Insecticidal Activity of Tannins from Selected Brown Macroalgae against the Cotton Leafhopper Amrasca devastans
Abstract
:1. Introduction
2. Results
2.1. Qualitative Profiling of Tannin Crude Extracts
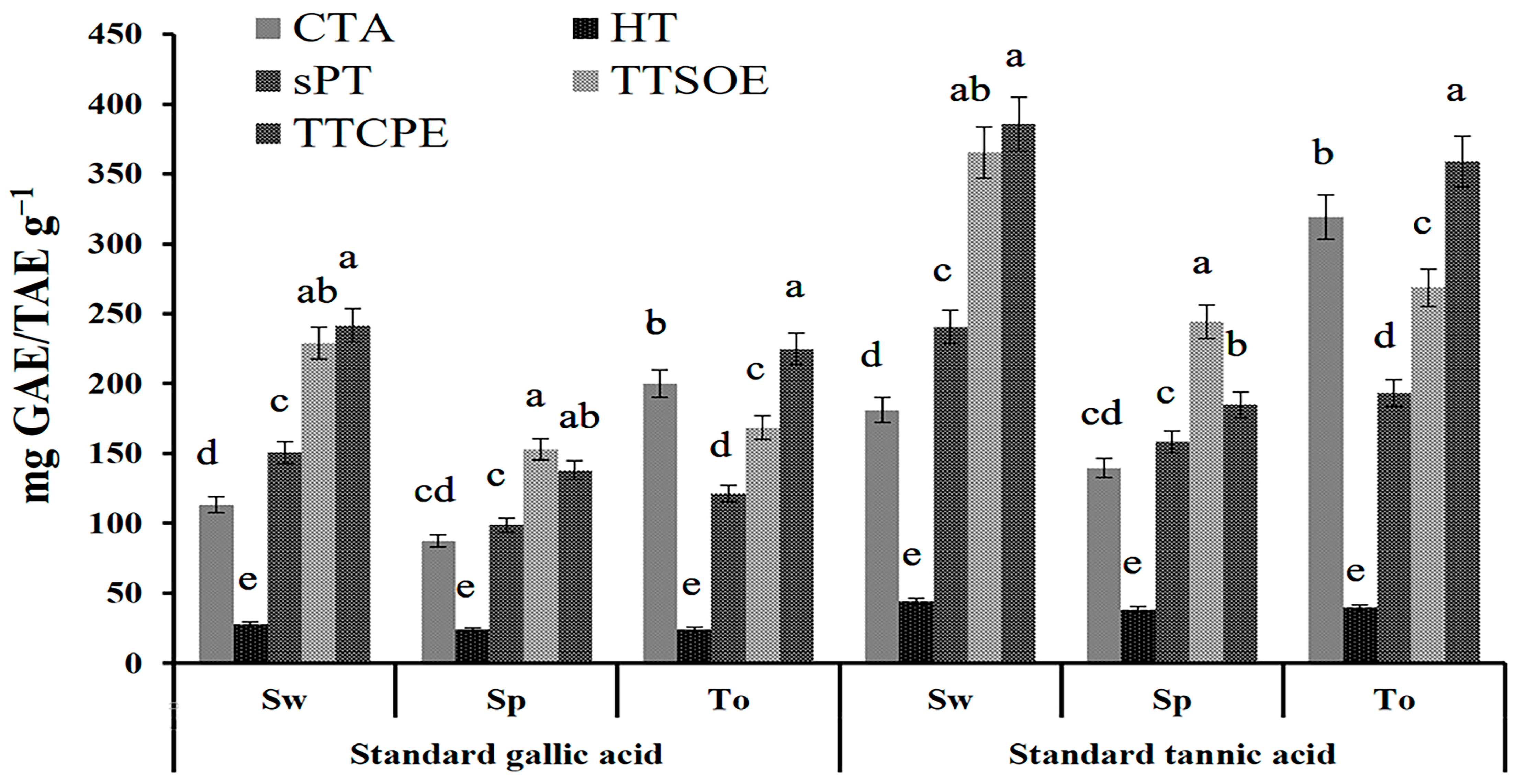
2.2. Quantitative Profiling of Crude Tannin
2.3. Phytochemical Profiling of Seaweed Tannins
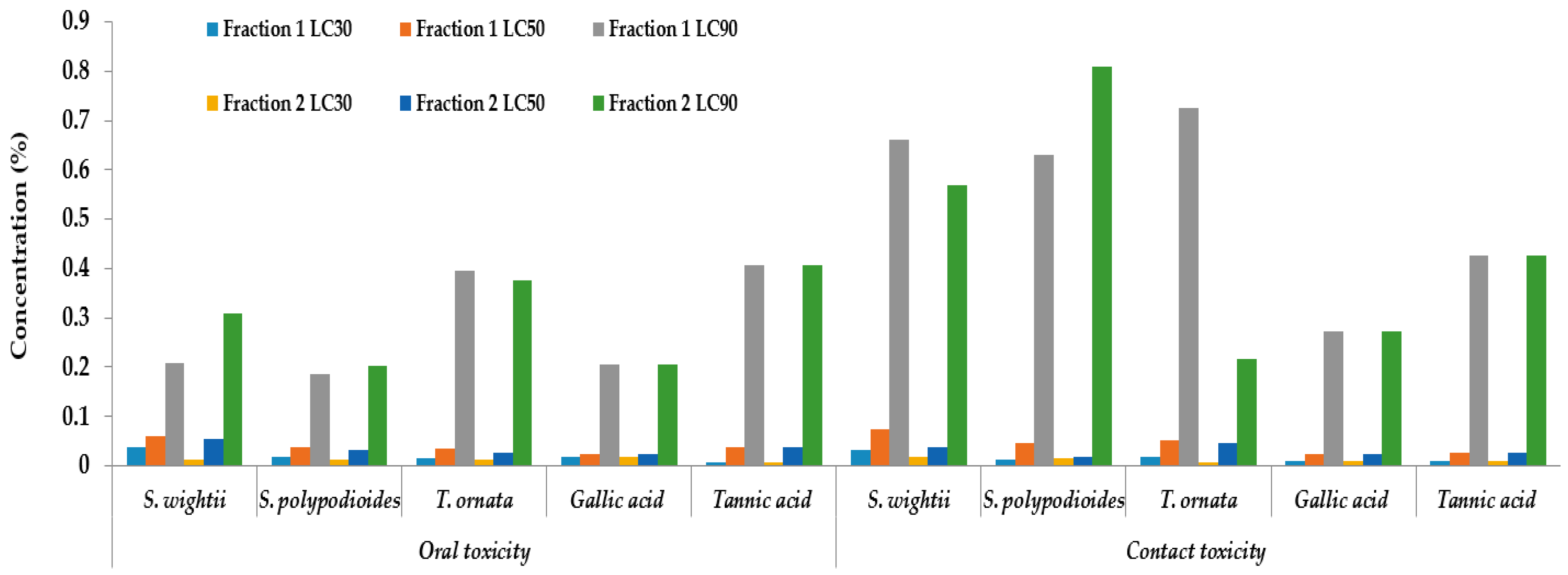
2.4. Insecticidal Activity of Crude Tannin Extract against A. devastans Adults
2.5. Insecticidal Activity of Crude Tannin Extracts against A. devastans Nymphs
2.6. Insecticidal Activity of Column Chromatographic Fractions of Crude Tannin Extracts against A. devastans Adults
2.7. Insecticidal Activity of Column Chromatographic Fractions of Crude Tannin Extracts against A. devastans Nymphs
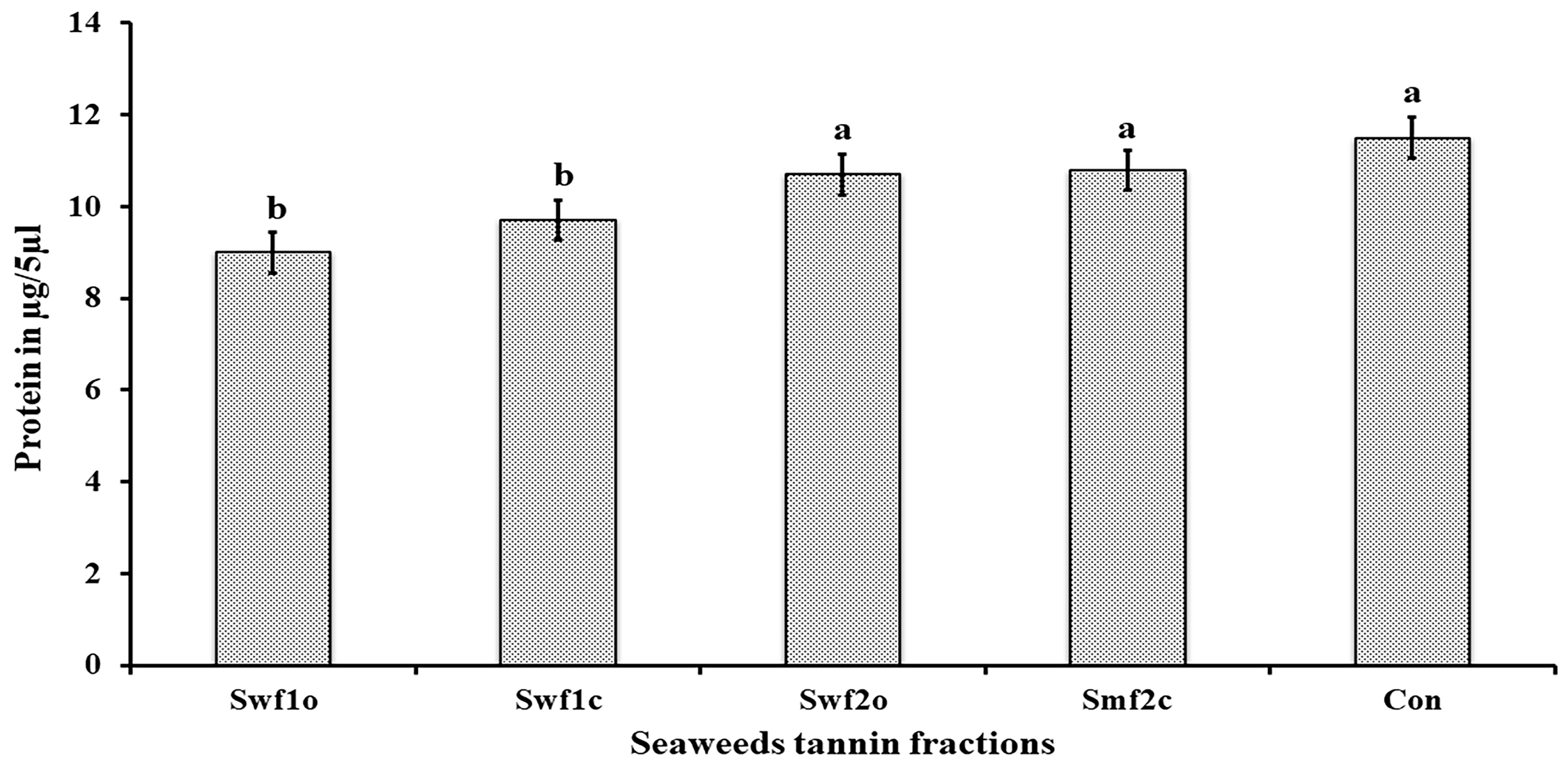
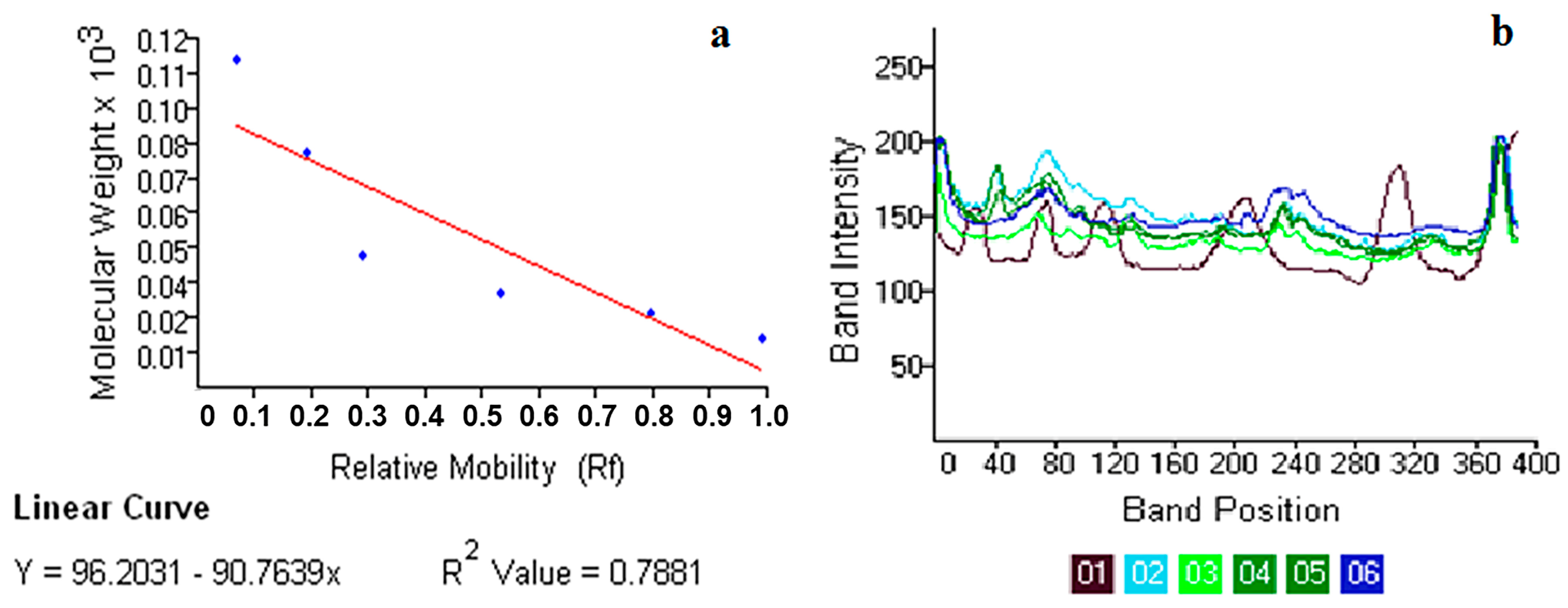
2.8. Insecticidal Mechanism of Action of Seaweed Tannin Extracts
3. Discussion
3.1. Qualitative Tannin Profiling
3.2. Quantitative Tannins Profiling
3.3. Tannin Analysis via HPLC
3.4. Tannin Analysis via GC-MS
3.5. Insecticidal Activity of Seaweed Tannins
3.6. Mechanism of Action of Insecticidal Activity of Seaweed Tannins
4. Materials and Methods
4.1. Seaweed Collection and Preparation
4.2. Extraction of Different Types of Tannins
4.3. Qualitative and Quantitative Profiling of Tannins
4.4. Fractionations of Crude Tannins Using a Sephadex LH-20 Column
4.5. TLC Analyses of Tannin Crude Extracts and Their Fractions
4.6. Pest Collection and Maintenance
4.7. Determination of Insecticidal Activity via Oral Toxicity Bioassay
4.8. Determination of Insecticidal Activity via the Contact Toxicity Method
4.9. Insecticidal Mechanism of Action
4.9.1. Preparation of Enzyme Sources and Their Quantifications
4.9.2. Total Body Protein Extraction and Estimation
4.9.3. Electrophoretic Analysis of Total Body Proteins
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nedumaran, T.; Arulbalachandran, D. Seaweeds: A promising source for sustainable development. In Environmental Sustainability; Thangavel, P., Sridevi, G., Eds.; Springer: New Delhi, India, 2015. [Google Scholar] [CrossRef]
- The Seaweed Anoneymous. Site: Information on Marine Algae. Available online: https://www.seaweed.ie/algae/seaweeds.php (accessed on 22 June 2023).
- Mora, J.; Pott, M.D.; Osorio, S.; Vallarino, G.J. Regulation of plant tannin synthesis in crop species. Front. Genet. 2022, 13, 870976. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Jain, S.; Pareek, A.; Sharma, S. A comprehensive study on the natural plantphenols: Perception to current scenario. Bull. Pharm. Res. 2013, 3, 90–106. [Google Scholar]
- Song, W.; Qin, S.T.; Fang, F.X.; Gao, Z.J.; Liang, D.D.; Liu, L.L.; Yang, H.B. Isolation and purification of condensed tannin from the leaves and branches of Prunus cerasifera and its structure and bioactivities. Appl. Biochem. Biotechnol. 2018, 185, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.l.; Lee, W.; Ahn, G. Marine algal flavonoids and phlorotannins; an intriguing frontier of biofunctional secondary metabolites. Crit. Rev. Biotechnol. 2021, 42, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Metting, B.; Zimmerman, W.J.; Crouch, I.; Van Staden, J. Agronomic uses of seaweed and microalgae. In Introduction to Applied Phycology; Akatsuka, I., Ed.; SPB Academic Publishing: The Hague, The Netherlands, 1990; pp. 589–627. [Google Scholar]
- Suganya, S.; Ishwarya, R.; Jayakumar, R.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Vaseeharan, B. New insecticides and antimicrobials derived from Sargassum wightii and Halimeda gracillis seaweeds: Toxicity against mosquito vectors and antibiofilm activity against microbial pathogens. S. Afr. J. Bot. 2019, 125, 466–480. [Google Scholar] [CrossRef]
- Gonzalez-Castro, A.L.; Muñoz-Ochoa, M.; Hernández-Carmona, G.; Lopez-Vivas, J.M. Evaluation of seaweed extracts for the control of the Asian citrus psyllid Diaphorina citri. J. Appl. Psychol. 2019, 31, 3815–3838. [Google Scholar] [CrossRef]
- Elbrense, H.; Gheda, S. Evaluation of the insecticidal and antifeedant activities of some seaweed extracts against the Egyptian cotton leaf worm, Spodoptera littoralis, and the lesser grain borer Rhyzopertha dominica. Egypt. J. Exp. Biol. (Zool.) 2021, 17, 1–17. [Google Scholar] [CrossRef]
- Song, C.; Yang, J.; Zhang, M.; Ding, G.; Jia, C.; Qin, J.; Guo, L. Marine natural products: The important resource of biological insecticide. Chem. Biodivers. 2021, 18, e2001020. [Google Scholar] [CrossRef] [PubMed]
- Thawfeeq Ahamed, J.; Srinivasan, G.; Shanthi, M.; Mini, M.L. Insecticidal activity of brown and red seaweed extracts against cowpea bean aphid, Aphis craccivora Koch. Pharma Innov. J. 2022, SP-11, 4223–4225. [Google Scholar]
- Thawfeeq Ahamed, J.; Srinivasan, G.; Shanthi, M.; Mini, M.L. Multifaceted effects of seaweed extracts against cowpea aphid, Aphis craccivora Koch, by evaluating four macroalgae. J. Appl. Phycol. 2023, 35, 1397–1406. [Google Scholar]
- Sahayaraj, K.; Mary Jeeva, Y. Nymphicidal and ovipositional efficacy of seaweed Sargassum tenerrimum (J. Agardh) against Dysdercus cingulatus (Fab.) (Pyrrhocoridae). Chil. J. Agric. Res. 2012, 72, 152–156. [Google Scholar] [CrossRef]
- Asaraja, A.; Sahayaraj, K. Screening of insecticidal activity of brown macro algal extracts against Dysdercus cingulatus (Fab.) (Hemiptera: Pyrrhocoridae). J. Biopestic. 2013, 6, 193–203. [Google Scholar]
- Punia, A.; Chauhan, N.S.; Singh, D.; Kesavan, A.K.; Kaur, S.; Sohal, S.K. Effect of gallic acid on the larvae of Spodoptera litura and its parasitoid Bracon hebetor. Sci. Rep. 2021, 11, 531. [Google Scholar] [CrossRef]
- Bernays, E.A.; Chamberlain, D.; McCarthy, P. The differential effects of ingested tannic acid on different species of Acridoidea. Entomol. Exp. Appl. 1980, 28, 158–166. [Google Scholar] [CrossRef]
- Bernays, E.A. Tannins: An alternative viewpoint. Entomol. Exp. Appl. 1978, 24, 244–253. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Martin, M.M. The protective role of the peritrophic membrane in the tannin-tolerant larvae of Orgyia leucostigma (Lepidoptera). J. Insect Physiol. 1992, 38, 973–980. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, L.; Zhao, J.; Chen, M. Effect of tannic acid on nutrition and activities of detoxification enzymes and acetylcholinesterase of the fall webworm (Lepidoptera: Arctiidae). J. Insect Sci. 2020, 20, 8. [Google Scholar] [CrossRef] [PubMed]
- Parthiban, C.; Anantharaman, P. Diversity and biomass of drift seaweeds from the Tuticorin coast India. Species 2018, 19, 72–86. [Google Scholar]
- Halappa, B.; Patil, R.K. Detoxifying enzyme studies on cotton leafhopper, Amrasca biguttula biguttula (Ishida), resistance to neonicotinoid insecticides in field populations in Karnataka, India. J. Plant Prot. Res. 2016, 54, 346–352. [Google Scholar] [CrossRef]
- Arunkumara, C.G.; Jagadish, K.S.; Mohan, M.; Venkatesan, T.; Narayanaswamy, K.C.; Peter, A. Biochemical basis of insecticides resistance in cotton leafhopper, Amrasca biguttula biguttula (Ishida) (Hemiptera: Cicadellidae). Int. J. Chem. Stud. 2020, 8, 2298–2301. [Google Scholar]
- Mahalakshmi, M.S.; Prasad, N.V.V.S.D. Insecticide resistance in field population of cotton leaf hopper, Amrasca devastans (Dist.) in Guntur, Andhra Pradesh, India. Int. J. Curr. Microbiol. App. Sci. 2020, 9, 3006–3011. [Google Scholar] [CrossRef]
- Silva, H.H.G.; Silva, I.G.; Santos, R.M.G.; Filho, E.R.; Elias, C.N. Larvicidal activity of tannins isolated of Magonia pubescens St. Hil. (Sapindaceae) against Aedes aegypti (Diptera, Culicidae). Rev. Soc. Bras. Med. Trop. 2004, 37, 396–399. [Google Scholar] [CrossRef]
- Li, E.; Wu, H.; Wang, Z.; Li, K.; Zhang, S.; Cao, Y.; Yin, J. Implication of antioxidant and detoxifying enzymes in the resistance of Holotrichia parallela larvae to EPN-Bt infection. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Ferreira, T.N.; Barufi, J.B.; Horta, P.A.; Castro, D.P.; Genta, F.A. Beta-1,3-glucanase inhibitors in Brazilian brown seaweed. An. Acad. Bras. De Cienc. 2021, 93, e20191402. [Google Scholar] [CrossRef]
- Yuan, W.; Li, W.J.; Lu, Y.H.; Wu, K.M. Combination of plant and insect eggs as food sources facilities ovarian development in an omnivorous bug Apolygus lucorum (Hemiptera: Miridae). J. Econ. Entomol. 2013, 106, 1200–1208. [Google Scholar] [CrossRef]
- Petchidurai, G.; Amruthraj Nagoth, J.; Sindhura John, M.; Sahayaraj, K.; Murugesan, N.; Pucciarelli, S. Standardization and quantification of total tannins, condensed tannin and soluble phlorotannins extracted from thirty-two drifted coastal macroalgae using high performance liquid chromatography. Bioresour. Technol. Rep. 2019, 7, 100273. [Google Scholar] [CrossRef]
- Melone, F.; Saladino, R.; Lange, H.; Crestini, C. Tannin structural elucidation and quantitative 31P NMR analysis. 1. model compounds. J. Agric. Food Chem. 2013, 61, 9307–9315. [Google Scholar] [CrossRef] [PubMed]
- Farvin, K.H.S.; Surendraraj, A.; Al-Ghunaim, A.; Al-Yamani, F. Chemical profile and antioxidant activities of 26 selected species of seaweeds from Kuwait coast. J. Appl. Phycol. 2019, 31, 2653–2668. [Google Scholar] [CrossRef]
- Paga, A.; Agus, A.; Kustantinah, K.; Budisatria, I.G.S. Secondary metabolites content of seaweed (Sargassum sp.) based on the different drying methods. Adv. Biol. Res. 2021, 21, 219–223. [Google Scholar]
- Monrroy, M.; Arauz, O.; Garcıa, J.R. Active compound identification in extracts of N. lappaceum peel and evaluation of antioxidant capacity. J. Chem. 2020, 2020, 4301891. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci. Technol. 2011, 22, 315–326. [Google Scholar] [CrossRef]
- Rani, P.M.J.; Kannan, P.S.M.; Kumaravel, S. Screening of antioxidant activity, total phenolics and gas chromatograph and mass spectrometer (GC-MS) study of Delonix regia. Afr. J. Biochem. Res. 2011, 5, 341–347. [Google Scholar]
- Nawaz, A.; Gogi, M.D.; Naveed, M.; Arshad, M.; Sufyan, M.; Binyameen, M.; Islam, S.U.; Waseem, M.; Ayyub, M.B.; Arif, M.J.; et al. In vivo and in vitro assessment of Trichoderma species and Bacillus thuringiensis integration to mitigate insect pests of brinjal (Solanumm elongena L.). Egypt. J. Biol. Pest Control 2020, 30, 60. [Google Scholar] [CrossRef]
- Fiaz, M.; Hameed, A.; Hasan, M.U.; Wakil, W. Efficacy of plant extracts on some cotton (Gossypium hirsutum) Pests: Amrasca bigutulla bigutulla Ishida and Thrips tabaci Lindeman. Pakistan J. Zool. 2012, 44, 277–283. [Google Scholar]
- Vijayabaskar, P.; Shiyamala, V. Antioxidant properties of seaweed polyphenol from Turbinaria ornata (Turner). Asian Pac. J. Trop. Biomed. 2012, 2, 90–98. [Google Scholar] [CrossRef]
- Remya, R.R.; Julius, A.; Ramadoss, R.; Parthiban, S.; Bharath, N.; Pavana, B.; Samrot, A.V.; Kanwal, S.V.; Vinayagam, M. Pharmacological activities of natural products from marine seaweed Turbinaria ornata: A review. J. Nanomater. 2022, 2022, 4784608. [Google Scholar] [CrossRef]
- Kannan, R.; Priya, N.D. Studies on methanolic extract of brown algal seaweed Liagora ceranoides J.V. Lamouroux from Southern coast of Tamil Nadu: In vitro anti-insect properties and phytochemicals. Nat. Prod. Chem. Res. 2019, 7, 1000354. [Google Scholar] [CrossRef]
- Niroja, D.; Kannan, R. Larvicidal and insect growth regulatory activities of methanol extract of Sargassum cinereum J. Agardh against Spodoptera litura Fabricius. J. Interdiscip. Cycle Res. 2020, 12, 1323–1333. [Google Scholar] [CrossRef]
- Chanthini, K.M.; Senthil-Nathan, S.; Stanley-Raja, V.; Karthi, S.; Sivanesh, H.; Ramasubramanian, R.; Abdel-Megeed, A.; El Maghraby, D.M.; Ghaith, A.; Alwahibi, M.S.; et al. Biologically active toxin from macroalgae Chaetomorpha antennina Bory, against the lepidopteran Spodoptera litura Fab. and evaluation of toxicity to earthworm Eudrilus eugeniae Kinb. Chem. Biol. Technol. Agric. 2021, 8, 49. [Google Scholar] [CrossRef]
- Yu, K.X.; Jantan, I.; Ahmad, R.R.; Wong, C.L. The major bioactive components of seaweeds and their mosquitocidal potential. Parasitol. Res. 2015, 113, 3121–3141. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef]
- Yu, K.X.; Wong, C.L.; Ahmad, R.; Jantan, I. Larvicidal activity, inhibition effect on development, histopathological alteration and morphological aberration induced by seaweed extracts in Aedes aegypti (Diptera: Culicidae). Asian Pac. J. Trop. Med. 2015, 8, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Sahayaraj, K. Modulation of botanicals on pest’s biochemistry. In Short Views on Insect Biochemistry and Molecular Biology; Chandrasekar, R., Tyagi, B.K., Gui, Z.Z., Reeck, G.R., Eds.; International Book Mission©, Academic Publisher: Kolkata, India, 2014; Chapter 2; pp. 57–74. [Google Scholar]
- Li, W.; Zhao, X.; Yuan, W.; Wu, K. Activates of digestive enzyme in the omnivorous pest Apolygus lucorum (Hemiptera: Miridae). J. Econ. Entomol. 2017, 110, 101–110. [Google Scholar] [CrossRef]
- Hafez, A.E.M.M.; El-Naby, A.M.S. Relationship between resistance level and some biochemical parameters to Spodoptera littoralis against some insect growth regulators (IGRs). Egypt. Acad. J. Biol. 2014, 6, 123–130. [Google Scholar] [CrossRef]
- Zibaee, A.; Bandani, A.R. Effects of Artemisia annua L. (Asteracea) on digestive enzymes profiles and cellular immune reactions of sunn pest, Eurygaster integriceps (Heteroptera: Scutellaridae), against Beauvaria bassiana. Bull. Entomol. Res. 2010, 100, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Sabeghi Khosroshahi, Z.; Abbasipour, H.; Rezazadeh, A. Inhibitory effect of aqueous bean extract, Phaseolus vulgaris (fabaceae), on α-amylase of the cabbage aphid, Brevicoryne brassicae. Arch. Agron. Soil Sci. 2020, 67, 1425–1433. [Google Scholar] [CrossRef]
- Hemmingi, J.D.C.; Lindroth, R.L. Effects of phenolic glycosides and protein on gypsy moth (Lepidoptera: Lymantriidae) and forest tent caterpillar (Lepidoptera: Lasiocampidae) performance and detoxication activities. Environ. Entomol. 2000, 29, 1108–1115. [Google Scholar] [CrossRef]
- Saadati, F.; Bandani, A.R. Effects of serine protease inhibitors on growth and development and digestive serine proteinases of the Sunn pest, Eurygaster integriceps. J. Insect Sci. 2011, 11, 72. [Google Scholar] [CrossRef]
- Kansal, R.; Kalika, K.; Niwas, G.R.; Kumar, G.V.; Ram, K.K. Screening of indigenous legumes for trypsin inhibitor protein activity. Indian J. Agric. Biochem. 2008, 21, 54–56. [Google Scholar]
- Aghaali, N.; Ghadamyari, M.; Hosseininaveh, V.; Riseh, N.S. Protease inhibitor from the crude extract of plant seeds affects the digestive proteases in Hyphantria cunea (Lep.: Arctiidae). J. Plant Prot. Res. 2013, 53, 338–346. [Google Scholar] [CrossRef]
- Ebeid, A.R. The efficiency of some plant extracts against Agrotis ipsilon (Lepidoptera: Noctuidae) regarding to their activity on vital biochemical parameters. GSC Biol. Pharm. Sci. 2020, 12, 240–248. [Google Scholar] [CrossRef]
- Senthil Nathan, S.; Kalaivani, K.; Murugan, K. Effect of biopesticides on the lactate dehydrogenase (LDH) of the rice leaffolder, Cnaphalocrocis medinalis (Guene´e) (Insecta: Lepidoptera: Pyralidae). Ecotoxicol. Environ. Saf. 2006, 65, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xi, K.; Wang, Y.; Ma, J.; Huang, X.; Liu, R.; Cai, X.; Zhu, Y.; Yin, J.; Jia, Q. Evaluation of the contact toxicity and physiological mechanisms of ginger (Zingiber officinale) shoot extract and selected major constituent compounds against Melanaphis sorghi Theobald. Horticulturae 2022, 8, 944. [Google Scholar] [CrossRef]
- Sahayaraj, K.; Antony, K. Impact of five plant extracts on the digestive and detoxification enzymes of Spodoptera litura (Fab.) (Lepidoptera: Noctuidae). Hexapoda 2006, 13, 53–57. [Google Scholar]
- Zulhussnain, M.; Zahoor, K.; Rizvi, H.; Zahoor, M.A.; Rasul, A.; Ahmad, A.; Majeed, H.N.; Rasul, A.; Ranian, K.; Jabeen, F. Insecticidal and genotoxic effects of some indigenous plant extracts in Culex quinquefasciatus Say mosquitoes. Sci. Rep. 2020, 10, 6826. [Google Scholar] [CrossRef]
- Yooboon, T.; Pengsook, A.; Ratwatthananon, A.; Pluempanupat, W.; Bullangpoti, V. A plant-based extract mixture for controlling Spodoptera litura (Lepidoptera: Noctuidae). Chem. Biol. Technol. Agric. 2019, 6, 5. [Google Scholar] [CrossRef]
- Senthil-Nathan, S. Physiological and biochemical effect of neem and other Meliaceae plants secondary metabolites against Lepidopteran insects. Front. Physiol. 2013, 4, 359. [Google Scholar] [CrossRef]
- Riaz, B.; Zahoor, M.K.; Zahoor, M.A.; Majeed, H.N.; Javed, I.; Ahmad, A.; Jabeen, F.; Zulhussnain, M.; Sultana, K. Toxicity, phytochemical composition, and enzyme inhibitory activities of some indigenous weed plant extracts in fruit fly, Drosophila melanogaster. Evid.-Based Complement. Altern. Med. 2018, 2018, 2325659. [Google Scholar] [CrossRef]
- Sofi, M.A.; Nanda, A.; Sofi, M.A.; Maduraiveeran, R.; Nazir, S.; Siddiqui, N.; Nadeem, A.; Shah, Z.A.; Rehman, M.U. Larvicidal activity of Artemisia absinthium extracts with special reference to inhibition of detoxifying enzymes in larvae of Aedes aegypti L. J. King Saud Univ. Sci. 2022, 34, 102248. [Google Scholar] [CrossRef]
- Hassam, U.A.; Gulzar, A.; Rasool, B.; Zafar, S.; Younis, T.; Shakeel, M.; Khan, D.; Ullah, S.; Khaliq, S.; Ahmad, S.F.; et al. Efficacy of Citrullus colocynthis seed extract on Earias vittella, Fabricius, (Lepidoptera: Noctuidae): Environment sustainable approach. Braz. J. Biol. 2021, 84, e254479. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.T.; Emerald, M.D.; Sethuraman, S. Impact of heavy metallic zinc on enzyme studies in selected tissues of Odontopus varicornis (Dist.) (Hemiptera: Pyrrhocordiae). Int. J. Recent Sci. Res. 2014, 5, 1717–1728. [Google Scholar]
- Dolma, S.K.; Singh, P.P.; Reddy, S.G.E. Insecticidal and enzyme inhibition activities of leaf/bark extracts, fractions, seed oil and isolated compounds from Triadica sebifera (L.) Small against Aphis craccivora Koch. Molecules 2022, 27, 1967. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.W.; Dillwith, W.J.; Puterka, J.G. Comparisons of salivary proteins from five aphid (Hemiptera: Aphididae) species. Environ. Entomol. 2011, 40, 152–156. [Google Scholar] [CrossRef]
- Fauzi, A.; Lamma, S.; Ruslin, M. Total tannin levels analysis of brown algae (Sargassum sp. and Padina sp.) to prevent blood loss in surgery. J. Dentomaxillofac. Sci. 2018, 3, 37–40. [Google Scholar] [CrossRef]
- Terrill, T.H.; Rowan, A.M.; Douglas, G.B.; Barry, T.N. Determinations of extractable and bounded condensed tannin concentrations of forage plants, protein concentrate meals and cereal grains. J. Sci. Food Agric. 1992, 58, 321–329. [Google Scholar] [CrossRef]
- Rhazi, N.; Hannache, H.; Oumam, M.; Sesbou, A.; Charrier, B.; Pizzi, A.; Bouhtoury, F.C. Green extraction process of tannins obtained from Moroccan Acacia mollissima barks by microwave: Modeling and optimization of the process using the response surface methodology RSM. Arab. J. Chem. 2019, 12, 2668–2684. [Google Scholar] [CrossRef]
- Koivikko, R.; Loponen, J.; Honkanen, T.; Jormalainen, V. Contents of soluble, cell-wall-bound and exuded phlorotannins in the brown alga Fucus vesiculosus, with implications on their ecological functions. J. Chem. Ecol. 2005, 31, 195–212. [Google Scholar] [CrossRef]
- Rajratna, B.K.; Nemade, S.N. Extraction of tannic acid from Emblica officinalis (Avala) by hot continuous extraction method. Int. J. Res. Eng. Sci. Manag. 2019, 2, 542–546. [Google Scholar]
- Mailoa, M.N.; Mahendradatta, M.; Laga, A.; Djide, N. Tannin extract of Guavaleaves (Psidium guajava L) variation with concentration organic solvents. Int. J. Sci. Technol. Res. 2013, 2, 106–110. [Google Scholar]
- Janarthanan, M.; Senthilkumar, M.S. Qualitative and quantitative analysis of phytochemical studies on selected seaweeds Acanthopora spicifera and Sargassum wightii. Int. J. Eng. Res. Dev. 2013, 7, 11–15. [Google Scholar]
- Harbourne, J.B. Metode Fitokimia: Penuntun Cara Modern Menganalisis Tumbuhan; Penerbit ITB: Bandung, Indonesia, 1987; Volume 78. [Google Scholar]
- Mamta, S.; Jyoti, S. Phytochemical screening of Acorus calamus and Lantana camara. Int. Res. J. Pharm. 2012, 3, 324–326. [Google Scholar]
- Elgailani, I.E.H.; Isha, K.C.Y. Methods for extraction and characterization of tannins from some Acacia Species of Sudan. Pak. J. Anal. Environ. 2016, 17, 43–49. [Google Scholar] [CrossRef]
- Makkar, H.P.S. Effects of fate of tannins in ruminant animals, adaptations to tannins and strategies to overcome detrimental effect of feeding tannin-rich feeds. Small Rumin. Res. 2003, 49, 241–256. [Google Scholar] [CrossRef]
- Gall, E.A.; Lelchat, F.; Hupel, M.; Jegou, C.; Stiger-Pouvreau, V. Extraction and purification of phlorotannins from brown algae. Natural product from marine algae: Methods and protocols. Methods Mol. Boil. 2015, 131–143. [Google Scholar]
- Yang, J.; Zhao, l.; Sun, R.; Shen, Y.; Wang, N.; Liu, X. Purification of Epothilones A and B with column chromatography on a Sephadex LH-20. Adv. Mat. Res. 2014, 904, 64–169. [Google Scholar] [CrossRef]
- Helen, L.R.; Jyothilakshmi, M.; Latha, M.S. Isolation and quantification of tannins from the root bark of Clerodendrum infortunatum Linn. and assessment of their antioxidant potential and antiproliferative effect on hct-15 cells. Int. J. Pharm. Pharm. Sci. 2015, 7, 70–175. [Google Scholar]
- Gupta, M.; Sasmal, S.; Majumdar, S.; Mukherjee, A. HPLC profiles of standard phenolic compounds present in medicinal plants. Int. J. Pharmacogn. Phytochem. 2012, 4, 162–167. [Google Scholar]
- Jayarao, B.; Abulkhader, S.B.; Naik, L.K.; Vinaykumar, M.M. Assessment of Biology and morphometric characteristics of different stages of leafhopper, Amrasca biguttula biguttula (Ishida) on okra. BioScan 2015, 10, 671–674. [Google Scholar]
- Vimala, V.; Bheemanna, M.; Rajesh, R.; Ronda, R.S. Biochemical characterization of insecticide resistance in geographic population of Amrasca biguttula biguttula (Ishida). Res. J. Biotechnol. 2016, 11, 32–38. [Google Scholar]
- King, J. The dehydrogenases or oxidoreductases. Lactate dehydrogenase. In Practical Clinical Enzymology; Van Nostrand, D., Ed.; London Publishers: London, UK, 1965; pp. 83–93. [Google Scholar]
- Darvishzadeh, A.; Bandani, A.R.; Mousavi, S.Q. Biochemical characterisation of α-amylase in two aphid species, Aphis fabae Scopoli (Hemiptera: Aphididae) and A. gossypii Glover (Hemiptera: Aphididae). Plant Prot. Sci. 2014, 50, 84–89. [Google Scholar] [CrossRef]
- Nigam, C.S.; Omker, M. Experimental Animal Physiology and Biochemistry; New Age International (P) Limited, Publishers: New Delhi, India, 2003; pp. 93–97. [Google Scholar]
- Morihara, K.; Tsuzuki, H. Production of protease and elastase by Pseudomonoas aeruginosa strains isolated from patients. Infect. Immun. 1977, 15, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.B.; Fragal, P.; Fleur, L.F.; Macedo, G.A. Lipase and esterase-to what extent can this classification be applied accurately Lipases e esterases: Comodefinir e classificar. Cienc. Tecnol. Aliment. Campinas 2011, 31, 608–613. [Google Scholar] [CrossRef]
- Beaufay, H.; Hers, H.C.; Berthet, J.; de Duve, C. Acid phosphatase activity. Bull. Soc. Chim. Biol. 1954, 36, 1539–1550. [Google Scholar]
- Bose, U.; Broadbent, A.J.; Juhasz, A.; Karnaneedi, S.; Johnston, B.E.; Stockwell, S.; Byrne, K.; Limviphuvadh, V.; Maurer-Stroh, S.; Lopata, L.A.; et al. Protein extraction protocols for optimal proteome measurement and arginine kinase quantitation from cricket Acheta domesticus for food safety assessment. Food Chem. 2021, 348, 129110. [Google Scholar] [CrossRef]
- Sulistyarsi, A.; Suranto, S.; Supriyadi, S. The total protein band profile of the green leafhoppers (Nephotettix virescens) and the leaves of rice (Oryza sativa) infected by tungro virus. Nusant. Biosci. 2012, 4, 32–35. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: London, UK, 1971; p. 383. [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 65–266. [Google Scholar] [CrossRef]

| Tannins | S. wightii | S. polypodioides | T. ornata | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FCT | AFCT | LAT | FSSPT | FCT | AFCT | LAT | FSSPT | FCT | AFCT | LAT | FSSPT | |
| CTA | + | + | +++ | ++ | ++ | + | +++ | ++ | ++ | + | +++ | ++ |
| HT | ++ | ++ | +++ | ++ | ++ | ++ | +++ | ++ | ++ | + | +++ | ++ |
| SPT | ++ | ++ | +++ | ++ | ++ | + | +++ | +++ | +++ | ++ | +++ | ++ |
| TT—Soxhlet method | ++ | +++ | +++ | ++ | ++ | +++ | ++ | +++ | ++ | +++ | + | ++ |
| TT—cold percolation method | ++ | + | +++ | ++ | ++ | + | +++ | + | ++ | + | +++ | ++ |
| Seaweeds | Soxhlet Method | Cold Percolation Method | ||
|---|---|---|---|---|
| RT | Area | RT | Area | |
| Gallic acid | 0.671 | 16,505,777 | - | - |
| Tannic acid | 0.679 | 1,357,710 | - | - |
| S. wightii | 0.635 | 1,094,181 | 0.626 | 1,837,110 |
| S. polypodioides | 0.637 | 7,439,832 | 0.626 | 911,639 |
| T. ornata | 0.664 | 2,028,143 | 0.640 | 15,480,659 |
| Seaweeds | Fraction | RT | Area |
|---|---|---|---|
| Gallic acid | - | 0.671 | 49,057,953 |
| Tannic acid | - | 0.679 | 62,682,156 |
| S. wightii | F1 | 0.676 | 72,230,027 |
| F2 | 0.678 | 15,268,144 | |
| S. polypodioides | F1 | 0.678 | 21,539,604 |
| F2 | 0.671 | 56,315,135 | |
| T. ornata | F1 | 0.671 | 10,317,969 |
| F2 | 0.678 | 18,011,551 |
| Seaweeds | RT (min) | Area (%) | RT (min) | Area (%) |
|---|---|---|---|---|
| Standard | ||||
| Gallic acid | 5.775 | 17.41 | - | - |
| Tannic acid | 6.612 | 9.79 | - | - |
| Tannin crude extract | ||||
| S. wightii | 3.685 | 60.90 | - | - |
| S. polypodioides | 3.675 | 80.32 | - | - |
| T. ornata | 3.647 | 73.94 | - | - |
| Fraction 1 | Fraction 2 | |||
| S. wightii | 5.862 | 18.82 | 3.637 | 100.00 |
| S. polypodioides | 5.772 | 56.68 | 3.638 | 100.00 |
| T. ornata | 5.752 | 65.81 | 3.704 | 100.00 |
| Seaweeds Extracts | Adult | Nymph | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LC50 | CI | Χ2 | Slope | p | LC50 | CI | Χ2 | Slope | p | |
| Oral Toxicity | ||||||||||
| S.wightii | 0.071 | 0.024–0.316 | 3.820 | 1.35 ± 0.84 | 0.051 | 0.106 | 0.049–0.287 | 37.976 | 2.91 ± 0.14 | 0.005 |
| S. polypodioides | 0.044 | 0.023–0.432 | 0.022 | 1.17 ± 0.19 | 0.881 ns | 0.092 | 0.020–0.318 | 68.751 | 2.72 ± 0.17 | 0.005 |
| T. ornata | 0.058 | 0.028–0.722 | 0.493 | 1.26 ± 0.10 | 0.481 ns | 0.070 | 0.042–0.160 | 74.502 | 1.59 ± 0.62 | 0.005 |
| Gallic acid | 0.044 | 0.014–0.232 | 2.192 | 1.16 ± 0.58 | 0.139 ns | 0.083 | 0.021–0.397 | 81.371 | 1.77 ± 0.13 | 0.005 |
| Tannic acid | 0.122 | 0.067–1.000 | 0.035 | 1.82 ± 0.13 | 0.130 ns | 0.084 | 0.024–0.273 | 48.448 | 1.86 ± 0.50 | 0.005 |
| Contact toxicity | ||||||||||
| S. wightii | 0.075 | 0.021–0.344 | 46.499 | 1.97 ± 0.22 | 0.000 | 0.071 | 0.036–0.355 | 2.060 | 2.86 ± 0.89 | 0.151 ns |
| S. polypodioides | 0.067 | 0.006–0.325 | 58.115 | 1.86 ± 0.16 | 0.000 | 0.099 | 0.040–0.736 | 6.047 | 2.14 ± 0.52 | 0.014 ns |
| T. ornata | 0.057 | 0.037–0.284 | 85.368 | 1.72 ± 0.42 | 0.000 | 0.111 | 0.040–0.296 | 5.529 | 2.41 ± 0.54 | 0.019 ns |
| Gallic acid | 0.066 | 0.001–0.269 | 69.111 | 1.80 ± 0.18 | 0.000 | 0.066 | 0.026–0.103 | 0.616 | 1.48 ± 0.33 | 0.414 ns |
| Tannic acid | 0.096 | 0.015–0.350 | 59.423 | 2.64 ± 0.17 | 0.000 | 0.064 | 0.026–0.097 | 1.559 | 3.02 ± 0.71 | 0.212 ns |
| Seaweed Extract /Tannin Standards | Concentrations | Oral Toxicity—Nymph | Contact Toxicity—Nymph | ||
|---|---|---|---|---|---|
| F1 | F2 | F1 | F2 | ||
| S. wightii | 0.0075 | 6.7 ± 0.4 eA | 6.7 ± 0.4 eA | 23.3 ± 9.5 eA | 20.0 ± 7.3 deB |
| 0.015 | 16.7 ± 6.1 dB | 36.7 ± 8.0 dA | 36.7 ± 9.5 dA | 26.7 ± 6.7 dB | |
| 0.03 | 33.3 ± 11.1 cB | 50.0 ± 8.6 cA | 40.0 ± 8.9 cA | 40.0 ± 10.3 cA | |
| 0.06 | 43.3 ± 3.3 bB | 56.7 ± 9.5 bA | 50.0 ± 1.2 bB | 63.3 ± 9.5 bA | |
| 0.12 | 60.0 ± 5.2 aB | 63.3 ± 2.0 aA | 63.3 ± 6.1 aB | 80.0 ± 7.3 aA | |
| S. polypodioides | 0.0075 | 16.7 ± 6.1 eA | 10.0 ± 4.4 eB | 13.3 ± 4.2 eA | 06.7 ± 4.2 eB |
| 0.015 | 33.3 ± 12.3 dB | 40.0 ± 7.3 dA | 43.3 ± 9.5 cdA | 30.0 ± 8.5 dB | |
| 0.03 | 56.7 ± 10.8 cA | 50.0 ± 8.5 bcB | 46.7 ± 9.9 cA | 43.3 ± 2.0 cB | |
| 0.06 | 63.3 ± 8.0 abA | 53.3 ± 6.1 bB | 70.0 ± 1.0 bA | 70.0 ± 8.5 bA | |
| 0.12 | 63.3 ± 3.3 aA | 63.3 ± 1.1 aA | 80.0 ± 5.9 aB | 83.3 ± 6.1 aA | |
| T. ornata | 0.0075 | 13.3 ± 4.2 eB | 26.7 ± 6.7 eA | 20.0 ± 5.1 eA | 16.7 ± 6.1 eB |
| 0.015 | 30.0 ± 4.4 dB | 50.0 ± 6.8 dA | 36.7 ± 4.0 dA | 30.0 ± 4.5 dB | |
| 0.03 | 43.3 ± 6.1 cB | 60.0 ± 1.5 cA | 46.7 ± 6.6 cB | 50.0 ± 2.0 cA | |
| 0.06 | 50.0 ± 10.0 bB | 73.3 ± 4.2 bA | 63.3 ± 9.5 bA | 60.0 ± 8.9 bB | |
| 0.12 | 66.7 ± 6.7 aB | 83.3 ± 5.4 aA | 73.3 ± 1.1 aB | 76.7 ± 8.0 aA | |
| Gallic acid | 0.0075 | 23.3 ± 8.0 e | 20.0 ± 8.9 e | ||
| 0.015 | 46.6 ± 6.7 d | 33.3 ± 6.7 d | |||
| 0.03 | 56.6 ± 6.7 c | 46.7 ± 6.7 c | |||
| 0.06 | 70.0 ± 10.0 b | 70.0 ± 8.6 b | |||
| 0.12 | 80.0 ± 7.3 a | 83.3 ± 8.0 a | |||
| Tannic acid | 0.0075 | 20.0 ± 5.1 e | 30.0 ± 4.5 e | ||
| 0.015 | 50.0 ± 10.0 cd | 36.7 ± 6.1 d | |||
| 0.03 | 50.0 ± 12.4 c | 56.7 ± 9.5 c | |||
| 0.06 | 63.3 ± 9.5 b | 73.3 ± 1.1 ab | |||
| 0.12 | 76.7 ± 8.0 a | 73.3 ± 9.9 a | |||
| Vijayneem | 0.03 | 63.3 ± 13.1 b | 66.7 ± 2.3 b | ||
| Monocrotophos | 0.03 | 70.0 ± 16.9 a | 76.7 ± 6.1 a | ||
| Seaweed Name | Concentrations | Amylase | Protease | Lipase | Invertase | Glycosidase | Acid Phosphates |
|---|---|---|---|---|---|---|---|
| S. wightii | 0.025 | 0.83 ± 0.11 a | 80.15 ± 0.10 ab | 6.41 ± 0.07 b | 0.26 ± 0.01 b | 0.50 ± 0.01 b | 7.02 ± 0.05 b |
| 0.05 | 0.41 ± 0.01 c | 60.21 ± 0.02 bc | 5.30 ± 0.13 c | 0.15 ± 0.05 c | 0.22 ± 0.02 c | 5.03 ± 0.10 c | |
| 0.1 | 0.34 ± 0.02 d | 43.12 ± 0.08 cd | 3.11 ± 0.05 d | 0.12 ± 0.01 cd | 0.05 ± 0.01 d | 2.35 ± 0.02 d | |
| 0.2 | 0.23 ± 0.01 e | 35.62 ± 0.03 de | 1.63 ± 0.01 de | 0.06 ± 0.02 e | 0.03 ± 0.01 de | 1.08 ± 0.01 e | |
| S. polypodioides | 0.025 | 0.41 ± 0.01-b | 81.08 ± 0.01 b | 5.61 ± 0.07 b | 0.34 ± 0.03 b | 0.52 ± 0.03 b | 7.19 ± 0.04 b |
| 0.05 | 0.23 ± 0.02 c | 75.35 ± 0.04 c | 3.38 ± 0.03 c | 0.24 ± 0.05 b | 0.31 ± 0.01 c | 5.73 ± 0.03 c | |
| 0.1 | 0.15 ± 0.01 d | 52.96 ± 0.01 d | 1.15 ± 0.01 d | 0.18 ± 0.02 d | 0.23 ± 0.02 d | 3.72 ± 0.03 d | |
| 0.2 | 0.13 ± 0.01 e | 27.97 ± 0.02 e | 0.69 ± 0.01 de | 0.13 ± 0.01 de | 0.14 ± 0.01 e | 1.61 ± 0.01 e | |
| T. ornata | 0.025 | 0.57 ± 0.02 b | 122.10 ± 0.14 b | 5.17 ± 0.15 b | 0.22 ± 0.02 b | 0.32 ± 0.05 b | 8.07 ± 0.11 b |
| 0.05 | 0.31 ± 0.04 c | 83.35 ± 0.01 c | 3.93 ± 0.03 c | 0.18 ± 0.01 c | 0.21 ± 0.03 c | 6.74 ± 0.06 c | |
| 0.1 | 0.16 ± 0.01 d | 33.17 ± 0.02 d | 2.96 ± 0.02 d | 0.11 ± 0.03 d | 0.14 ± 0.01 d | 5.93 ± 0.02 d | |
| 0.2 | 0.06 ± 0.01 de | 20.81 ± 0.02 e | 2.84 ± 0.02 de | 0.06 ± 0.01 de | 0.10 ± 0.01 de | 3.15 ± 0.05 e | |
| Galic acid | 0.025 | 0.30 ± 0.01 b | 93.14 ± 0.02 b | 4.96 ± 0.02 b | 0.22 ± 0.01 b | 0.37 ± 0.02 b | 7.75 ± 0.05 b |
| 0.05 | 0.26 ± 0.01 c | 64.01 ± 0.01 c | 3.75 ± 0.01 c | 0.18 ± 0.03 c | 0.32 ± 0.01 bc | 6.26 ± 0.03 c | |
| 0.1 | 0.16 ± 0.01 d | 44.34 ± 0.04 d | 1.87 ± 0.03 d | 0.14 ± 0.05 cd | 0.28 ± 0.03 d | 4.65 ± 0.01 d | |
| 0.2 | 0.16 ± 0.01 de | 21.26 ± 0.02 e | 1.95 ± 0.00 de | 0.08 ± 0.01 e | 0.15 ± 0.01 e | 3.04 ± 0.05 e | |
| Tannic acid | 0.025 | 0.50 ± 0.02 b | 92.23 ± 0.05 b | 3.79 ± 0.09 b | 0.32 ± 0.05 b | 0.49 ± 0.01 b | 7.42 ± 0.30 b |
| 0.05 | 0.33 ± 0.03 c | 74.72 ± 0.03 c | 2.43 ± 0.10 c | 0.30 ± 0.02 c | 0.43 ± 0.04 c | 5.56 ± 0.02 c | |
| 0.1 | 0.20 ± 0.02 d | 51.37 ± 0.01 d | 1.95 ± 0.03 d | 0.21 ± 0.03 d | 0.38 ± 0.01 d | 3.87 ± 0.02 d | |
| 0.2 | 0.13 ± 0.01 e | 33.34 ± 0.01 e | 0.86 ± 0.01 e | 0.20 ± 0.05 e | 0.29 ± 0.02 e | 1.89 ± 0.02 e | |
| Vijayneem | 0.03 | 0.15 ± 0.01 c | 54.27 ± 0.07 b | 2.53 ± 0.03 bc | 0.13 ± 0.01 bc | 0.30 ± 0.01 b | 5.97 ± 0.02 b |
| Monocrotophos | 0.03 | 0.14 ± 0.02 bc | 43.97 ± 0.01 c | 2.58 ± 0.08 b | 0.15 ± 0.02 b | 0.17 ± 0.03 c | 4.56 ± 0.02 bc |
| Control | 0.00 | 0.66 ± 0.04 a | 135.24 ± 0.12 a | 8.19 ± 0.05 a | 0.47 ± 0.03 a | 0.47 ± 0.10 a | 9.25 ± 1.40 a |
| Seaweeds Name | Concentrations | Esterase | Lactate Dehydrogenase |
|---|---|---|---|
| S. wightii | 0.025 | 0.15 ± 0.02 a | 3.51 ± 0.03 a |
| 0.05 | 0.14 ± 0.01 ab | 2.44 ± 0.02 b | |
| 0.1 | 0.13 ± 0.03 c | 2.46 ± 0.02 bc | |
| 0.2 | 0.13 ± 0.02 cd | 1.28 ± 0.02 d | |
| S. polypodioides | 0.025 | 0.16 ± 0.02 a | 6.37 ± 0.07 a |
| 0.05 | 0.14 ± 0.01 ab | 5.66 ± 0.02 b | |
| 0.1 | 0.12 ± 0.01 c | 4.84 ± 0.01 c | |
| 0.2 | 0.11 ± 0.02 cd | 4.26 ± 0.05 cd | |
| T. ornata | 0.025 | 0.94 ± 0.21 a | 4.91 ± 0.01 a |
| 0.05 | 0.93 ± 0.11 ab | 4.44 ± 0.05 ab | |
| 0.1 | 0.83 ± 0.10 c | 4.28 ± 0.03 c | |
| 0.2 | 0.73 ± 0.14 d | 3.52 ± 0.05 d | |
| Galic acid | 0.025 | 0.15 ± 0.05 a | 2.50 ± 0.02 a |
| 0.05 | 0.12 ± 0.01 ab | 1.73 ± 0.04 b | |
| 0.1 | 0.12 ± 0.01 bc | 1.56 ± 0.01 c | |
| 0.2 | 0.11 ± 0.02 cd | 1.88 ± 0.01 cd | |
| Tannic acid | 0.025 | 0.15 ± 0.02 a | 4.64 ± 0.03 a |
| 0.05 | 0.14 ± 0.01 ab | 3.17 ± 0.05 b | |
| 0.1 | 0.12 ± 0.02 bc | 2.79 ± 0.04 c | |
| 0.2 | 0.11 ± 0.05 cd | 1.47 ± 0.02 d | |
| Vijayneem | 0.03 | 0.15 ± 0.03 bc | 2.15 ± 0.04 bc |
| Monocrotophos | 0.03 | 0.13 ± 0.01 b | 2.47 ± 0.03 b |
| Control | 0.00 | 0.98 ± 0.02 a | 5.62 ± 0.06 a |
| Marker | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Band/Line | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| Distance (pixels) | 27 | 74 | 113 | 206 | 309 | 385 | - | - | - |
| Rf | 0.070 | 0.191 | 0.292 | 0.532 | 0.798 | 0.995 | - | - | - |
| Area | 867 | 765 | 1173 | 1938 | 1734 | 1122 | - | - | - |
| Size | 113 | 81 | 46 | 33 | 26 | 17 | - | - | - |
| Raw volume | 147,415 | 133,002 | 195,130 | 308,315 | 268,945 | 186,601 | - | - | - |
| Band % | 11.89 | 10.73 | 15.74 | 24.88 | 21.7 | 15.06 | - | - | - |
| Control | |||||||||
| Distance (pixels) | 41 | 73 | 84 | 130 | 190 | 232 | 243 | 322 | 377 |
| Rf | 0.106 | 0.189 | 0.217 | 0.336 | 0.491 | 0.599 | 0.628 | 0.832 | 0.974 |
| Area | 1007 | 848 | 795 | 795 | 901 | 636 | 530 | 1484 | 1060 |
| Size | 87 | 79 | 77 | 66 | 52 | 42 | 39 | 21 | 8 |
| Raw volume | 183,762 | 164,300 | 144,290 | 140,081 | 156,148 | 120,430 | 98,952 | 242,149 | 206,103 |
| Band % | 12.62 | 11.28 | 9.91 | 9.62 | 10.72 | 8.27 | 6.8 | 16.63 | 14.15 |
| S. wightii tannin F1 (OT) | |||||||||
| Distance (pixels) | 67 | 89 | 128 | 188 | 228 | 241 | 336 | 376 | - |
| Rf | 0.173 | 0.23 | 0.331 | 0.486 | 0.589 | 0.623 | 0.868 | 0.972 | - |
| Area | 583 | 477 | 954 | 689 | 689 | 689 | 1484 | 1007 | - |
| Size | 81 | 75 | 66 | 52 | 43 | 40 | 17 | 8 | - |
| Raw volume | 105,928 | 84,023 | 161,879 | 114,650 | 119,240 | 117,036 | 232,630 | 191,000 | - |
| Band % | 9.4 | 7.46 | 14.37 | 10.18 | 10.59 | 10.39 | 20.65 | 16.96 | - |
| S. wightii tannin F1 (CT) | |||||||||
| Distance (pixels) | 41 | 75 | 88 | 130 | 190 | 232 | 242 | 330 | 374 |
| Rf | 0.106 | 0.194 | 0.227 | 0.336 | 0.491 | 0.599 | 0.625 | 0.853 | 0.966 |
| Area | 1311 | 1026 | 798 | 912 | 1026 | 570 | 684 | 1482 | 741 |
| Size | 87 | 79 | 76 | 66 | 52 | 42 | 39 | 19 | 9 |
| Raw volume | 238,608 | 197,107 | 145,182 | 160,235 | 175,741 | 107,944 | 123,505 | 237,000 | 150,656 |
| Band % | 15.53 | 12.83 | 9.45 | 10.43 | 11.44 | 7.03 | 8.04 | 15.43 | 9.81 |
| S. wightii tannin F2 (OT) | |||||||||
| Distance (pixels) | 43 | 73 | 92 | 129 | 192 | 232 | 244 | 377 | - |
| Rf | 0.111 | 0.189 | 0.238 | 0.333 | 0.496 | 0.599 | 0.63 | 0.974 | - |
| Area | 784 | 1120 | 896 | 1064 | 616 | 672 | 728 | 784 | - |
| Size | 86 | 79 | 75 | 66 | 51 | 42 | 39 | 8 | - |
| Raw volume | 142,491 | 204,140 | 157,857 | 180,277 | 108,466 | 123,369 | 129,120 | 155,170 | - |
| Band % | 11.87 | 17 | 13.15 | 15.01 | 9.03 | 10.27 | 10.75 | 12.92 | - |
| S. wightii tannin F2 (CT) | |||||||||
| Distance (pixels) | 75 | 88 | 131 | 186 | 232 | 245 | 329 | 377 | - |
| Rf | 0.194 | 0.227 | 0.339 | 0.481 | 0.599 | 0.633 | 0.85 | 0.974 | - |
| Area | 1218 | 638 | 1218 | 1044 | 1102 | 1160 | 928 | 1218 | - |
| Size | 79 | 76 | 65 | 53 | 42 | 39 | 19 | 8 | - |
| Raw volume | 230,090 | 118,225 | 213,365 | 181,762 | 203,929 | 204,665 | 155,950 | 233,939 | - |
| Band % | 14.92 | 7.67 | 13.84 | 11.79 | 13.23 | 13.27 | 10.11 | 15.17 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petchidurai, G.; Sahayaraj, K.; Al-Shuraym, L.A.; Albogami, B.Z.; Sayed, S.M. Insecticidal Activity of Tannins from Selected Brown Macroalgae against the Cotton Leafhopper Amrasca devastans. Plants 2023, 12, 3188. https://doi.org/10.3390/plants12183188
Petchidurai G, Sahayaraj K, Al-Shuraym LA, Albogami BZ, Sayed SM. Insecticidal Activity of Tannins from Selected Brown Macroalgae against the Cotton Leafhopper Amrasca devastans. Plants. 2023; 12(18):3188. https://doi.org/10.3390/plants12183188
Chicago/Turabian StylePetchidurai, Ganeshan, Kitherian Sahayaraj, Laila A. Al-Shuraym, Bader Z. Albogami, and Samy M. Sayed. 2023. "Insecticidal Activity of Tannins from Selected Brown Macroalgae against the Cotton Leafhopper Amrasca devastans" Plants 12, no. 18: 3188. https://doi.org/10.3390/plants12183188
APA StylePetchidurai, G., Sahayaraj, K., Al-Shuraym, L. A., Albogami, B. Z., & Sayed, S. M. (2023). Insecticidal Activity of Tannins from Selected Brown Macroalgae against the Cotton Leafhopper Amrasca devastans. Plants, 12(18), 3188. https://doi.org/10.3390/plants12183188








