Exploring Wild Hordeum spontaneum and Hordeum marinum Accessions as Genetic Resources for Fungal Resistance
Abstract
:1. Introduction
2. Results
2.1. Morphological Phenotyping of the Collection
2.2. Evaluation of Resistance against Fungal Pathogens
2.2.1. Field Resistance of the Evaluated Accessions to Blumeria graminis, Puccinia striiformis, Puccinia hordei, and Puccinia graminis
2.2.2. Resistance against Puccinia hordei, Puccinia graminis, Fusarium culmorum and Pyrenophora teres in Greenhouse under Artificial Infection
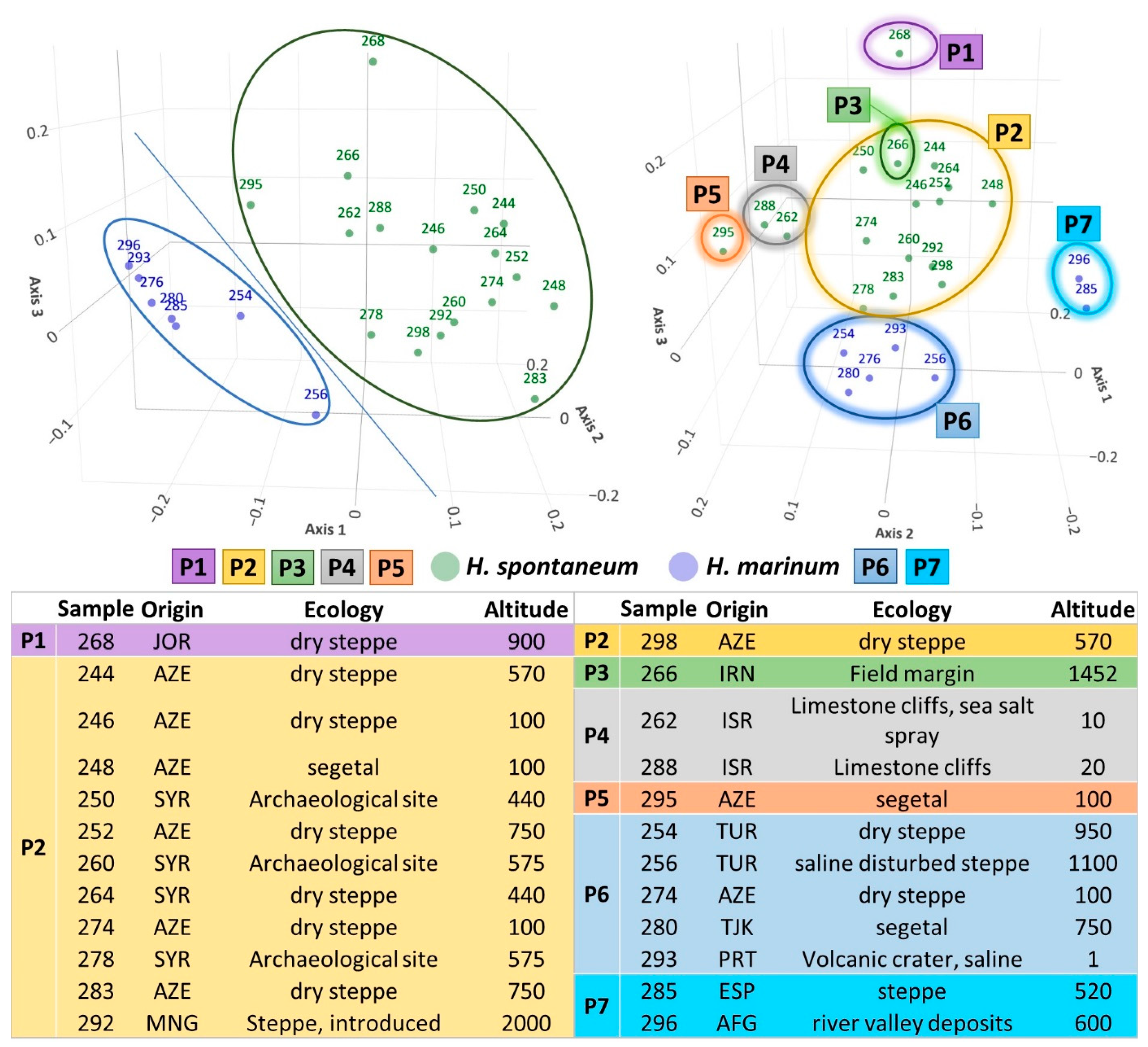
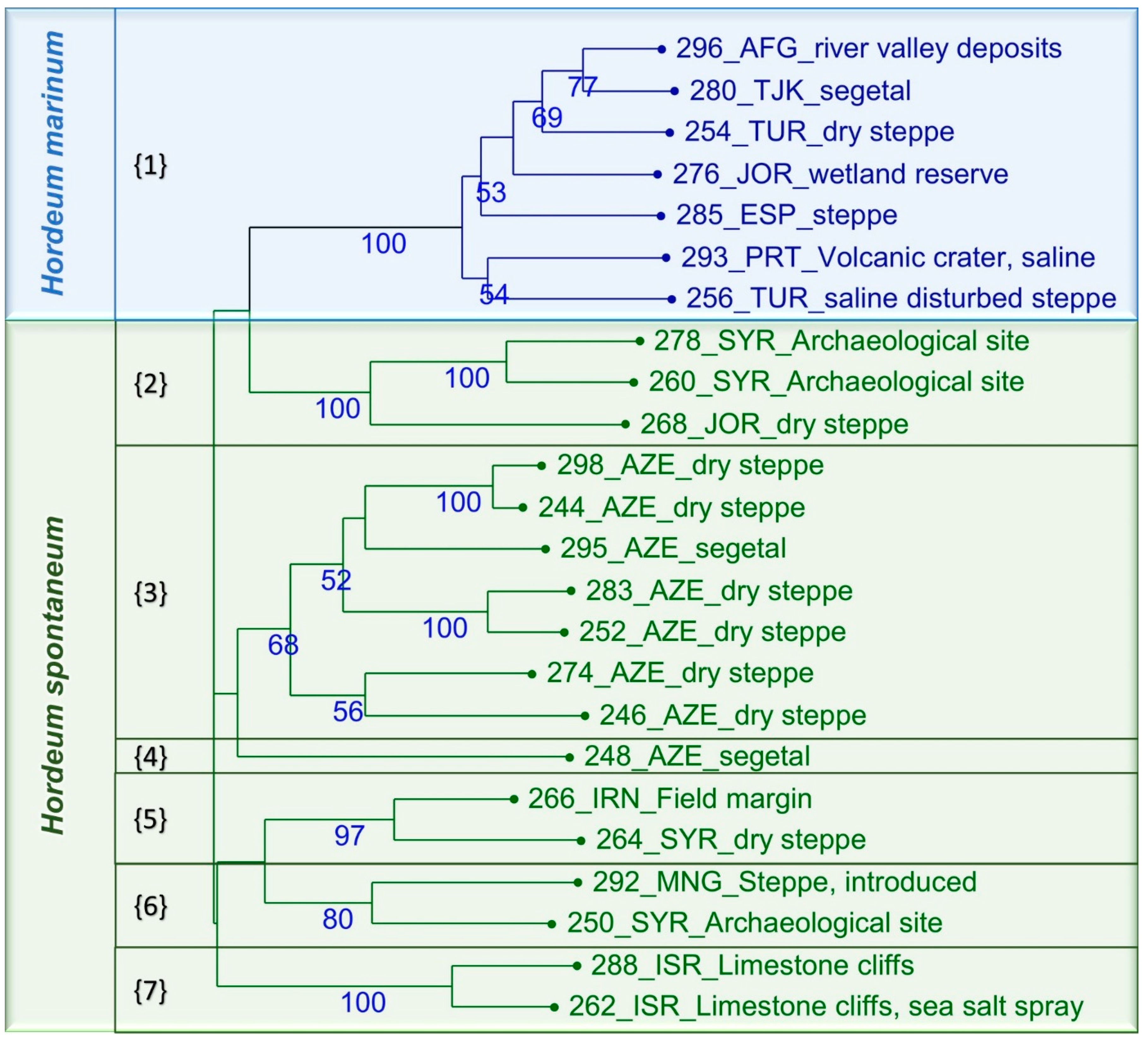
2.3. Evaluation of the Collection Using SSR Markers
2.4. Evaluation of CWRs Using SSR Markers Associated with Barley Resistance Loci
3. Discussion
4. Materials and Methods
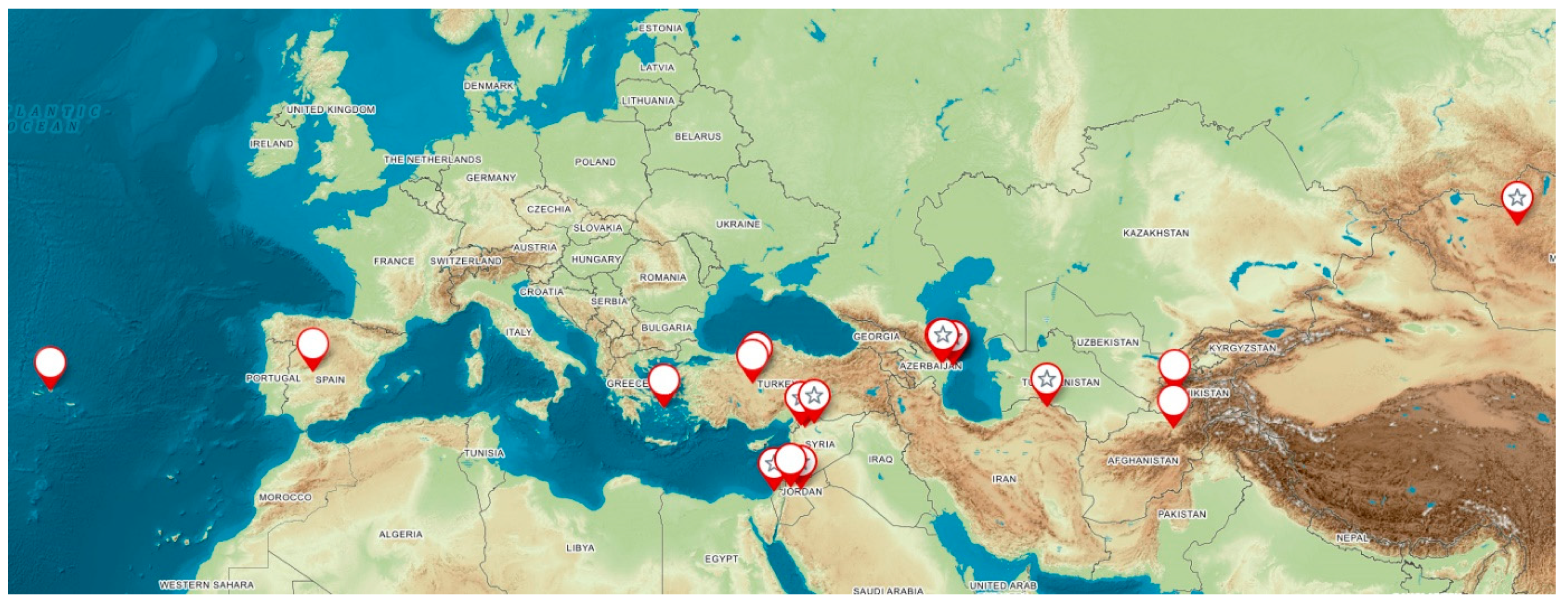
4.1. Plant Materials
Distribution of Species and Habitats
4.2. Field Evaluation of the CWR Collection
4.3. Evaluation of Brown Rust and Stem Rust Resistance in the Greenhouse
4.4. Evaluation of Fusarium Head Blight (FBH) Resistance in the Greenhouse
4.5. Evaluation of Pyrenophora Teres Resistance in the Greenhouse
4.6. Genetic Analysis of the CWR Collection
4.6.1. DNA Extraction
4.6.2. SSR Analysis
4.7. Screening for Length Variability at Rpg1- and Rpg5-Associated Loci
4.8. Statistical Analysis
4.9. Cluster and Dissimilarity Analyses
4.10. Phenotypic Data Analysis
4.11. SSR and Phenotypic Data Comparisons
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hajjar, R.; Hodgkin, T. The use of wild relatives in crop improvement: A survey of developments over the last 20 years. Euphytica 2007, 156, 1–13. [Google Scholar] [CrossRef]
- Dempewolf, H.; Baute, G.; Anderson, J.; Kilian, B.; Smith, C.; Guarino, L. Past and Future Use of Wild Relatives in Crop Breeding Crop Science. 2017, pp. 1070–1082. Available online: https://acsess.onlinelibrary.wiley.com/doi/abs/10.2135/cropsci2016.10.0885 (accessed on 7 September 2023).
- Hajjar, R.; Hodgkin, T. Using crop wild relatives for crop improvement: Trends and perspectives. In Crop Wild Relative Conservation and Use; Maxted, N.B.V.F., Kell, S.P., Iriondo, J., Dulloo, E., Turok, J., Eds.; CABI: Sicily, Italy, 2007; pp. 535–548. [Google Scholar]
- Maxted, N.; Scholten, M.; Codd, R.; Ford-Lloyd, B. Creation and use of a national inventory of crop wild relatives. Biol. Conserv. 2007, 140, 142–159. [Google Scholar] [CrossRef]
- Razzaq, A.; Saleem, F.; Wani, S.H.; Abdelmohsen, S.A.M.; Alyousef, H.A.; Abdelbacki, A.M.M.; Alkallas, F.H.; Tamam, N.; Elansary, H.O. De-novo Domestication for Improving Salt Tolerance in Crops. Front. Plant Sci. 2021, 12, 681367. [Google Scholar] [CrossRef] [PubMed]
- FAO. Faostat Database. Available online: https://www.fao.org/faostat/en/#search/FAO%202020%20FAO%20crop%20estimate%20data%20 (accessed on 27 January 2023).
- Badr, A.M.K.; Sch, R.; Rabey, H.E.; Effgen, S.; Ibrahim, H.H.; Pozzi, C.; Rohde, W.; Salamini, F. On the Origin and Domestication History of Barley (Hordeum vulgare). Mol. Biol. Evol. 2000, 17, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Morrell, P.L.; Clegg, M.T. Genetic evidence for a second domestication of barley (Hordeum vulgare) east of the Fertile Crescent. Proc. Natl. Acad. Sci. USA 2007, 104, 3289–3294. [Google Scholar] [CrossRef]
- Hammer, K. Das Domestikationssyndrom. Die Kult. 1984, 32, 11–34. [Google Scholar] [CrossRef]
- Barati, M.; Majidi, M.M.; Mirlohi, A.; Safari, M.; Mostafavi, F.; Karami, Z. Potential of Iranian Wild Barley (Hordeum vulgare ssp. spontaneum) in Breeding for Drought Tolerance. Cereal Res. Commun. 2018, 46, 707–716. [Google Scholar] [CrossRef]
- Ebrahim, F.; Arzani, A.; Rahimmalek, M.; Sun, D.F.; Peng, J.H. Salinity tolerance of wild barley Hordeum vulgare ssp. spontaneum. Plant Breed. 2020, 139, 304–316. [Google Scholar] [CrossRef]
- Kintzios, S.; Fischbeck, G. Identification of new sources for resistance to powdery mildew in H-spontaneum-derived winter barley lines. Genet. Resour. Crop Evol. 1996, 43, 25–31. [Google Scholar] [CrossRef]
- Global, G. GRIN Czech. Available online: https://grinczech.vurv.cz/gringlobal/search.aspx (accessed on 6 January 2022).
- Jakob, S.S.; Ihlow, A.; Blattner, F.R. Combined ecological niche modelling and molecular phylogeography revealed the evolutionary history of Hordeum marinum (Poaceae)—Niche differentiation, loss of genetic diversity, and speciation in Mediterranean Quaternary refugia. Mol. Ecol. 2007, 16, 1713–1727. [Google Scholar] [CrossRef]
- Harlan, J.R.; Zohary, D. Distribution of Wild Wheats and Barley. Science 1966, 153, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Bedada, G.; Westerbergh, A.; Müller, T.; Galkin, E.; Bdolach, E.; Moshelion, M.; Fridman, E.; Schmid, K.J. Transcriptome sequencing of two wild barley (Hordeum spontaneum L.) ecotypes differentially adapted to drought stress reveals ecotype-specific transcripts. BMC Genom. 2014, 15, 995. [Google Scholar] [CrossRef] [PubMed]
- Carmona, A.; Friero, E.; de Bustos, A.; Jouve, N.; Cuadrado, A. The evolutionary history of sea barley (Hordeum marinum) revealed by comparative physical mapping of repetitive DNA. Ann. Bot. 2013, 112, 1845–1855. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Sun, G.L.; Sun, D.K.; Ren, X.F. Phylogenetic analysis of two single-copy nuclear genes revealed origin of tetraploid barley Hordeum marinum. PLoS ONE 2020, 15, e0235475. [Google Scholar] [CrossRef]
- Huang, L.; Kuang, L.H.; Li, X.; Wu, L.Y.; Wu, D.Z.; Zhang, G.P. Metabolomic and transcriptomic analyses reveal the reasons why Hordeum marinum has higher salt tolerance than Hordeum vulgare. Environ. Exp. Bot. 2018, 156, 48–61. [Google Scholar] [CrossRef]
- Alamri, S.A.; Barrett-Lennard, E.G.; Teakle, N.L.; Colmer, T.D. Improvement of salt and waterlogging tolerance in wheat: Comparative physiology of Hordeum marinum-Triticum aestivum amphiploids with their H. marinum and wheat parents. Funct. Plant Biol. 2013, 40, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Liu, D. New hordeum-triticum hybrids. Cereal Res. Commun. 1987, 15, 95–99. [Google Scholar]
- Garthwaite, A.J.; von Bothmer, R.; Colmer, T.D. Salt tolerance in wild Hordeum species is associated with restricted entry of Na+ and Cl− into the shoots. J. Exp. Bot. 2005, 56, 2365–2378. [Google Scholar] [CrossRef]
- Efremova, T.; Arbuzova, V.; Trubacheeva, N.; Ocadchaya, T.; Chumanova, E.; Pershina, L. Substitution of Hordeum marinum ssp gussoneanum chromosome 7HL into wheat homoeologous group-7. Euphytica 2013, 192, 251–257. [Google Scholar] [CrossRef]
- Mano, Y.; Omori, F. Flooding tolerance in interspecific introgression lines containing chromosome segments from teosinte (Zea nicaraguensis) in maize (Zea mays subsp mays). Ann. Bot. 2013, 112, 1125–1139. [Google Scholar] [CrossRef]
- Salomon, B.; Vonbothmer, R.; Jacobsen, N. Intergeneric crosses between Hordeum and north-american Elymus (Poaceae, Triticeae). Hereditas 1991, 114, 35–39. [Google Scholar] [CrossRef]
- Riaz, A.; Kanwal, F.; Borner, A.; Pillen, K.; Dai, F.; Alqudah, A.M. Advances in Genomics-Based Breeding of Barley: Molecular Tools and Genomic Databases. Agronomy 2021, 11, 894. [Google Scholar] [CrossRef]
- Thudi, M.; Palakurthi, R.; Schnable, J.C.; Chitikineni, A.; Dreisigacker, S.; Mace, E.; Srivastava, R.K.; Satyavathi, C.T.; Odeny, D.; Tiwari, V.K.; et al. Genomic resources in plant breeding for sustainable agriculture. J. Plant Physiol. 2021, 257, 153351. [Google Scholar] [CrossRef]
- Alqudah, A.M.; Sallam, A.; Baenziger, P.S.; Borner, A. GWAS: Fast-forwarding gene identification and characterization in temperate Cereals: Lessons from Barley—A review. J. Adv. Res. 2020, 22, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Zenda, T.; Liu, S.T.; Dong, A.Y.; Duan, H.J. Advances in Cereal Crop Genomics for Resilience under Climate Change. Life 2021, 11, 502. [Google Scholar] [CrossRef]
- Sovova, T.; Kerins, G.; Demnerova, K.; Ovesna, J. Genome Editing with Engineered Nucleases in Economically Important Animals and Plants: State of the Art in the Research Pipeline. Curr. Issues Mol. Biol. 2017, 21, 41–61. [Google Scholar]
- Bilz, M.; Kell, S.; Maxted, N.; Lansdown, R. European Red. List. of Vascular Plants; Publications Office of the European Union: Luxembourg, 2011. [Google Scholar]
- Kell, S.; Maxted, N.; Bilz, M. European crop wild relative threat assessment: Knowledge gained and lessons learnt. In Agrobiodiversity Conservation: Securing the Diversity of Crop Wild Relatives and Landraces; CABI International: Wallingford, UK, 2012; pp. 218–242. [Google Scholar]
- Jia, Q.J.; Zhu, J.H.; Wang, J.M.; Yang, J.M. Fusarium Head Blight Evaluation and Genetic Diversity Assessment by Simple Sequence Repeats in 88 Barley Cultivars and Landraces. In Proceedings of the 10th International Barley Genetics Symposium, Alexandria, Egypt, 5–10 April 2008; Ceccarelli, S., Grando, S., Eds.; ICARDA: Alexandria, Egypt, 2008; pp. 298–310. [Google Scholar]
- Russell, J.; Fuller, J.; Young, G.; Thomas, B.; Taramino, G.; Macaulay, M.; Waugh, R.; Powell, W. Discriminating between barley genotypes using microsatellite markers. Genome 1997, 40, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, L.; Macaulay, M.; Ivanissevich, S.d.; MacLean, K.; Cardle, L.; Fuller, J.; Edwards, K.J.; Tuvesson, S.; Morgante, M.; Massari, A.; et al. A Simple Sequence Repeat-Based Linkage Map of Barley. Genetics 2000, 156, 1997–2005. [Google Scholar] [CrossRef]
- Sanchez-Martin, J.; Keller, B. Contribution of recent technological advances to future resistance breeding. Theor. Appl. Genet. 2019, 132, 713–732. [Google Scholar] [CrossRef]
- Osman, M.; He, X.; Capettini, F.; Helm, J.; Singh, P.K. Phenotypic Characterization of Canadian Barley Advanced Breeding Lines for Multiple Disease Resistance. Cereal Res. Commun. 2019, 47, 484–495. [Google Scholar] [CrossRef]
- von Korff, M.; Wang, H.; Léon, J.; Pillen, K. Development of candidate introgression lines using an exotic barley accession (Hordeum vulgare ssp. spontaneum) as donor. Theor. Appl. Genet. 2004, 109, 1736–1745. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Z.; Huang, X.Q.; Heinrichs, F.; Ganal, M.W.; Röder, M.S. Analysis of QTLs for yield components, agronomic traits, and disease resistance in an advanced backcross population of spring barley. Genome 2006, 49, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Rubiales, D.; Ramírez, M.C.; Niks, R.E. Avoidance of leaf rust fungi in wild relatives of cultivated cereals. Euphytica 1996, 87, 1–6. [Google Scholar] [CrossRef]
- Rehman, S.; Amouzoune, M.; Hiddar, H.; Aberkane, H.; Benkirane, R.; Filali-Maltouf, A.; Al-Jaboobi, M.; Acqbouch, L.; Tsivelikas, A.; Verma, R.P.S.; et al. Trait discovery in Hordeum vulgare sbsp. spontaneum accessions and in lines derived from interspecific crosses with wild Hordeum species for enhancing barley breeding efforts. Crop Sci. 2021, 61, 219–233. [Google Scholar] [CrossRef]
- Yu, X.H.; Casonato, S.; Jones, E.; Butler, R.C.; Johnston, P.A.; Chng, S. Phenotypic characterization of the Hordeum bulbosum derived leaf rust resistance genes Rph22 and Rph26 in barley. J. Appl. Microbiol. 2022, 133, 2083–2094. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kistler, H.; Ma, Z. Fusarium graminearum Trichothecene Mycotoxins: Biosynthesis, Regulation, and Management. Annu. Rev. Phytopathol. 2019, 57, 15–39. [Google Scholar] [CrossRef]
- Salacova, L.; Faltusova, Z.; Ovesna, J. Mechanisms Used by Plants for Defence against Fungal Pathogens. Chem. Listy 2015, 109, 613–618. [Google Scholar]
- Moretti, A.; Pascale, M.; Logrieco, A.F. Mycotoxin risks under a climate change scenario in Europe. Trends Food Sci. Technol. 2019, 84, 38–40. [Google Scholar] [CrossRef]
- Valverde-Bogantes, E.; Bianchini, A.; Herr, J.R.; Rose, D.J.; Wegulo, S.N.; Hallen-Adams, H.E. Recent population changes of Fusarium head blight pathogens: Drivers and implications. Can. J. Plant Pathol. 2020, 42, 315–329. [Google Scholar] [CrossRef]
- Kolawole, O.; Meneely, J.; Petchkongkaew, A.; Elliott, C. A review of mycotoxin biosynthetic pathways: Associated genes and their expressions under the influence of climatic factors. Fungal Biol. Rev. 2021, 37, 8–26. [Google Scholar] [CrossRef]
- Spunarova, M.; Ovesna, J.; Tvaruzek, L.; Kucera, L.; Spunar, J.; Hollerova, I. The use of molecular markers for characterisation of spring barley for breeding to Fusarium head blight resistance. Plant Soil. Environ. 2005, 51, 483–490. [Google Scholar] [CrossRef]
- Sato, K.; Hori, K.; Takeda, K. Detection of Fusarium head blight resistance QTLs using five populations of top-cross progeny derived from two-row x two-row crosses in barley. Mol. Breed. 2008, 22, 517–526. [Google Scholar] [CrossRef]
- Liu, M.; Li, Y.; Ma, Y.L.; Zhao, Q.; Stiller, J.; Feng, Q.; Tian, Q.L.; Liu, D.C.; Han, B.; Liu, C.J. The draft genome of a wild barley genotype reveals its enrichment in genes related to biotic and abiotic stresses compared to cultivated barley. Plant Biotechnol. J. 2020, 18, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Backes, G.; Madsen, L.H.; Jaiser, H.; Stougaard, J.; Herz, M.; Mohler, V.; Jahoor, A. Localisation of genes for resistance against Blumeria graminis f.sp hordei and Puccinia graminis in a cross between a barley cultivar and a wild barley (Hordeum vulgare ssp spontaneum) line. Theor. Appl. Genet. 2003, 106, 353–362. [Google Scholar] [CrossRef]
- Henningsen, E.; Sallam, A.H.; Matny, O.; Szinyei, T.; Figueroa, M.; Steffenson, B.J. Rpg7: A New Gene for Stem Rust Resistance from Hordeum vulgare ssp. spontaneum. Phytopathology 2021, 111, 548–558. [Google Scholar] [CrossRef]
- Tyryshkin, L.G. Genetic control of effective leaf rust resistance in collection accessions of barley Hordeum vulgare L. Russ. J. Genet. 2009, 45, 376–378. [Google Scholar] [CrossRef]
- Amouzoune, M.; Rehman, S.; Benkirane, R.; Verma, S.; Gyawali, S.; Al-Jaboobi, M.; Verma, R.P.S.; Kehel, Z.; Amri, A. Genome-Wide Association Study of Leaf Rust Resistance at Seedling and Adult Plant Stages in a Global Barley Panel. Agriculture 2022, 12, 1829. [Google Scholar] [CrossRef]
- Sallam, A.H.; Tyagi, P.; Brown-Guedira, G.; Muehlbauer, G.J.; Hulse, A.; Steffenson, B.J. Genome-Wide Association Mapping of Stem Rust Resistance in Hordeum vulgare subsp spontaneum. G3-Genes Genomes Genet. 2017, 7, 3491–3507. [Google Scholar] [CrossRef]
- Clare, S.J.; Oguz, A.C.; Effertz, K.; Poudel, R.S.; See, D.; Karakaya, A.; Brueggeman, R.S. Genome-wide association mapping of Pyrenophora teres f. maculata and Pyrenophora teres f. teres resistance loci utilizing natural Turkish wild and landrace barley populations. G3-Genes Genomes Genet. 2021, 11, jkab280. [Google Scholar] [CrossRef]
- Vasighzadeh, A.; Sharifnabi, B.; Javan-Nikkhah, M.; Stukenbrock, E.H. Infection experiments of Pyrenophora teres f. maculata on cultivated and wild barley indicate absence of host specificity. Eur. J. Plant Pathol. 2022, 163, 749–759. [Google Scholar] [CrossRef]
- Afanasenko, O.; Rozanova, I.; Gofman, A.; Lashina, N.; Novakazi, F.; Mironenko, N.; Baranova, O.; Zubkovich, A. Validation of Molecular Markers of Barley Net Blotch Resistance Loci on Chromosome 3H for Marker-Assisted Selection. Agriculture 2022, 12, 439. [Google Scholar] [CrossRef]
- Backes, A.; Guerriero, G.; Barka, E.A.; Jacquard, C. Pyrenophora teres: Taxonomy, Morphology, Interaction With Barley, and Mode of Control. Front. Plant Sci. 2021, 12, 614951. [Google Scholar] [CrossRef] [PubMed]
- Novakazi, F.; Afanasenko, O.; Anisimova, A.; Platz, G.J.; Snowdon, R.; Kovaleva, O.; Zubkovich, A.; Ordon, F. Genetic analysis of a worldwide barley collection for resistance to net form of net blotch disease (Pyrenophora teres f. teres). Theor. Appl. Genet. 2019, 132, 2633–2650. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Takeda, K. Net blotch resistance in wild species of Hordeum. Euphytica 1997, 95, 179–185. [Google Scholar] [CrossRef]
- Davila, J.A.; Loarce, Y.; Ramsay, L.; Waugh, R.; Ferrer, E. Comparison of RAMP and SSR markers for the study of wild barley genetic diversity. Hereditas 1999, 131, 5–13. [Google Scholar] [CrossRef]
- Jilal, A.; Grando, S.; Henry, R.J.; Lee, L.S.; Rice, N.; Hill, H.; Baum, M.; Ceccarelli, S. Genetic diversity of ICARDA’s worldwide barley landrace collection. Genet. Resour. Crop Evol. 2008, 55, 1221–1230. [Google Scholar] [CrossRef]
- Shakhatreh, Y.; Baum, M.; Haddad, N.; Alrababah, M.; Ceccarelli, S. Assessment of genetic diversity among Jordanian wild barley (Hordeum spontaneum) genotypes revealed by SSR markers. Genet. Resour. Crop Evol. 2016, 63, 813–822. [Google Scholar] [CrossRef]
- Trubacheeva, N.V.; Badaeva, E.D.; Osadchaya, T.S.; Pershina, L.A. Use of H. vulgare EST Markers, GISH and C-banding to Study Bread Wheat—H. marinum subsp. gussoneanum (2n = 28) Introgression Lines. Cereal Res. Commun. 2019, 47, 593–603. [Google Scholar] [CrossRef]
- Ben Romdhane, M.; Riahi, L.; Yazidi, R.; Mliki, A.; Zoghlami, N. Cross transferability of barley nuclear SSRs to pearl millet genome provides new molecular tools for genetic analyses and marker assisted selection. Open Agric. 2022, 7, 668–678. [Google Scholar] [CrossRef]
- Löve, Á. Conspectus of the Triticeae. Feddes Repert. 1984, 95, 425–521. [Google Scholar] [CrossRef]
- Jacobsen, N.; Vonbothmer, R. Supraspecific groups in the genus hordeum. Hereditas 1992, 116, 21–24. [Google Scholar] [CrossRef]
- Celik Oguz, A. Resistance of wild barley (Hordeum spontaneum) and barley landraces to leaf stripe (Drechslera graminea). Phytopathol. Mediterr. 2019, 58, 485–495. [Google Scholar]
- Sanei, M.; Pickering, R.; Fuchs, J.; Moghaddam, A.M.B.; Dziurlikowska, A.; Houben, A. Interspecific Hybrids of Hordeum marinum ssp marinum x H. bulbosum Are Mitotically Stable and Reveal No Gross Alterations in Chromatin Properties. Cytogenet. Genome Res. 2010, 129, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Bohra, A.; Kilian, B.; Sivasankar, S.; Caccamo, M.; Mba, C.; McCouch, S.R.; Varshney, R.K. Reap the crop wild relatives for breeding future crops. Trends Biotechnol. 2022, 40, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Lekeš, J.Z.P.; Bareš, I.; Sehnalová, J.; Vlasák, M. Descriptor list genus Hordeum L. 2023. Available online: https://www.gzr.cz/descriptor-lists/?lang=en (accessed on 15 January 2022).
- Stakman, E.C.; Stewart, D.M.; Loegering, W.Q. Identification of Physiologic Races of Puccinia Graminis Var. Tritici.; United States Department of Agriculture, Agricultural Research Service: Beltsville, MD, USA, 1962. [Google Scholar]
- Chrpová, J.; Šíp, V.; Štočková, L.; Stemberková, L.; Tvarůžek, L. Resistance to Fusarium head blight in spring barley. Czech J. Genet. Plant Breed. 2011, 47, 58–63. [Google Scholar] [CrossRef]
- Šíp, V.S.S.; Stuchlíková, E.; Chrpová, J. The effect of infection with Fusarium culmorum L. on deoxynivalenol content in grain of selected winter wheat varieties. J. Appl. Genet. 2002, 43A, 319–332. [Google Scholar]
- Palicová-Šárová, J.; Hanzalová, A. Reaction of 50 Winter Wheat Cultivars Grown in the Czech Republic to Pyrenophora tritici-repentis Races 1, 3, and 6. Czech J. Genet. Plant Breed. 2006, 42, 31–37. [Google Scholar] [CrossRef]
- Saghai-Maroof, M.A.; Soliman, K.M.; Jorgensen, R.A.; Allard, R.W. Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc. Natl. Acad. Sci. USA 1984, 81, 8014–8018. [Google Scholar] [CrossRef]
- Becker, J.; Heun, M. Barley microsatellites: Allele variation and mapping. Plant Mol. Biol. 1995, 27, 835–845. [Google Scholar] [CrossRef]
- Liu, Z.W.; Biyashev, R.M.; Maroof, M.A. Development of simple sequence repeat DNA markers and their integration into a barley linkage map. Theor. Appl. Genet. 1996, 93, 869–876. [Google Scholar] [CrossRef]
- Brunner, S.; Keller, B.; Feuillet, C. Molecular mapping of the Rph7.g leaf rust resistance gene in barley (Hordeum vulgare L.). Theor. Appl. Genet. 2000, 101, 783–788. [Google Scholar] [CrossRef]
- Derevnina, L.; Fetch, T.; Singh, D.; Brueggeman, R.; Dong, C.; Park, R.F. Analysis of Stem Rust Resistance in Australian Barley Cultivars. Plant Dis. 2014, 98, 1485–1493. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, P.R.B.; Scoles, G. Allele-Specific Markers within the Barley Stem Rust Resistance Gene (Rpg1); Department of Plant Sciences/Crop Development Centre, University of Saskatchewan: Saskatoon, SK, Canada, 2003; Volume 33. [Google Scholar]
- Nei, M. Analysis of Gene Diversity in Subdivided Populations. Proc. Natl. Acad. Sci. USA 1973, 70, 3321–3323. [Google Scholar] [CrossRef] [PubMed]
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 1980, 32, 314–331. [Google Scholar]
- Tessier, C.; David, J.; This, P.; Boursiquot, J.M.; Charrier, A. Optimization of the choice of molecular markers for varietal identification in Vitis vinifera L. Theor. Appl. Genet. 1999, 98, 171–177. [Google Scholar] [CrossRef]
- Belaj, A.; Satovic, Z.; Cipriani, G.; Baldoni, L.; Testolin, R.; Rallo, L.; Trujillo, I. Comparative study of the discriminating capacity of RAPD, AFLP and SSR markers and of their effectiveness in establishing genetic relationships in olive. Theor. Appl. Genet. 2003, 107, 736–744. [Google Scholar] [CrossRef]
- Sokal, R.R.; Michener, C.D. A statistical method for evaluating systematic relationships. Univ. Kans. Sci. Bull. 1958, 38, 1409. [Google Scholar]
- Farris, J.S. On the Cophenetic Correlation Coefficient. Syst. Biol. 1969, 18, 279–285. [Google Scholar] [CrossRef]
- Paradis, E.; Schliep, K. ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2018, 35, 526–528. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org (accessed on 13 June 2022).
- Galili, T. dendextend: An R package for visualizing, adjusting and comparing trees of hierarchical clustering. Bioinformatics 2015, 31, 3718–3720. [Google Scholar] [CrossRef]


| An | I | I/Ns | P | P% | Pu | Pu/Ns | Dj | Cj | DL | xj | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BMS02 | 5 | 9 | 0.375 | 5.747 | 0.639 | - | - | 0.862 | 0.138 | 0.826 | 38 |
| Bmag0135 | 7 | 8 | 0.333 | 3.788 | 0.474 | - | - | 0.768 | 0.232 | 0.736 | 64 |
| BMS90 | 6 | 7 | 0.292 | 3.65 | 0.521 | - | - | 0.757 | 0.243 | 0.726 | 67 |
| BLYRCAB | 14 | 15 | 0.625 | 10.309 | 0.687 | - | - | 0.942 | 0.058 | 0.903 | 16 |
| HVM62 | 5 | 9 | 0.375 | 5.988 | 0.665 | - | - | 0.87 | 0.13 | 0.833 | 36 |
| Bmac0310 | 10 | 15 | 0.625 | 9.259 | 0.617 | - | - | 0.931 | 0.069 | 0.892 | 19 |
| HVM74 | 5 | 6 | 0.25 | 3.344 | 0.557 | - | - | 0.732 | 0.268 | 0.701 | 74 |
| EBmac0906 | 7 | 9 | 0.375 | 6.41 | 0.712 | - | - | 0.88 | 0.12 | 0.844 | 33 |
| BMS32 | 11 | 12 | 0.5 | 9.901 | 0.825 | - | - | 0.938 | 0.062 | 0.899 | 17 |
| BMS18 | 4 | 6 | 0.25 | 3.509 | 0.585 | - | - | 0.746 | 0.254 | 0.715 | 70 |
| HVM60 | 9 | 10 | 0.417 | 6.41 | 0.641 | - | - | 0.88 | 0.12 | 0.844 | 33 |
| EBmac0874 | 8 | 8 | 0.333 | 3.891 | 0.486 | - | - | 0.775 | 0.225 | 0.743 | 62 |
| Bmag0500 | 6 | 7 | 0.292 | 3.597 | 0.514 | - | - | 0.754 | 0.246 | 0.722 | 68 |
| Bmag0173 | 7 | 8 | 0.333 | 5.747 | 0.718 | - | - | 0.862 | 0.138 | 0.826 | 38 |
| BMS40 | 13 | 18 | 0.75 | 15.152 | 0.842 | - | - | 0.975 | 0.025 | 0.934 | 7 |
| HVM30 | 5 | 7 | 0.292 | 4.292 | 0.613 | - | - | 0.801 | 0.199 | 0.767 | 55 |
| Bmac0181 | 9 | 13 | 0.542 | 8 | 0.615 | - | - | 0.913 | 0.087 | 0.875 | 24 |
| HVM67 | 5 | 8 | 0.333 | 5.236 | 0.654 | - | - | 0.844 | 0.156 | 0.809 | 43 |
| HVM43 | 7 | 7 | 0.292 | 5.051 | 0.722 | - | - | 0.837 | 0.163 | 0.802 | 45 |
| Bmac0067 | 9 | 13 | 0.542 | 5.747 | 0.442 | - | - | 0.862 | 0.138 | 0.826 | 38 |
| Bmag0120 | 6 | 9 | 0.375 | 5.525 | 0.614 | - | - | 0.855 | 0.145 | 0.819 | 40 |
| Bmag0136 | 3 | 4 | 0.167 | 2.551 | 0.638 | - | - | 0.634 | 0.366 | 0.608 | 101 |
| Bmag0496 | 11 | 14 | 0.583 | 11.111 | 0.794 | - | - | 0.949 | 0.051 | 0.91 | 14 |
| HVM40 | 8 | 12 | 0.5 | 7.576 | 0.631 | - | - | 0.906 | 0.094 | 0.868 | 26 |
| Bmag0306 | 12 | 11 | 0.458 | 5.236 | 0.476 | - | - | 0.844 | 0.156 | 0.809 | 43 |
| Bmag0112 | 9 | 11 | 0.458 | 7.042 | 0.64 | - | - | 0.895 | 0.105 | 0.858 | 29 |
| HVCMA | 3 | 5 | 0.208 | 3.472 | 0.694 | - | - | 0.743 | 0.257 | 0.712 | 71 |
| Bmag0394 | 6 | 10 | 0.417 | 5.747 | 0.575 | - | - | 0.862 | 0.138 | 0.826 | 38 |
| EBmac0679 | 10 | 12 | 0.5 | 7.576 | 0.631 | - | - | 0.906 | 0.094 | 0.868 | 26 |
| HVBKASI | 4 | 8 | 0.333 | 5.051 | 0.631 | - | - | 0.837 | 0.163 | 0.802 | 45 |
| EBmac0602 | 11 | 17 | 0.708 | 9.901 | 0.582 | - | - | 0.938 | 0.062 | 0.899 | 17 |
| Bmag0125 | 16 | 18 | 0.75 | 14.493 | 0.805 | - | - | 0.971 | 0.029 | 0.931 | 8 |
| HVRCABG | 2 | 3 | 0.125 | 1.292 | 0.431 | - | - | 0.236 | 0.764 | 0.226 | 211 |
| Bmag0807 | 6 | 8 | 0.333 | 5.525 | 0.691 | - | - | 0.855 | 0.145 | 0.819 | 40 |
| EBmac0415 | 13 | 14 | 0.583 | 5.747 | 0.41 | - | - | 0.862 | 0.138 | 0.826 | 38 |
| HVM54 | 9 | 13 | 0.542 | 8.197 | 0.631 | - | - | 0.917 | 0.083 | 0.878 | 23 |
| EBmac0603 | 11 | 12 | 0.5 | 5.988 | 0.499 | - | - | 0.87 | 0.13 | 0.833 | 36 |
| Bmag0692 | 8 | 9 | 0.375 | 5.319 | 0.591 | - | - | 0.848 | 0.152 | 0.812 | 42 |
| Lrk3SNP_Lrk3S4 | 2 | 3 | 0.125 | 1.988 | 0.663 | - | - | 0.518 | 0.482 | 0.497 | 133 |
| MTP 1 | 23 | 22 | 0.917 | 19.231 | 0.874 | 19.231 | 0.801 | 0.989 | 0.011 | 0.948 | 3 |
| MTP 2 | 20 | 19 | 0.792 | 14.493 | 0.763 | 14.493 | 0.604 | 0.971 | 0.029 | 0.931 | 8 |
| MTP 3 | 31 | 19 | 0.792 | 16.949 | 0.892 | 16.949 | 0.706 | 0.982 | 0.018 | 0.941 | 5 |
| MTP 4 | 33 | 22 | 0.917 | 20.408 | 0.928 | 20.408 | 0.85 | 0.993 | 0.007 | 0.951 | 2 |
| MTP 5 | 26 | 21 | 0.875 | 19.231 | 0.916 | 19.231 | 0.801 | 0.989 | 0.011 | 0.948 | 3 |
| MTP 6 | 18 | 15 | 0.625 | 7.812 | 0.521 | 7.812 | 0.326 | 0.909 | 0.091 | 0.872 | 25 |
| MTP 7 | 30 | 23 | 0.958 | 22.222 | 0.966 | 22.222 | 0.926 | 0.996 | 0.004 | 0.955 | 1 |
| MTP 8 | 16 | 17 | 0.708 | 12.5 | 0.735 | 12.5 | 0.521 | 0.96 | 0.04 | 0.92 | 11 |
| MTP 9 | 38 | 24 | 1 | 23.81 | 0.992 | 23.81 | 0.992 | 1 | 0 | 0.958 | 0 |
| MTP 10 | 30 | 22 | 0.917 | 20.408 | 0.928 | 20.408 | 0.85 | 0.993 | 0.007 | 0.951 | 2 |
| MTP 11 | 19 | 17 | 0.708 | 13.699 | 0.806 | 13.699 | 0.571 | 0.967 | 0.033 | 0.927 | 9 |
| Accession Number | Our Number | Rpg1-R | Rpg1-S | Rpg5-R | Rpg5-S |
|---|---|---|---|---|---|
| 01C0509028 | 244 | - | - | + | - |
| 01C0509031 | 246 | - | + | - | - |
| 01C0509033 | 248 | - | - | + | - |
| 01C0509055 | 250 | - | - | - | + |
| 01C0509057 | 252 | - | - | + | - |
| 01C0509067 | 254 | - | + | - | + |
| 01C0509068 | 256 | - | + | - | - |
| 01C0509089 | 260 | + | - | - | + |
| 01C0509047 | 262 | - | - | + | - |
| 01C0509090 | 264 | - | - | - | + |
| 01C0509101 | 266 | - | - | + | + |
| 01C0509106 | 268 | + | - | - | + |
| 01C0509031 | 274 | - | + | + | - |
| 01C0509109 | 276 | - | + | - | + |
| 01C0509089 | 278 | + | - | - | + |
| 01C0509107 | 280 | - | + | - | + |
| 01C0509057 | 283 | - | - | + | - |
| 01C0509111 | 285 | - | + | - | + |
| 01C0509047 | 288 | - | - | + | - |
| 01C0509053 | 292 | - | - | - | - |
| 01C0509112 | 293 | - | + | - | + |
| 01C0509033 | 295 | - | - | + | - |
| 01C0509108 | 296 | - | + | - | - |
| 01C0509028 | 298 | - | - | + | - |
| Acc. No. | Regen. No | Taxon | Origin | Locality | Latitude | Longitude | Altitude | Ecology | Habitat Type: Primary/Secondary | Coll. No./Donor No. |
|---|---|---|---|---|---|---|---|---|---|---|
| 01C0509028 | 244 | H. spontaneum | AZE | Shemacha-Maraza | 40°33′37.633″ N | 48°45′45.465″ E | 570 | dry steppe | P | E200 |
| 01C0509028 | 298 | H. spontaneum | AZE | Shemacha-Maraza | 40°33′37.633″ N | 48°45′45.465″ E | 570 | dry steppe | P | E200 |
| 01C0509031 | 246 | H. spontaneum | AZE | Baku-Chanlar | 40°21′48.870″ N | 49°47′55.906″ E | 100 | dry steppe | S | E215 |
| 01C0509031 | 274 | H. spontaneum | AZE | Baku-Chanlar | 40°21′48.870″ N | 49°47′55.906″ E | 100 | dry steppe | S | E215 |
| 01C0509033 | 248 | H. spontaneum | AZE | Baku, city | 40°25′12.789″ N | 49°49′18.252″ E | 100 | segetal | S | E216 |
| 01C0509033 | 295 | H. spontaneum | AZE | Baku, city | 40°25′12.789″ N | 49°49′18.252″ E | 100 | segetal | S | E216 |
| 01C0509046 | 262 | H. spontaneum | ISR | Ashkelon, prostrate | 31°39′48.799″ N | 34°32′42.246″ E | 10 | Limestone cliffs, sea salt spray | P | E509 |
| 01C0509047 | 288 | H. spontaneum | ISR | Ashkelon, erect | 31°39′48.799″ N | 34°32′42.246″ E | 20 | Limestone cliffs | P | E512 |
| 01C0509053 | 292 | H. spontaneum | MNG | Zagastai, Zavchan | 48°48′0.000″ N | 97°58′0.000″ E | 2000 | Steppe, introduced | S | E786 |
| 01C0509055 | 250 | H. spontaneum | SYR | Aleppo, Citadel | 36°11′57.108″ N | 37°9′43.965″ E | 440 | Archaeological site | S | E926 |
| 01C0509057 | 252 | H. spontaneum | AZE | Maraza | 40°30′33.780″ N | 48°57′1.615″ E | 750 | dry steppe | P | JAN2/35 |
| 01C0509057 | 283 | H. spontaneum | AZE | Maraza | 40°30′33.780″ N | 48°57′1.615″ E | 750 | dry steppe | P | JAN2/35 |
| 01C0509089 | 260 | H. spontaneum | SYR | St.Simeon | 36°20′0.850″ N | 36°50′37.439″ E | 575 | Archaeological site | S | E1292 |
| 01C0509089 | 278 | H. spontaneum | SYR | St.Simeon | 36°20′0.850″ N | 36°50′37.439″ E | 575 | Archaeological site | S | E1292 |
| 01C0509090 | 264 | H. spontaneum | SYR | Manbij | 36°30′35.466″ N | 37°57′37.566″ E | 440 | dry steppe | P | E1342 |
| 01C0509101 | 266 | H. spontaneum | IRN | Chukanlu | 37°35′24.200″ N | 57°51′36.500″ E | 1452 | Field margin | S | 18/56 |
| 01C0509106 | 268 | H. spontaneum | JOR | Amman, E of Belem | 31°57′5.650″ N | 35°55′26.265″ E | 900 | dry steppe | S | |
| 01C0509067 | 254 | H. marinum ssp. gussoneanum | TUR | Tolkoy, Ankara Prov. | 39°38′12.501″ N | 33°3′9.550″ E | 950 | dry steppe | P | E937 |
| 01C0509068 | 256 | H. marinum ssp. gussoneanum | TUR | Pinarbasi SW, Kayseri Prov. | 39°12′27.762″ N, | 32°43′12.546″ E | 1100 | saline disturbed steppe | P | E955 |
| 01C0509070 | 258 | H. marinum ssp. gussoneanum | TUR | Tutak, Nehri R., Agri Prov. | 39°32′21.681″ N | 42°46′21.900″ E | 1550 | saline disturbed steppe | P | E971 |
| 01C0509107 | 280 | H. marinum ssp. gussoneanum | TAI | Sharora, W Dushambe | 38°30′29″ N | 68°42′29″ E | 750 | segetal | S | N6507_1 |
| 01C0509108 | 296 | H. marinum ssp. gussoneanum | AFG | Kataghan Prov. | 36°7′12.338″ N, | 68°40′54.702″ E | 600 | river valley deposits | P | N6509_2 |
| 01C0509109 | 276 | H. marinum ssp. marinum | JOR | Azraq Oasis | 31°50′1.574″ N | 36°49′16.644″ E | 540 | wetland reserve | P | N6820_4 |
| 01C0509110 | 290 | H. marinum ssp. marinum | GRC | Tinos Island | 37°32′40.976″ N | 25°9′12.919″ E | 5 | coastland | P | N6824_5 |
| 01C0509111 | 285 | H. marinum ssp. marinum | ESP | Arroyo de Los Fraeles, S of Talavera de La Reina | 39°54′29″ N | 4°48′29″ W | 520 | steppe | P | N7295_6 |
| 01C0509112 | 293 | H. marinum ssp. gussoneanum | PRT | Terceira Isl., Acores | 38°43′16.298″ N, | 27°13′3.490″ W | 450 | Volcanic crater, saline | P | N90237_7 |
| Marker Name | Product Length (bp) | Primer Sequence | Annealing Temperature | Association with Resistance (Pt, Ph, F) | Reference |
|---|---|---|---|---|---|
| BMS32 | 186–228 | F: 5′-GGA TCA AAG TCC GGC TAG-3′ | 55 °C | - | [34] |
| R: 5′-TGC GGG CCT CAT ACT GAC-3′ | |||||
| BMS40 | 176–225 | F: 5′-AGC CCG ATC AGA TTT ACG-3′ | 55 °C | - | [34] |
| R: 5′-TTC TCC CTT TGG TCC TTG-3′ | |||||
| BMS90 | 178–225 | F: 5′-ACA TCA ACC CTC CTG CTC-3′ | 60 °C | - | [34] |
| R: 5′-CCG CAC ATA GTG GTT ACA TC-3′ | |||||
| EBmac0415 | 225–285 | F: 5′-GAA ACC CAT CAT AGC AGC-3′ | 60 °C | F | [34] |
| R: 5′-AAA CAG CAG CAA GAG GAG-3′ | |||||
| EBmac0602 | 187–221 | F: 5′-GAT TGG AGC TTC GGA TCA C-3′ | 60 °C | F | [34] |
| R: 5′-CCG TCT AGG GAG AGG TTC TC-3′ | |||||
| EBmac0603 | 134–184 | F: 5′-ACC GAA ACT AAA TGA ACT ACT TCG-3′ | 58 °C | Ph | [34] |
| R: 5′-TGC AAA CTG TGC TAT TAA GGG-3′ | |||||
| EBmac0679 | 95–148 | F: 5′-ATT GGA GCG GAT TAG GAT-3′ | 60 °C | F | [34] |
| R: 5′-CCC TAT GTC ATG TAG GAG ATG-3′ | |||||
| EBmac0874 | 147–205 | F: 5′-AAC CAT TCC TCA CCC AGG-3′ | 55 °C | Pt | [34] |
| R: 5′-GTG AAT GAT GTT GAG GAC ATT G-3′ | |||||
| EBmac0906 | 140–156 | F: 5′-CAA ATC AAT CAA GAG GCC-3′ | 55 °C | Pt | [35] |
| R: 5′-TTT GAA GTG AGA CAT TTC CA-3′ | |||||
| HVBKASI | 179–201 | F: 5′-ATT GGC GTG ACC GAT ATT TAT GTT CA-3′ | 60 °C | F | [78] |
| R: 5′-CAA AAC TGC AGC TAA GCA GGG GAA CA-3′ | |||||
| HVCMA | 125–139 | F: 5′-GCC TCG GTT TGG ACA TAT AAA G-3′ | 60 °C | - | [78] |
| R: 5′-GTA AAG CAA ATG TTG AGC AAC G-3′ | |||||
| HVM30 | 118–154 | F: 5′-AGT GGG GAA TGA GAG AAT GG-3′ | 55 °C | - | [79] |
| R: 5′-TGC TTG TGG GTC ATC ACA C-3′ | |||||
| HVM40 | 141–216 | F: 5′-CGA TTC CCC TTT TCC CAC-3′ | 60 °C | - | [79] |
| R: 5′-ATT CTC CGC CGT CCA CTC-3′ | |||||
| HVM43 | 200–235 | F: 5′-GGA TTT TCT CAA GAA CAC TT-3′ | 55 °C | Pt | [35] |
| R: 5′-GCG TGA GTG CAT AAC ATT-3′ | |||||
| HVM54 | 155–178 | F: 5′-AAC CCA GTA ACA CCG TCC TG-3′ | 60 °C | F | [79] |
| R: 5′-AGT TCC CTG ACC CGA TGT C-3′ | |||||
| HVM60 | 99–120 | F: 5′-CAA TGA TGC GGT GAA CTT TG-3′ | 55 °C | - | [79] |
| R: 5′-CCT CGG ATC TAT GGG TCC TT-3′ | |||||
| HVM62 | 231–265 | F: 5′-TCG CGA CCA GAC GAG AAG-3′ | 60 °C | - | [79] |
| R: 5′-AGC TAG CCG ACG ACG CAC-3′ | |||||
| HVM67 | 97–129 | F: 5′-GTC GGG CTC CAT TGC TCT-3′ | 55 °C | - | [79] |
| R: 5′-CCG GTA CCC AGT GAC GAC-3′ | |||||
| HVM74 | 180–196 | F: 5′-AGGAAGTCATTGCGTGAG-3′ | 60 °C | Pt | [79] |
| R: 5′-TGATCAAGAATGATAACATGG-3′ | |||||
| HVRCABG | 120–124 | F: 5′-TTT AAA AGA AAA GTG AAT GGC-3′ | 60 °C | F | [79] |
| R: 5′-TAA TGA AGA ATG AGG AGA AGC-3′ | |||||
| Lrk3SNP/ Lrk3S4 | 261–297 | F: 5′-ATC CGC AGG ATG CCC-3′ | 58 °C | Ph | [80] |
| R: 5′-TTG GCC CAA TCT CTT GC-3′ | |||||
| BMS02 | 207–225 | F: 5′-AGA GTA GTG GAA AGA AAG TT-3′ | 60 °C | - | [34] |
| R: 5′-TGG TAG TGA GAT GAG GTG AC-3′ | |||||
| BLYRCAB | 136–209 | F: 5′-ACA CCT TCC CAG GAC AAT CCA TTG-3′ | 60 °C | - | [34] |
| R: 5′-AGC ACG CAG AGC ACC GAA AAA GTC-3′ | |||||
| Bmac0067 | 149–216 | F: 5′-AAC GTA CGA GCT CTT TTT CTA-3′ | 60 °C | Pt | [35] |
| R: 5′-ATG CCA ACT GCT TGT TTA G-3′ | |||||
| Bmac0181 | 158–186 | F: 5′-ATA GAT CAC CAA GTG AAC CAC-3′ | 55 °C | Pt | [35] |
| R: 5′-GGT TAT CAC TGA GGC AAA TAC-3′ | |||||
| Bmac0310 | 135–188 | F: 5′-CTA CCT CTG AGA TAT CAT GCC-3′ | 60 °C | Pt | [35] |
| R: 5′-ATC TAG TGT GTG TTG CTT CCT-3′ | |||||
| Bmag0112 | 155–203 | F: 5′-CCC GTG ATA TAT TAA GAT CAT G-3′ | 60 °C | Pt | [35] |
| R: 5′-AGG GGG AGA TCT TCT CTG-3′ | |||||
| Bmag0120 | 219–258 | F: 5′-ATT TCA TCC CAA AGG AGA C-3′ | 60 °C | Pt | [35] |
| R: 5′-GTC ACA TAG ACA GTT GTC TTC C-3′ | |||||
| Bmag0125 | 119–152 | F: 5′-AAT TAG CGA GAA CAA AAT CAC-3′ | 60 °C | F | [35] |
| R: 5′-AGA TAA CGA TGC ACC ACC-3′ | |||||
| Bmag0135 | 138–168 | F: 5′-ACG AAA GAG TTA CAA CGG ATA-3′ | 60 °C | - | [35] |
| R: 5′-GTT TAC CAC AGA TCT ACA GGT G-3′ | |||||
| Bmag0136 | 197–201 | F: 5′-GTA CGC TTT CAA ACC TGG-3′ | 60 °C | - | [35] |
| R: 5′-GTA GGA GGA AGA ATA AGG AGG-3′ | |||||
| Bmag0173 | 109–153 | F: 5′-CAT TTT TGT TGG TGA CGG-3′ | 55 °C | Pt | [35] |
| R: 5′-ATA ATG GCG GGA GAG ACA-3′ | |||||
| Bmag0306 | 126–176 | F: 5′-ATG TAC AAG TAG CTA TGT GTT TGA-3′ | 60 °C | Pt | [35] |
| R: 5′-CAC ATC AAG ATA ACA AGA GAA GA-3′ | |||||
| Bmag0394 | 160–182 | F: 5′-AAT TCA TCA CAA CAA GAT AGG A-3′ | 60 °C | - | [35] |
| R: 5′-AAT TGA TCT CCC TCT CTC TAT G-3′ | |||||
| Bmag0496 | 173–229 | F: 5′-AGTATAACCAACAGCCGTCTA-3′ | 60 °C | Pt | [35] |
| R: 5′-CTATAGCACGCCTTTGAGA-3′ | |||||
| Bmag0500 | 142–191 | F: 5′-GGG AAC TTG CTA ATG AAG AG-3′ | 55 °C | - | [35] |
| R: 5′-AAT GTA AGG GAG TGT CCA TAG-3′ | |||||
| Bmag0692 | 158–185 | F: 5′-GCA AGG TAT CTC TTG TAT TTT G-3′ | 58 °C | Ph | [35] |
| R: 5′-TGG CAT CTA CAA TCT AAA ACA-3′ | |||||
| Bmag0807 | 97–113 | F: 5′-GGATATAAGGGTCCATAGCA-3′ | 60 °C | Pt, F | [35] |
| R: 5′-AATTACATCAAATAGGCTCCA-3′ | |||||
| BMS18 | 133–141 | F: 5′-GTC CTT TAC GCA TGA ACC GT-3′ | 55 °C | - | [34] |
| R: 5′-ACA TAC GCC AGA CTC GTG TG-3′ |
| Multiplex Number | Markers Included | Multiplex Number | Markers Included |
|---|---|---|---|
| MTP 1 | BMS02 | MTP 6 | Bmac0067 |
| Bmag0135 | Bmag0120 | ||
| BMS90 | Bmag0136 | ||
| BLYRCAB | |||
| MTP 7 | Bmag0496 | ||
| MTP 2 | HVM62 | HVM40 | |
| Bmac0310 | Bmag0306 | ||
| HVM74 | |||
| MTP 8 | Bmag0112 | ||
| MTP 3 | EBmac0906 | HVCMA | |
| BMS32 | Bmag0394 | ||
| BMS18 | |||
| HVM60 | MTP 9 | EBmac0679 | |
| HVBKASI | |||
| MTP 4 | EBmac0874 | EBmac0602 | |
| Bmag0500 | Bmag0125 | ||
| Bmag0173 | |||
| BMS40 | MTP 10 | HVRCABG | |
| Bmag0807 | |||
| MTP 5 | HVM30 | EBmac0415 | |
| Bmac0181 | HVM54 | ||
| HVM67 | |||
| HVM43 | MTP 11 | EBmac0603 | |
| Lrk3SNP/Lrk3S4 | |||
| Bmag0692 |
| Marker Name | Product Length (bp) | Primer Sequence | Annealing Temperature | Reference |
|---|---|---|---|---|
| Rpg1-R | 669 | F: 5′-CGGCTAATCACATCAAGTAA-3′ | 60 °C | [81] |
| R: 5′-AGCCCATCATCAATAGACAA-3′ | 60 °C | |||
| Rpg1-S | 487 | F: 5′-GGCTAATCACATCAAGGTT-3′ | 60 °C | [82] |
| R: 5′-CCACGACCAATTATGTTCTG-3′ | 60 °C | |||
| Rpg5-R | 1046 | F: 5′-CTGCTGGCACAGAGTCTGCCTTGAG-3′ | 60 °C | [81] |
| R: 5′-ACTCTCGGGTCTGAAGTTCCGTGTG-3′ | 60 °C | |||
| Rpg5-S | 840 | F: 5′-CTGCTGGCACAGAGTCTGCCTTGAG-3′ | 60 °C | [81] |
| R: 5′-CCCGAGGTTTGCGATGAAGAGAGTC-3′ | 60 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ovesna, J.; Chrpova, J.; Kolarikova, L.; Svoboda, P.; Hanzalova, A.; Palicova, J.; Holubec, V. Exploring Wild Hordeum spontaneum and Hordeum marinum Accessions as Genetic Resources for Fungal Resistance. Plants 2023, 12, 3258. https://doi.org/10.3390/plants12183258
Ovesna J, Chrpova J, Kolarikova L, Svoboda P, Hanzalova A, Palicova J, Holubec V. Exploring Wild Hordeum spontaneum and Hordeum marinum Accessions as Genetic Resources for Fungal Resistance. Plants. 2023; 12(18):3258. https://doi.org/10.3390/plants12183258
Chicago/Turabian StyleOvesna, Jaroslava, Jana Chrpova, Lucia Kolarikova, Pavel Svoboda, Alena Hanzalova, Jana Palicova, and Vojtech Holubec. 2023. "Exploring Wild Hordeum spontaneum and Hordeum marinum Accessions as Genetic Resources for Fungal Resistance" Plants 12, no. 18: 3258. https://doi.org/10.3390/plants12183258
APA StyleOvesna, J., Chrpova, J., Kolarikova, L., Svoboda, P., Hanzalova, A., Palicova, J., & Holubec, V. (2023). Exploring Wild Hordeum spontaneum and Hordeum marinum Accessions as Genetic Resources for Fungal Resistance. Plants, 12(18), 3258. https://doi.org/10.3390/plants12183258






