Study on the Key Autotoxic Substances of Alfalfa and Their Effects
Abstract
:1. Introduction
2. Results
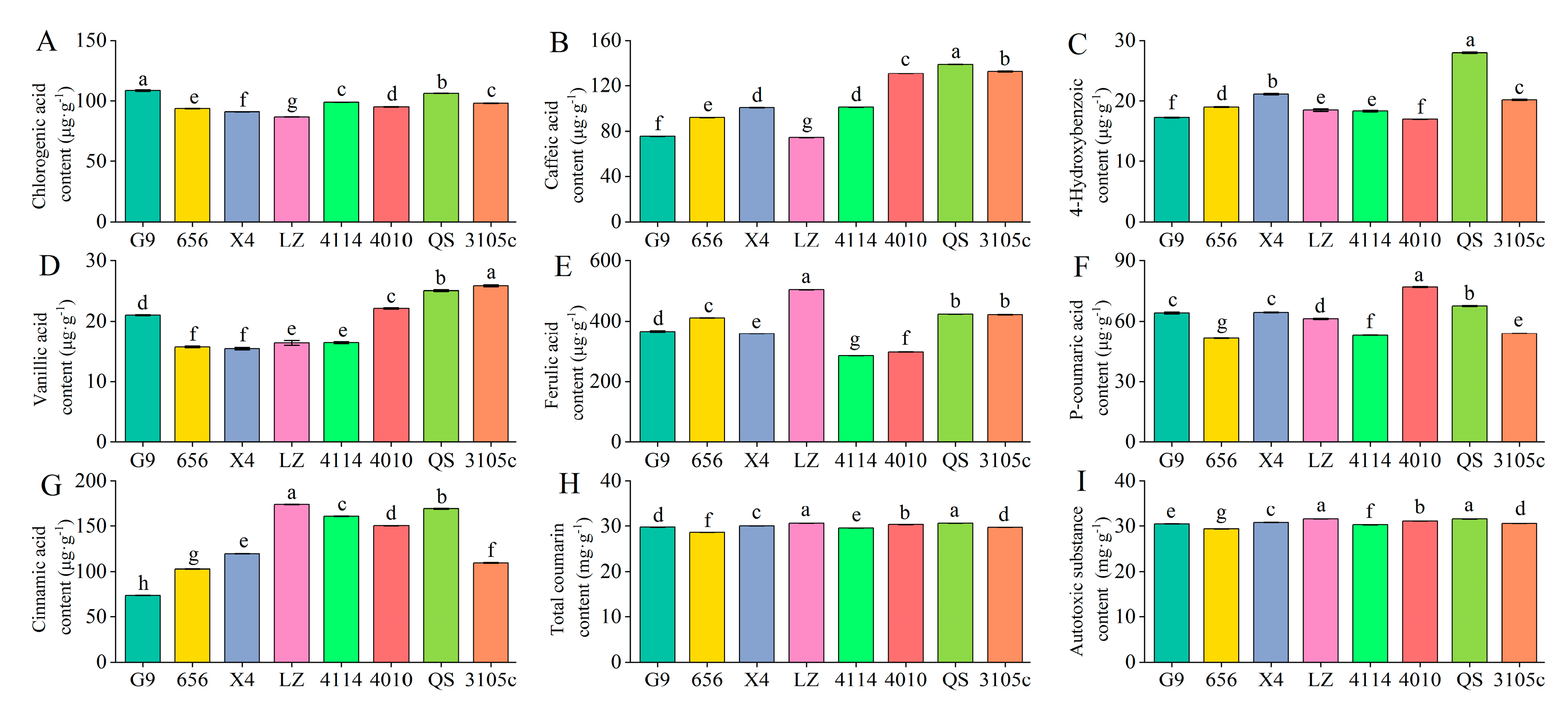
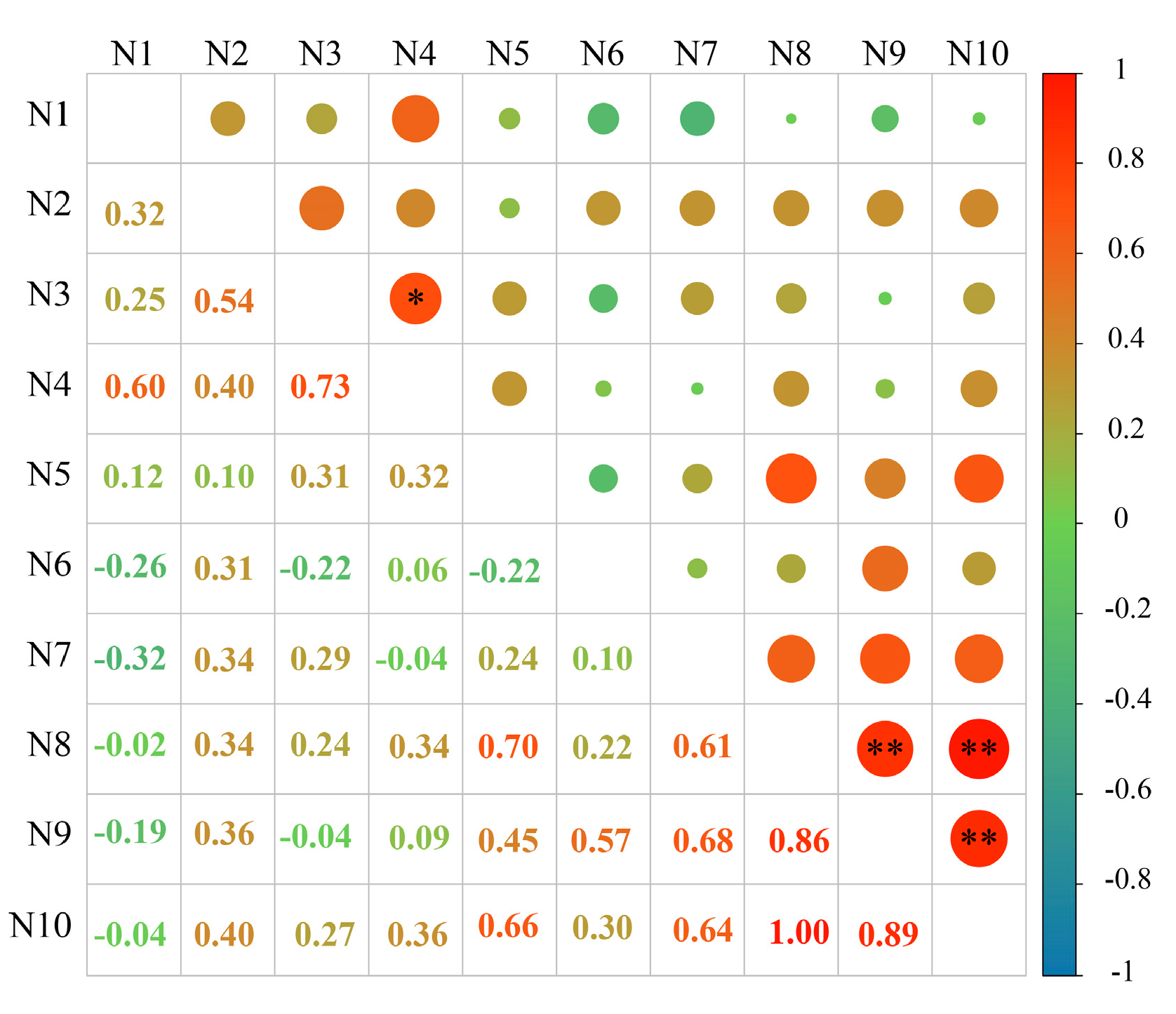
2.1. Differences in the Content of Phenolic Autotoxic Substances in Alfalfa
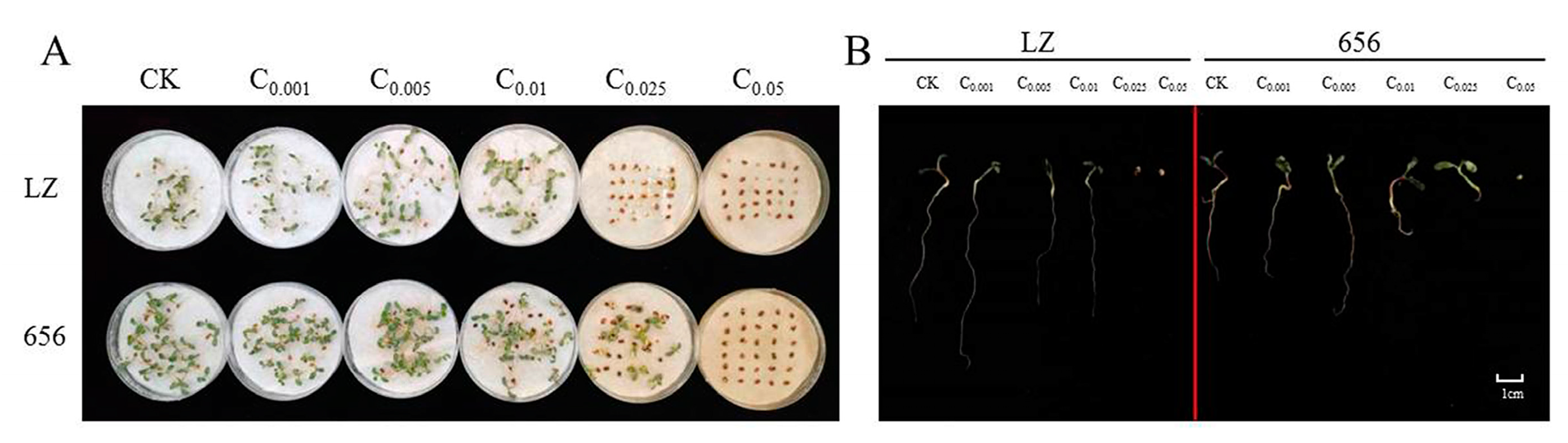
2.2. Effect of Alfalfa Extracts on Seed Germination and Seedling Growth
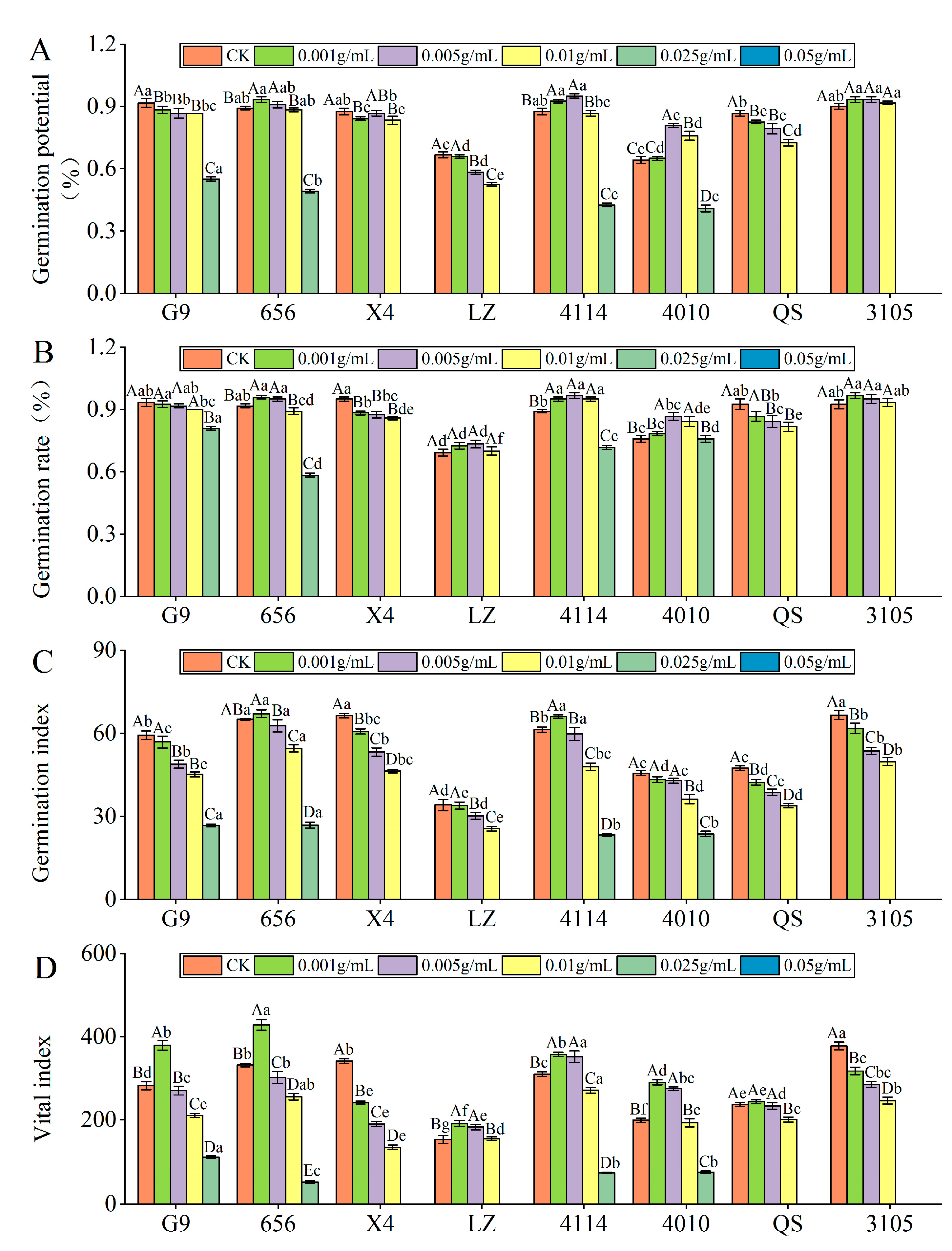
2.2.1. Effect of Alfalfa Extracts on Seed Germination
- Germination potential
- Germination rate
- Germination index
- Vital index
2.2.2. Effect of Alfalfa Extracts on Seedling Growth
- Root length
- Seedling length
- Fresh weight
- Dry weight
2.2.3. The Autotoxicity of Alfalfa Extracts on Seed Germination and Seedling Growth
- RI (GP)
- RI (GR)
- RI (GI)
- RI (VI)
- RI (RL)
- RI (SL)
- RI (FW)
- RI (DW)
2.3. Effect of Alfalfa Extracts on Seedling Physiology
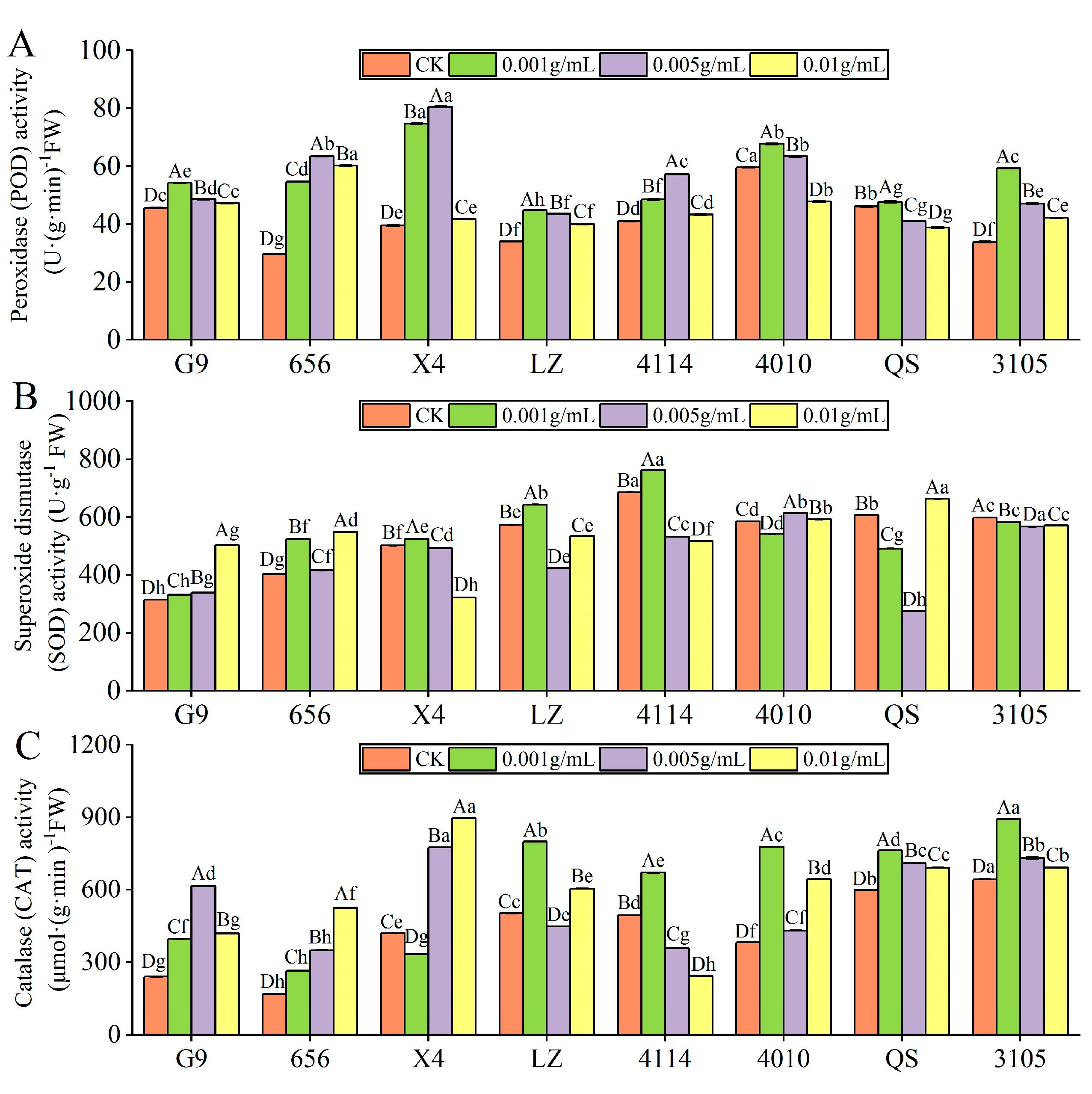
2.3.1. Effect of Alfalfa Extracts on Antioxidant Enzyme Activity of Seedlings
- POD activity
- SOD activity
- CAT activity
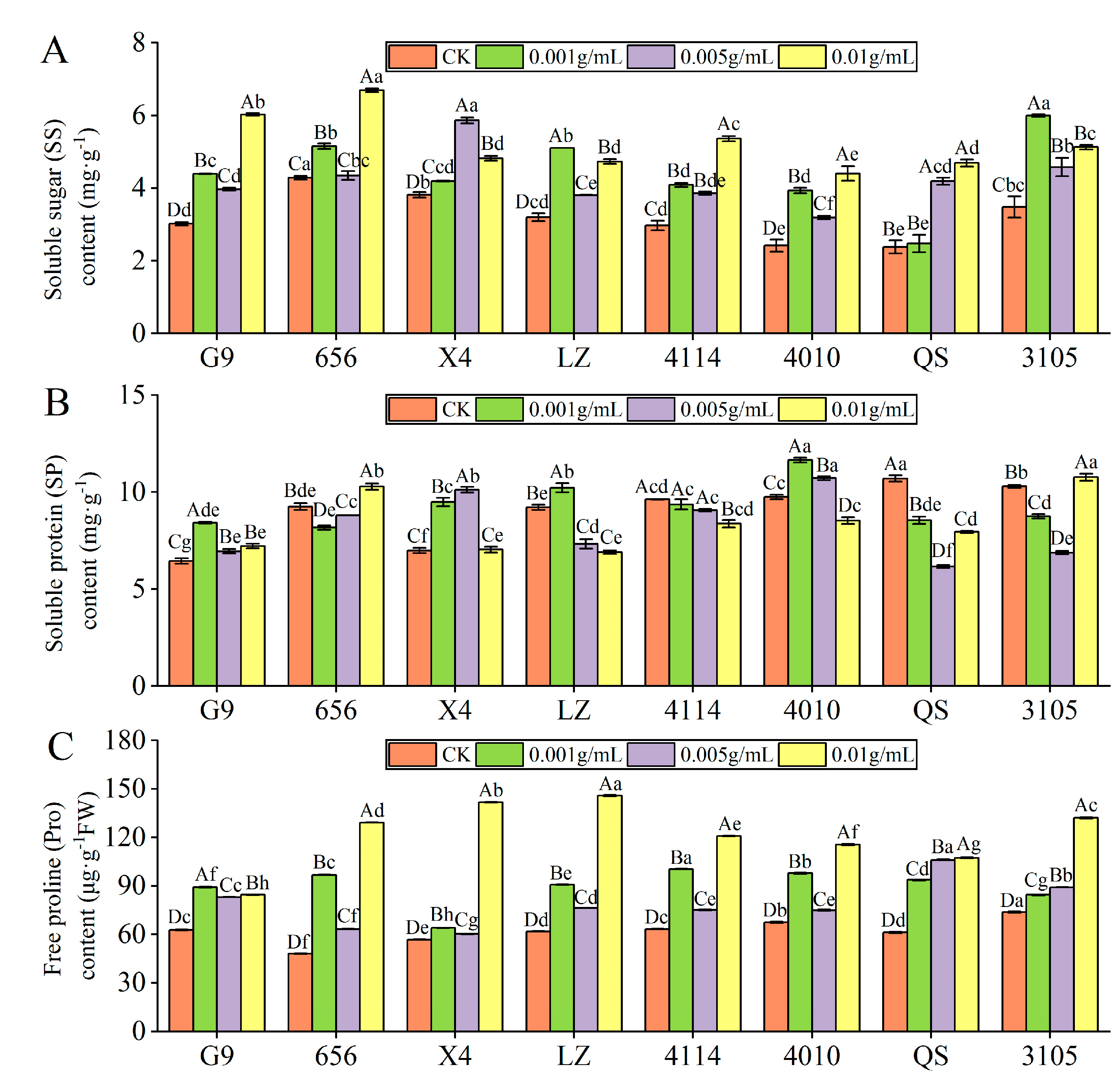
2.3.2. Effect of Alfalfa Extracts on the Content of Osmotic Adjustment Substances in Seedlings
- SS content
- SP content
- Pro content
2.3.3. Effect of Alfalfa Extracts on MDA and H2O2 Content in Seedlings
- MDA content
- H2O2 content
2.3.4. The Autotoxicity of Alfalfa Extracts on Seedling Physiology
- RI (POD)
- RI (SOD)
- RI (CAT)
- RI (SS)
- RI (SP)
- RI (Pro)
- RI (MDA)
- RI (H2O2)
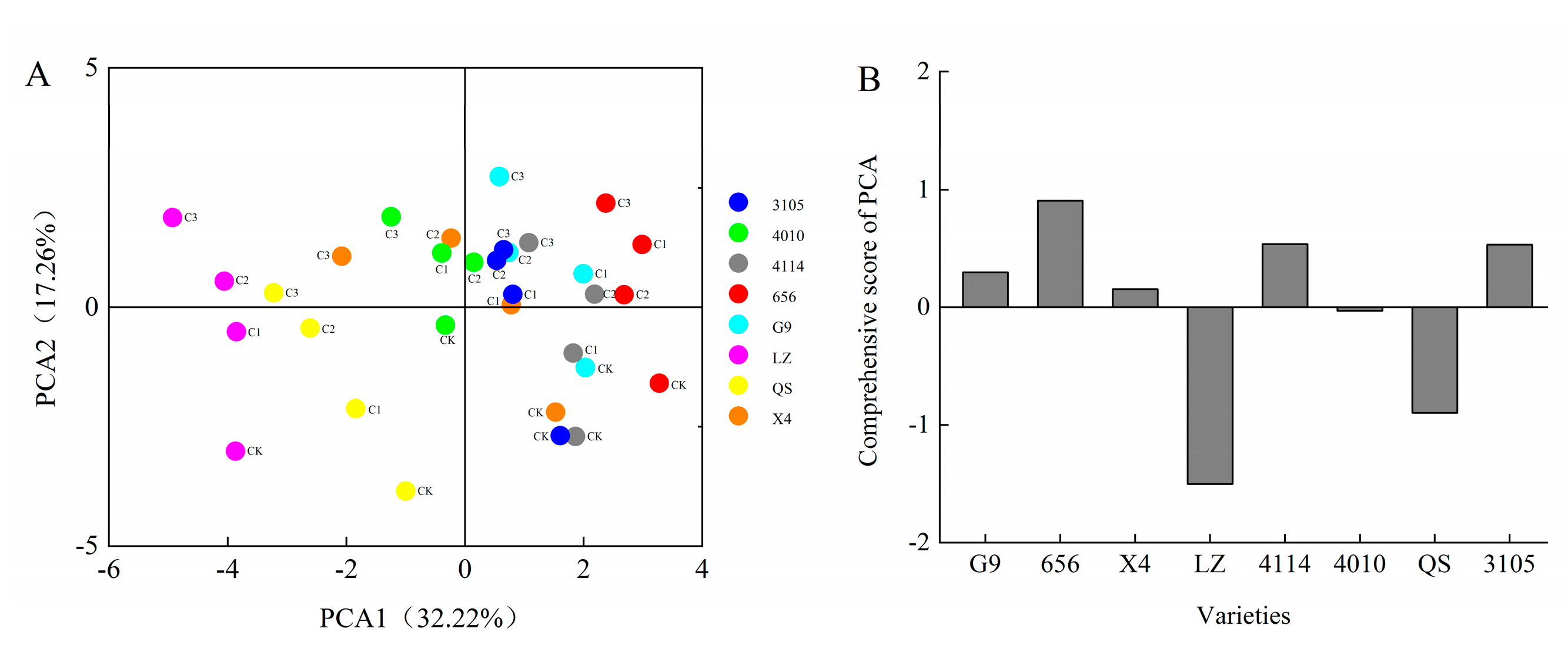
2.4. Comprehensive Analysis of Alfalfa Autotoxicity
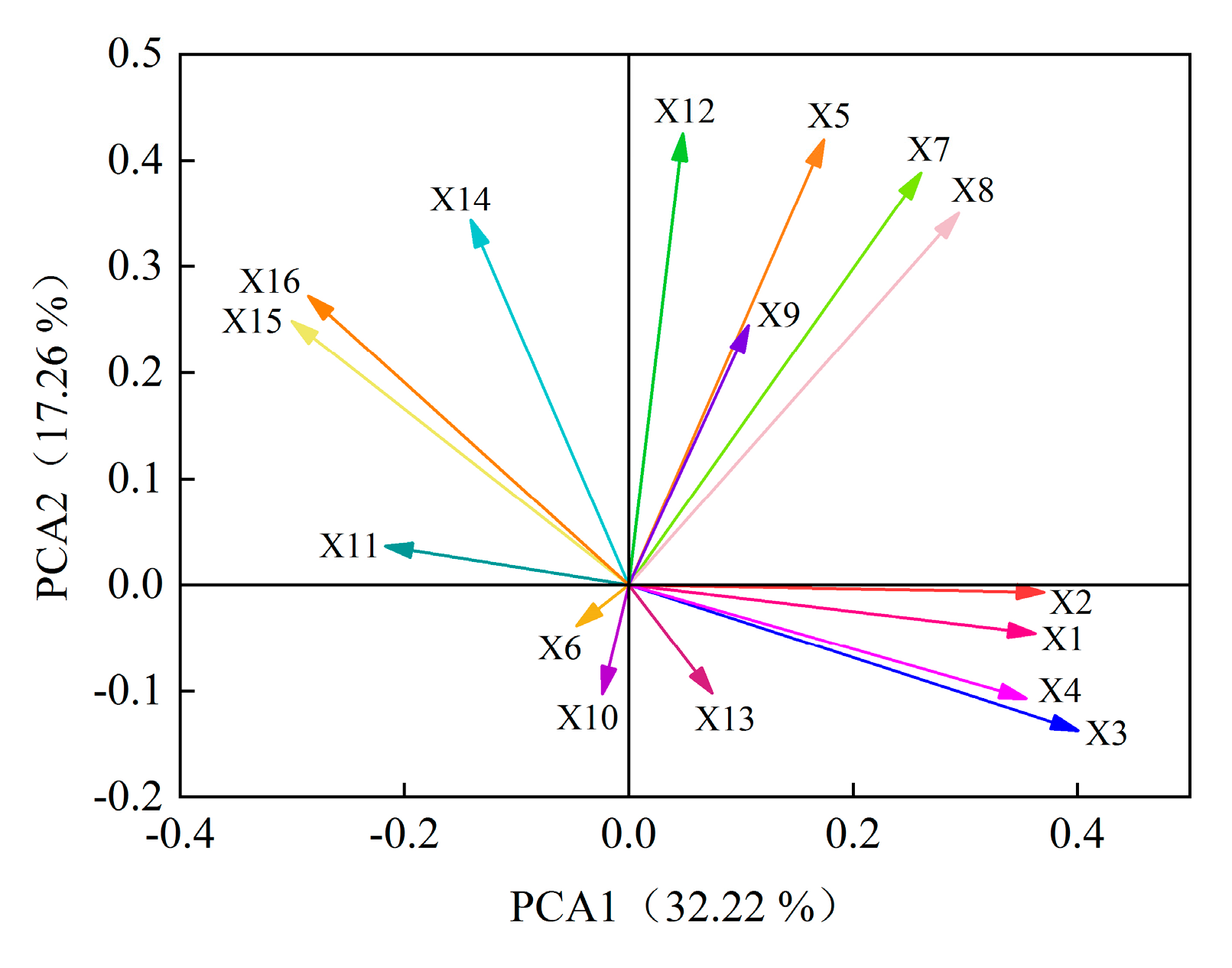
2.5. Study on the Relationship between the Content of Phenolic Autotoxic Substances and Autotoxicity in Alfalfa
2.5.1. Correlation Analysis between the Content of Phenolic Autotoxic Substances and Autotoxicity in Different Alfalfa Varieties
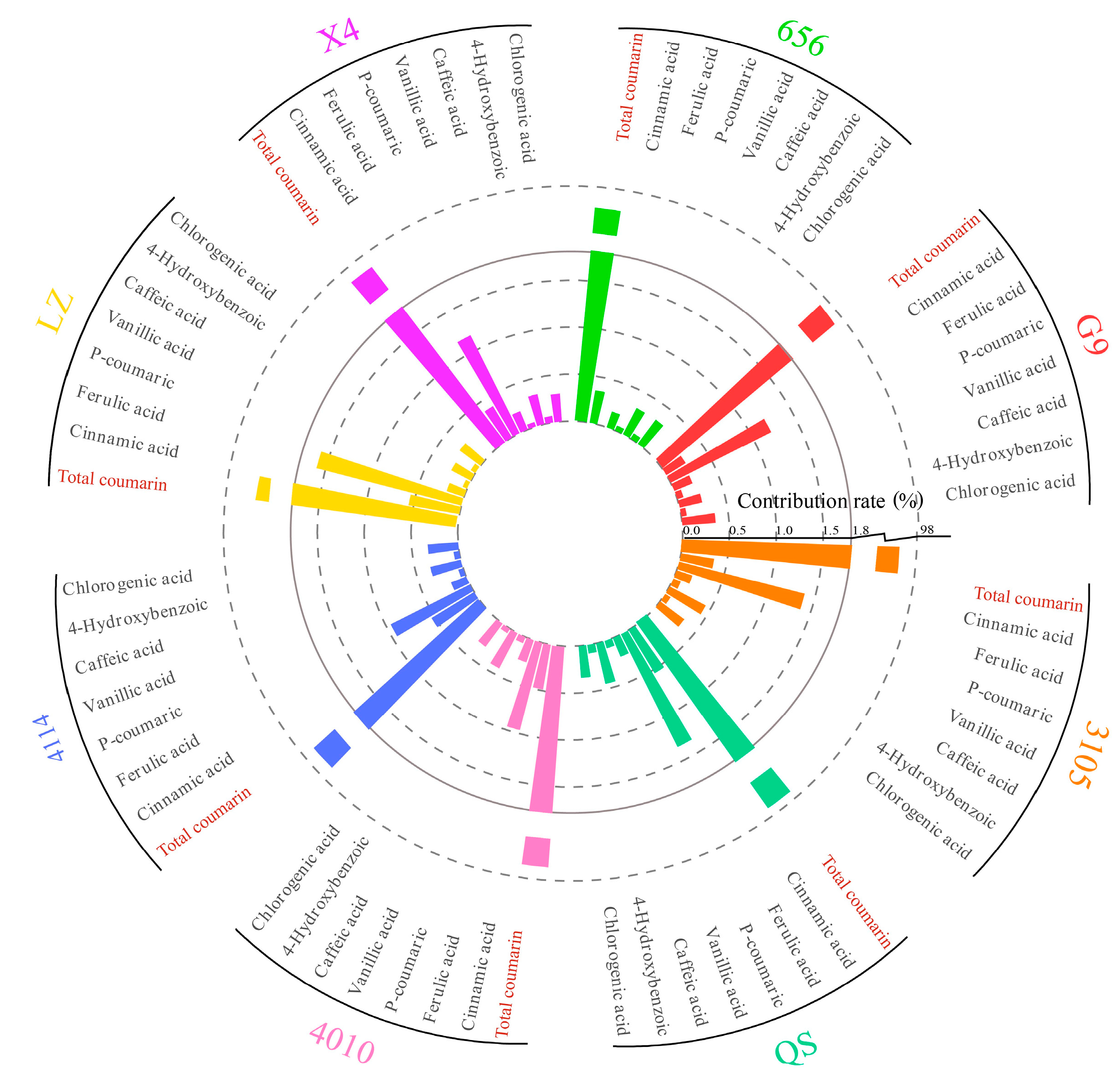
2.5.2. Analysis of the Contribution of Alfalfa Single Autotoxic Substances to Autotoxicity
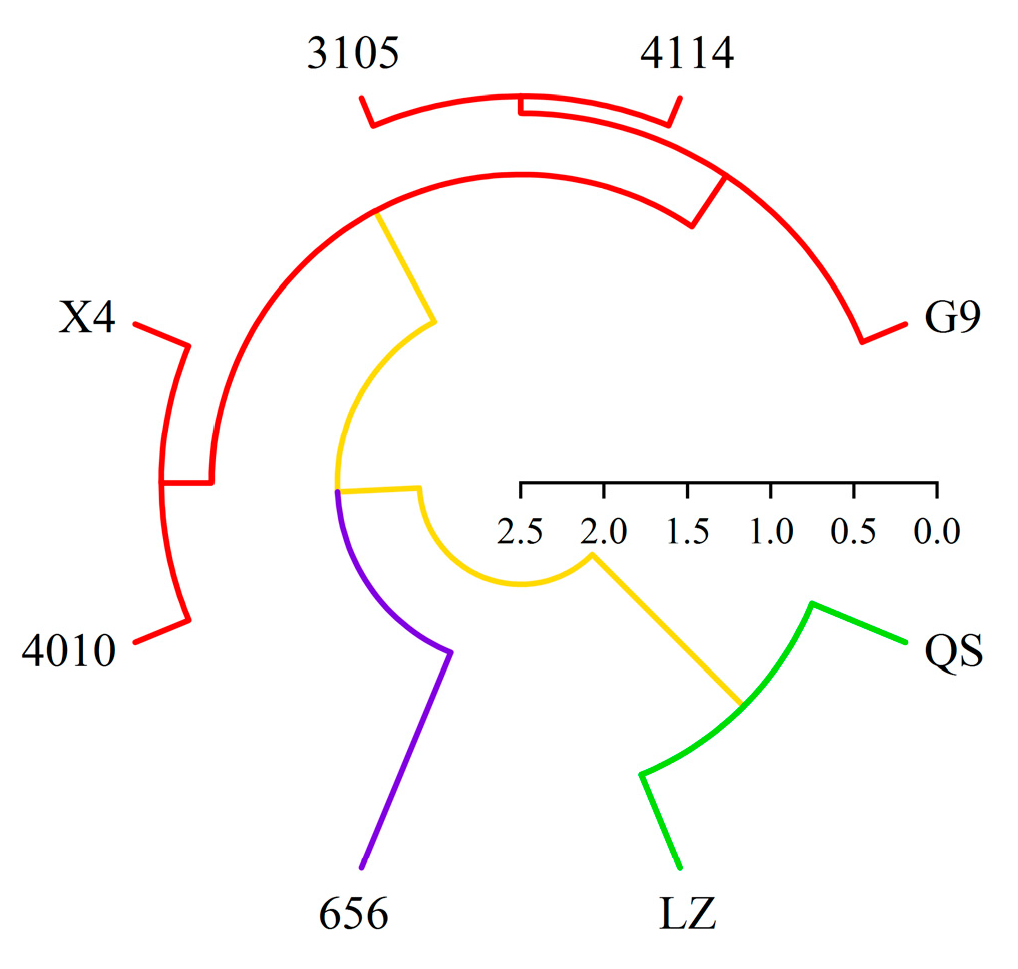
2.6. Cluster Analysis of Alfalfa Varieties
2.7. Construction of Autotoxicity Mechanism Model of Alfalfa
3. Discussion
3.1. The Response of Alfalfa Seed Germination, Seedling Growth and Seedling Physiology to Extracts Treatment
3.1.1. The Response of Alfalfa Seed Germination and Seedling Growth to Extracts Treatment
3.1.2. The Physiological Response of Alfalfa Seedlings to Extracts Treatment
- Osmotic adjustment ability
- Antioxidant system
3.2. The Relationship between the Key Autotoxic Substances and Autotoxicity of Alfalfa
4. Materials and Methods
4.1. Plant Material
4.2. Growth Conditions and Treatments
4.3. Preparation of Extracts from the Leaves of Alfalfa
4.4. The Seed Germination Test
4.5. Measurement Indexes
4.5.1. Determination of Phenolic Autotoxic Substances in Alfalfa Leaf Extracts
- Determination of chlorogenic acid, cinnamic acid, p-coumaric acid, caffeic acid, ferulic acid, p-hydroxybenzoic acid and vanillic acid content
- Determination of total coumarin content
4.5.2. Determination of Seed Germination and Seedling Growth Indexes
4.5.3. Determination of Seedling Physiological Indexes
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, D.A. Allelopathic effects of alfalfa. J. Chem. Ecol. 1983, 9, 1059–1072. [Google Scholar] [CrossRef] [PubMed]
- Chon, S.U.; Jennings, J.A.; Nelson, C.J. Alfalfa (Medicago sativa L.) autotoxicity: Current status. Allelopath. J. 2006, 18, 57–80. [Google Scholar]
- Rice, E.L. Allelopathy, 2nd ed.; AcademicPress: New York, NY, USA, 1984; p. 424. [Google Scholar]
- Wang, C.; Liu, Z.; Wang, Z.; Pang, W.; Zhang, L.; Wen, Z.; Yang, Z. Effects of autotoxicity and allelopathy on seed germination and seedling growth in Medicago truncatula. Front. Plant Sci. 2022, 13, 908426. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.A. Allelopathy in forage crop systems. Agron. J. 1996, 88, 854–859. [Google Scholar] [CrossRef]
- Rong, S.C. Study on the Autotoxin Contents and Its Autotoxicity in Medicago sativa L. Master’s Thesis, Gansu Agriculture University, Lanzhou, China, 2017. [Google Scholar]
- Cheng, J.; Wang, F.Q.; Li, X.S.; Du, Y.D.; Xie, Y. Research progress on types, extraction, separation and detection of allelochemicals of phenolic acids. Jiangsu Agric. Sci. 2022, 50, 8–15. [Google Scholar]
- Kong, C.H.; Xu, X.H.; Liang, W.J.; Zhou, Y.J.; Hu, F. Non-phenolic allelochemicals in root exudates of an allelopathic rice variety and their identification and weed-suppressive activity. Acta Ecol. Sin. 2004, 7, 1317–1322. [Google Scholar]
- Wang, K.J.; Chen, F.; Fan, J.W.; Li, Q.; Sun, Z.W.; Guo, M.M.; Zhang, G.X. Research Progress and Prospect of Wheat Allelopathy. Bull. Agric. Sci. Technol. 2018, 2, 7–11. [Google Scholar]
- Qin, Y.M.; Nie, L.W.; Huang, Y.Q.; Wang, Q.; Liu, X.; Zhou, K. Detection of Paeonia ostii autotoxins and their mechanism. Acta Ecol. Sin. 2009, 29, 1153–1161. [Google Scholar]
- Ghimire, B.K.; Ghimire, B.; Yu, C.Y.; Chung, I.M. Allelopathic and autotoxic effects of Medicago sativa-Derived allelochemicals. Plants 2019, 8, 233. [Google Scholar] [CrossRef]
- Lu, C.; Zeng, Z.H.; Zheng, S.Z.; Qi, Z.Q.; Hu, Y.G. The Difference of Autotoxic Compounds Content in Alfalfa Cultivars. Acta Agron. Sin. 2007, 33, 578–582. [Google Scholar]
- Sun, Y.; Lin, S.; Huang, L.; Zhang, X.; Guo, L. Review: Autotoxicity in medicinal plants and means to overcome. China J. Chin. Mater. Medica 2011, 36, 387–390. [Google Scholar] [CrossRef]
- Li, Z.F.; Zhang, Z.G.; Xie, D.F.; Dai, L.Q.; Zhu, L.F.; Li, J.; Lin, W.X. Positive allelopathic stimulation and underlying molecular mechanism of achyranthe under continuous monoculture. Acta Physiol. Plant. 2011, 33, 2339–2347. [Google Scholar] [CrossRef]
- Rong, S.C.; Shi, S.L.; Sun, C.C. Determination of Coumarins and Major Phenolic Acids in Plant and Rhizosphere Soil of Alfalfa (Medicago sativa L.). Soils 2016, 48, 931–938. [Google Scholar]
- Long, J.L.; Chen, S.S.; Wang, H.L.; Liu, H.C.; Zhao, Z.; Li, J.L. Effects of Polygonatum cyrtonema Plants and Rhizosphere Soil Water Extract on Seed Germination and Seed Bud Growth. J. Chin. Med. Mater. 2021, 44, 269–272. [Google Scholar]
- Li, Z.F.; He, C.L.; Wang, Y.; Li, M.J.; Dai, Y.J.; Wang, T.; Lin, W. Enhancement of trichothecene mycotoxins of Fusarium oxysporum by ferulic acid aggravates oxidative damage in Rehmannia glutinosa Libosch. Sci. Rep. 2016, 6, 33962. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y. Membrane lipid peroxidation and plant adversity stress. Chin. Bull. Bot. 1989, 4, 211–217. [Google Scholar]
- Yang, M.; Lin, S.Z.; Huang, Y.H.; Cao, G.Q. Allelopathic Effects of Salicylic Acid Stress on Membrane Lipid Peroxidation and Osmosis-regulating Substances in Different Chinese Fir Clones. Acta Bot. Boreali-Occident. Sin. 2006, 10, 2088–2093. [Google Scholar]
- Yang, L.F.; Zhu, Y.L.; Hu, C.M.; Liu, Z.L.; Zhang, G.W. Effects of NaCl Stress on the Contents of the Substances Regulating Membrane Lipid Oxidation and Osmosis and Photosynthetic Characteristics of Grafted Cucumber. Acta Bot. Boreali-Occident. Sin. 2006, 6, 1195–1200. [Google Scholar]
- Zhang, Z.; Zhang, Z.; Han, X.; Wu, J.; Zhang, L.; Wang, J.; Wang-Pruski, G. Specific response mechanism to autotoxicity in melon (Cucumis melo L.) root revealed by physiological analyses combined with transcriptome profiling. Ecotoxicol. Environ. Saf. 2020, 200, 110779. [Google Scholar] [CrossRef]
- Li, Z.H. Study on Allelopathic Effect of Alfalfa. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, 2005. [Google Scholar]
- Zhang, X.Y. Physiological and Molecular Mechanisms of Response to Autotoxicity Courmain in Alfalfa (Medicago sativa L.) Varieties. Ph.D. Thesis, Gansu Agriculture University, Lanzhou, China, 2022. [Google Scholar]
- Yang, T.T.; Du, H.R.; Chen, G.; Deng, P.; Zhen, W.W. Current research on plant allelopathy and its application in agricultural production. Acta Agric. Zhejiangensis 2012, 2, 343–348. [Google Scholar]
- Li, X.; Duan, Z.Q. Progress on the Research Methods for Root Exudates. Genom. Appl. Biol. 2013, 32, 540–547. [Google Scholar]
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed germination and vigor. Annu. Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef] [PubMed]
- Matías, L.; González-Díaz, P.; Jump, A.S. Larger investment in roots in southern range-edge populations of Scots pine is associated with increased growth and seedling resistance to extreme drought in response to simulated climate change. Environ. Exp. Bot. 2014, 105, 32–38. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, M.; Li, Y.; Shui, J.; Zhou, Y. Allelopathy of rice (Oryza sativa L.) root exudates and its relations with Orobanche cumana Wallr. and Orobanche minor Sm. germination. J. Plant Interact. 2014, 9, 722–730. [Google Scholar] [CrossRef]
- Zeng, R.S. Reviewonbioassaymethodsforallelopathyresearch. Chin. J. Appl. Ecol. 1999, 1, 125–128. [Google Scholar]
- Tibugari, H.; Chiduza, C.; Mashingaidze, A.B.; Mabasa, S. High sorgoleone autotoxicity in sorghum (Sorghum bicolor (L.) Moench) varieties that produce high sorgoleone content. S. Afr. J. Plant Soil 2020, 37, 160–167. [Google Scholar] [CrossRef]
- Wu, H.; Pratley, J.; Lemerle, D.; An, M.; Liu, D.L. Autotoxicity of wheat (Triticum aestivum L.) as determined by laboratory bioassays. Plant Soil 2007, 296, 85–93. [Google Scholar] [CrossRef]
- Kulan, E.; Ergin, N.; Kaya, M. Determination of allelopathic and autotoxic effects of root and stem extracts of some safflower (Carthamus tinctorius L.) varieties. Yuz. Yil Univ. J. Agric. Sci. 2020, 30, 801–809. [Google Scholar]
- Hegde, R.S.; Miller, D.A. Concentration dependency and stage of crop growth in alfalfa autotoxicity. Agron. J. 1992, 84, 940–946. [Google Scholar] [CrossRef]
- Luo, X.Y.; Sun, J. A study on allelopathic activities of different organs of 23 alfalfa species. Acta Prataculturae Sin. 2012, 21, 83–91. [Google Scholar]
- Shao, H.; Peng, S.L. Allelopathic effects in agroecosystems. Chin. J. Eco-Agric. 2002, 3, 106–108. [Google Scholar]
- Zhang, X.Y.; Shi, S.L.; Li, X.L.; Li, C.N.; Zhang, C.M.; Kang, W.J.; Yin, G.L. Effects of autotoxicity on alfalfa (Medicago sativa): Seed germination, oxidative damage and lipid peroxidation of seedlings. Agronomy 2021, 11, 1027. [Google Scholar] [CrossRef]
- Yang, C.Y.; Liu, S.J.; Zhou, S.W.; Wu, H.F.; Yu, J.B.; Xia, C.H. Allelochemical ethyl 2-methyl acetoacetate (EMA) induces oxidative damage and antioxidant responses in Phaeodactylum tricornutum. Pestic. Biochem. Physiol. 2011, 100, 93–103. [Google Scholar] [CrossRef]
- Lara-Nuñez, A.; Romero-Romero, T.; Ventura, J.L.; Blancas, V.; Anaya, A.L.; Cruz-Ortega, R. Allelochemical stress causes inhibition of growth and oxidative damage in Lycopersicon esculentum Mill. Plant Cell Environ. 2006, 29, 2009–2016. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Zhang, X.L.; Pan, Z.G.; Zhou, X.F.; Ni, W.Z. Autotoxicity and continuous cropping obstacles: A review. Chin. J. Soil Sci. 2007, 38, 781–784. [Google Scholar]
- Wang, R.L.; Liu, S.W.; Xin, X.W.; Chen, S.; Peng, G.X.; Su, Y.J.; Song, Z.K. Phenolic acids contents and allelopathic potential of 10-cultivars of alfalfa and their bioactivity. Allelopath. J. 2017, 40, 63–70. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Wang, L.Y.; Ding, G.H.; Li, L. Progress in Synthesis and Metabolism of Proline. Nat. Sci. J. Harbin Norm. 2010, 26, 84–89. [Google Scholar]
- Li, J.; Hong, T.; Lin, Y.M.; Xie, A.Q.; Chen, C.; Wu, C.Z.; Hong, W. Influences of Quercetin-3-α-araboside on Osmolytes in Branchlets and Roots of Casuarina equisetifolia L. J. Xiamen Univ. Nat. Sci. 2014, 53, 883–891. [Google Scholar]
- Li, G.Y.; Cao, Y.F.; Liu, R.Q.; Yu, W.W. Allelopathic Effects of Extracts from Metasequoia Metasequoia Leaves on the Physiology and Biochemistry of Ginkgo Biloba Seedlings. For. Sci. Technol. 2022, 47, 17–20. [Google Scholar]
- Wang, J.; Li, D.Q. The accumulation of plant osmoticum and activated oxygen metabolism under stress. Chin. Bull. Bot. 2001, 4, 459–465. [Google Scholar]
- Chen, S.L.; Zhou, B.L.; Lin, S.S.; Li, X.; Ye, X.L. Effects of cinnamic acid and vanillin on grafted eggplant root growth and physiological characteristics. Chin. J. Appl. Ecol. 2010, 21, 1446–1452. [Google Scholar]
- Yang, M. Analysis on the Allelopathic Effects and Differential Proteomeof the Different Chinese Fir Clones under Salicylic Acid Stress. Ph.D. Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2007. [Google Scholar]
- Cui, J.J. Effects of Two Typical Allelochemicals on Growth and Microorganism of Lanzhou Lily. Ph.D. Thesis, Gansu Agricultural University, Lanzhou, China, 2021. [Google Scholar]
- Luo, X. Study on the Biochemical Mechanism of Picea schrenkiana Autotoxicity. Master’s Thesis, Xinjiang Agricultural University, Urumqi, China, 2006. [Google Scholar]
- Hong, Y.; Hu, H.Y.; Xie, X.; Li, F.M. Responses of enzymatic antioxidants and non-enzymatic antioxidants in the cyanobacterium Microcystis aeruginosa to the allelochemical ethyl 2-methyl acetoacetate (EMA) isolated from reed (Phragmites communis). J. Plant Physiol. 2008, 165, 1264–1273. [Google Scholar] [CrossRef]
- Caverzan, A.; Casassola, A.; Brammer, S.P. Antioxidant responses of wheat plants under stress. Genet. Mol. Biol. 2016, 39, 1–6. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.A.D.; Tognetti, V.B.; Vandepoele, K.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, W.L.; Li, J. Study on root exudates of allelopathic autotoxicity of Astragalus membranaceus Bge. var. mongholicus Bge. Hsiao. Soils Crops 2019, 8, 102–109. [Google Scholar]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Breusegem, F.V. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Shahidi, R.; Yoshida, J.; Cougnon, M.; Reheul, D.; Van Labeke, M.C. Morpho-physiological responses to dehydration stress of perennial ryegrass and tall fescue genotypes. Funct. Plant Biol. 2017, 44, 612–623. [Google Scholar] [CrossRef]
- Song, L.B. Effect of Autotoxicity on the Growth of Rehmannia glutinosa Libosch. Master’s Thesis, Shandong Normal University, Jinan, China, 2009. [Google Scholar]
- Hu, S.K. Study on Effect of Rice Straw Allelochemicals to Rice Growth Andeliminate the Impact of Allelopathic. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2012. [Google Scholar]
- Zhang, R.M.; Wang, Y.Z.; Hou, P.; Wen, G.S.; Gao, Y. Physiological responses to allelopathy of aquatic stem and leaf extract of Artemisia frigida in seedling of several pasture plants. Acta Ecol. Sin. 2010, 8, 2197–2204. [Google Scholar]
- Li, M.; Yan, X.F.; Ma, L.; Ma, L.; Shi, R. Allelopathic inhibition of phenolic acids on germination of Wolfberry (Lycium barbarum Linn.). Acta Ecol. Sin. 2020, 40, 2072–2079. [Google Scholar]
- Yeasmin, R.; Nakamatsu, K.; Matsumoto, H.; Motoki, S.; Yamamoto, S. Inference of allelopathy and autotoxicity to varietal resistance of asparagus (Asparagus officinalis L.). Aust. J. Crop Sci. 2014, 8, 251–256. [Google Scholar]
- Han, X.; Yang, W.X.; Qin, F.Y.; Fan, X.S.; Li, L.W.; Huang, K.; Yang, T.Y.; Shen, Z.Y.; Wei, M.G. Extraction and Characterization of Root Exudates of Different Cassava Varieties. Chin. J. Trop. Crops 2022, 43, 1248. [Google Scholar]
- Zheng, R.; Shi, S.L.; Ma, S.C. Effects of exogenous autotoxic substances on alfalfa and wheat growth and development. Grassl. Turf. 2018, 38, 1–11. [Google Scholar]
- Song, L.; Pan, K.W.; Wang, J.C.; Ma, Y.H. Effects of phenolic acids on seed germination and seedling antioxidant enzyme activity of alfalfa. Acta Ecol. Sin. 2006, 26, 3393–3403. [Google Scholar]
- Chen, J.Y. Study on the Phenolic Acids in Winesand Phenylpropanoid Metabolism in Grape Berries. Ph.D. Thesis, China Agricultural University, Beijing, China, 2005. [Google Scholar]
- Tang, C.N. Study on Extraction, Separation and Purification Technology of Coumarin from Melilotus. Ph.D. Thesis, Northwest University, Xi’an, China, 2010. [Google Scholar]
- Buysse, J.A.N.; Merckx, R. An improved colorimetric method to quantify sugar content of plant tissue. J. Exp. Bot. 1993, 44, 1627–1629. [Google Scholar] [CrossRef]
- Zou, Q. Experimental Guidance of Plant Physiology; China Agricultural Publishing House: Beijing, China, 2007. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Draper, H.; Hadley, M. Malondialdehyde determination as index of lipid peroxidation. Oxyg. Radic. Biol. Syst. Part B Oxyg. Radic. Antioxid. 1990, 186, 421–431. [Google Scholar]



| Forage Extracts | Response Indexes (RI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Varieties | Concentrations | Seed Germination Indexes | Seedling Growth Indexes | ||||||
| GP | GR | GI | VI | RL | SL | FW | DW | ||
| G9 | C0.001 | −0.04 | −0.01 | −0.04 | 0.26 | 0.35 | 0.07 | 0.12 | 0.03 |
| C0.005 | −0.05 | −0.02 | −0.18 | −0.04 | 0.16 | 0.08 | 0.17 | 0.08 | |
| C0.01 | −0.05 | −0.04 | −0.24 | −0.25 | −0.08 | 0.12 | 0.15 | 0.19 | |
| C0.025 | −0.40 | −0.13 | −0.55 | −0.61 | −0.22 | 0.06 | 0.10 | 0.34 | |
| C0.05 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | |
| 656 | C0.001 | 0.04 | 0.04 | 0.03 | 0.23 | 0.25 | 0.08 | 0.10 | 0.01 |
| C0.005 | 0.02 | 0.04 | −0.04 | −0.09 | −0.08 | −0.02 | 0.10 | 0.02 | |
| C0.01 | −0.01 | −0.03 | −0.16 | −0.23 | −0.10 | −0.04 | 0.16 | 0.19 | |
| C0.025 | −0.45 | −0.36 | −0.59 | −0.85 | −0.87 | −0.07 | −0.08 | 0.06 | |
| C0.05 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | |
| X4 | C0.001 | −0.04 | −0.07 | −0.09 | −0.29 | −0.31 | 0.02 | 0.05 | 0.20 |
| C0.005 | −0.01 | −0.08 | −0.20 | −0.44 | −0.45 | 0.11 | 0.00 | 0.16 | |
| C0.01 | −0.05 | −0.10 | −0.30 | −0.61 | −0.61 | 0.06 | −0.09 | 0.07 | |
| C0.025 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | |
| C0.05 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | |
| LZ | C0.001 | −0.01 | 0.05 | 0.00 | 0.20 | 0.25 | 0.06 | 0.19 | 0.19 |
| C0.005 | −0.13 | 0.06 | −0.11 | 0.16 | 0.30 | 0.12 | 0.34 | 0.28 | |
| C0.01 | −0.21 | 0.01 | −0.25 | 0.01 | 0.29 | 0.16 | 0.37 | 0.26 | |
| C0.025 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | |
| C0.05 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | |
| 4114 | C0.001 | 0.05 | 0.06 | 0.07 | 0.13 | 0.08 | 0.02 | 0.01 | 0.06 |
| C0.005 | 0.08 | 0.08 | −0.02 | 0.12 | 0.17 | 0.05 | 0.19 | 0.11 | |
| C0.01 | −0.01 | 0.06 | −0.22 | −0.13 | 0.07 | 0.18 | 0.14 | −0.07 | |
| C0.025 | −0.51 | −0.20 | −0.62 | −0.77 | −0.54 | −0.01 | −0.03 | −0.08 | |
| C0.05 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | |
| 4010 | C0.001 | 0.01 | 0.03 | −0.05 | 0.31 | 0.44 | 0.03 | 0.08 | 0.02 |
| C0.005 | 0.21 | 0.13 | −0.06 | 0.28 | 0.39 | 0.11 | 0.01 | −0.06 | |
| C0.01 | 0.15 | 0.10 | −0.21 | −0.03 | 0.19 | 0.16 | −0.06 | −0.12 | |
| C0.025 | −0.36 | 0.00 | −0.48 | −0.63 | −0.47 | 0.08 | −0.14 | −0.15 | |
| C0.05 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | |
| QS | C0.001 | −0.05 | −0.06 | −0.11 | 0.03 | 0.12 | 0.16 | 0.05 | 0.03 |
| C0.005 | −0.09 | −0.09 | −0.18 | −0.02 | 0.17 | 0.16 | 0.14 | 0.05 | |
| C0.01 | −0.16 | −0.12 | −0.28 | −0.15 | 0.12 | 0.25 | 0.19 | 0.09 | |
| C0.025 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | |
| C0.1 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | |
| 3105 | C0.001 | 0.04 | 0.04 | −0.07 | −0.16 | −0.13 | 0.03 | 0.04 | 0.03 |
| C0.005 | 0.04 | 0.03 | −0.19 | −0.24 | −0.14 | 0.17 | 0.16 | 0.06 | |
| C0.01 | 0.02 | 0.01 | −0.25 | −0.35 | −0.23 | 0.18 | 0.13 | 0.12 | |
| C0.025 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | |
| C0.05 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | −1.00 | |
| Forage Extracts | Response Indexes (RI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Varieties | Concentrations | POD | SOD | CAT | SS | SP | PRO | MDA | H2O2 |
| G9 | C0.001 | 0.16 | 0.05 | 0.39 | 0.31 | 0.24 | 0.30 | 0.18 | 0.04 |
| C0.005 | 0.06 | 0.07 | 0.61 | 0.24 | 0.07 | 0.25 | 0.21 | 0.07 | |
| C0.01 | 0.03 | 0.37 | 0.43 | 0.50 | 0.11 | 0.26 | 0.24 | 0.11 | |
| 656 | C0.001 | 0.46 | 0.23 | 0.37 | 0.17 | −0.12 | 0.50 | 0.30 | 0.13 |
| C0.005 | 0.53 | 0.03 | 0.52 | 0.01 | −0.05 | 0.24 | 0.23 | 0.07 | |
| C0.01 | 0.51 | 0.27 | 0.68 | 0.36 | 0.10 | 0.63 | 0.15 | 0.01 | |
| X4 | C0.001 | 0.47 | 0.04 | −0.21 | 0.09 | 0.26 | 0.11 | 0.15 | 0.08 |
| C0.005 | 0.51 | −0.02 | 0.46 | 0.35 | 0.31 | 0.06 | 0.21 | 0.11 | |
| C0.01 | 0.05 | −0.36 | 0.53 | 0.21 | 0.01 | 0.60 | 0.19 | 0.07 | |
| LZ | C0.001 | 0.24 | 0.11 | 0.37 | 0.37 | 0.10 | 0.32 | 0.07 | 0.08 |
| C0.005 | 0.22 | −0.26 | −0.11 | 0.16 | −0.20 | 0.19 | 0.15 | 0.15 | |
| C0.01 | 0.15 | −0.07 | 0.17 | 0.32 | −0.25 | 0.58 | 0.12 | 0.12 | |
| 4114 | C0.001 | 0.15 | 0.10 | 0.27 | 0.27 | −0.03 | 0.37 | 0.05 | 0.13 |
| C0.005 | 0.28 | −0.22 | −0.28 | 0.23 | −0.06 | 0.16 | 0.25 | 0.16 | |
| C0.01 | 0.05 | −0.25 | −0.51 | 0.45 | −0.13 | 0.48 | 0.11 | 0.16 | |
| 4010 | C0.001 | 0.12 | −0.08 | 0.51 | 0.38 | 0.16 | 0.31 | 0.12 | 0.02 |
| C0.005 | 0.06 | 0.05 | 0.11 | 0.24 | 0.09 | 0.10 | 0.30 | 0.03 | |
| C0.01 | −0.20 | 0.01 | 0.41 | 0.45 | −0.13 | 0.42 | 0.24 | 0.03 | |
| QS | C0.001 | 0.03 | −0.19 | 0.22 | 0.04 | −0.20 | 0.35 | 0.07 | 0.01 |
| C0.005 | −0.11 | −0.55 | 0.16 | 0.43 | −0.42 | 0.42 | 0.20 | 0.03 | |
| C0.01 | −0.16 | 0.08 | 0.13 | 0.49 | −0.26 | 0.43 | 0.24 | 0.07 | |
| 3105 | C0.001 | 0.43 | −0.03 | 0.28 | 0.42 | −0.15 | 0.13 | 0.25 | 0.05 |
| C0.005 | 0.28 | −0.05 | 0.12 | 0.24 | −0.33 | 0.17 | 0.35 | 0.03 | |
| C0.01 | 0.20 | −0.05 | 0.07 | 0.32 | 0.04 | 0.44 | 0.21 | 0.02 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, B.; Shi, S.; Zhang, H.; Du, Y.; Jing, F. Study on the Key Autotoxic Substances of Alfalfa and Their Effects. Plants 2023, 12, 3263. https://doi.org/10.3390/plants12183263
Wu B, Shi S, Zhang H, Du Y, Jing F. Study on the Key Autotoxic Substances of Alfalfa and Their Effects. Plants. 2023; 12(18):3263. https://doi.org/10.3390/plants12183263
Chicago/Turabian StyleWu, Bei, Shangli Shi, Huihui Zhang, Yuanyuan Du, and Fang Jing. 2023. "Study on the Key Autotoxic Substances of Alfalfa and Their Effects" Plants 12, no. 18: 3263. https://doi.org/10.3390/plants12183263
APA StyleWu, B., Shi, S., Zhang, H., Du, Y., & Jing, F. (2023). Study on the Key Autotoxic Substances of Alfalfa and Their Effects. Plants, 12(18), 3263. https://doi.org/10.3390/plants12183263





