Laser-Induced Fluorescence for Monitoring Environmental Contamination and Stress in the Moss Thuidium plicatile
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Procedure
2.2. LIF Imaging Using the CoCoBi
2.3. Data Analysis
2.3.1. Single-Color Comparison
2.3.2. Multi-Color Comparison
3. Results
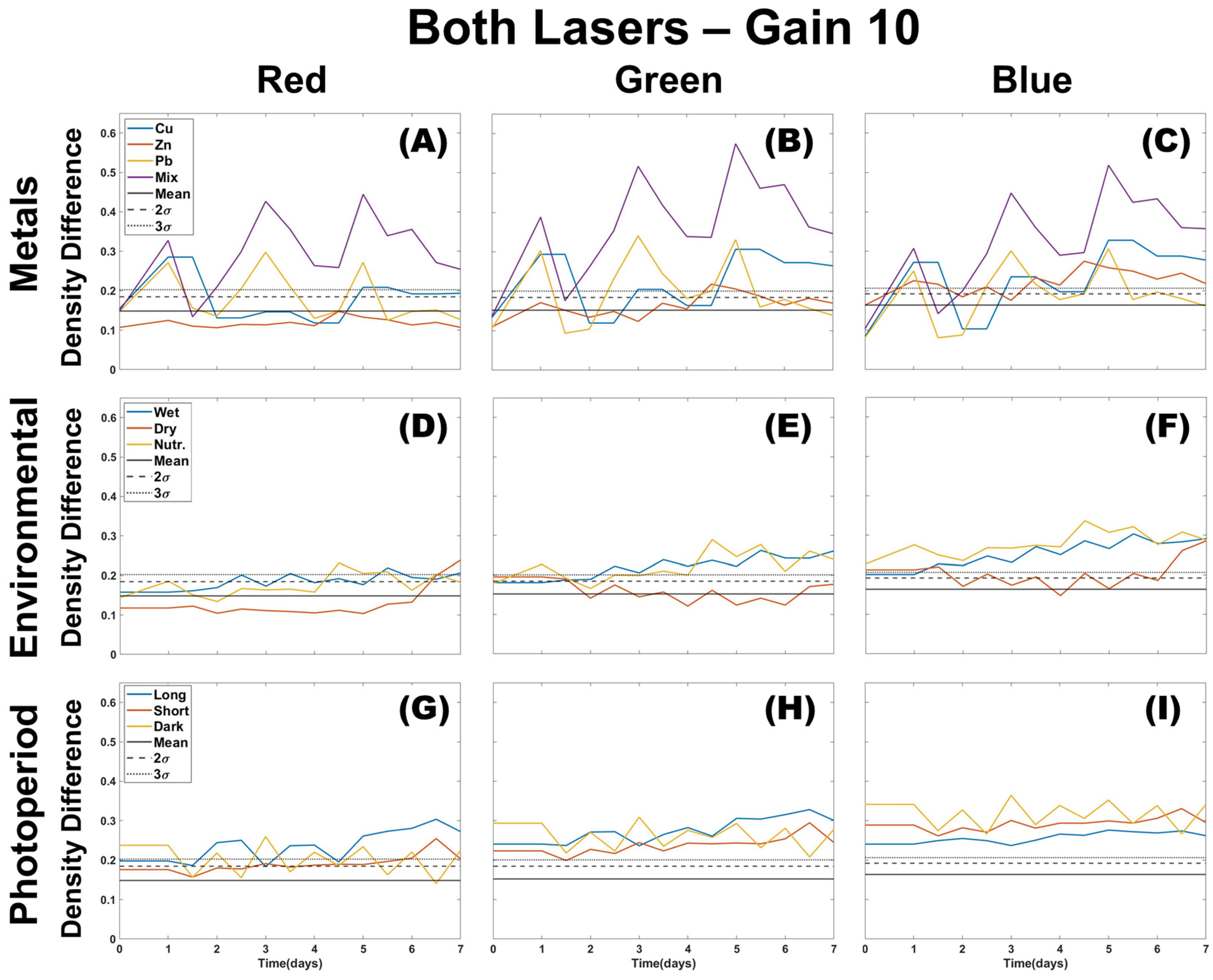
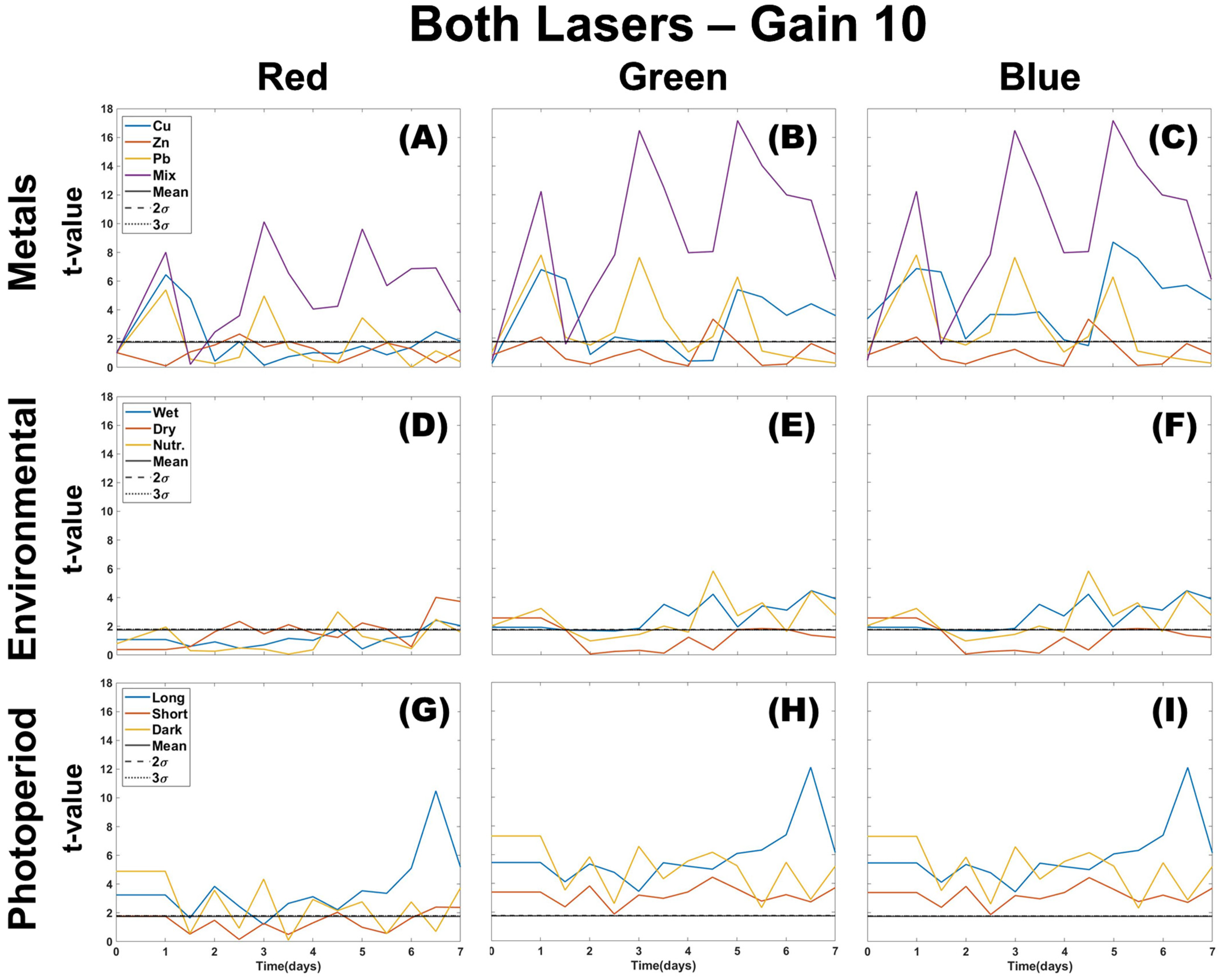
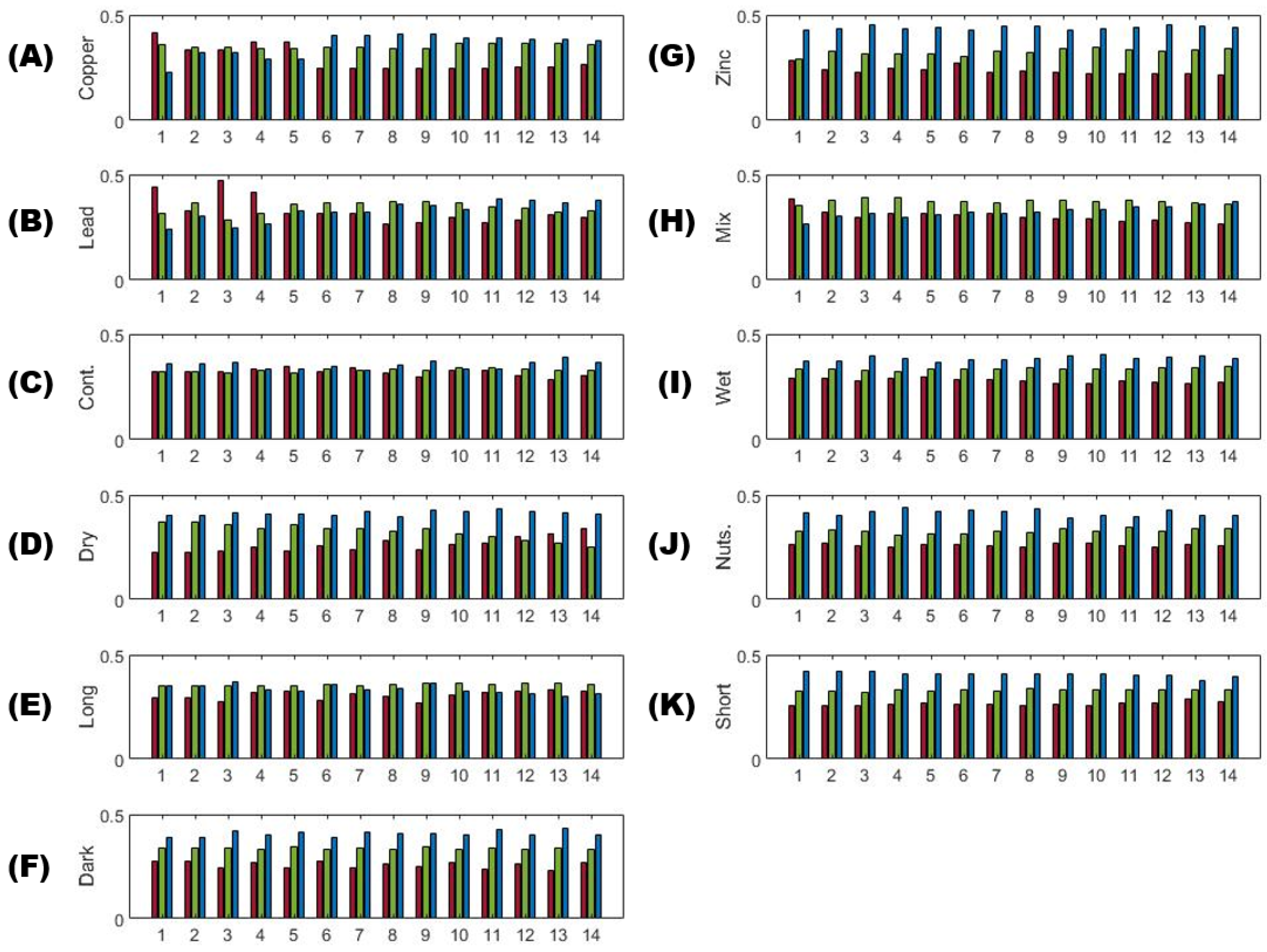
3.1. Single Color Analysis Using Density Difference
3.2. Two Color Analysis Using DTW
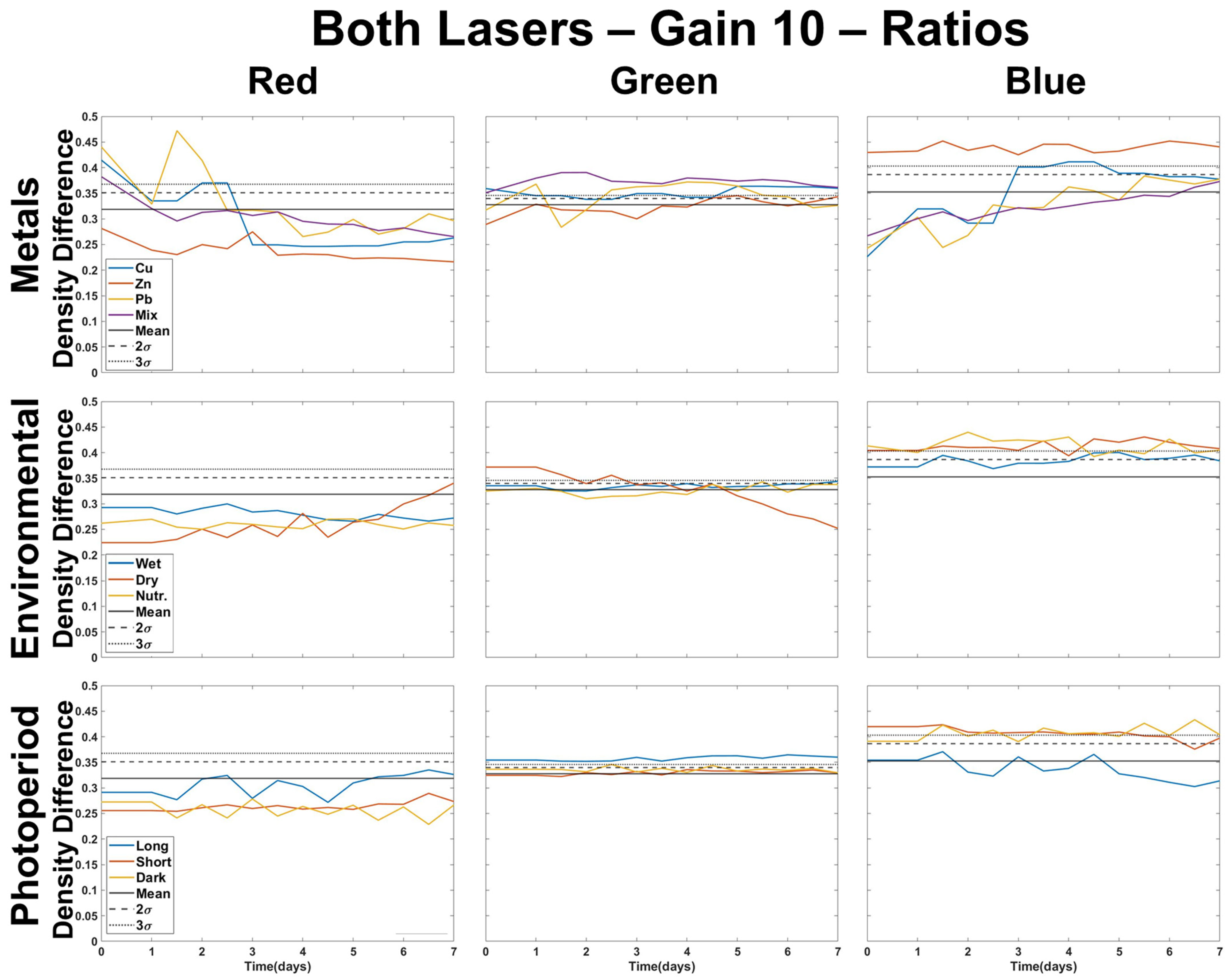
3.3. Multi-Color Ratios as a Means of Stressor Determination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix B
| Miracle-Gro AeroGarden Liquid Plant Food 4-3-6 |
| Total Nitrogen ……………………………. 4% 1% Ammoniacal Nitrogen 3% Nitrate Nitrogen Available Phosphate (P2O5) ……………. 3% Soluble Potash (K2O) …….…………….. 6% Calcium (Ca) ……………………………. 1% Magnesium (Mg) ……………………….. 0.5% 0.5% Water-Soluble Magnesium |
| Derived from: Potassium Nitrate, Calcium Nitrate, Mono Potassium Phosphate, Ammonium Nitrate, Magnesium Sulfate |
References
- Stanković, J.D.; Sabovljević, A.D.; Sabovljević, M. Bryophytes and heavy metals: A review. Acta Bot. Croat. 2018, 77, 109–118. [Google Scholar] [CrossRef]
- Norgate, T.E.; Jahanshahi, S.; Rankin, W.J. Assessing the environmental impact of metal production processes. J. Clean. Prod. 2007, 15, 838–848. [Google Scholar] [CrossRef]
- Berg, T.; Røyset, O.; Steinnes, E.; Vadset, M. Atmospheric trace element deposition: Principal component analysis of ICP-MS data from moss samples. Environ. Pollut. 1995, 88, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Wolterbeek, B. Biomonitoring of trace element air pollution: Principles, possibilities and perspectives. Environ. Pollut. 2002, 120, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Tremper, A.H.; Agneta, M.; Burton, S.; Higgs, D.E. Field and laboratory exposures of two moss species to low level metal pollution. J. Atmos. Chem. 2004, 49, 111–120. [Google Scholar] [CrossRef]
- Berg, T.; Steinnes, E. Use of mosses (Hylocomium splendens and Pleuroziumschreberi) as biomonitors of heavy metal deposition: From relative to absolute deposition values. Environ. Pollut. 1997, 98, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Degola, F.; De Benedictis, M.; Petraglia, A.; Massimi, A.; Fattorini, L.; Sorbo, S.; Basile, A.; di Toppi, L.S. A Cd/Fe/Zn responsive phytochelatin synthase is constitutively present in the ancient liverwort Luruciatenularia cruciata (L.) Dumort. Plant Cell Physiol. 2014, 55, 1884–1891. [Google Scholar] [CrossRef]
- Sun, S.Q.; He, M.; Cao, T.; Zhang, Y.C.; Han, W. Response mechanisms of antioxidants in bryophyte (Hypnum plumaeforme) under the stress of single or combined Pb and/or Ni. Environ. Monit. Assess. 2009, 149, 291–302. [Google Scholar] [CrossRef]
- Suchara, I.; Sucharova, J.; Hola, M.; Reimann, C.; Boyd, R.; Filzmoser, P.; Englmaier, P. The performance of moss, grass, and 1- and 2-year old spruce needles as bioindicators of contamination: A comparative study at the scale of the Czech Republic. Sci. Total Environ. 2011, 409, 2281–2297. [Google Scholar] [CrossRef]
- Serbula, M.S.; Miljkovic, D.D.; Kovacevic, M.R.; Ilic, A.A. Assessment of airborne heavy metal pollution using plant parts and topsoil. Ecotoxicol. Environ. Saf. 2012, 76, 209–214. [Google Scholar] [CrossRef]
- Chakrabortty, S.; Paratkar, G.T. Biomonitoring of trace element air pollution using mosses. Aerosol Air Qual. Res. 2006, 6, 247–258. [Google Scholar] [CrossRef]
- Vázquez, M.D.; Lopez, J.; Carballeira, A. Uptake of heavy metals to the extracellular and intracellular compartments in three species of aquatic bryophyte. Ecotoxicol. Environ. Saf. 1999, 44, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Panda, S.K. Toxic effects, oxidative stress and ultrastructural changes in moss Taxithelium nepalense (Schwaegr.) both under chromium and lead phytotoxicity. Water Air Soil Pollut. 2005, 167, 73–90. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Shakya, K.; Chettri, M.K.; Sawidis, T. Impact of heavy metals (copper, zinc, and lead) on the chlorophyll content of some mosses. Arch. Environ. Contam. Toxicol. 2008, 54, 412–421. [Google Scholar] [CrossRef]
- Vernon, L.P. Spectrophotometric determination of chlorophylls and pheophytins in plant extracts. Anal. Chem. 1960, 32, 1144–1150. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Mantoura, R.F.C.; Wright, S.W. Phytoplankton Pigments in Oceanography: Guidelines to Modern Methods; UNESCO Pub.: Paris, France, 1997; ISBN 9231032755. [Google Scholar]
- Han, S.G.; Kang, S.B.; Moon, Y.I.; Park, J.H.; Park, K.J.; Choi, Y.H. Establishment of analytical method for chlorophyll using the N,N-dimethylformamide and dimethylsulfoxide in citrus leaves. Korean J. Environ. Agric. 2014, 33, 344–349. [Google Scholar] [CrossRef][Green Version]
- Sun, H.; Liu, S.; Chen, K.; Li, G. Spectrophotometric determination of chlorophylls in different solvents related to the leaf traits of the main tree species in Northeast China. IOP Conference Series, Bristol. Earth Environ. Sci. 2021, 836, 012008. [Google Scholar] [CrossRef]
- Schreiber, U. Pulse-Amplitude-Modulation (PAM) fluorometry and saturation pulse method: An overview. In Advances in Photosynthesis and Respiration, 19; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004. [Google Scholar] [CrossRef]
- Brooks, M.D.; Niyogi, K.K. Use of a pulse-amplitude modulated chlorophyll fluorometer to study the efficiency of photosynthesis in Arabidopsis plants. Methods Mol. Biol. 2011, 775, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Haidekker, M.A.; Dong, K.; Mattos, E.; van Iersel, M.W. A very low-cost pulse-amplitude modulated chlorophyll fluorometer. Comput. Electron. Agric. 2022, 203, 107438. [Google Scholar] [CrossRef]
- Truax, K.; Dulai, H.; Misra, A.; Kuhne, W.; Fulkey, P. Quantifying moss response to metal contaminant exposure using laser induced fluorescence. Appl. Sci. 2022, 12, 11580. [Google Scholar] [CrossRef]
- Al-Radady, A.S.; Davies, B.E.; French, M.J. A new design of moss bag to monitor metal deposition both indoors and outdoors. Sci. Total Environ. 1993, 133, 275–283. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Hák, R.; Rinderle, U. The chlorophyll fluorescence ratio F690/F730 in leaves of different chlorophyll contents. Photosynth. Res. 1990, 25, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Subhash, N.; Mohanan, C.N. Curve fit analysis of chlorophyll fluorescence spectra: Application to nutrient stress detection in sunflower. Remote Sens. Environ. 1997, 60, 347–356. [Google Scholar] [CrossRef]
- Yang-Er, C.; Zhong-Wei, Z.; Ming, Y.; Shu, Y. Perspective of monitoring heavy metals by moss visible chlorophyll fluorescence parameters. Front. Plant Sci. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- McMurtrey, J.E.; Chappelle, E.W.; Kim, M.S.; Meisinger, J.J.; Corp, L.A. Distinguishing nitrogen fertilization levels in field corn (Zea mays L.) with actively induced fluorescence and passive reflectance measurements. Remote Sens. Environ. 1994, 47, 36–44. [Google Scholar] [CrossRef]
- Lavrov, A.; Utkin, A.B.; Marques da Silva, J.; Vilar, R.; Santos, N.M.; Alves, B. Water stress assessment of cork oak leaves and maritime pine needles based on LIF spectra. Opt. Spectrosc. 2012, 112, 271–279. [Google Scholar] [CrossRef]
- Gameiro, C.; Utkin, A.B.; Cartaxana, P.; Marques da Silva, J.; Matos, A.R. The use of laser induced chlorophyll fluorescence (LIF) as a fast and non-destructive method to investigate water deficit in Arabidopsis. Agric. Water Manag. 2016, 164, 127–136. [Google Scholar] [CrossRef]
- Lefsrud, M.G.; Kopsell, D.A.; Augé, R.M.; Both, A.J. Biomass production and pigment accumulation in kale grown under increasing photoperiods. HortScience 2006, 41, 603–606. [Google Scholar] [CrossRef]
- Brach, E.J.; Molnar, J.M.; Jasmin, J.J. Detection of lettuce maturity and variety by remote sensing techniques. J. Agric. Eng. Res. 1977, 22, 45–54. [Google Scholar] [CrossRef]
- Buschmann, C. Variability and application of the chlorophyll fluorescence emission ratio red/far red of leaves. Photosynth. Res. 2007, 92, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Kinsey, J.L. Laser-Induced Fluorescence. Annu. Rev. Phys. Chem. 1977, 28, 349–372. [Google Scholar] [CrossRef]
- Maarek, J.I.; Kim, S. Multispectral excitation of time-resolved fluorescence of biological compounds: Variation of fluorescence lifetime with excitation and emission wavelengths. In Proceedings SPIE 4252, Advances in Fluorescence Sensing Technology V; SPIE: Bellingham, WA, USA, 2001. [Google Scholar] [CrossRef]
- Manzar Abbas, M.; Melesse, A.M.; Scinto, L.J.; Rehage, J.S. Satellite estimation of chlorophyll-a using Moderate Resolution Imaging Spectroradiometer (MODIS) sensor in shallow coastal water bodies: Validation and improvement. Water 2019, 11, 1621. [Google Scholar] [CrossRef]
- Papenfus, M.; Schaeffer, B.; Pollard, A.I.; Loftin, K. Exploring the potential value of satellite remote sensing to monitor chlorophyll-a for US lakes and reservoirs. Environ. Monit. Assess. 2020, 192, 808. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, F.; Galvez-Sola, L.; Martínez-Nicolás, J.J.; Muelas-Domingo, R.; Nieves, M. Using Near-Infrared Spectroscopy in Agricultural Systems; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Marques da Silva, J.; Borissovitch Utkin, A. Application of Laser-Induced Fluorescence in functional studies of photosynthetic biofilms. Processes 2018, 6, 227. [Google Scholar] [CrossRef]
- Tan, J.Y.; Ker, P.J.; Lau, K.Y.; Hannan, M.A.; Tang, S.G.H. Applications of Photonics in Agriculture Sector: A Review. Molecules 2019, 24, 2025. [Google Scholar] [CrossRef]
- Truax, K.; Dulai, H.; Misra, A.; Kuhne, W.; Fulkey, P. Quantifying Moss Response to Contaminant Exposure using Laser Induced Fluorescence. Master’s Thesis, University of Hawaii at Manoa, Honolulu, HI, USA, 2020. Available online: http://hdl.handle.net/10125/73329 (accessed on 25 June 2023).
- Misra, A.K.; Acosta-Maeda, T.E.; Porter, J.N.; Egan, M.J.; Sandford, M.; Gasda, P.J.; Sharma, S.K.; Lucey, P.; Garmire, D.; Zhou, J.; et al. Standoff Biofinder: Powerful search for life instrument for planetary exploration. In Proceedings of the Lidar Remote Sensing for Environmental Monitoring XVI, Honolulu, HI, USA, 24–25 September 2018. [Google Scholar] [CrossRef]
- Misra, A.K.; Acosta-Maeda, T.E.; Zhou, J.; Egan, M.J.; Dasilveira, L.; Porter, J.N.; Rowley, S.J.; Trimble, A.Z.; Boll, P.; Sandford, M.W.; et al. Compact Color Biofinder (CoCoBi): Fast, standoff, sensitive detection of biomolecules and polyaromatic hydrocarbons for the detection of life. Appl. Spectrosc. 2021, 75, 1427–1436. [Google Scholar] [CrossRef]
- Nriagu, O.A. A history of global metal pollution. Science 1996, 272, 223. [Google Scholar] [CrossRef]
- WHO. Health Risks of Heavy Metals from Long-Range Transboundary Air Pollution; World Health Organization 2007; WHO Regional Office for Europe Copenhagen: Copenhagen, Denmark, 2007; ISBN 978-92-890-7179-6. [Google Scholar]
- Wong CS, C.; Li, X.; Thornton, I. Urban environmental geochemistry of trace metals: A review. Environ. Pollut. 2006, 142, 1–16. [Google Scholar] [CrossRef]
- Staples, G.W.; Imada, C.T.; Hoe, W.J.; Smith, C.W. A revised checklist of Hawaiian mosses. Trop. Bryol. 2004, 25, 35–69. [Google Scholar] [CrossRef]
- Beckett, R.P.; Brown, D.H. The control of cadmium uptake in the lichen genus Peltigera. J. Exp. Bot. 1984, 35, 1071–1082. [Google Scholar] [CrossRef]
- Jekel, C.F.; Venter, G.; Venter, M.P.; Stander, N.; Haftka, R.T. Similarity measures for identifying material parameters from hysteresis loops using inverse analysis. Int. J. Mater. Form. 2018, 12, 355–378. [Google Scholar] [CrossRef]
- Lu, Z.; Yuan, K. Welch’s t Test; SAGE: Thousand Oaks, CA, USA, 2010. [Google Scholar] [CrossRef]
- Bardi, U. Extracting minerals from seawater: An energy analysis. Sustainability 2010, 2, 980–992. [Google Scholar] [CrossRef]
- Reinfelder, J.R.; Totten, L.A.; Eisenreich, S.J.; Aucott, M. Research Project Summary. New Jersey Atmospheric Deposition Network (April, 2005). 2005. Available online: https://www.nj.gov/dep/dsr/nutrients/NJ%20Atmospheric%20Deposition%20Network_RPS.pdf (accessed on 20 June 2023).
- Willoughby, T.C. Quality of Wet Deposition in the Grand Calumet River Watershed, Northwestern Indiana, 30 June 1992–August 31, 1993: U.S. Geological Survey Water-Resources Investigations Report 95-4172, 55. 1995. Available online: https://pubs.usgs.gov/wri/1995/4172/report.pdf (accessed on 20 June 2023).
- Colman, J.A.; Rice, K.C.; Willoughby, T.C. Methodology and Significance of Studies of Atmospheric Deposition in Highway Runoff. Open-File Report 01-259; US Geological Survey: Northborough, MA, USA, 2001. Available online: https://pubs.usgs.gov/of/2001/ofr01-259/pdf/ofr01259.pdf (accessed on 20 June 2023).
- Vermette, S.J.; Peden, M.E.; Willoughby, T.C.; Lindberg, S.E.; Weiss, A.D. Methodology for the sampling of metals in precipitation: Results of the national atmospheric deposition pilot network. Atmos. Environ. 1995, 29, 1221–1229. [Google Scholar] [CrossRef]
- Pan, Y.P.; Wang, Y.S. Atmospheric wet and dry deposition of trace elements at 10 sites in Northern China. Atmos. Chem. Phys. 2015, 15, 951–972. [Google Scholar] [CrossRef]
- Paode, R.D.; Sofuoglu, S.C.; Sivadechathep, J.; Noll, K.E.; Holsen, T.M.; Keeler, G.J. Dry deposition fluxes and mass size distributions of Pb, Cu, and Zn measured in Southern Lake Michigan during AEOLUS. Environ. Sci. Technol. 1998, 32, 1629–1635. [Google Scholar] [CrossRef]
- Swoczyna, T.; Kalaji, H.M.; Bussotti, F.; Mojski, J.; Pollastrini, M. Environmental stress-what can we learn from chlorophyll a fluorescence analysis in woody plants? A review. Front. Plant Sci. 2022, 13, 1048582. [Google Scholar] [CrossRef]
- Rastogi, A.; Antala, M.; Gąbka, M.; Rosadziński, S.; Stróżecki, M.; Brestic, M.; Juszczak, R. Impact of warming and reduced precipitation on morphology and chlorophyll concentration in peat mosses (Sphagnum angustifolium and S. fallax). Sci. Rep. 2020, 10, 8592. [Google Scholar] [CrossRef]
- Świsłowski, P.; Nowak, A.; Rajfur, M. Is your moss alive during active biomonitoring study? Plants 2021, 10, 2389. [Google Scholar] [CrossRef]
- Malenovský, Z.; Turnbull, J.D.; Lucieer, A.; Robinson, S.A. Antarctic moss stress assessment based on chlorophyll content and leaf density retrieved from imaging spectroscopy data. New Phytol. 2015, 208, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Świsłowski, P.; Nowak, A.; Rajfur, M. The influence of environmental conditions on the lifespan of mosses under long-term active biomonitoring. Atmos. Pollut. Res. 2021, 12, 101203. [Google Scholar] [CrossRef]
- Segura, A.; de Wit, P.; Preston, G.M. Life of microbes that interact with plants. Microb. Biotechnol. 2009, 2, 412–415. [Google Scholar] [CrossRef]
- Chaudhry, V.; Runge, P.; Sengupta, P.; Doehlemann, G.; Parker, J.E.; Kemen, E. Shaping the leaf microbiota: Plant–microbe–microbe interactions. J. Exp. Bot. 2021, 72, 36–56. [Google Scholar] [CrossRef] [PubMed]
- Crum, H.; Mueller-Dombois, D. Two new mosses from Hawaii. J. Hattori Bot. Lab. 1968, 31, 293–296. [Google Scholar]
- Hoe, W.J. Annotated checklist of Hawaiian mosses. Lyonia 1974, 1, 1–45. [Google Scholar]
- Touw, A. A taxonomic revision of the Thuidiaceae (Musci) of tropical Asia, the western Pacific, and Hawaii. J. Hattori Bot. Lab. 2001, 91, 1–136. [Google Scholar] [CrossRef]
- World Flora Online. Thuidium Schimp. June 2023. Available online: https://wfoplantlist.org/plant-list/taxon/wfo-4000038297-2023-06?page=1 (accessed on 1 July 2023).



| Group | Tray | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 |
|---|---|---|---|---|---|---|---|---|
| Control | DI | DI | DI | DI | DI | DI | DI | |
| Metals | Copper | DI | 1 nmol/cm2 | DI | 10 nmol/cm2 | DI | 100 nmol/cm2 | DI |
| Zinc | DI | 1 nmol/cm2 | DI | 10 nmol/cm2 | DI | 100 nmol/cm2 | DI | |
| Lead | DI | 1 nmol/cm2 | DI | 10 nmol/cm2 | DI | 100 nmol/cm2 | DI | |
| Mix | DI | 1 nmol Cu/cm2 1 nmol Zn/cm2 1 nmol Pb/cm2 | DI | 10 nmol Cu/cm2 10 nmol Zn/cm2 10 nmol Pb/cm2 | DI | 100 nmol Cu/cm2 100 nmol Zn/cm2 100 nmol Pb/cm2 | DI | |
| Environmental | Nutrients | 4-3-6 3 mL diluted with 27 mL DI * | DI | DI | DI | DI | DI | DI |
| Drought | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Flood | 2xDI | 2xDI | 2xDI | 2xDI | 2x DI | 2xDI | 2xDI | |
| Photoperiod | Long | DI 14 h | DI 14 h | DI 14 h | DI 14 h | DI 14 h | DI 14 h | DI 14 h |
| Short | DI 6 h | DI 6 h | DI 6 h | DI 6 h | DI 6 h | DI 6 h | DI 6 h | |
| Dark | DI N/A | DI N/A | DI N/A | DI N/A | DI N/A | DI N/A | DI N/A | |
| Cu | Zn | Pb | |
|---|---|---|---|
| Soil | 50–140 mg/kg | 10–300 mg/kg | 5–30 mg/kg |
| Water | - | - | 10 lg/L 0.001–0.06 mg/L (uncontaminated) |
| Air | - | - | 0.5 lg/m3 4–20 mg/g (dust) |
| Lowest Level Tested | 0.34 mg/m2 | - | - |
| Highest Level Tested | 34 mg/m2 | - | - |
| Wet Deposition | ||
| Cu | Zn | Pb |
| 0.49–2.2 mg/m2/yr New Jersey [52] | 2.41 mg/m2/yr Gary, Indiana [53] | 1.06 mg/m2/yr Gary, Indiana [53] |
| 0.70 mg/m2/yr Reston, Virginia [54] | - | 2.20 mg/m2/yr Chicago, Illinois [55] |
| 1.06 mg/m2/yr Chicago, Illinois [54] | - | - |
| 0.8 ± 0.7 mg/m2/yr Urban China [56] | - | - |
| 4.7 mg/m2/yr Hong Kong, China [56] | - | - |
| 14.6 mg/m2/yr Singapore [56] | - | - |
| Dry Deposition | ||
| Cu | Zn | Pb |
| 3.65 mg/m2/yr Michigan [57] | - | 1.10 mg/m2/yr Michigan [57] |
| 21.9 mg/m2/yr Chicago, Illinois [57] | - | 25.55 mg/m2/yr Chicago, Illinois [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truax, K.; Dulai, H.; Misra, A.; Kuhne, W.; Fuleky, P.; Smith, C.; Garces, M. Laser-Induced Fluorescence for Monitoring Environmental Contamination and Stress in the Moss Thuidium plicatile. Plants 2023, 12, 3124. https://doi.org/10.3390/plants12173124
Truax K, Dulai H, Misra A, Kuhne W, Fuleky P, Smith C, Garces M. Laser-Induced Fluorescence for Monitoring Environmental Contamination and Stress in the Moss Thuidium plicatile. Plants. 2023; 12(17):3124. https://doi.org/10.3390/plants12173124
Chicago/Turabian StyleTruax, Kelly, Henrietta Dulai, Anupam Misra, Wendy Kuhne, Peter Fuleky, Celia Smith, and Milton Garces. 2023. "Laser-Induced Fluorescence for Monitoring Environmental Contamination and Stress in the Moss Thuidium plicatile" Plants 12, no. 17: 3124. https://doi.org/10.3390/plants12173124
APA StyleTruax, K., Dulai, H., Misra, A., Kuhne, W., Fuleky, P., Smith, C., & Garces, M. (2023). Laser-Induced Fluorescence for Monitoring Environmental Contamination and Stress in the Moss Thuidium plicatile. Plants, 12(17), 3124. https://doi.org/10.3390/plants12173124







