Fine Mapping and Identification of a Candidate Gene for the Glossy Green Trait in Cabbage (Brassica oleracea var. capitata)
Abstract
:1. Introduction
2. Results
2.1. Obtaining the Homozygous Glossy Green Mutant
2.2. Phenotypic Characterization of the Glossy Green Mutant and Its Wild Type
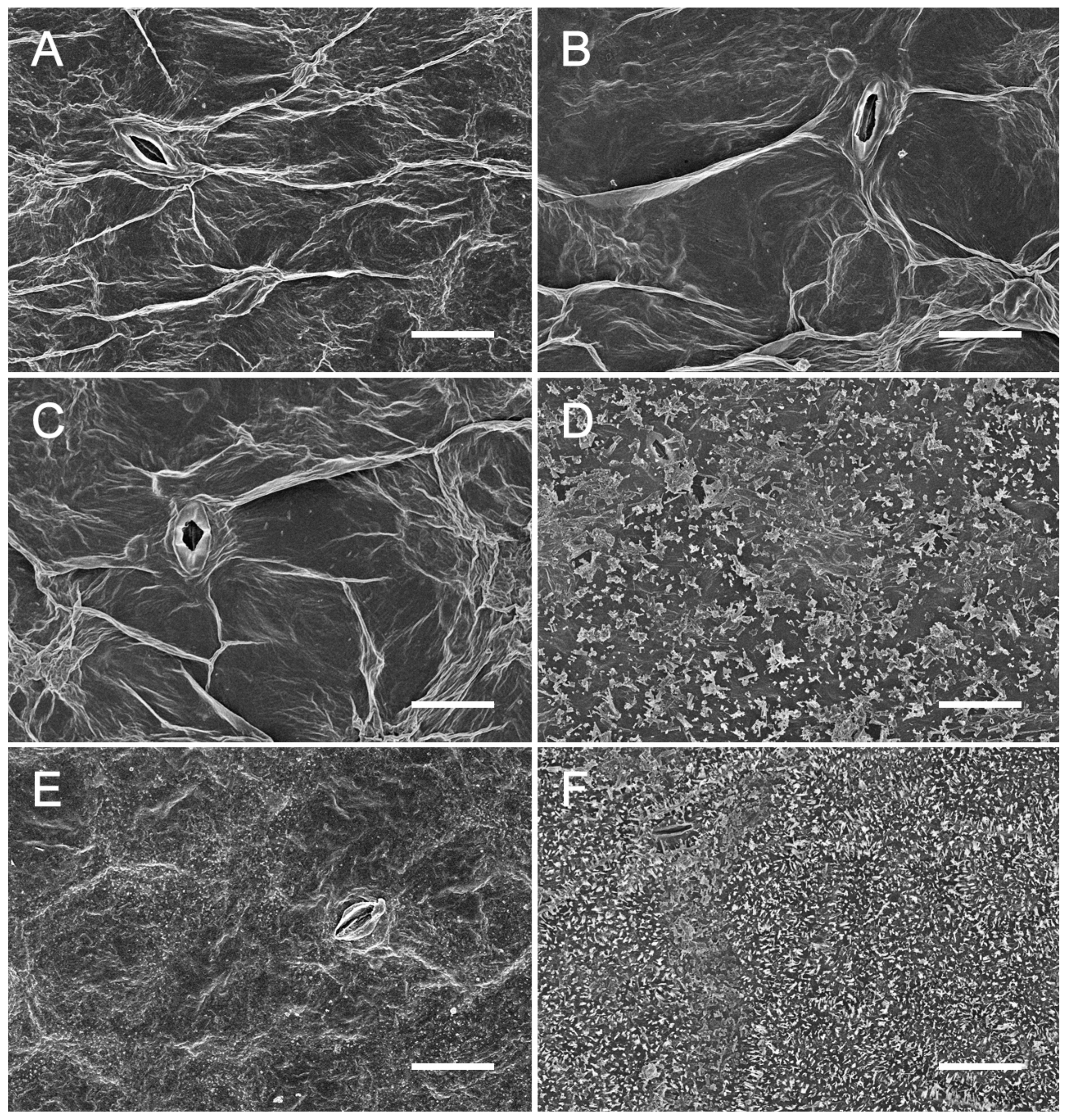
2.3. Observation of Wax Crystal on the Surface of 98-1030gl and WT Leaves
2.4. Genetic Analysis of the Glossy Green Trait
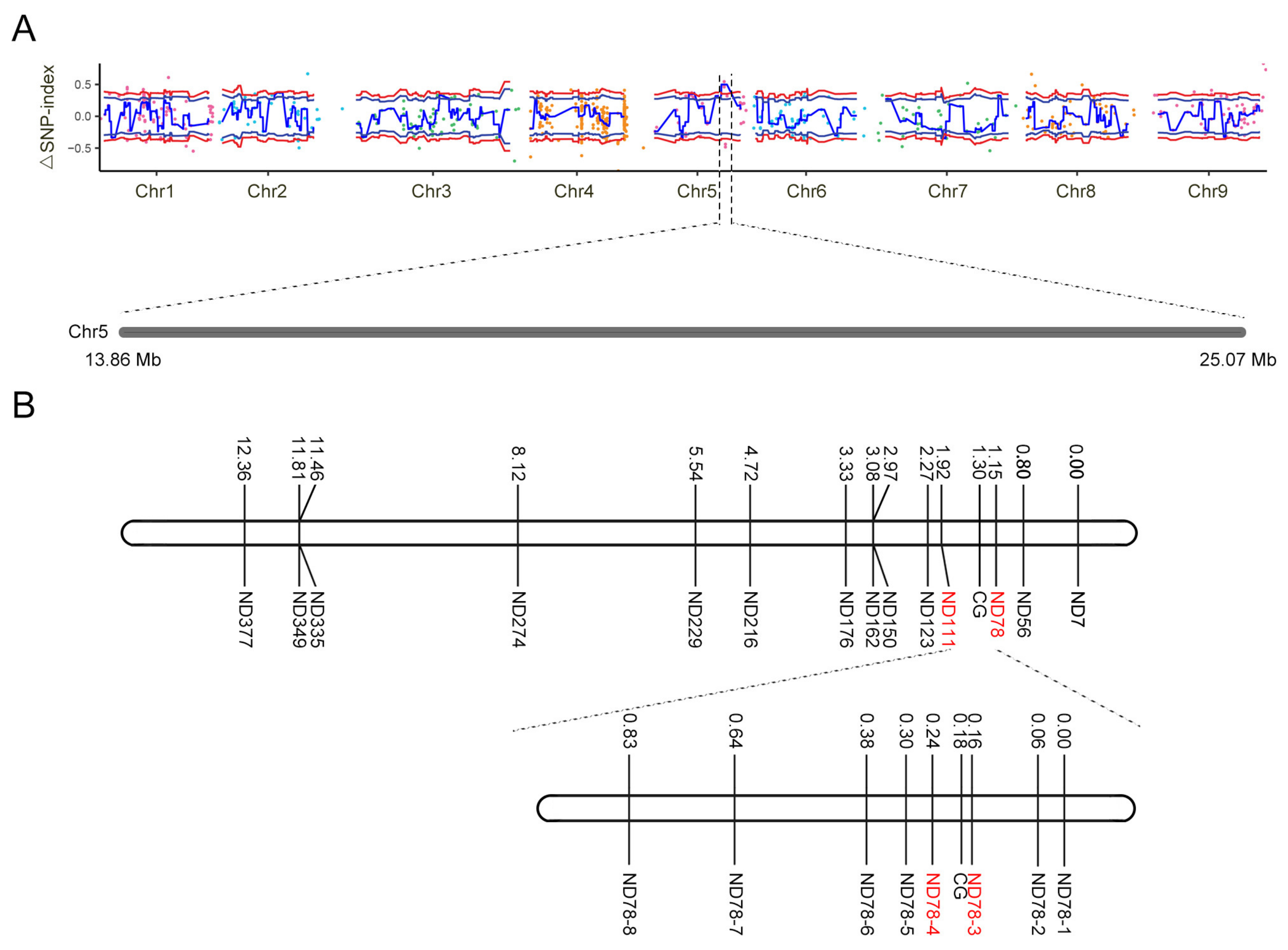
2.5. Fine Mapping of the Candidate Gene
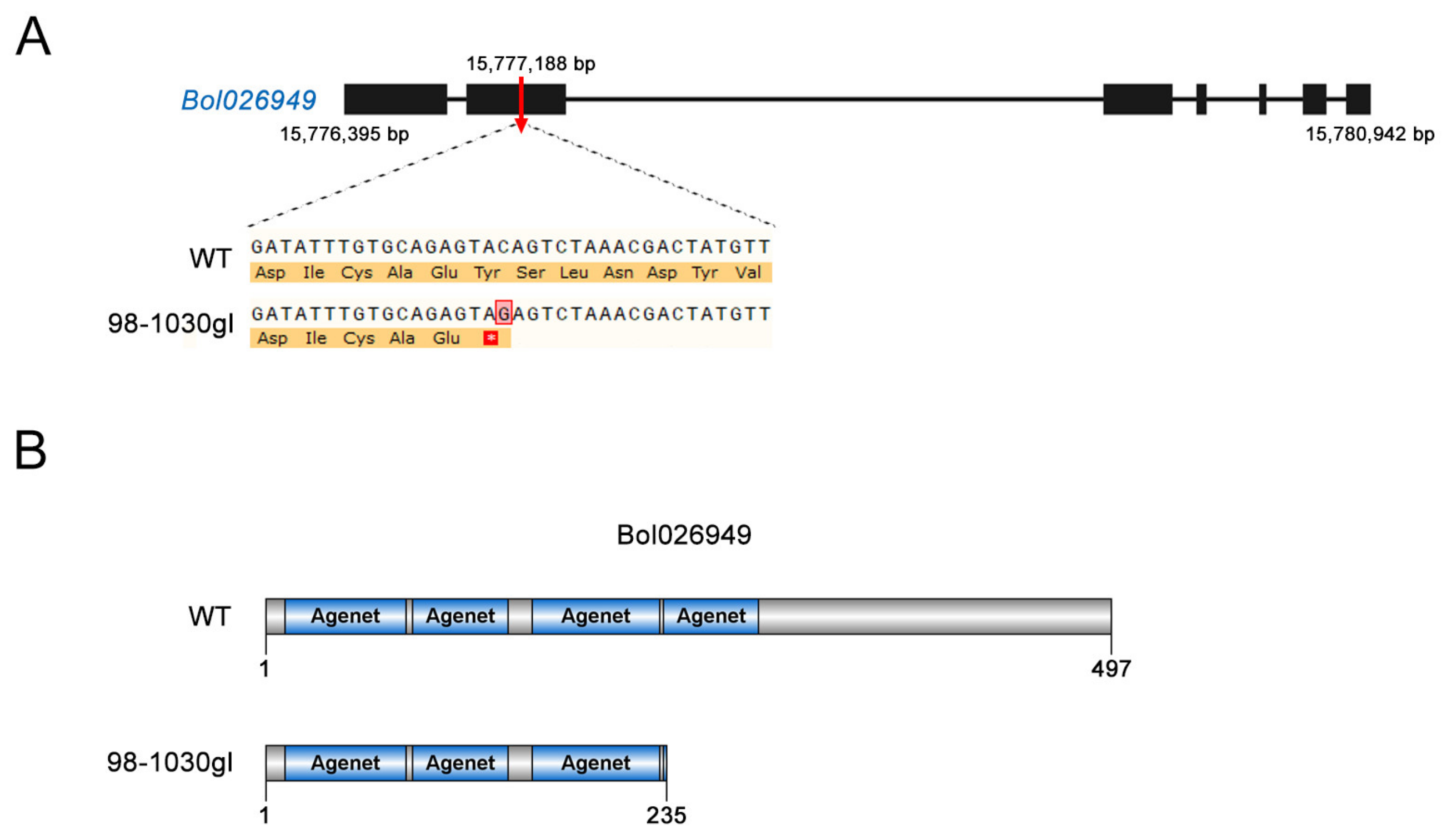
2.6. Identification of the Candidate Gene
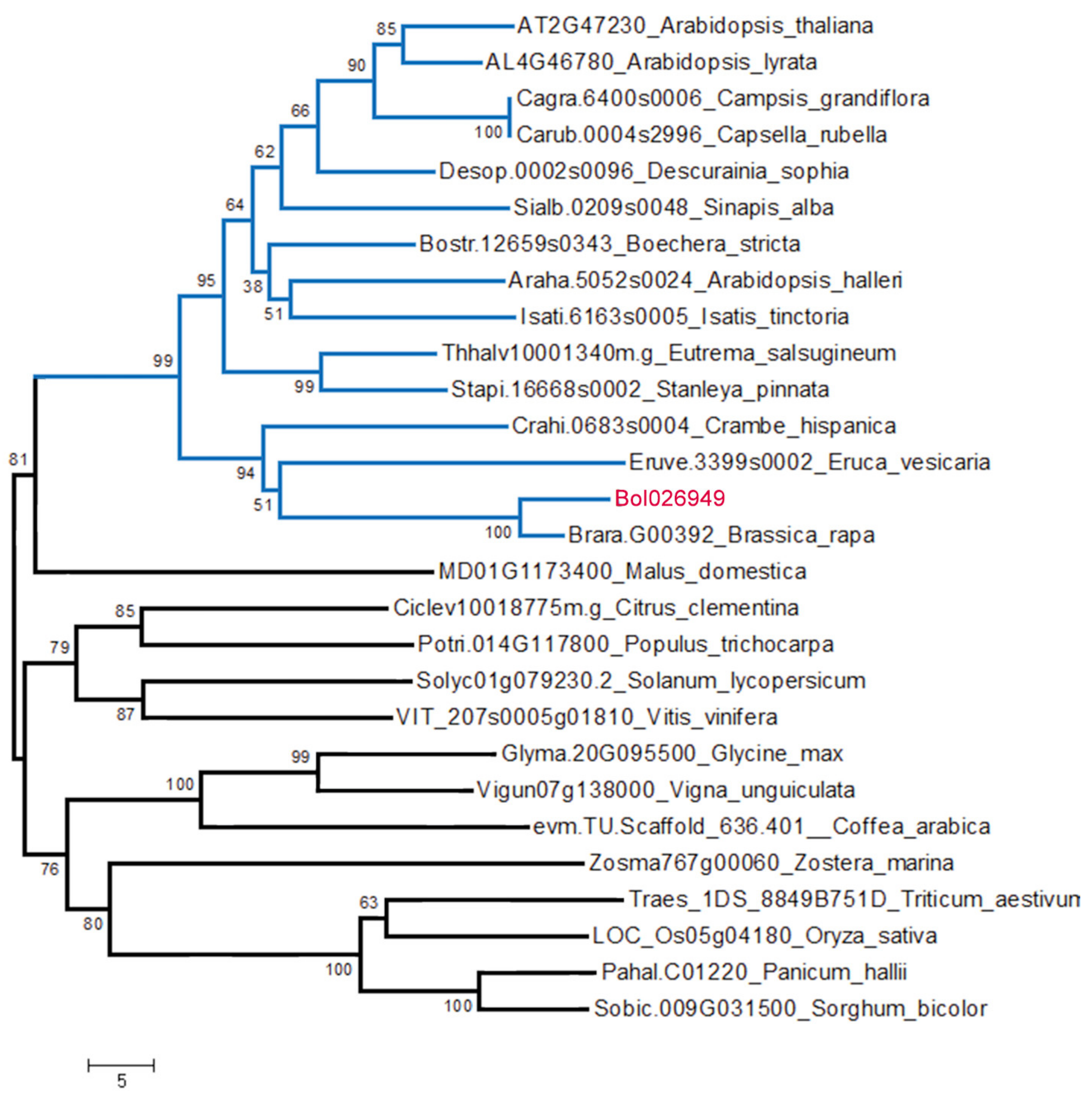
2.7. Phylogenic Analysis
2.8. Transcriptome Profiling
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Phenotype Identification
4.2. SEM Analysis
4.3. DNA Isolation
4.4. BSA-seq Analysis
4.5. Marker Development and Fine Mapping
4.6. Functional Annotation and Sequence Analysis
4.7. Phylogenetic Analysis
4.8. RNA-seq
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samuels, L.; Kunst, L.; Jetter, R. Sealing plant surfaces: Cuticular wax formation by epidermal cells. Annu. Rev. Plant Biol. 2008, 59, 683–707. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Suh, M.C. Advances in the understanding of cuticular waxes in Arabidopsis thaliana and crop species. Plant Cell Rep. 2015, 34, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, P.; Qiang, S.; Xu, L.L. Interaction of plant epicuticular waxes and extracellular esterases of Curvularia eragrostidis during infection of Digitaria sanguinalis and Festuca arundinacea by the fungus. Int. J. Mol. Sci. 2006, 7, 346–357. [Google Scholar] [CrossRef]
- Roeland, E.V.; Greet, S.B.; Marjolein, T.H.; Edith, T.L.B. Plant traits associated with resistance to Thrips tabaci in cabbage (Brassica oleracea var capitata). Euphytica 2008, 163, 409–415. [Google Scholar]
- Seo, P.J.; Lee, S.B.; Suh, M.C.; Park, M.J.; Go, Y.S.; Park, C.M. The MYB96 transcription factor regulates cuticular wax biosynthesis under drought conditions in Arabidopsis. Plant Cell 2011, 23, 1138–1152. [Google Scholar] [CrossRef]
- Rahman, T.; Shao, M.; Pahari, S.; Venglat, P.; Soolanayakanahally, R.; Qiu, X.; Rahman, A.; Tanino, K. Dissecting the roles of cuticular wax in plant resistance to shoot dehydration and low-temperature stress in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 1554. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, T.; Wynne Griffiths, D. The effects of stress on plant cuticular waxes. New Phytol. 2006, 171, 469–499. [Google Scholar] [CrossRef]
- Weng, H.; Molina, I.; Shockey, J.; Browse, J. Organ fusion and defective cuticle function in a lacs1 lacs2 double mutant of Arabidopsis. Planta 2010, 231, 1089–1100. [Google Scholar] [CrossRef]
- McFarlane, H.E.; Watanabe, Y.; Yang, W.; Huang, Y.; Ohlrogge, J.; Samuels, A.L. Golgi- and trans-golgi network-mediated vesicle trafficking is required for wax secretion from epidermal cells. Plant Physiol. 2014, 164, 1250–1260. [Google Scholar] [CrossRef]
- Lü, S.; Zhao, H.; Des Marais, D.L.; Parsons, E.P.; Wen, X.; Xu, X.; Bangarusamy, D.K.; Wang, G.; Rowland, O.; Juenger, T.; et al. Arabidopsis ECERIFERUM9 involvement in cuticle formation and maintenance of plant water status. Plant Physiol. 2012, 159, 930–944. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wan, L.; Zhang, L.; Zhang, Z.; Zhang, H.; Quan, R.; Zhou, S.; Huang, R. An ethylene response factor OsWR1 responsive to drought stress transcriptionally activates wax synthesis related genes and increases wax production in rice. Plant Mol. Biol. 2012, 78, 275–288. [Google Scholar] [CrossRef]
- Li, L.; Li, D.; Liu, S.; Ma, X.; Dietrich, C.R.; Hu, H.C.; Zhang, G.; Liu, Z.; Zheng, J.; Wang, G.; et al. The maize glossy13 gene, cloned via BSR-seq and Seq-walking encodes a putative ABC transporter required for the normal accumulation of epicuticular waxes. PLoS ONE 2013, 8, e82333. [Google Scholar] [CrossRef]
- Smirnova, A.; Leide, J.; Riederer, M. Deficiency in a very-long-chain fatty acid β-ketoacyl-coenzyme a synthase of tomato impairs microgametogenesis and causes floral organ fusion. Plant Physiol. 2013, 161, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Gao, J.; Guo, Y.; Liu, T.; Zhu, L.; Xu, P.; Yi, B.; Wen, J.; Tu, J.; Ma, C.; et al. A novel dominant glossy mutation causes suppression of wax biosynthesis pathway and deficiency of cuticular wax in Brassica napus. BMC Plant Biol. 2013, 13, 215. [Google Scholar] [CrossRef]
- Huo, Z.; Xu, Y.; Yuan, S.; Chang, J.; Li, S.; Wang, J.; Zhao, H.; Xu, R.; Zhong, F. The AP2 transcription factor BrSHINE3 regulates wax accumulation in nonheading Chinese cabbage. Int. J. Mol. Sci. 2022, 23, 13454. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fang, Z.; Zhuang, M.; Zhang, Y.; Lv, H.; Liu, Y.; Li, Z.; Sun, P.; Tang, J.; Liu, D.; et al. Fine-mapping and analysis of Cgl1, a gene conferring glossy trait in cabbage (Brassica oleracea L. var. capitata). Front. Plant Sci. 2017, 8, 239. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Yang, X.; Tong, C.; Edwards, D.; Parkin, I.A.; Zhao, M.; Ma, J.; Yu, J.; Huang, S.; et al. The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat. Commun. 2014, 5, 3930. [Google Scholar] [CrossRef]
- Xiao, Z.; Hu, Y.; Zhang, X.; Xue, Y.; Fang, Z.; Yang, L.; Zhang, Y.; Liu, Y.; Li, Z.; Liu, X.; et al. Fine mapping and transcriptome analysis reveal candidate genes associated with hybrid lethality in cabbage (Brassica oleracea). Genes 2017, 8, 147. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Peng, A.; Yang, J.; Zheng, S.; Li, Z.; Mu, Y.; Chen, L.; Si, J.; Ren, X.; Song, H. A 215-bp indel at intron I of BoFLC2 affects flowering time in Brassica oleracea var. capitata during vernalization. Theor. Appl. Genet. 2022, 135, 2785–2797. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.E.; Afrin, K.S.; Rahim, M.A.; Jung, H.J.; Nou, I.S. Inheritance of black rot resistance and development of molecular marker linked to Xcc races 6 and 7 resistance in cabbage. Plants 2021, 10, 1940. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Ji, J.; Yang, L.; Fang, Z.; Zhuang, M.; Zhang, Y.; Lv, H.; Wang, Y.; Sun, P.; Tang, J.; et al. Fine-mapping and transcriptome analysis of BoGL-3, a wax-less gene in cabbage (Brassica oleracea L. var. capitata). Mol. Genet. Genom. 2019, 294, 1231–1239. [Google Scholar] [CrossRef]
- Ji, J.; Cao, W.; Tong, L.; Fang, Z.; Zhang, Y.; Zhuang, M.; Wang, Y.; Yang, L.; Lv, H. Identification and validation of an ECERIFERUM2-LIKE gene controlling cuticular wax biosynthesis in cabbage (Brassica oleracea L. var. capitata L.). Theor. Appl. Genet. 2021, 134, 4055–4066. [Google Scholar] [CrossRef] [PubMed]
- Brasil, J.N.; Cabral, L.M.; Eloy, N.B.; Primo, L.M.; Barroso-Neto, I.L.; Grangeiro, L.P.; Gonzalez, N.; Inzé, D.; Ferreira, P.C.; Hemerly, A.S. AIP1 is a novel Agenet/Tudor domain protein from Arabidopsis that interacts with regulators of DNA replication, transcription and chromatin remodeling. BMC Plant Biol. 2015, 15, 270. [Google Scholar] [CrossRef]
- Koch, K.; Ensikat, H.J. The hydrophobic coatings of plant surfaces: Epicuticular wax crystals and their morphologies, crystallinity and molecular self-assembly. Micron 2008, 39, 759–772. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C.; Cutler, D.; Ditsch, F.; Meusel, I.; Theisen, I.; Wilhelmi, H. Classification and terminology of plant epicuticular waxes. Bot. J. Linn. Soc. 1998, 126, 237–260. [Google Scholar] [CrossRef]
- Rashotte, A.M.; Jenks, M.A.; Nguyen, T.D.; Feldmann, K.A. Epicuticular wax variation in ecotypes of Arabidopsis thaliana. Phytochemistry 1997, 45, 251–255. [Google Scholar] [CrossRef]
- O’Toole, J.C.; Cruz, R.T. Genotypic variation in epicuticular wax of rice. Crop Sci. 1983, 23, 392–400. [Google Scholar] [CrossRef]
- Bernard, A.; Joubès, J. Arabidopsis cuticular waxes: Advances in synthesis, export and regulation. Prog. Lipid Res. 2013, 52, 110–129. [Google Scholar] [CrossRef] [PubMed]
- Jenks, M.A.; Tuttle, H.A.; Eigenbrode, S.D.; Feldmann, K.A. Leaf epicuticular waxes of the eceriferum mutants in Arabidopsis. Plant Physiol. 1995, 108, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, P.; Lu, S.; Ou-Yang, Q.; Zhu-Ge, Y.; Tian, R.; Jia, H.; Fang, J. Comparative analysis of cuticular wax in various grape cultivars during berry development and after storage. Front. Nutr. 2021, 8, 817796. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mao, H.; Lv, Y.; Chen, G.; Jiang, Y. Comparative analysis of total wax content, chemical composition and crystal morphology of cuticular wax in Korla pear under different relative humidity of storage. Food Chem. 2021, 339, 128097. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.A. The influence of environment on leaf wax development in Brassica oleracea var. gemmifera. New Phytol. 1974, 73, 955–966. [Google Scholar] [CrossRef]
- Kosma, D.K.; Bourdenx, B.; Bernard, A.; Parsons, E.P.; Lü, S.; Joubès, J.; Jenks, M.A. The impact of water deficiency on leaf cuticle lipids of Arabidopsis. Plant Physiol. 2009, 151, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Dellaert, L.M.W. Eceriferum mutants in Arabidopsis thaliana (L.) Heynh: I. Induction by x-rays and fast neutrons. Arab. Inf. Serv. 1979, 16, 1–9. [Google Scholar]
- Jung, K.H.; Han, M.J.; Lee, D.Y.; Lee, Y.S.; Schreiber, L.; Franke, R.; Faust, A.; Yephremov, A.; Saedler, H.; Kim, Y.W.; et al. Wax-deficient anther1 is involved in cuticle and wax production in rice anther walls and is required for pollen development. Plant Cell 2006, 18, 3015–3032. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, G.; Avato, P.; Salamini, F. Glossy mutants of maize. Viii. Accumulation of fatty aldehydes in surface waxes of gl5 maize seedlings. Biochem. Genet. 1978, 16, 1015–1021. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, C.; Mishina, K.; Zhao, J.; Zhang, J.; Duan, R.; Ma, X.; Wang, A.; Meng, Q.; Komatsuda, T.; et al. Characterization and genetic mapping of the β-diketone deficient eceriferum-b barley mutant. Theor. Appl. Genet. 2017, 130, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Dong, X.; Liu, Z.; Tang, J.; Zhuang, M.; Zhang, Y.; Lv, H.; Liu, Y.; Li, Z.; Fang, Z.; et al. Fine mapping and candidate gene identification for wax biosynthesis locus, BoWAX1 in Brassica oleracea L. var. capitata. Front. Plant Sci. 2018, 9, 309. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Tang, J.; Liu, Z.; Dong, X.; Zhuang, M.; Zhang, Y.; Lv, H.; Sun, P.; Liu, Y.; Li, Z.; et al. Fine mapping of BoGL1, a gene controlling the glossy green trait in cabbage (Brassica oleracea L. var. capitata). Mol. Breed. 2017, 37, 69. [Google Scholar] [CrossRef]
- Shaheenuzzamn, M.; Shi, S.; Sohail, K.; Wu, H.; Liu, T.; An, P.; Wang, Z.; Hasanuzzaman, M. Regulation of cuticular wax biosynthesis in plants under abiotic stress. Plant Biotechnol. Rep. 2021, 15, 1–12. [Google Scholar]
- Aharoni, A.; Dixit, S.; Jetter, R.; Thoenes, E.; van Arkel, G.; Pereira, A. The SHINE clade of AP2 domain transcription factors activates wax biosynthesis, alters cuticle properties, and confers drought tolerance when overexpressed in Arabidopsis. Plant Cell 2004, 16, 2463–2480. [Google Scholar] [CrossRef] [PubMed]
- Broun, P.; Poindexter, P.; Osborne, E.; Jiang, C.Z.; Riechmann, J.L. WIN1, a transcriptional activator of epidermal wax accumulation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2004, 101, 4706–4711. [Google Scholar] [CrossRef]
- Lee, S.B.; Kim, H.; Kim, R.J.; Suh, M.C. Overexpression of Arabidopsis MYB96 confers drought resistance in Camelina sativa via cuticular wax accumulation. Plant Cell Rep. 2014, 33, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.S.; Kim, H.; Kim, H.J.; Suh, M.C. Arabidopsis cuticular wax biosynthesis is negatively regulated by the DEWAX gene encoding an AP2/ERF-type transcription factor. Plant Cell 2014, 26, 1666–1680. [Google Scholar] [CrossRef]
- Kim, H.; Go, Y.S.; Suh, M.C. DEWAX2 transcription factor negatively regulates cuticular wax biosynthesis in Arabidopsis leaves. Plant Cell Physiol. 2018, 59, 966–977. [Google Scholar] [CrossRef]
- Ménard, R.; Verdier, G.; Ors, M.; Erhardt, M.; Beisson, F.; Shen, W.H. Histone H2B monoubiquitination is involved in the regulation of cutin and wax composition in Arabidopsis thaliana. Plant Cell Physiol. 2014, 55, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, F.; Castro-Cegrí, A.; Jiménez-Muñoz, R.; Jamilena, M.; Garrido, D.; Palma, F. Changes in morphology, metabolism and composition of cuticular wax in zucchini fruit during postharvest cold storage. Front. Plant Sci. 2021, 12, 778745. [Google Scholar] [CrossRef] [PubMed]
- Springer, N.M. Isolation of plant DNA for PCR and genotyping using organic extraction and CTAB. Cold Spring Harb. Protoc. 2010, 2010, pdb.prot5515. [Google Scholar] [CrossRef] [PubMed]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, M.; Hakonarson, H. Annovar: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic. Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef]
- Meng, L.; Li, H.; Zhang, L.; Wang, J. QTL IciMapping: Integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J. 2015, 3, 269–283. [Google Scholar] [CrossRef]
- Kosambi, D.D. The estimation of map distances from recombination values. Ann. Eugen. 1943, 12, 172–175. [Google Scholar] [CrossRef]
- Sievers, F.; Higgins, D.G. Clustal omega. Curr. Protoc. Bioinform. 2014, 48, 3.13.1–3.13.16. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Varet, H.; Brillet-Guéguen, L.; Coppée, J.Y.; Dillies, M.A. SARTools: A DESeq2- and EdgeR-based R pipeline for comprehensive differential analysis of RNA-Seq data. PLoS ONE 2016, 11, e0157022. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The gene ontology consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Tatusov, R.L.; Fedorova, N.D.; Jackson, J.D.; Jacobs, A.R.; Kiryutin, B.; Koonin, E.V.; Krylov, D.M.; Mazumder, R.; Mekhedov, S.L.; Nikolskaya, A.N.; et al. The COG database: An updated version includes eukaryotes. BMC Bioinform. 2003, 4, 41. [Google Scholar] [CrossRef] [PubMed]


| Population | Total | Glaucous | Glossy | Segregation Ratio | χ2 | p Value |
|---|---|---|---|---|---|---|
| P1 (98-1030) | 30 | 30 | 0 | — | — | |
| P2 (98-1030gl) | 30 | 0 | 30 | — | — | |
| F1 | 132 | 132 | 0 | — | — | |
| F2 | 875 | 650 | 225 | 2.89:1 | 0.22 | 0.64 |
| BC1 (F1 × 98-1030) | 204 | 204 | 0 | — | — | |
| BC2 (F1 × 98-1030gl) | 168 | 92 | 76 | 1.21:1 | 1.52 | 0.22 |
| Gene ID | Start Position | Stop Position | DNA Strand | Homologous Gene in Arabidopsis | Function Annotation |
|---|---|---|---|---|---|
| Bol026947 | 15767582 | 15769050 | reverse | AT1G54680.4 | unknown protein |
| Bol026948 | 15773097 | 15774439 | reverse | — | unknown protein |
| Bol026949 | 15776395 | 15780942 | forward | AT1G26540 | Agenet domain-containing protein-related |
| Bol026950 | 15803605 | 15804112 | reverse | AT1G20340 | plastocyanin (petE) |
| Bol026952 | 15815490 | 15817138 | reverse | AT1G20370 | tRNA pseudouridylate synthase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Li, Z.; Zhu, L.; Cheng, M.; Chen, X.; Wang, A.; Wang, C.; Zhang, X. Fine Mapping and Identification of a Candidate Gene for the Glossy Green Trait in Cabbage (Brassica oleracea var. capitata). Plants 2023, 12, 3340. https://doi.org/10.3390/plants12183340
Wang P, Li Z, Zhu L, Cheng M, Chen X, Wang A, Wang C, Zhang X. Fine Mapping and Identification of a Candidate Gene for the Glossy Green Trait in Cabbage (Brassica oleracea var. capitata). Plants. 2023; 12(18):3340. https://doi.org/10.3390/plants12183340
Chicago/Turabian StyleWang, Peiwen, Ziheng Li, Lin Zhu, Mozhen Cheng, Xiuling Chen, Aoxue Wang, Chao Wang, and Xiaoxuan Zhang. 2023. "Fine Mapping and Identification of a Candidate Gene for the Glossy Green Trait in Cabbage (Brassica oleracea var. capitata)" Plants 12, no. 18: 3340. https://doi.org/10.3390/plants12183340
APA StyleWang, P., Li, Z., Zhu, L., Cheng, M., Chen, X., Wang, A., Wang, C., & Zhang, X. (2023). Fine Mapping and Identification of a Candidate Gene for the Glossy Green Trait in Cabbage (Brassica oleracea var. capitata). Plants, 12(18), 3340. https://doi.org/10.3390/plants12183340





