petiveria alliacea extract on hematopoiesis, which may be responsible for the increased resistance of mice to Listeria monocytogenes infection. |
| Hernandez et al., 2014 [31] | Pre-clinical in vitro study (breast adenocarcinoma cell line) and in vivo study (female mice) | Leaves and stems | Maceration method; Solvent: ethanol (96%). The fractionation process was started with ethyl acetate and later with a methanol and water solution | Benzaldehyde, Leridol, Petiveral, Petiveral 4-ethyl, Pinitol, Dibenzyl disulfide, and Dibenzyl trisulfide | 125 to 0.95 μg/mL (cytotoxicity); 29.3, 2.9, and 0.29 μg/mL (clonogenic assays); 29.3, 14.6, and 7.3 μg/mL (membrane potential) | Not applicable | Cytotoxicity study | In this study, the authors evaluated the potential biological activity of a P. alliacea fraction and its effects in a model of metastatic breast adenocarcinoma (4T1). The authors report that in the study, the fraction of P. alliacea in vitro induced apoptosis of 4T1 cells, as well as reporting that there was regression of primary breast tumors. The authors suggest that the glycolytic pathway contributes to elucidating the action of the fraction in antitumor and antiproliferative activity. |
| Hernandez et al., 2017 [25] | Pre-clinical in vitro (cancer cell lines) and in vivo study (female mice) | Leaves and stems | Reflux extraction; Solvent: ethanol (96%). The ethanolic extract was partitioned with ethyl acetate, yielding a fraction that, in turn, was extracted with methanol and water, producing a dry extract | Benzaldehyde, Leridol, Petiveral, Myricetin, Petiveral 4-ethyl, Pinitol, Dibenzyl disulfide, and Dibenzyl trisulfide | For the cytotoxicity assay, doses ranged from 250 to 0.95 μg/mL. For the in vivo tumor model, the dose used was 250 mg/kg | Parenteral administration (Intraperitoneal); Twice a week for 56 days | Anticancer study | In this study, the authors evaluated the antitumor capacity of P. alliacea. To evaluate one of its traditional uses, a murine model of breast cancer was used. The authors reported that P. alliacea promoted a decrease in breast cancer cells in the in vitro and in vivo model; mice that were transplanted with tumor and were treated with P. alliacea demonstrated a reduction in primary tumor growth. |
| Zavala-Ocampo et al., 2017 [24] | Pre-clinical in vitro (Entamoeba histolytica trophozoite) and in vivo study (male mice) | Leaves | Maceration method; Solvents: methanol and water. A hexane fraction was obtained from the methanolic extract, which was sub-fractionated into three other subfractions (hexane, methanolic, and ethyl acetate) | Hexane subfractions were selected to isolate Isoarborine | The aqueous and methanolic extracts were tested for antiamebic activity and cell viability assay. In addition, the hexane fraction and its subfractions were also tested. Concentrations of all types of extraction range from 0.1 to 1.8 mg/mL. The isolated substance Isoarborinol was also used for Antiamebic activity and cell viability assay at a concentration ranging from 0.05 to 0.3 mg/kg. The hexane fraction was chosen for acute oral toxicity at a dose of 2000 mg/kg | Oral administration (gavage); Single dose | Antiamoebic and Toxicological study | In this study, the authors carried out an investigation of the antiamebic activity of P. alliacea in different extracts and fractions. The authors reported that the extracts and fractions demonstrated antiamebic activity, emphasizing that this result is possibly associated with the presence of an important metabolite called isoarborinol. |
| Ileke et al., 2021 [9] | Pre-clinical in vitro (mosquito larvae and pups) and in vivo study (adult mosquito and male rat) | Leaves | Maceration method; Solvent: absolute ethanol | Not investigated | For all tests performed (Oviposition deterrent, Larvicidal, Pupicidal, Adulticidal, and Repellency tests), concentrations of 50, 100, 200, 400, and 800 mg/L of the ethanolic extract of P. alliacea were used | Antimalarial study | In this study, the authors investigated the potency of P. alliacea against mosquitoes of the genus Anopheles gambiae in different stages of life. The authors also reported that the use of P. alliaceae was highly effective, which strengthens the idea of replacing chemical insecticides and reducing the harmful effects related to the use of these substances. | |
| In vivo/in silico study | ||||||||
| Olajubutu et al., 2022 [19] | Pre-clinical in vivo study (female and male rats) and in silico study | Leaves | Maceration method; Solvent: ethanol. Fractionation process with hexane and water | Ethanolic extract: Not investigated; Aqueous fraction: Not investigated; Hexanic fraction: 2,6-Pyridinedicarboxylic acid, Neophytadiene, 2-Pentadecanone, 6,10,14-trimethyl-, 9,12-Octadecadienoic acid (Z,Z)-, n-Hexadecanoic acid, 4,8,12,16-Tetramethylheptadecan-4-olide, n-Eicosane, Hexadecanoic acid, methyl ester, 9-Octadecenoic acid, (E)-, Octadecanoic acid, 9,12-Octadecadienoic acid (Z,Z)-, methyl ester, Hexadecanoic acid, ethyl ester, Oxirane, tetradecyl-, 13-Docosenamide, (Z), 11-Octadecenoic acid, methyl ester, Methyl stearate, Vitamin E, 1,19-Eicosadiene, and Stigmasterol | Ethanolic extract: 1, 2.5 | Topical administration | Anti-inflammatory study | In this study, the authors carried out an investigation of the topical anti-inflammatory activity of P. alliacea using the paw edema model. The authors state that there was strong topical anti-inflammatory activity and comment that in the evaluation of the molecular docking of some of the most abundant compounds present in the composition of the constituents, some of them proved to be potential topical anti-inflammatory agents. The study also further recommended in vivo studies with isolated compounds to confirm such activities. |
| Cruz-Salomon et al., 2022 [8] | Pre-clinical in vivo study (male mice) and in silico study | Leaves | Sonication method; Solvents: methanol, hexane, and water | Methanolic extract: Ethyl palmitate, Phytol, Ethyl linoleate, and Squalene; Hexanoic extract: Butylated hydroxytoluene, Methyl oleate, Eicosane, and Squalene; Aqueous extract: Methyl 14-methylpentadecanoate, Methyl oleate, Bis(2-ethylhexyl) maleate, Octadecyl acetate, and 2,4-Bis(1-phenylethyl)phenol | 10, 31.6, 100, and 316 mg/kg (for each type of extract) | Oral administration; Single dose | Antinociceptive study | In this study, the authors evaluated the effects of some P. alliacea extracts on the antinociceptive activity through the formalin test and on the toxicity of the extracts through the acute oral toxicity test. The authors related the phytochemical composition of the extracts with positive results in the antinociceptive evaluation, where there was a reduction in pain. They also stated that the extracts proved to be safe, with no risk to health. |
| Literature review | ||||||||
| Luz et al., 2016 [27] | Literature review | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable | Neuropharmacological study | In this study, the authors reviewed aspects of P. alliacea, including a compilation of neuropharmacological information about the plant. The authors reported that, based on their research, P. alliacea has demonstrated activity in several central nervous system disorders, including depression, anxiety, memory, pain, and epilepsy. |
| Castañeda et al., 2022 [12] | Literature review | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable | Neuropsychiatric disorders study | In this study, the authors performed a review with the aim of compiling information on the use of medicinal plants in Mesoamerica as a traditional use for the treatment of CNS disorders. The authors provide information about the traditional use of P. alliacea as a treatment for dementia, epilepsy, and some nervous treatments. The study reports some information from pharmacological tests performed on animals, pointing out anticonvulsant and anxiolytic activities and effects on memory. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conceição, B.C.d.; Silva, T.A.d.; Pantoja, L.V.P.d.S.; Luz, D.A.d.; Cardoso, E.K.S.; Reis, L.D.d.S.; Raiol-da-Silva, M.C.; Kussler, M.S.; Maia, C.S.F.; Fontes-Júnior, E.A. Amazonian Plants: A Global Bibliometric Approach to Petiveria alliacea L. Pharmacological and Toxicological Properties. Plants 2023, 12, 3343. https://doi.org/10.3390/plants12183343
Conceição BCd, Silva TAd, Pantoja LVPdS, Luz DAd, Cardoso EKS, Reis LDdS, Raiol-da-Silva MC, Kussler MS, Maia CSF, Fontes-Júnior EA. Amazonian Plants: A Global Bibliometric Approach to Petiveria alliacea L. Pharmacological and Toxicological Properties. Plants. 2023; 12(18):3343. https://doi.org/10.3390/plants12183343
Chicago/Turabian StyleConceição, Brenda Costa da, Thales Andrade da Silva, Lucas Villar Pedrosa da Silva Pantoja, Diandra Araújo da Luz, Eloise Karoline Serrão Cardoso, Laryssa Danielle da Silva Reis, Maria Carolina Raiol-da-Silva, Monique Silva Kussler, Cristiane Socorro Ferraz Maia, and Enéas Andrade Fontes-Júnior. 2023. "Amazonian Plants: A Global Bibliometric Approach to Petiveria alliacea L. Pharmacological and Toxicological Properties" Plants 12, no. 18: 3343. https://doi.org/10.3390/plants12183343
APA StyleConceição, B. C. d., Silva, T. A. d., Pantoja, L. V. P. d. S., Luz, D. A. d., Cardoso, E. K. S., Reis, L. D. d. S., Raiol-da-Silva, M. C., Kussler, M. S., Maia, C. S. F., & Fontes-Júnior, E. A. (2023). Amazonian Plants: A Global Bibliometric Approach to Petiveria alliacea L. Pharmacological and Toxicological Properties. Plants, 12(18), 3343. https://doi.org/10.3390/plants12183343









