Inoculation with Azospirillum brasilense Strains AbV5 and AbV6 Increases Nutrition, Chlorophyll, and Leaf Yield of Hydroponic Lettuce
Abstract
:1. Introduction
2. Results
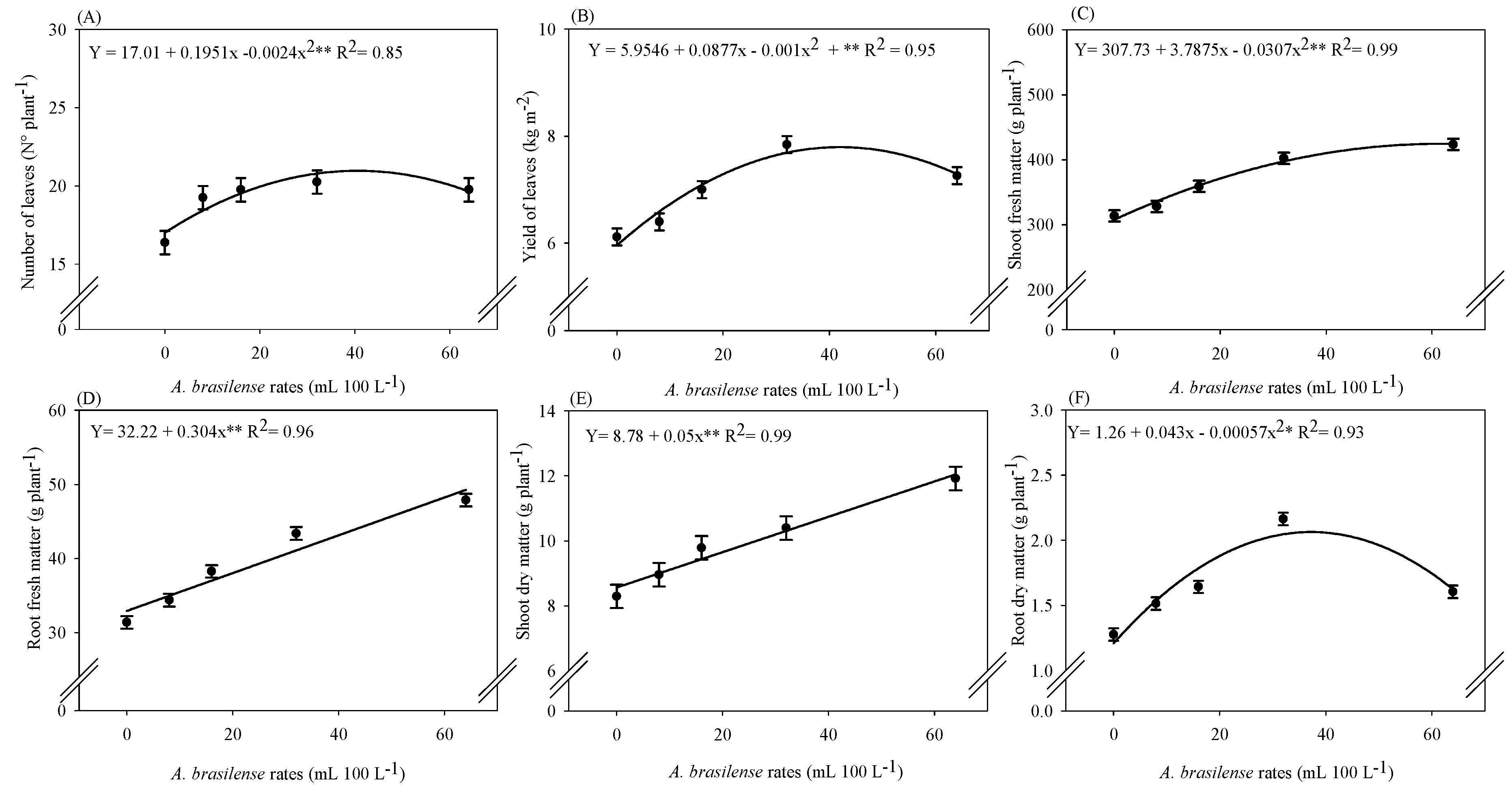
2.1. Productive Components
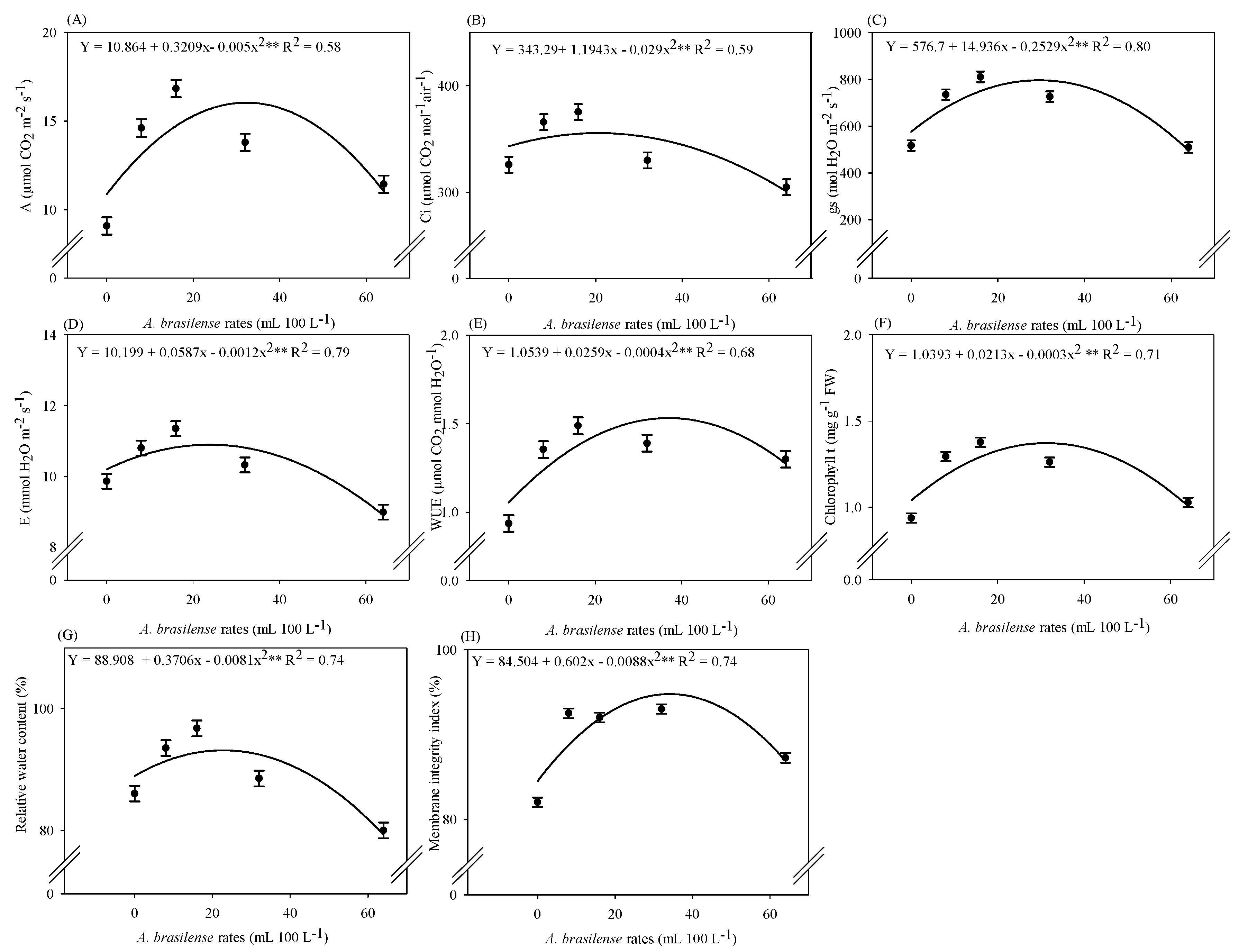
2.2. Inoculation with A. brasilense Improves Plant Physiology
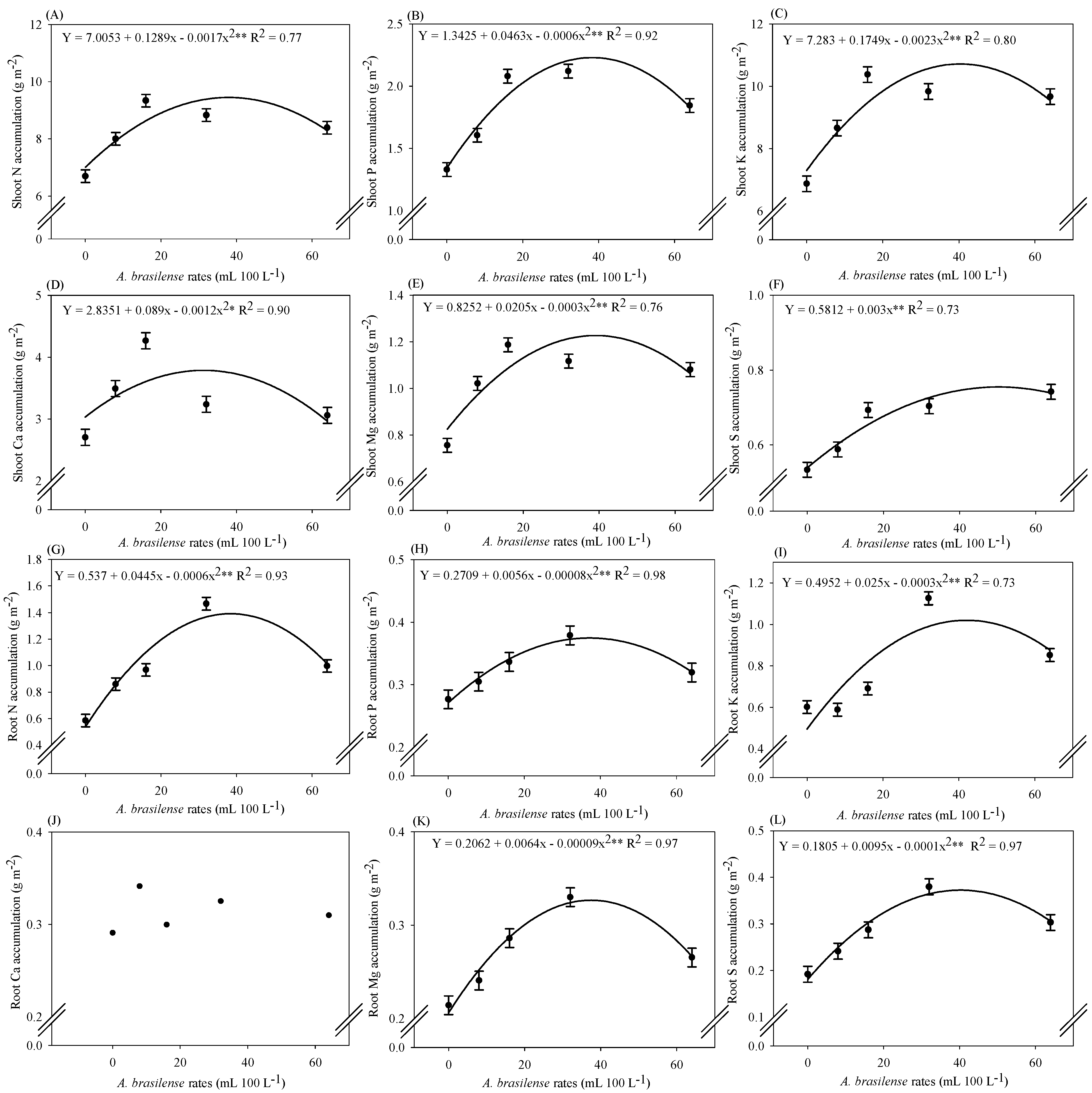
2.3. Macronutrient Accumulation in Lettuce Plants
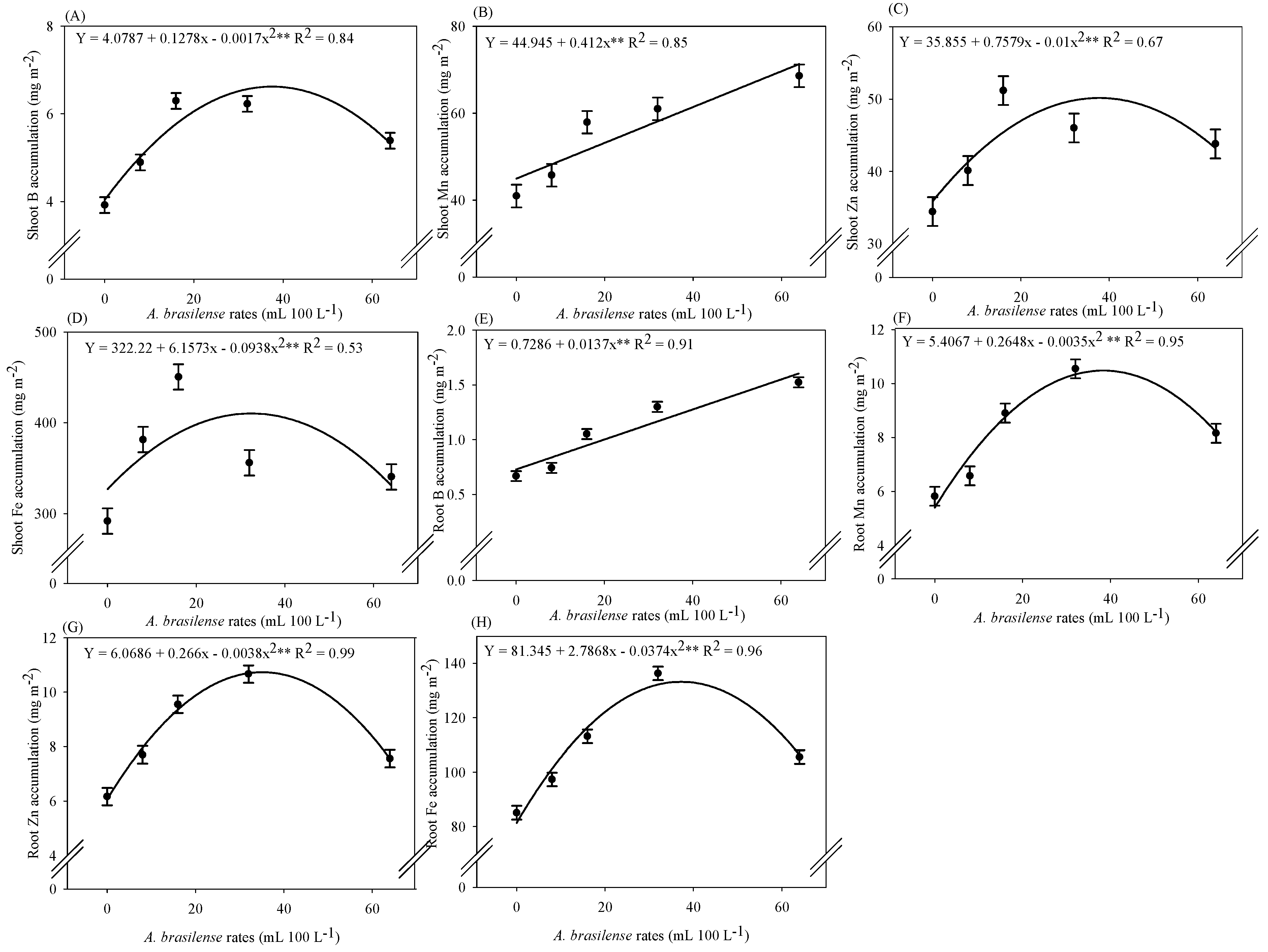
2.4. Micronutrient Accumulation in Lettuce Plants
3. Discussion
4. Materials and Methods
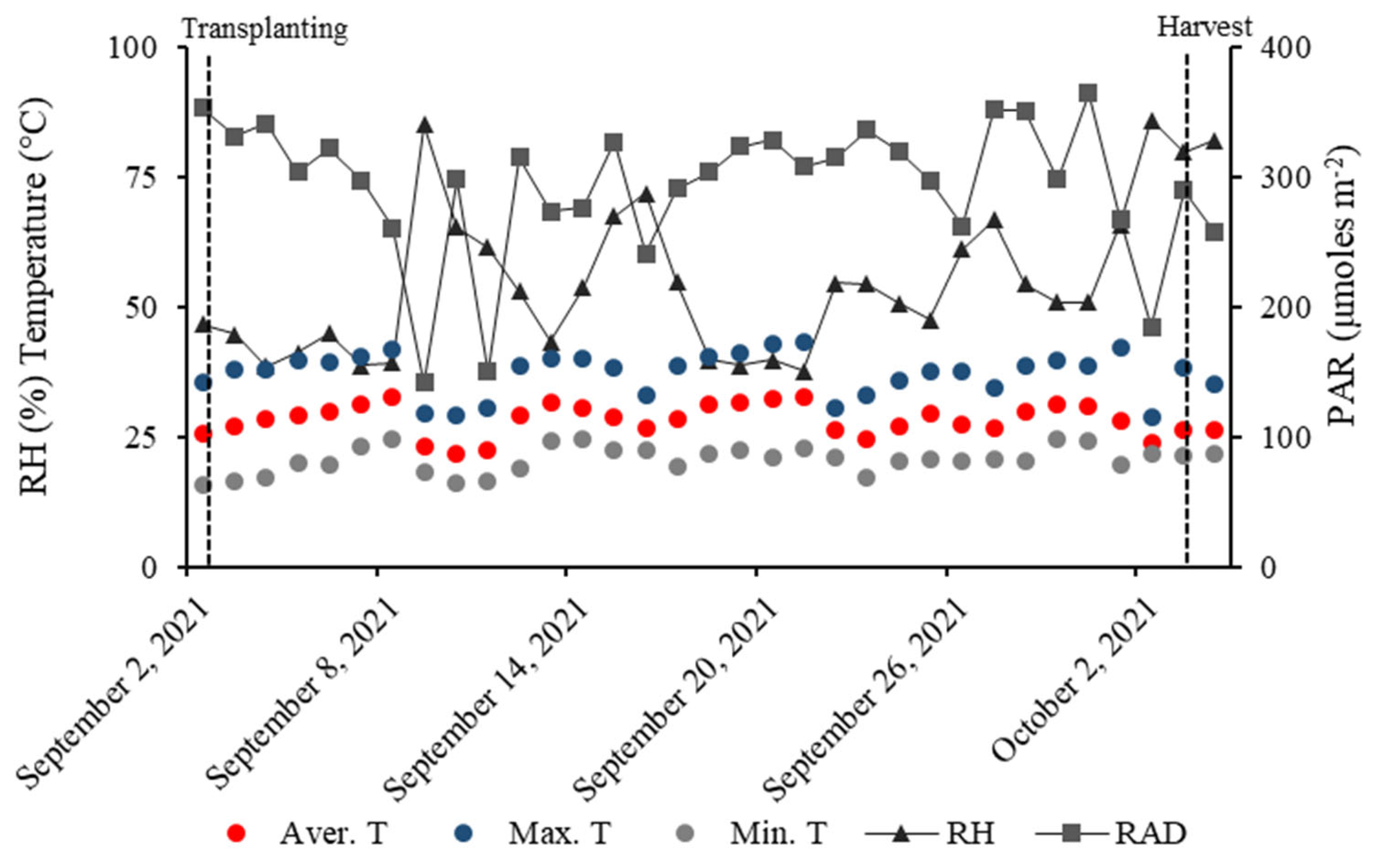
4.1. Environmental Characterization
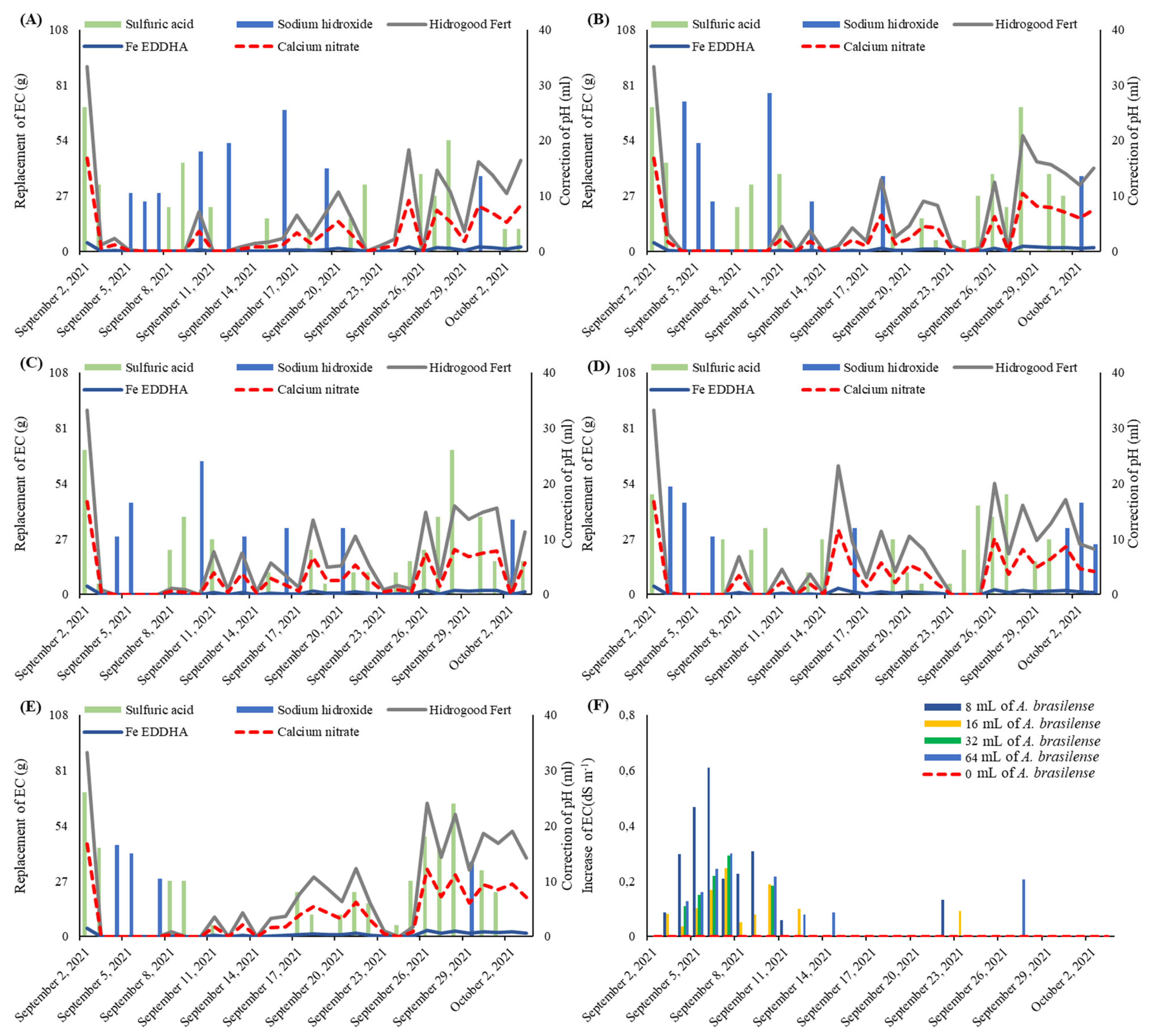
4.2. Experimental Delineation
4.3. Experimental Characterization
4.4. Biometric and Yield Evaluations
4.5. Nutritional Evaluations
4.6. Physiological Evaluations
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dalastra, C.; Teixeira Filho, M.C.M.; Silva, M.R.; Nogueira, T.A.R.; Fernandes, G.C. Head lettuce production and nutrition in relation to nutrient solution flow. Hortic. Bras. 2020, 38, 21–26. [Google Scholar] [CrossRef]
- FAOSTAT—Food and Agriculture Organization of the United Nations, Statistics Division. Forestry Production and Trade; FAO: Rome, Italy, 2022; Available online: http://www.fao.org/faostat/en/#data/FO (accessed on 4 September 2022).
- Artés, F.; Allende, A. Minimal fresh processing of vegetables, fruits and juices. In Emerging Technologies for Food Processing; Elsevier: London, UK, 2014; pp. 583–597. [Google Scholar]
- Wang, Q.Y.; Zhao, M.R.; Wang, J.Q.; Hu, B.Y.; Chen, Q.J.; Qin, Y.; Zhang, G.Q. Effects of microbial inoculants on agronomic characters, physicochemical properties and nutritional qualities of lettuce and celery in hydroponic cultivation. Sci. Hortic. 2023, 320, 112202. [Google Scholar] [CrossRef]
- Majid, M.; Khan, J.N.; Shah, Q.M.A.; Masoodi, K.Z.; Afroza, B.; Parvaze, S. Evaluation of hydroponic systems for the cultivation of lettuce (Lactuca sativa L. var. Longifolia) and comparison with protected soil-based cultivation. Agric. Water. Manag. 2021, 245, 106572. [Google Scholar] [CrossRef]
- Dalastra, C.; Teixeira Filho, M.C.M.; Vargas, P.F. Periodicity of exposure of hydroponic lettuce plants to nutrient solution. Rev. Caatinga 2020, 33, 81–89. [Google Scholar] [CrossRef]
- Fukami, J.; Nogueira, M.A.; Araujo, R.S.; Hungria, M. Accessing inoculation methods of maize and wheat with Azospirillum brasilense. AMB Express 2016, 6, 3. [Google Scholar] [CrossRef]
- Meza, B.; Bashan, L.E.; Bashan, Y. Involvement of indole-3-acetic acid produced by Azospirillum brasilense in accumulating intracellular ammonium in Chlorella vulgaris. Res. Microbiol. 2015, 166, 72–83. [Google Scholar] [CrossRef]
- Oliveira, C.E.S.; Jalal, A.; Oliveira, J.R.; Tamburi, K.V.; Teixeira Filho, M.C.M. Leaf inoculation of Azospirillum brasilense and Trichoderma harzianum in hydroponic arugula improve productive components and plant nutrition and reduce leaf nitrate. Pesqui. Agrop. Trop. 2022, 52, e72755. [Google Scholar] [CrossRef]
- Moreira, V.D.A.; Oliveira, C.E.S.; Jalal, A.; Gato, I.M.B.; Oliveira, T.J.S.S.; Boleta, G.H.M.; Giolo, V.M.; Vitória, L.S.; Tamburi, K.V.; Teixeira Filho, M.C.M. Inoculation with Trichoderma harzianum and Azospirillum brasilense increases nutrition and yield of hydroponic lettuce. Arch. Microbiol. 2022, 204, e440. [Google Scholar] [CrossRef]
- Aini, N.; Yamika, W.S.D.; Pahlevi, R.W. The effect of nutrient concentration and inoculation of PGPR and AMF on the yield and fruit quality of hydroponic cherry tomatoes (Lycopersicon esculentum Mill. var. cerasiforme). J. Appl. Hortic. 2019, 21, 116–122. [Google Scholar] [CrossRef]
- Sela Saldinger, S.; Rodov, V.; Kenigsbuch, D.; Bar-Tal, A. Hydroponic Agriculture and Microbial Safety of Vegetables: Promises, Challenges, and Solutions. Horticulturae 2023, 9, 51. [Google Scholar] [CrossRef]
- Oliveira, C.E.S.; Gato, I.M.B.; Moreira, V.A.; Jalal, A.; Oliveira, T.J.S.S.; Oliveira, J.R.; Fernandes, G.C.; Teixeira Filho, M.C.M. Inoculation methods of Azospirillum brasilense in lettuce and arugula in the hydroponic system. Rev. Bras. Eng. Agric. Amb. 2023, 27, 653–662. [Google Scholar] [CrossRef]
- Oliveira, T.J.S.S.; Oliveira, C.E.S.; Jalal, A.; Gato, I.M.B.; Rauf, K.; Moreira, V.A.; Lima, B.H.; Vitória, L.S.; Giolo, V.M.; Teixeira Filho, M.C.M. Inoculation reduces nitrate accumulation and increases growth and nutrient accumulation in hydroponic arugula. Sci. Hortic. 2023, 320, 112213. [Google Scholar] [CrossRef]
- Pereira, L.C.; Bertuzzi Pereira, C.; Correia, L.V.; Matera, T.C.; Santos, R.F.D.; Carvalho, C.D.; Osipi, E.A.F.; Braccini, A.L. Corn Responsiveness to Azospirillum: Accessing the Effect of Root Exudates on the Bacterial Growth and Its Ability to Fix Nitrogen. Plants 2020, 9, 923. [Google Scholar] [CrossRef] [PubMed]
- Kudoyarova, G.; Arkhipova, T.; Korshunova, T.; Bakaeva, M.; Loginov, O.; Dodd, I.C. Phytohormone Mediation of Interactions Between Plants and Non-Symbiotic Growth Promoting Bacteria Under Edaphic Stresses. Front. Plant Sci. 2019, 10, 1368. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.J.O.; Araujo, B.A.; Bezerra, M.A.; Silva, C.F.B.; Sousa, A.B.O.; Nogueira, C.M.R. Gas exchange and leaf area of banana plants under salt stress inoculated with growth-promoting bacteria. Rev. Bras. Eng. Agric. Amb. 2021, 25, 779–786. [Google Scholar] [CrossRef]
- Buckley, T.N. How do stomata respond to water status? New Phytol. 2019, 224, 21–36. [Google Scholar] [CrossRef]
- Thor, K. Calcium—Nutrient and Messenger. Front. Plant Sci. 2019, 10, 440. [Google Scholar] [CrossRef]
- Wimmer, M.A.; Abreu, I.; Bell, R.W.; Bienert, M.D.; Brown, P.H.; Dell, B.; Fujiwara, T.; Goldbach, H.E.; Lehto, T.; Mock, H.P.; et al. Boron: And essential element for vascular plants. New Phytol. 2020, 226, 1232–1237. [Google Scholar] [CrossRef]
- Lewis, D.H. Boron: The essential element for vascular plants that never was. New Phytol. 2019, 221, 1685–1690. [Google Scholar] [CrossRef]
- Fukami, J.; Cerezin, P.; Hungria, M. Azospirillum: Benefits that go far beyond biological nitrogen fixation. AMB Express 2018, 8, 73. [Google Scholar] [CrossRef]
- Besen, M.R.; Goes Neto, A.F.; Esper Neto, M.; Zampar, E.J.O.; Costa, E.J.O.; Cordioli, V.R.; Inoue, T.T.; Batista, M.A. Nitrogen fertilization and leaf spraying with Azospirillum brasilense in wheat: Effects on mineral nutrition and yield. Rev. Cienc. Agrovet. 2020, 19, 483–493. [Google Scholar] [CrossRef]
- Galindo, F.S.; Pagliari, P.H.; Buzetti, S.; Rodrigues, W.L.; Fernandes, G.C.; Biagini, A.L.C.; Marega, E.M.R.; Tavanti, R.F.R.; Jalal, A.; Teixeira Filho, M.C.M. Corn shoot and grain nutrient uptake affected by silicon application combined with Azospirillum brasilense inoculation and nitrogen rates. J. Plant Nutr. 2021, 45, 168–184. [Google Scholar] [CrossRef]
- Jalal, A.; Galindo, F.S.; Boleta, E.H.M.; Oliveira, C.E.D.S.; Reis, A.R.D.; Nogueira, T.A.R.; Moretti Neto, M.J.; Mortinho, E.S.; Fernandes, G.C.; Teixeira Filho, M.C.M. Common Bean Yield and Zinc Use Efficiency in Association with Diazotrophic Bacteria Co-Inoculations. Agronomy 2021, 11, 959. [Google Scholar] [CrossRef]
- Galindo, F.S.; Bellotte, J.L.M.; Santini, J.M.K.; Buzetti, S.; Rosa, P.A.L.; Jalal, A.; Teixeira Filho, M.C.M. Zinc use efficiency of maize-wheat cropping after inoculation with Azospirillum brasilense. Nutr. Cycl. Agroecosys. 2021, 120, 205–221. [Google Scholar] [CrossRef]
- Jalal, A.; Oliveira, C.E.S.; Freitas, L.A.; Galindo, F.S.; Lima, B.H.; Boleta, E.H.M.; Da Silva, E.C.; Do Nascimento, V.; Nogueira, T.A.; Buzetti, S.; et al. Agronomic biofortification and productivity of wheat with soil zinc and diazotrophic bacteria in tropical savannah. Crop Pasture Sci. 2022, 73, 817–830. [Google Scholar] [CrossRef]
- Scott, S.; Housh, A.; Powell, G.; Anstaett, A.; Gerheart, A.; Benoit, M.; Wilder, S.; Schueller, M.; Ferrieri, R. Crop Yield, Ferritin and Fe (II) boosted by Azospirillum brasilense (HM053) in Corn. Agronomy 2020, 10, 394. [Google Scholar] [CrossRef]
- Gato, I.M.B.; Oliveira, C.E.S.; Oliveira, T.J.S.S.; Jalal, A.; Moreira, V.A.; Giolo, V.M.; Vitoria, L.S.; Lima, B.H.; Vargas, P.F.; Teixeira Filho, M.C.M. Nutrition and yield of hydroponic arugula under inoculation of beneficial microorganisms. Hortic. Environ. Biotechnol. 2023, 63, 193–208. [Google Scholar] [CrossRef]
- Lade, S.B.; Román, C.; Cueto-Ginzo, A.I.; Serrano, L.; Sin, E.; Achón, M.A.; Medina, V. Host-specific proteomic and growth analysis of maize and tomato seedlings inoculated with Azospirillum brasilense Sp7. Plant Physiol. Biochem. 2018, 129, 381–393. [Google Scholar] [CrossRef]
- Singh, S.K.; Reddy, V.R. Potassium starvation limits soybean growth more than the photosynthetic processes across CO2 levels. Front. Plant Sci. 2017, 8, 991. [Google Scholar] [CrossRef]
- Jákli, B.; Tavakol, E.; Tränkner, M.; Senbayram, M.; Dittert, K. Quantitative limitations to photosynthesis in K deficient sunflower and their implications on water-use efficiency. J. Plant Physiol. 2017, 209, 20–30. [Google Scholar] [CrossRef]
- Tränkner, M.; Travakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef]
- Karpagam, T.; Shanmugapriya, A.; Suganya, B.; Firdous, J. Chapter 10—Advanced study of plant-microbe interactions in photosynthesis. In Plant-Microbe Interaction—Recent Advances in Molecular and Biochemical Approaches; Swapnil, P., Meena, M., Harish, M.A., Vijayalakshmi, S., Zehra, A., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 205–228. [Google Scholar] [CrossRef]
- Pankievicz, V.C.S.; Amaral, F.P.; Santos, K.F.D.N.; Agtuca, B.; Xu, Y.; Schueller, M.J.; Arisi, A.C.M.; Steffens, M.B.R.; Souza, E.M.; Pedrosa, F.O.; et al. Robust biological nitrogen fixation in a model grass-bacterial association. Plant J. 2015, 81, 907–919. [Google Scholar] [CrossRef]
- Hungria, M.; Ribeiro, R.A.; Nogueira, M.A. Draft genome sequences of Azospirillum brasilense strains Ab-V5 and Ab-V6, commercially used in inoculants for grasses and legumes in Brazil. Genome Announc. 2018, 6, e00393-18. [Google Scholar] [CrossRef]
- Pii, Y.; Aldrighetti, A.; Valentinuzzi, F.; Mimmo, T.; Cesco, S. Azospirillum brasilense inoculation counteracts the induction of nitrate uptake in maize plants. J. Exp. Bot. 2019, 70, 1313–1324. [Google Scholar] [CrossRef] [PubMed]
- Santos, K.F.D.N.; Moure, V.R.; Hauer, V.; Santos, A.R.S.; Donatti, L.; Galvao, C.W.; Pedrosa, F.O.; Souza, E.M.; Wassem, R.; Steffens, M.B.R. Wheat colonization by an Azospirillum brasilense ammonium-excreting strain reveals upregulation of nitrogenase and superior plant growth promotion. Plant Soil 2017, 415, 245–255. [Google Scholar] [CrossRef]
- Bashan, Y.; Bashan, L.E. How the Plant Growth-Promoting Bacterium Azospirillum Promotes Plant Growth—A Critical Assessment. In Advances in Agronomy; Sparks, D.L., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2010; pp. 78–122. [Google Scholar]
- Cruz-Hernández, M.A.; Mendoza-Herrera, A.; Bocanegra-García, V.; Rivera, G. Azospirillum spp. from Plant Growth-Promoting Bacteria to Their Use in Bioremediation. Microorganisms 2022, 10, 1057. [Google Scholar] [CrossRef]
- Biotrop. Technical Information on the Liquid Inoculant Azospirillum brasilense Strains AbV5 and AbV6 (Azotrop). Available online: https://biotrop.com/produto/azotrop/ (accessed on 10 July 2023).
- Sakata. Technical Information on the Cultivar of Iceberg Lettuce Angelina. Available online: https://www.sakata.com.br/hortalicas/folhosas (accessed on 20 March 2023).
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Assessment of the Nutritional Status of Plants: Principles and Applications, 2nd ed.; Brazilian Association for the Research of Potash and Phosphate: Piracicaba, Brazil, 1997; p. 319. [Google Scholar]
- Hiscox, J.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. Effects of salt stress on growth, mineral nutrition and proline accumulation in relation to osmotic adjustment in rice (Oryza sativa L.) cultivars differing in salinity resistance. J. Plant Growth Regul. 1996, 19, 207. [Google Scholar] [CrossRef]
- Systat Software SigmaPlot 15. Available online: http://www.sigmaplot.com (accessed on 10 July 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva Oliveira, C.E.; Jalal, A.; Vitória, L.S.; Giolo, V.M.; Oliveira, T.J.S.S.; Aguilar, J.V.; de Camargos, L.S.; Brambilla, M.R.; Fernandes, G.C.; Vargas, P.F.; et al. Inoculation with Azospirillum brasilense Strains AbV5 and AbV6 Increases Nutrition, Chlorophyll, and Leaf Yield of Hydroponic Lettuce. Plants 2023, 12, 3107. https://doi.org/10.3390/plants12173107
da Silva Oliveira CE, Jalal A, Vitória LS, Giolo VM, Oliveira TJSS, Aguilar JV, de Camargos LS, Brambilla MR, Fernandes GC, Vargas PF, et al. Inoculation with Azospirillum brasilense Strains AbV5 and AbV6 Increases Nutrition, Chlorophyll, and Leaf Yield of Hydroponic Lettuce. Plants. 2023; 12(17):3107. https://doi.org/10.3390/plants12173107
Chicago/Turabian Styleda Silva Oliveira, Carlos Eduardo, Arshad Jalal, Letícia Schenaide Vitória, Victoria Moraes Giolo, Thaissa Julyanne Soares Sena Oliveira, Jailson Vieira Aguilar, Liliane Santos de Camargos, Matheus Ribeiro Brambilla, Guilherme Carlos Fernandes, Pablo Forlan Vargas, and et al. 2023. "Inoculation with Azospirillum brasilense Strains AbV5 and AbV6 Increases Nutrition, Chlorophyll, and Leaf Yield of Hydroponic Lettuce" Plants 12, no. 17: 3107. https://doi.org/10.3390/plants12173107
APA Styleda Silva Oliveira, C. E., Jalal, A., Vitória, L. S., Giolo, V. M., Oliveira, T. J. S. S., Aguilar, J. V., de Camargos, L. S., Brambilla, M. R., Fernandes, G. C., Vargas, P. F., Zoz, T., & Filho, M. C. M. T. (2023). Inoculation with Azospirillum brasilense Strains AbV5 and AbV6 Increases Nutrition, Chlorophyll, and Leaf Yield of Hydroponic Lettuce. Plants, 12(17), 3107. https://doi.org/10.3390/plants12173107









