Early Detection of Phenotypic Diversity of Alfalfa (Medicago sativa L.) in Response to Temperature
Abstract
:1. Introduction
2. Results
2.1. Germination
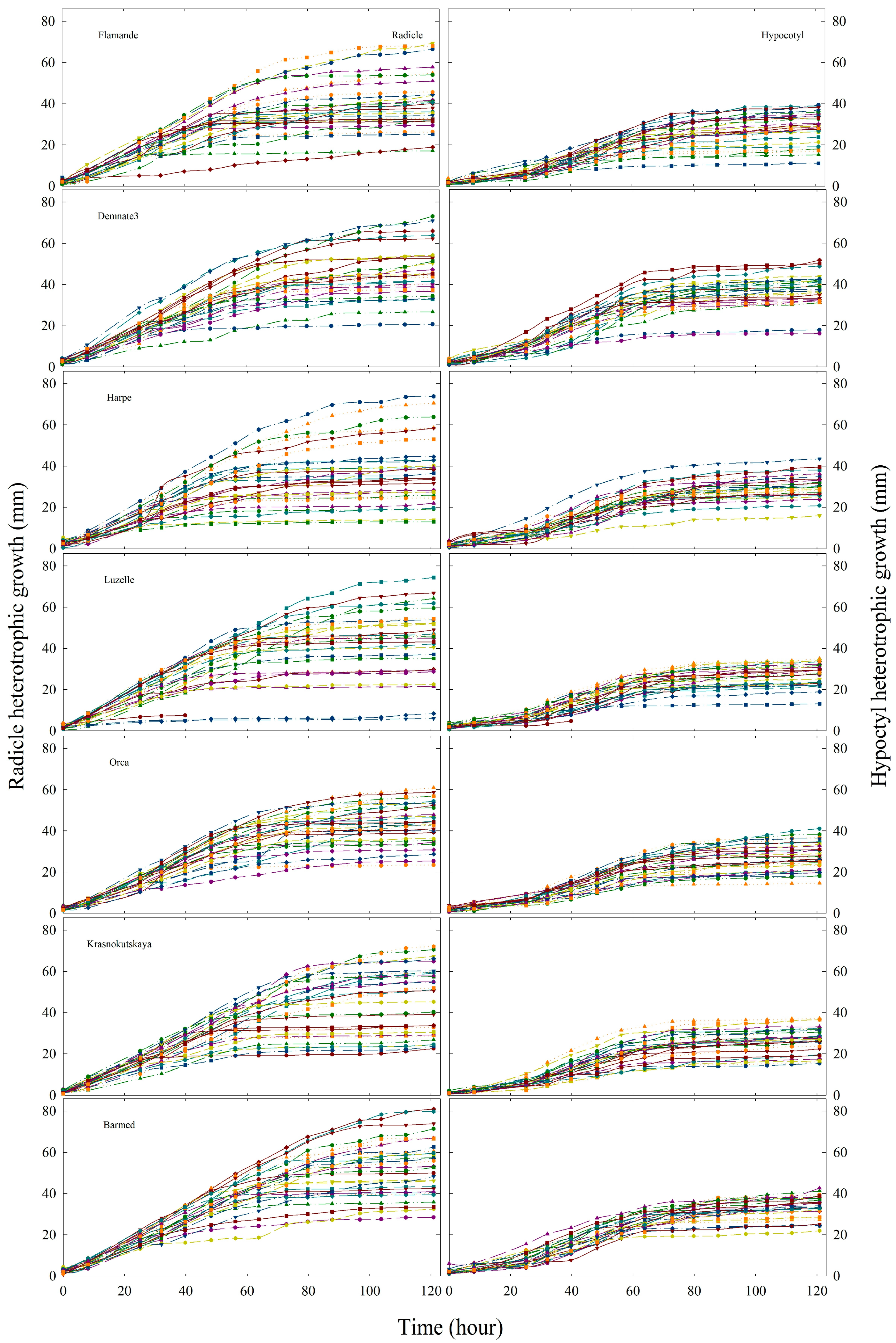
2.2. Initial Heterotrophic Growth
2.3. Dynamics of Heterotrophic Growth
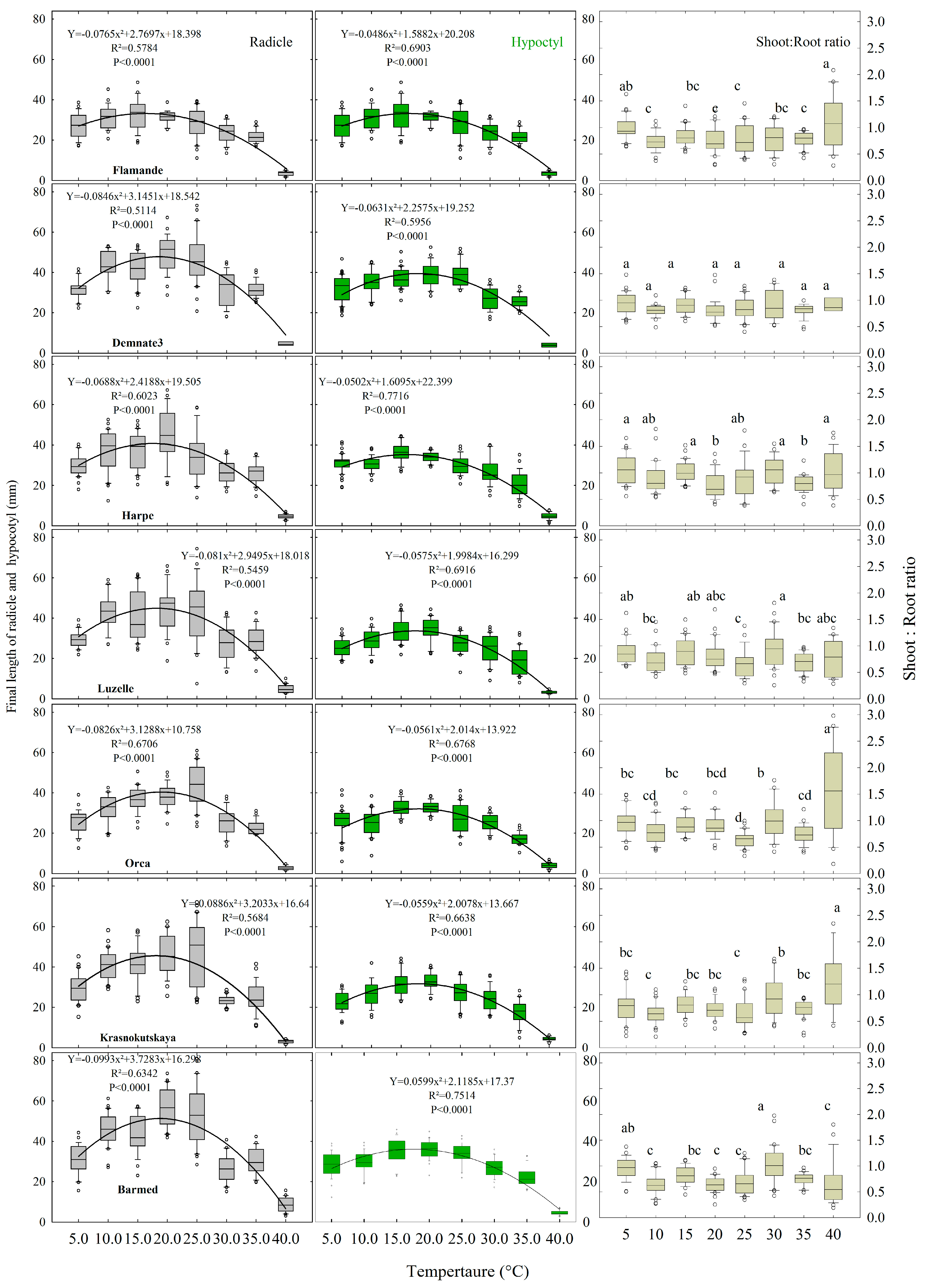
2.4. Embryo Axes Final Lengths
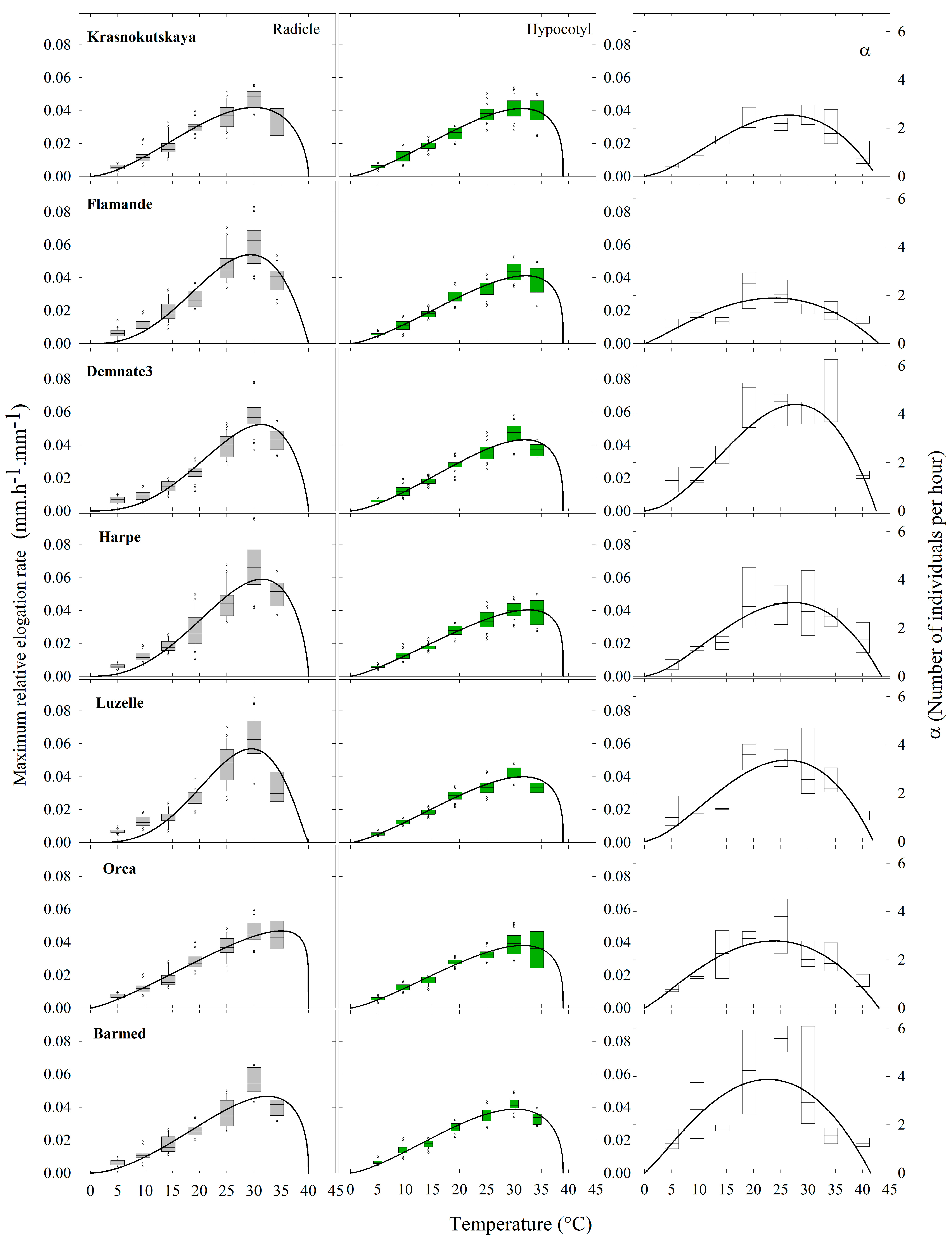
2.5. Relative Elongation Rate
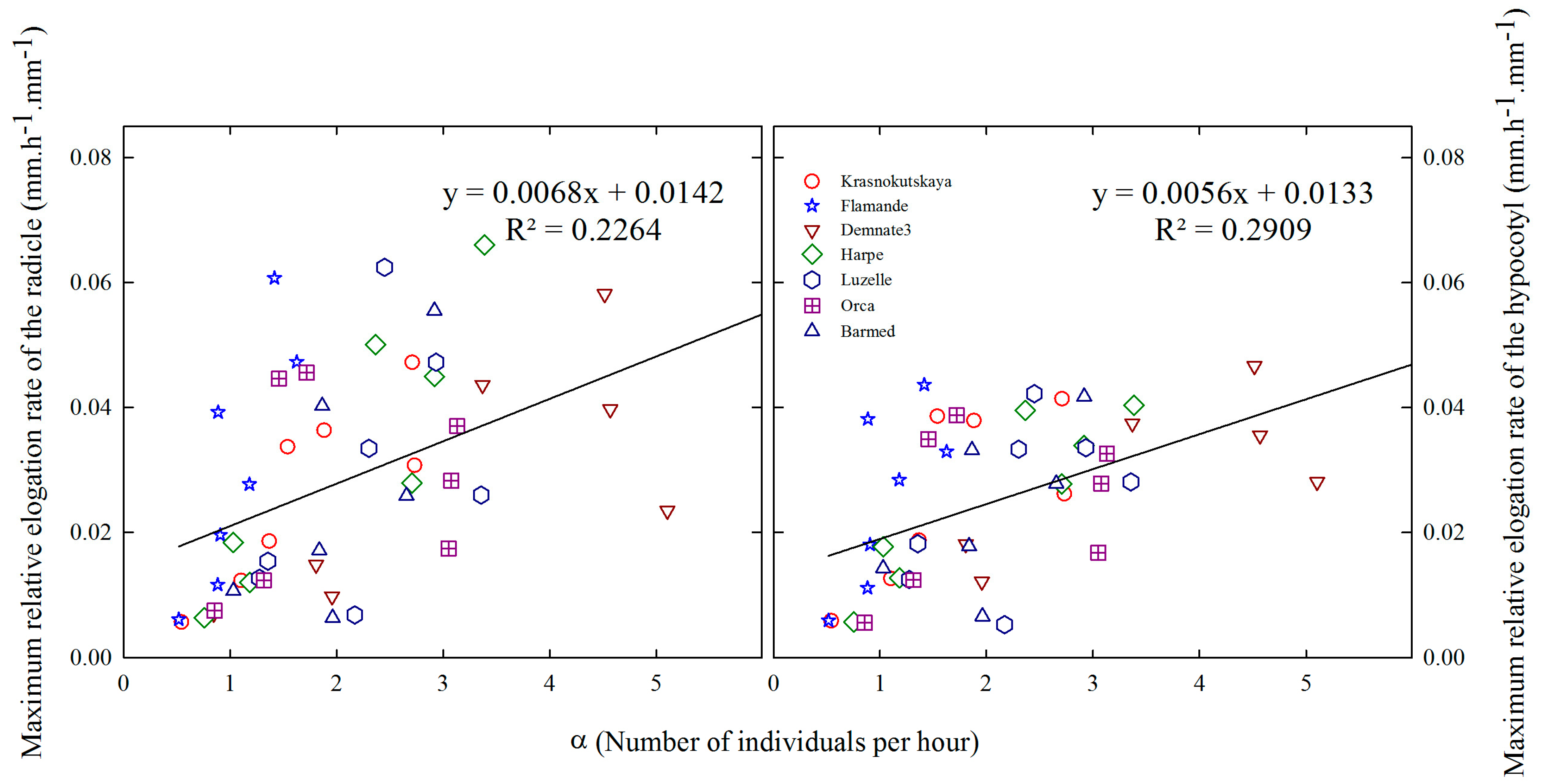
2.6. Relationships between Relative Elongation Rates and Germination Rates
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Germination Analysis
4.3. Heterotrophic Growth Measurement
4.4. Heterotrophic Growth Modelling and Calculations
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Chang. 2013, 3, 52–58. [Google Scholar] [CrossRef]
- Blanco-Pastor, J.L.; Mane, S.; Barre, P.; Roschanski, A.M.; Willner, E.; Dehmer, K.J.; Hegarty, M.; Muylle, H.; Ruttink, T.; Roldán-Ruiz, I.; et al. Pleistocene climate changes, and not agricultural spread, accounts for range expansion and admixture in the dominant grassland species Lolium perenne L. J. Biogeogr. 2019, 46, 1451–1465. [Google Scholar] [CrossRef]
- Keep, T.; Sampoux, J.P.; Blanco-Pastor, J.L.; Dehmer, K.J.; Hegarty, M.J.; Ledauphin, T.; Litrico, I.; Muylle, H.; Roldán-Ruiz, I.; Roschanski, A.M.; et al. High-throughput genome-wide genotyping to optimize the use of natural genetic resources in the grassland species perennial ryegrass (Lolium perenne L.). Genes Genomes Genet. 2020, 10, 3347–3364. [Google Scholar] [CrossRef]
- Blanco-Pastor, J.; Liberal, I.; Sakiroglu, M.; Wei, Y.; Charles Brummer, E.; Andrew, R.; Pfeil, B. Annual and perennial Medicago show signatures of parallel adaptation to climate and soil in highly conserved genes. Mol. Ecol. 2021, 30, 4448–4465. [Google Scholar] [CrossRef]
- Maleki, K.; Soltani, E.; Seal, C.E.; Pritchard, H.W.; Lamichhane, J.R. The seed germination spectrum of 528 plant species: A global meta-regression in relation to temperature and water potential. BioRxiv 2022. [Google Scholar] [CrossRef]
- Ghaleb, W.; Ahmed, L.Q.; Escobar-Gutiérrez, A.J.; Julier, B. The History of Domestication and Selection of Lucerne: A New Perspective from the Genetic Diversity for Seed Germination in Response to Temperature and Scarification. Front. Plant Sci. 2021, 11, 578121. [Google Scholar] [CrossRef]
- Blanco-Pastor, L.; Barre, P.; Keep, T.; Ledauphin, T.; Escobar-Gutièrrez, A.; Roschaski, A.M.; Willner, E.; Dehmer, K.J.; Hegarty, M.; Muylle, H.; et al. Canonical correlations reveal adaptive loci and phenotypic responses to climate in perennial ryegrass. Mol. Ecol. Resour. 2020, 21, 849–870. [Google Scholar] [CrossRef] [PubMed]
- Ghaleb, W.; Barre, P.; Teulat, B.; Ahmed, L.Q.; Escobar-Gutiérrez, A.J. Divergent Selection for Seed Ability to Germinate at Extreme Temperatures in Perennial Ryegrass (Lolium perenne L.). Front. Plant Sci. 2022, 12, 794488. [Google Scholar] [CrossRef]
- de Candolle, A. Geographie Botanique Raisonée; Masson: Paris, France, 1855; p. 606. [Google Scholar]
- Ahmed, L.Q.; Escobar-Gutiérrez, A.J. Analysis of intra-specific variability of cocksfoot (Dactylis glomerata L.) in response to temperature during germination. Acta Physiol. Plant. 2022, 44, 117. [Google Scholar] [CrossRef]
- Ahmed, L.Q.; Escobar-Gutiérrez, A.J. Unexpected intraspecific variability of perennial ryegrass (Lolium perenne L.) in response to constant temperature during germination and initial heterotrophic growth. Front. Plant Sci. Plant Breed. 2022, 13, 856099. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, P.J.H.; de Michele, D.W. Reaction kinetics of poikilotherm development. J. Theor. Biol. 1977, 64, 649–670. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seed Ecology, Biogeography and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Finch-Savage, W.E.; Bassels, G.H. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Phelps, K.; Steckel, J.R.A.; Whalley, W.R.; Rowse, H.R. Seed reserve-dependent growth responses to temperature and water potential in carrot (Daucus carota L.). J. Exp. Bot. 2001, 52, 2187–2197. [Google Scholar] [CrossRef]
- Bettey, M.; Finch-Savage, W.E.; King, G.J.; Lynn, J.R. Quantitative genetic analysis of seed vigour and preemergence seedling growth traits in Brassica oleracea. New Phytol. 2000, 148, 277–286. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Clay, H.A.; Lynn, J.R.; Morris, K. Towards a genetic understanding of seed vigour in small seeded crops using natural variation in Brassica oleracea. Plant Sci. 2010, 179, 582–589. [Google Scholar] [CrossRef]
- Mohan, A.; Schillinger, W.F.; Gill, K.S. Wheat seedling emergence from deep planting depths and its relationship with coleoptile length. PLoS ONE 2013, 8, e73314. [Google Scholar] [CrossRef] [PubMed]
- Mouttet, R.; Escobar-Gutiérrez, A.J.; Esquibet, M.; Gentzbittel, L.; Mugniéry, D.; Reignault, P.; Sarniguet, C.; Castagnone-Sereno, P. Banning of methyl bromide for seed treatment: Could Ditylenchus dipsaci again become a major threat to alfalfa production in Europe? Pest Manag. Sci. 2014, 70, 1017–1022. [Google Scholar] [CrossRef]
- Prosperi, J.-M.; Jenczewski, E.; Muller, M.-H.; Fourtier, S.; Sampoux, J.-P.; Ronfort, J. Alfalfa domestication history, genetic diversity and genetic resources. Legume Perspect. 2014, 4, 13–14. [Google Scholar]
- Lamichhane, J.R.; Debaeke, P.; Steinberg, C.; You, M.P.; Barbetti, M.J.; Aubertot, J.-N. Abiotic and biotic factors affecting crop seed germination and seedling emergence: A conceptual framework. Plant Soil 2018, 432, 1–28. [Google Scholar] [CrossRef]
- Ahmed, L.Q.; Durand, J.-L.; Escobar-Gutiérrez, A.J. Genetic diversity of alfalfa (Medicago sativa) in response to temperature during germination. Seed Sci. Technol. 2019, 47, 351–356. [Google Scholar] [CrossRef]
- Shafii, B.; Price, W.J. Estimation of cardinal temperatures in germination data analysis. J. Agric. Biol. Environ. Stat. 2001, 6, 356–366. [Google Scholar] [CrossRef]
- Escobar-Gutiérrez, A.J.; Daudet, F.A.; Gaudillère, J.-P.; Maillard, P.; Frossard, J.-S. Modelling of allocation and balance of carbon in walnut (Juglans regia L.) seedlings during heterotrophy-autotrophy transition. J. Theor. Biol. 1998, 194, 29–47. [Google Scholar] [CrossRef]
- Ghaleb, W.; Ahmed, L.Q.; Wagner, M.-H.; Eprinchard-Ciesla, A.; Olivares-Rodríguez, W.E.; Perrot, C.; Chenu, K.; Norton, M.; EscobarGutiérrez, A.J. The Concepts of Seed Germination Rate and Germinability: A Re-Evaluation for Cool-Season Grasses. Agronomy 2022, 12, 1291. [Google Scholar] [CrossRef]
- Stanisavljevic, R.; Ðjokic, D.; Jasmina Milenkovic, J.; Ðukanovic, L.; Stevovic, V.; Simic, A.; Dodig, D. Seed germination and seedling vigour of italian ryegrass, cocksfoot and timothy following harvest and storage. Ciênc. Agrotec., Lavras 2011, 35, 1141–1148. [Google Scholar] [CrossRef]
- Kamkar, B.; Koocheki, A.; Mahallati, M.N.; Moghaddam, P.R. Cardinal Temperatures for Germination in Three Millet Specieses (Panicum miliaceum, Pennisetum glaucum and Setaria italica). Asian J. Plant Sci. 2006, 5, 316–319. [Google Scholar] [CrossRef]
- Lonati, M.; Moot, D.J.; Aceto, P.; Cavallero, A.; Lucs, R.J. Thermal time requirements for germination, emergence and seedling development of adventive legume and grass species. N. Z. J. Agric. Res. 2009, 52, 17–29. [Google Scholar] [CrossRef]
- Sampayo-Maldonado, S.; Ordoñez-Salanueva, C.A.; Mattana, E.; Ulian, T.; Way, M.; Castillo-Lorenzo, E.; Dávila-Aranda, P.D.; Lira-Saade, R.; Téllez-Valdéz, O.; Rodriguez-Arevalo, N.I.; et al. Thermal Time and Cardinal Temperatures for Germination of Cedrela odorata L. Forests 2019, 10, 841. [Google Scholar] [CrossRef]
- Moreira de Oliveira, G.M.; Santos da Silva, F.F.; Araujo, M.N.; Campos da Costa, D.C.; Gomes, S.E.V.; Matias, J.R.; Angelotti, F.; Cruz, C.R.P.; Seal, C.E.; Dantas, B.F. Environmental stress, future climate, and germination of Myracrodruon urundeuva seeds. J. Seed Sci. 2019, 41, 32–43. [Google Scholar] [CrossRef]
- Masiunas, J.B.; Carpenter, P.L. Radicle growth of gasses and legumes in response to temperature. HortScience 1984, 19, 298–300. [Google Scholar] [CrossRef]
- Zhang, R.; Luo, K.; Chen, D.; Baskin, J.; Bskin, C.; Wang, Y.; Hu, X. Comparison of Thermal and Hydrotime Requirements for Seed Germination of Seven Stipa Species from Cool and Warm Habitats. Front. Plant Sci. 2020, 11, 560714. [Google Scholar] [CrossRef]
- Bakhshandeh, E.; Jamali, M. Halothermal and hydrothermal time models describe germination responses of canola seeds to ageing. Plant Biol. 2021, 23, 621–629. [Google Scholar] [CrossRef]
- Alinia, M.; Jalali, A.H.; Kazemeini, S.A.; Bakhshandeh, E. Modeling seed germination response of maize with different shapes and sizes using halotime and halothermal time concept. Acta Physiol. Plant. 2022, 44, 1–13. [Google Scholar] [CrossRef]
- Zaka, S.; Ahmed, L.Q.; Escobar-Gutiérrez, A.J.; Gastal, F.; Julier, B.; Louarn, G. How variable are non-linear developmental responses to temperature in two perennial forage species? Agric. For. Meteorol. 2017, 232, 433–442. [Google Scholar] [CrossRef]
- Schnute, J. A versatile growth model with statistically stable parameters. Can. J. Fish. Aquat. Sci. 1981, 38, 1128–1140. [Google Scholar] [CrossRef]
- Steel, R.G.; Torrie, J.H. Principles and Procedures of Statistics: A Biometrical Approach, 2nd ed.; McGraw-Hill: New York, NY, USA, 1980. [Google Scholar]
- Yin, X.; Kropff, M.J.; McLaren, G.; Visperas, R.M. A nonlinear model for crop development as a function of temperature. Agric. For. Meteorol. 1995, 77, 1–16. [Google Scholar] [CrossRef]
- Körner, C. Plant adaptation to cold climates. F1000Research 2016. [Google Scholar] [CrossRef] [PubMed]
- Husson, F.; Josse, J.; Pagès, J. Principal Component Methods—Hierarchical Clustering—Partitional Clustering: Why Would We Need to Choose for Visualizing Data? 2010. Available online: http://www.sthda.com/english/upload/hcpc_husson_josse.pdf (accessed on 10 September 2023).
- R Software. R-Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
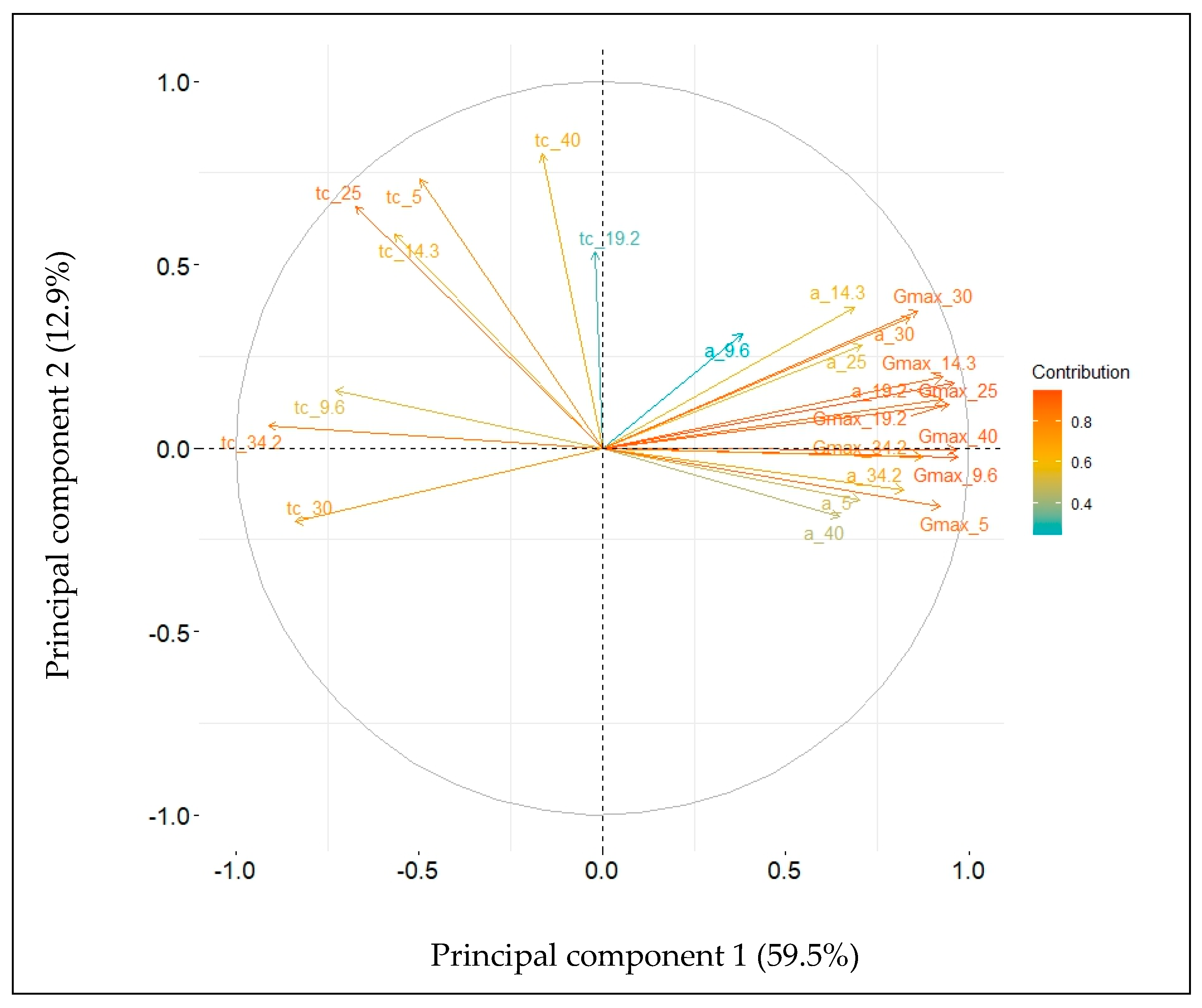
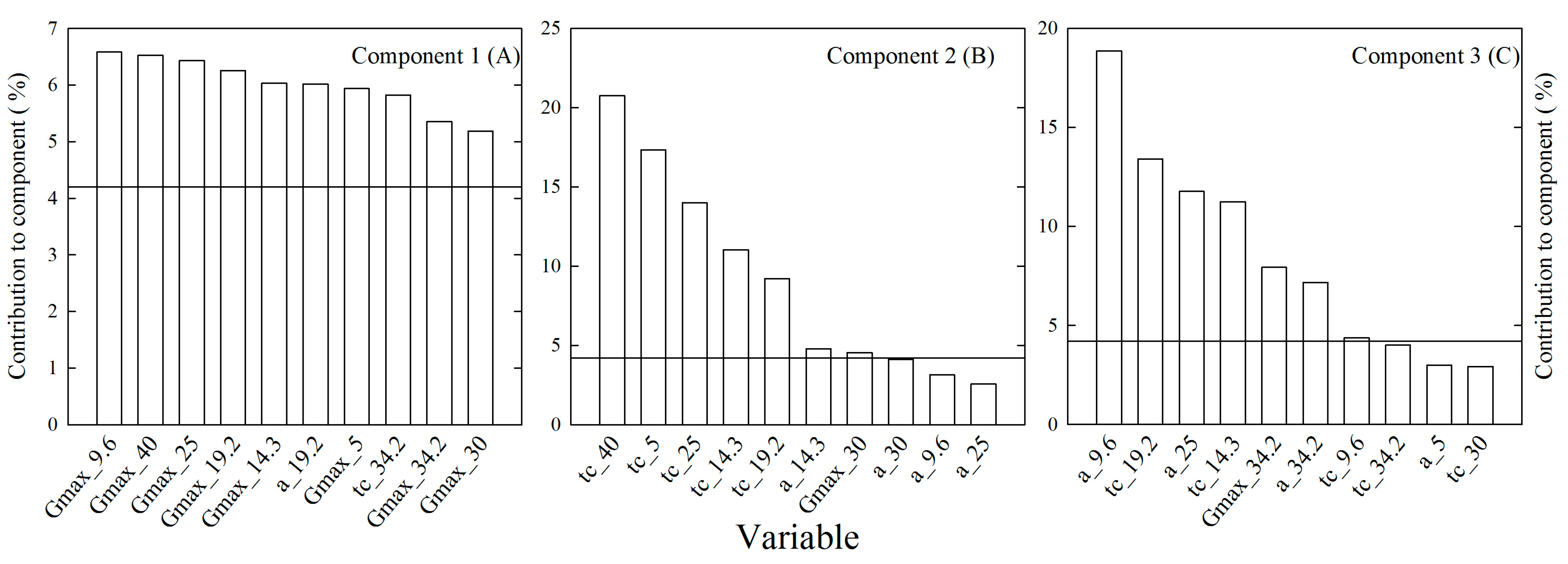


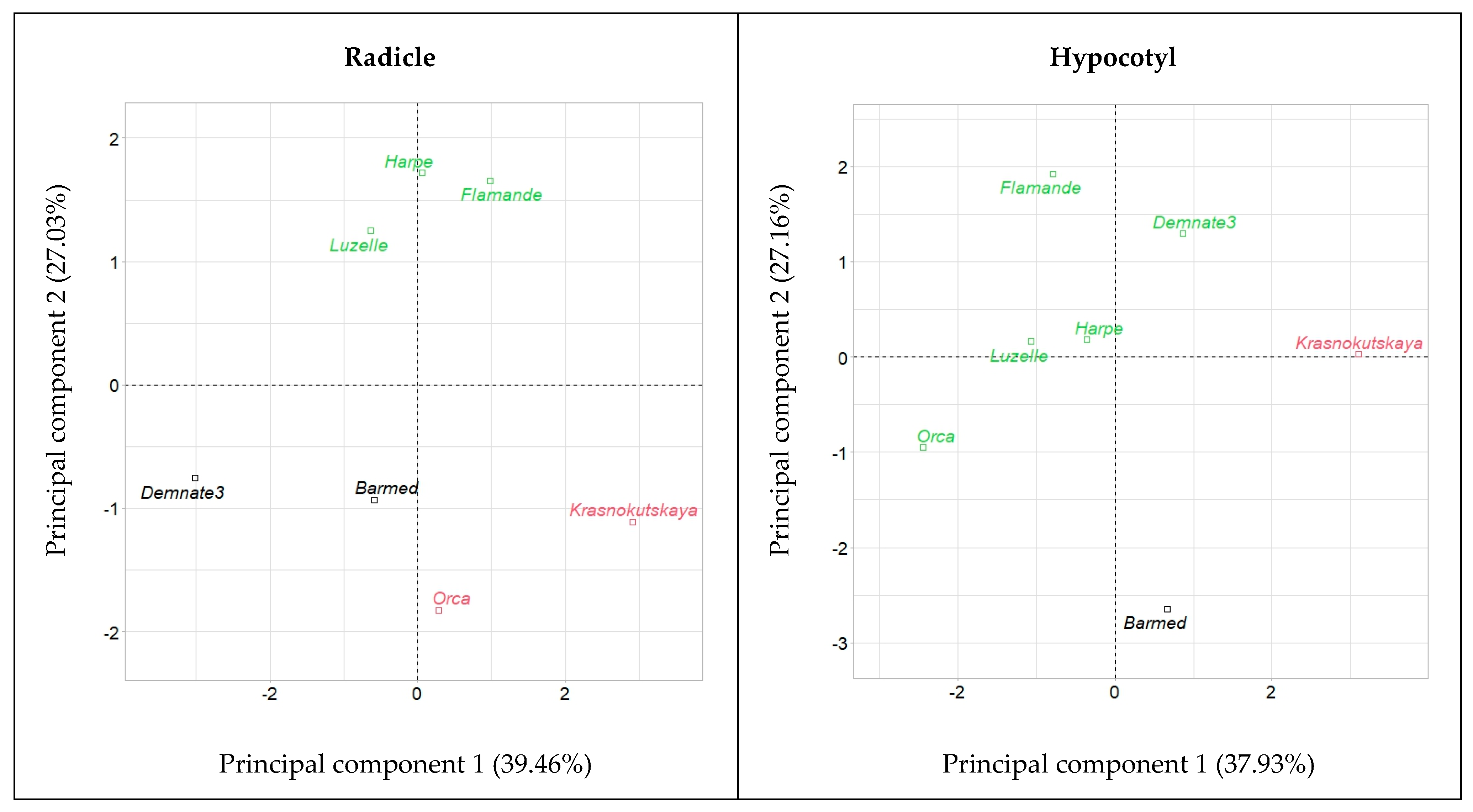
| Component | Eigenvalue | Percentage of Variability | |
|---|---|---|---|
| Component | Cumulative | ||
| 1 | 14.288 | 59.5 | 59.5 |
| 2 | 3.099 | 12.9 | 72.4 |
| 3 | 2.882 | 12.0 | 84.4 |
| 4 | 1.744 | 7.3 | 91.7 |
| Component | Eigenvalue | Percentage of Variability | |
|---|---|---|---|
| Component | Cumulative | ||
| 1 | 4.127 | 29.48 | 29.48 |
| 2 | 3.329 | 23.78 | 53.26 |
| 3 | 2.848 | 20.34 | 73.61 |
| 4 | 1.795 | 12.82 | 86.43 |
| Accession | Taxon | Autumn Dormancy | Type | Collection Site | Latitude and Longitude | Altitude above Sea Level (m) | Mean Temperature Warmest Quarter (°C) | Mean Temperature Coldest Quarter (°C) | Precipitation Warmest Quarter (mm) | Precipitation Coldest Quarter (mm) |
|---|---|---|---|---|---|---|---|---|---|---|
| Flamande | M. sativa spp. sativa L. (4×) | 4 | Landrace | Calais, France | 50°57′04.64″ N, 1°51′31.27″ E | 27 | 16.6 | 3.3 | 181 | 154 |
| Demnate3 | Idem | 9 | Landrace | Demnate, Marocco | 31°43′55.51″ N, 7°00′41.25″ W | 923 | 24.7 | 9.4 | 17 | 154 |
| Harpe | Idem | 4 | Variety | |||||||
| Luzelle | Idem | 3 | Variety | |||||||
| Orca | Idem | 4.5 | Variety | |||||||
| Barmed | Idem | 7 | Variety | |||||||
| Krasnokutskaya | M. sativa spp. falcata (L.) Arcang 1 (4×) | Variety | Russia |
| Temperature Treatment (°C) | Actual Temperature (°C) | Relative Humidity (%) | VPD (kPa) | Sampling Frequency (h) | Sampling Period (h) | Equivalent Growing Degree-Day |
|---|---|---|---|---|---|---|
| 5 | 5.0 | 74 | 0.23 | 48 | 672 | 140 |
| 10 | 9.6 | 84 | 0.20 | 16 | 224 | 90 |
| 15 | 14.3 | 76 | 0.41 | 12 | 168 | 100 |
| 20 | 19.2 | 74 | 0.61 | 8 | 112 | 90 |
| 25 | 25.0 | 80 | 0.63 | 8 | 112 | 117 |
| 30 | 30.0 | 50 | 2.12 | 8 | 112 | 140 |
| 35 | 34.2 | 65 | 1.97 | 12 | 168 | 239 |
| 40 | 40.0 | 55–100 | 3.3–0.0 | 168 | 1344 | 2240 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobar-Gutiérrez, A.J.; Ahmed, L.Q. Early Detection of Phenotypic Diversity of Alfalfa (Medicago sativa L.) in Response to Temperature. Plants 2023, 12, 3224. https://doi.org/10.3390/plants12183224
Escobar-Gutiérrez AJ, Ahmed LQ. Early Detection of Phenotypic Diversity of Alfalfa (Medicago sativa L.) in Response to Temperature. Plants. 2023; 12(18):3224. https://doi.org/10.3390/plants12183224
Chicago/Turabian StyleEscobar-Gutiérrez, Abraham J., and Lina Q. Ahmed. 2023. "Early Detection of Phenotypic Diversity of Alfalfa (Medicago sativa L.) in Response to Temperature" Plants 12, no. 18: 3224. https://doi.org/10.3390/plants12183224
APA StyleEscobar-Gutiérrez, A. J., & Ahmed, L. Q. (2023). Early Detection of Phenotypic Diversity of Alfalfa (Medicago sativa L.) in Response to Temperature. Plants, 12(18), 3224. https://doi.org/10.3390/plants12183224







