Comparative Transcriptome Analysis of Defense Response of Potato to Phthorimaea operculella Infestation
Abstract
1. Introduction
2. Results
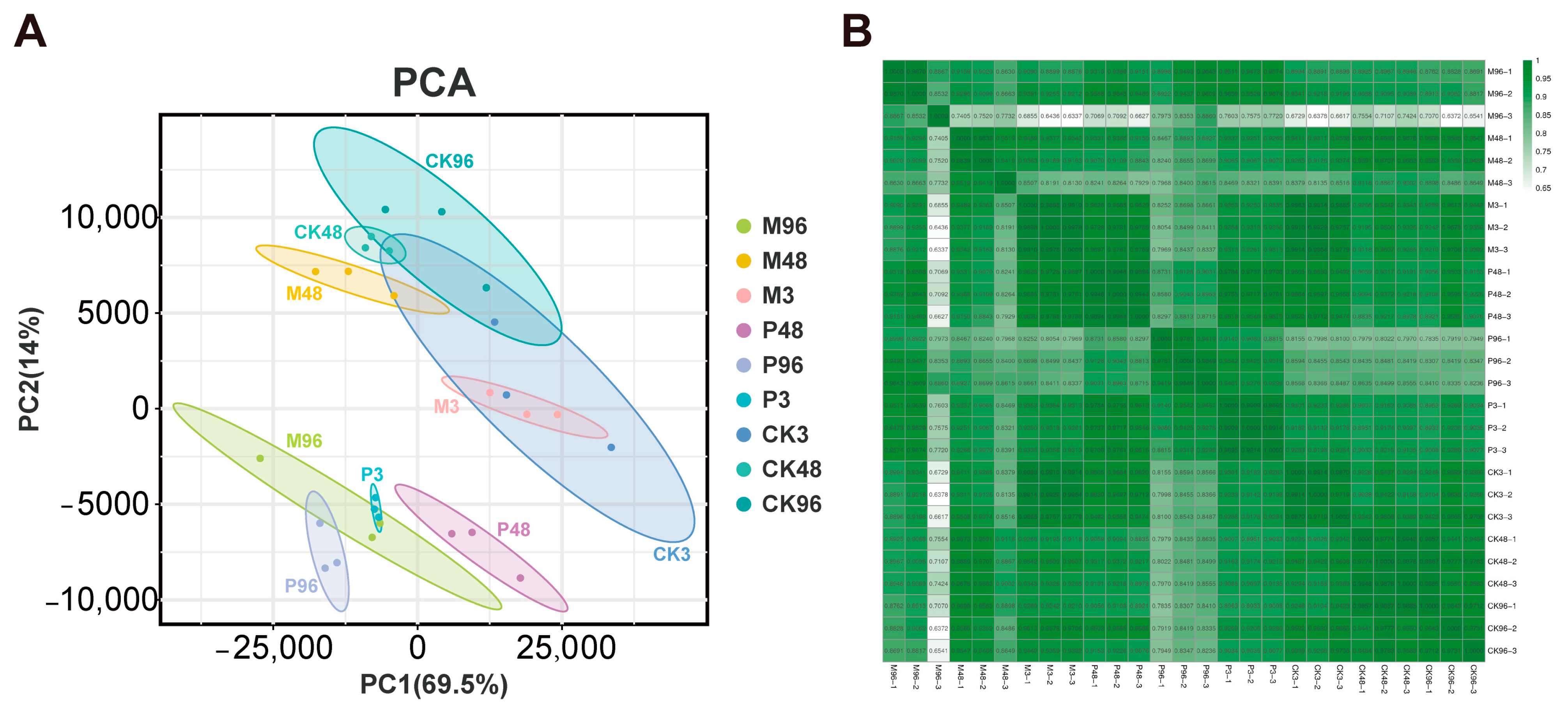
2.1. Overview of Illumina Sequencing within Different Treatment Samples
2.2. Identification and Functional Annotation of Differentially Expressed Genes (DEGs)
2.2.1. Transcriptomic Changes in Response to Mechanical Damage (CK-vs-M)
2.2.2. Transcriptomic Changes in Response to PTM Infestation (CK-vs-P)
2.2.3. Transcriptomic Comparison Analysis between the Two Treatments (M-vs-P)
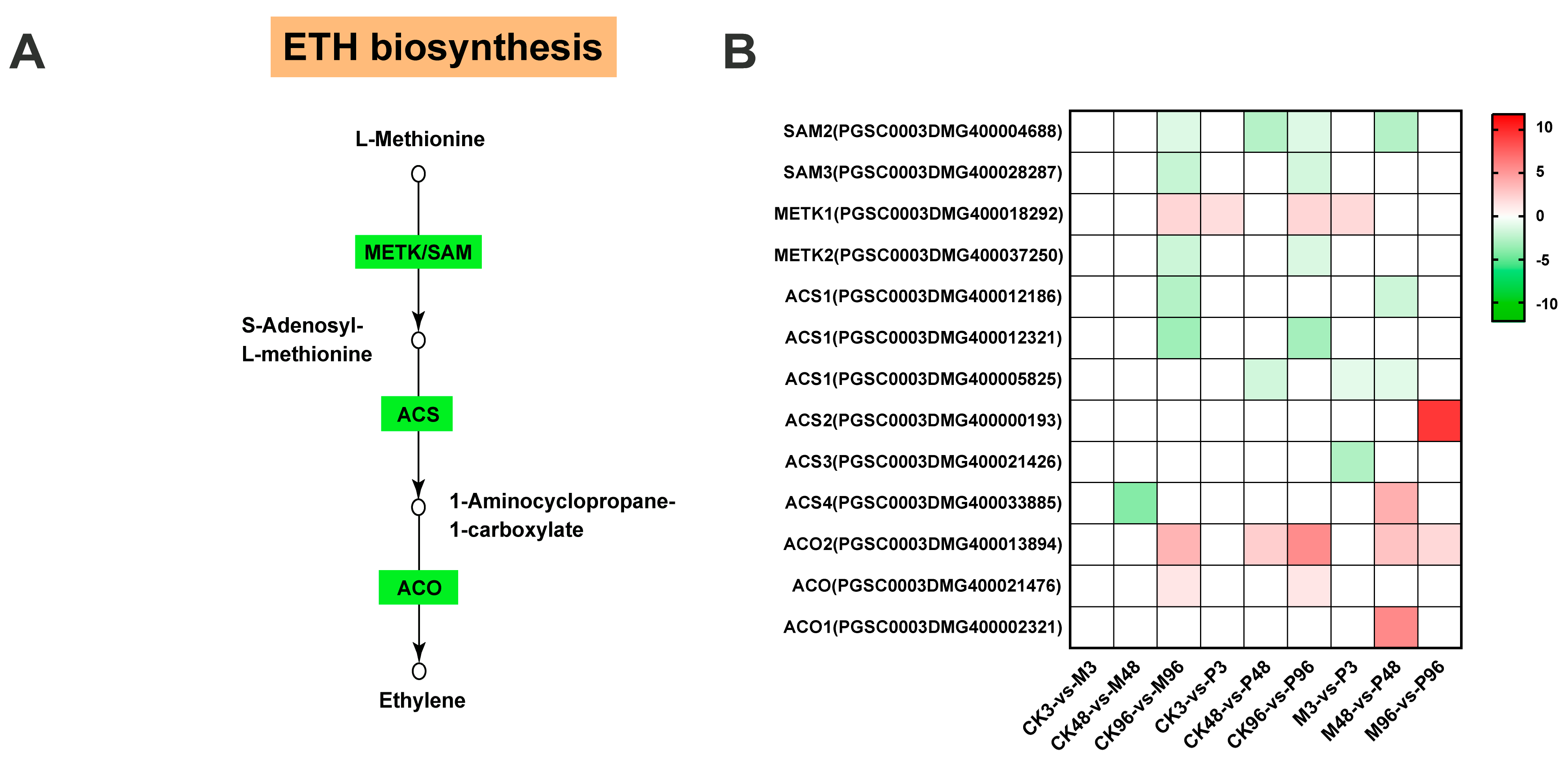
2.3. Expression Analysis of DEGs Involved in Plant Hormone Biosynthesis
2.4. Expression Analysis of DEGs Involved in Reactive Oxygen Species (ROS) Signaling
2.5. Expression Analysis of DEGs Involved in Transcription Factors (TFs)
2.6. Expression Analysis of DEGs Involved in Plant Secondary Metabolites (PSMs)
2.7. Expression Analysis of DEGs Involved in Plant–Pathogen Interactions and Defense Response
3. Discussion
4. Materials and Methods
4.1. Insect Colony
4.2. Plant Growth, Treatment and Sample Collection
4.3. RNA Extraction, Illumina Library Construction and Sequencing
4.4. Processing and Analysis of RNA-Seq Data
4.5. GO Functional and KEGG Pathway Enrichment Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar]
- Pingault, L.; Basu, S.; Vellichirammal, N.N.; Williams, W.P.; Sarath, G.; Louis, J. Co-Transcriptomic Analysis of the Maize-Western Corn Rootworm Interaction. Plants 2022, 11, 2335. [Google Scholar]
- Gibson, R.W. Glandular hairs providing resistance to aphids in certain wild potato species. Ann. Appl. Biol. 1971, 68, 113–119. [Google Scholar] [CrossRef]
- Hettenhausen, C.; Schuman, M.C.; Wu, J. MAPK signaling: A key element in plant defense response to insects. Insect Sci. 2015, 22, 157–164. [Google Scholar] [PubMed]
- Paiva, N.L. An Introduction to the Biosynthesis of Chemicals Used in Plant-Microbe Communication. J. Plant Growth Regul. 2000, 19, 131–143. [Google Scholar] [PubMed]
- Gatehouse, J.A. Plant resistance towards insect herbivores: A dynamic interaction. New Phytol. 2002, 156, 145–169. [Google Scholar]
- Zhan, X.; Chen, Z.; Chen, R.; Shen, C. Environmental and Genetic Factors Involved in Plant Protection-Associated Secondary Metabolite Biosynthesis Pathways. Front. Plant Sci. 2022, 13, 877304. [Google Scholar]
- Mosolov, V.V.; Valueva, T.A. Proteinase inhibitors and their function in plants: A review. Appl. Biochem. Microbiol. 2005, 41, 227–246. [Google Scholar] [CrossRef]
- Vandenborre, G.; Smagghe, G.; Van Damme, E.J. Plant lectins as defense proteins against phytophagous insects. Phytochemistry 2011, 72, 1538–1550. [Google Scholar]
- Leitner, M.; Boland, W.; Mithöfer, A. Direct and indirect defences induced by piercing-sucking and chewing herbivores in Medicago truncatula. New Phytol. 2005, 167, 597–606. [Google Scholar] [PubMed]
- Chen, C.Y.; Mao, Y.B. Research advances in plant-insect molecular interaction. F1000Research 2020, 9, F1000 Faculty Rev-198. [Google Scholar]
- Erb, M.; Reymond, P. Molecular Interactions between Plants and Insect Herbivores. Annu. Rev. Plant Biol. 2019, 70, 527–557. [Google Scholar] [CrossRef]
- Maffei, M.E.; Mithöfer, A.; Boland, W. Before gene expression: Early events in plant-insect interaction. Trends Plant Sci. 2007, 12, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Vincent, T.R.; Avramova, M.; Canham, J.; Higgins, P.; Bilkey, N.; Mugford, S.T.; Pitino, M.; Toyota, M.; Gilroy, S.; Miller, A.J.; et al. Interplay of Plasma Membrane and Vacuolar Ion Channels, Together with BAK1, Elicits Rapid Cytosolic Calcium Elevations in Arabidopsis during Aphid Feeding. Plant Cell 2017, 29, 1460–1479. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E.; Mithöfer, A.; Arimura, G.; Uchtenhagen, H.; Bossi, S.; Bertea, C.M.; Starvaggi Cucuzza, L.; Novero, M.; Volpe, V.; Quadro, S.; et al. Effects of feeding Spodoptera littoralis on lima bean leaves. III. Membrane depolarization and involvement of hydrogen peroxide. Plant Physiol. 2006, 140, 1022–1035. [Google Scholar] [CrossRef] [PubMed]
- Vadassery, J.; Reichelt, M.; Hause, B.; Gershenzon, J.; Boland, W.; Mithöfer, A. CML42-mediated calcium signaling coordinates responses to Spodoptera herbivory and abiotic stresses in Arabidopsis. Plant Physiol. 2012, 159, 1159–1175. [Google Scholar] [PubMed]
- Kandoth, P.K.; Ranf, S.; Pancholi, S.S.; Jayanty, S.; Walla, M.D.; Miller, W.; Howe, G.A.; Lincoln, D.E.; Stratmann, J.W. Tomato MAPKs LeMPK1, LeMPK2, and LeMPK3 function in the systemin-mediated defense response against herbivorous insects. Proc. Natl. Acad. Sci. USA 2007, 104, 12205–12210. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Z.; Hua, H.; Ma, W. Comparative transcriptome analysis of defense response of rice to Nilaparvata lugens and Chilo suppressalis infestation. Int. J. Biol. Macromol. 2020, 163, 2270–2285. [Google Scholar] [CrossRef]
- Wang, D.; Li, C.-F.; Ma, C.-L.; Chen, L. Novel insights into the molecular mechanisms underlying the resistance of Camellia sinensis to Ectropis oblique provided by strategic transcriptomic comparisons. Sci. Hortic. 2015, 192, 429–440. [Google Scholar] [CrossRef]
- Mao, Z.; Ge, Y.; Zhang, Y.; Zhong, J.; Munawar, A.; Zhu, Z.; Zhou, W. Disentangling the Potato Tuber Moth-Induced Early-Defense Response by Simulated Herbivory in Potato Plants. Front. Plant Sci. 2022, 13, 902342. [Google Scholar] [CrossRef]
- Li, R.; Zhang, J.; Li, J.; Zhou, G.; Wang, Q.; Bian, W.; Erb, M.; Lou, Y. Prioritizing plant defence over growth through WRKY regulation facilitates infestation by non-target herbivores. eLife 2015, 4, e04805. [Google Scholar] [CrossRef] [PubMed]
- Meraj, T.A.; Fu, J.; Raza, M.A.; Zhu, C.; Shen, Q.; Xu, D.; Wang, Q. Transcriptional Factors Regulate Plant Stress Responses through Mediating Secondary Metabolism. Genes 2020, 11, 346. [Google Scholar] [CrossRef] [PubMed]
- Reymond, P.; Weber, H.; Damond, M.; Farmer, E.E. Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 2000, 12, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Korth, K.L.; Dixon, R.A. Evidence for Chewing Insect-Specific Molecular Events Distinct from a General Wound Response in Leaves. Plant Physiol. 1997, 115, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Q.; Xu, X.; Guo, H. UHPLC-MS/MS Analysis of the Accumulation and Excretion of Steroidal Glycoalkaloids Consumed by Potato Tuber Moth (Phthorimaea operculella) Larvae under Different Feeding Treatments. Insects 2022, 14, 26. [Google Scholar] [CrossRef]
- Zhang, M.; Yan, J.; Ali, A.; Gao, Y. Potato plant variety affects the performance and oviposition preference of Phthorimaea operculella Zeller (Lepidoptera: Gelechiidae). Pest Manag. Sci. 2022, 78, 3912–3919. [Google Scholar] [CrossRef] [PubMed]
- Gallego, J.R.; Caicedo, O.; Gamez, M.; Hernandez, J.; Cabello, T. Selection of Predatory Mites for the Biological Control of Potato Tuber Moth in Stored Potatoes. Insects 2020, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.E.; Rose, D.J.W. Chemical control of potato tuber moth, phthorimaea operculella (zell.), in rhodesia. Bull. Entomol. Res. 1967, 57, 271. [Google Scholar] [CrossRef]
- del Pulgar, J.S.; Lucarini, M.; Aguzzi, A.; Gabrielli, P.; Parisi, B.; Pacifico, D.; Mandolino, G.; Lombardi-Boccia, G. Glycoalkaloid Content in Italian Potato Breeding Clones Improved for Resistance against Potato Tuber Moth (Phthorimaea operculella Zeller). Potato Res. 2021, 64, 229–240. [Google Scholar] [CrossRef]
- Meiyalaghan, S.; Barrell, P.J.; Jacobs, J.M.; Conner, A.J. Regeneration of multiple shoots from transgenic potato events facilitates the recovery of phenotypically normal lines: Assessing a cry9Aa2 gene conferring insect resistance. BMC Biotechnol. 2011, 11, 93. [Google Scholar] [CrossRef]
- Ito, Y.; Sasaki, K.; Ogihara, T.; Kitaoka, N.; Takahahi, K.; Matsuura, H. Verification of the versatility of the in vitro enzymatic reaction giving (+)-cis-12-Oxo-phytodienoic acid. Bioorganic Med. Chem. Lett. 2021, 49, 128284. [Google Scholar] [CrossRef]
- Royo, J.; Vancanneyt, G.; Pérez, A.G.; Sanz, C.; Störmann, K.; Rosahl, S.; Sánchez-Serrano, J.J. Characterization of three potato lipoxygenases with distinct enzymatic activities and different organ-specific and wound-regulated expression patterns. J. Biol. Chem. 1996, 271, 21012–21019. [Google Scholar] [CrossRef]
- Wasternack, C. Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Ding, Y. Stories of Salicylic Acid: A Plant Defense Hormone. Trends Plant Sci. 2020, 25, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.M.; Zhang, Y. Plant Immunity: Danger Perception and Signaling. Cell 2020, 181, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic Acid Signaling and Abiotic Stress Tolerance in Plants: A Review on Current Knowledge and Future Prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef]
- Park, H.Y.; Seok, H.Y.; Park, B.K.; Kim, S.H.; Goh, C.H.; Lee, B.H.; Lee, C.H.; Moon, Y.H. Overexpression of Arabidopsis ZEP enhances tolerance to osmotic stress. Biochem. Biophys. Res. Commun. 2008, 375, 80–85. [Google Scholar] [CrossRef]
- Al-Babili, S.; Hugueney, P.; Schledz, M.; Welsch, R.; Frohnmeyer, H.; Laule, O.; Beyer, P. Identification of a novel gene coding for neoxanthin synthase from Solanum tuberosum. FEBS Lett. 2000, 485, 168–172. [Google Scholar] [CrossRef]
- Tan, B.C.; Schwartz, S.H.; Zeevaart, J.A.; McCarty, D.R. Genetic control of abscisic acid biosynthesis in maize. Proc. Natl. Acad. Sci. USA 1997, 94, 12235–12240. [Google Scholar] [CrossRef]
- Seo, M.; Peeters, A.J.; Koiwai, H.; Oritani, T.; Marion-Poll, A.; Zeevaart, J.A.; Koornneef, M.; Kamiya, Y.; Koshiba, T. The Arabidopsis aldehyde oxidase 3 (AAO3) gene product catalyzes the final step in abscisic acid biosynthesis in leaves. Proc. Natl. Acad. Sci. USA 2000, 97, 12908–12913. [Google Scholar] [CrossRef]
- Cheng, W.H.; Endo, A.; Zhou, L.; Penney, J.; Chen, H.C.; Arroyo, A.; Leon, P.; Nambara, E.; Asami, T.; Seo, M.; et al. A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signaling and abscisic acid biosynthesis and functions. Plant Cell 2002, 14, 2723–2743. [Google Scholar] [CrossRef]
- Rock, C.D.; Heath, T.G.; Gage, D.A.; Zeevaart, J.A. Abscisic alcohol is an intermediate in abscisic Acid biosynthesis in a shunt pathway from abscisic aldehyde. Plant Physiol. 1991, 97, 670–676. [Google Scholar] [CrossRef][Green Version]
- Rodrigues, M.A.; Bianchetti, R.E.; Freschi, L. Shedding light on ethylene metabolism in higher plants. Front. Plant Sci. 2014, 5, 665. [Google Scholar] [CrossRef] [PubMed]
- Adie, B.; Chico, J.M.; Rubio-Somoza, I.; Solano, R. Modulation of Plant Defenses by Ethylene. J. Plant Growth Regul. 2007, 26, 160–177. [Google Scholar] [CrossRef]
- Czarny, J.C.; Grichko, V.P.; Glick, B.R. Genetic modulation of ethylene biosynthesis and signaling in plants. Biotechnol. Adv. 2006, 24, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Singh, S.; Parihar, P.; Mishra, R.K.; Tripathi, D.K.; Singh, V.P.; Chauhan, D.K.; Prasad, S.M. Reactive Oxygen Species (ROS): Beneficial Companions of Plants’ Developmental Processes. Front. Plant Sci. 2016, 7, 1299. [Google Scholar] [CrossRef]
- Hrmova, M.; Hussain, S.S. Plant Transcription Factors Involved in Drought and Associated Stresses. Int. J. Mol. Sci. 2021, 22, 5662. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Plant Secondary Metabolites Modulate Insect Behavior-Steps Toward Addiction? Front. Physiol. 2018, 9, 364. [Google Scholar] [CrossRef]
- Donze-Reiner, T.; Palmer, N.A.; Scully, E.D.; Prochaska, T.J.; Koch, K.G.; Heng-Moss, T.; Bradshaw, J.D.; Twigg, P.; Amundsen, K.; Sattler, S.E.; et al. Transcriptional analysis of defense mechanisms in upland tetraploid switchgrass to greenbugs. BMC Plant Biol. 2017, 17, 46. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.N.; Lee, W.H.; Won, S.Y.; Chang, S.; Hong, J.P.; Oh, T.J.; Lee, S.M.; Kang, S.H. Systemic Expression of Genes Involved in the Plant Defense Response Induced by Wounding in Senna tora. Int. J. Mol. Sci. 2021, 22, 10073. [Google Scholar] [CrossRef]
- Lawrence, P.K.; Koundal, K.R. Plant protease inhibitors in control of phytophagous insects. Electron. J. Biotechnol. 2002, 5, 5–6. [Google Scholar] [CrossRef]
- Vaghela, B.; Vashi, R.; Rajput, K.; Joshi, R. Plant chitinases and their role in plant defense: A comprehensive review. Enzym. Microb. Technol. 2022, 159, 110055. [Google Scholar] [CrossRef]
- Zhang, S.; Klessig, D.F. MAPK cascades in plant defense signaling. Trends Plant Sci. 2001, 6, 520–527. [Google Scholar] [CrossRef] [PubMed]
- De Vos, M.; Van Oosten, V.R.; Van Poecke, R.M.; Van Pelt, J.A.; Pozo, M.J.; Mueller, M.J.; Buchala, A.J.; Métraux, J.P.; Van Loon, L.C.; Dicke, M.; et al. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol. Plant-Microbe Interact. MPMI 2005, 18, 923–937. [Google Scholar] [CrossRef] [PubMed]
- De Vos, M.; Van Oosten, V.R.; Jander, G.; Dicke, M.; Pieterse, C.M. Plants under attack: Multiple interactions with insects and microbes. Plant Signal. Behav. 2007, 2, 527–529. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bellande, K.; Bono, J.J.; Savelli, B.; Jamet, E.; Canut, H. Plant Lectins and Lectin Receptor-Like Kinases: How Do They Sense the Outside? Int. J. Mol. Sci. 2017, 18, 1164. [Google Scholar] [CrossRef] [PubMed]
- Vaid, N.; Macovei, A.; Tuteja, N. Knights in action: Lectin receptor-like kinases in plant development and stress responses. Mol. Plant 2013, 6, 1405–1418. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Shah, A.; Karthik, K.; Rathinam, M.; Rai, V.; Chaudhary, N.; Sreevathsa, R. Reactive oxygen species in plants: An invincible fulcrum for biotic stress mitigation. Appl. Microbiol. Biotechnol. 2022, 106, 5945–5955. [Google Scholar] [CrossRef]
- Liu, Y.; He, C. A review of redox signaling and the control of MAP kinase pathway in plants. Redox Biol. 2017, 11, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Kovtun, Y.; Chiu, W.L.; Tena, G.; Sheen, J. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc. Natl. Acad. Sci. USA 2000, 97, 2940–2945. [Google Scholar] [CrossRef] [PubMed]
- Pitzschke, A.; Djamei, A.; Bitton, F.; Hirt, H. A major role of the MEKK1-MKK1/2-MPK4 pathway in ROS signalling. Mol. Plant 2009, 2, 120–137. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.; Ali Wani, O.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive Oxygen Species in Plants: From Source to Sink. Antioxidants 2022, 11, 225. [Google Scholar] [CrossRef] [PubMed]
- Sewelam, N.; Kazan, K.; Schenk, P.M. Global Plant Stress Signaling: Reactive Oxygen Species at the Cross-Road. Front. Plant Sci. 2016, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Raja, V.; Majeed, U.; Kang, H.; Andrabi, K.I.; John, R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ. Exp. Bot. 2017, 137, 142–157. [Google Scholar] [CrossRef]
- Lu, J.; Robert, C.A.; Riemann, M.; Cosme, M.; Mène-Saffrané, L.; Massana, J.; Stout, M.J.; Lou, Y.; Gershenzon, J.; Erb, M. Induced jasmonate signaling leads to contrasting effects on root damage and herbivore performance. Plant Physiol. 2015, 167, 1100–1116. [Google Scholar] [CrossRef] [PubMed]
- McConn, M.; Creelman, R.A.; Bell, E.; Mullet, J.E.; Browse, J. Jasmonate is essential for insect defense in Arabidopsis. Proc. Natl. Acad. Sci. USA 1997, 94, 5473–5477. [Google Scholar] [CrossRef] [PubMed]
- Glauser, G.; Dubugnon, L.; Mousavi, S.A.; Rudaz, S.; Wolfender, J.L.; Farmer, E.E. Velocity estimates for signal propagation leading to systemic jasmonic acid accumulation in wounded Arabidopsis. J. Biol. Chem. 2009, 284, 34506–34513. [Google Scholar] [CrossRef]
- Göbel, C.; Feussner, I.; Schmidt, A.; Scheel, D.; Sanchez-Serrano, J.; Hamberg, M.; Rosahl, S. Oxylipin profiling reveals the preferential stimulation of the 9-lipoxygenase pathway in elicitor-treated potato cells. J. Biol. Chem. 2001, 276, 6267–6273. [Google Scholar] [CrossRef]
- Woldemariam, M.G.; Ahern, K.; Jander, G.; Tzin, V. A role for 9-lipoxygenases in maize defense against insect herbivory. Plant Signal. Behav. 2018, 13, e1422462. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Bustos-Segura, C.; Degen, T.; Erb, M.; Turlings TC, J. Belowground and aboveground herbivory differentially affect the transcriptome in roots and shoots of maize. Plant Direct 2022, 6, e426. [Google Scholar] [CrossRef] [PubMed]
- Koo Abraham, J. Metabolism of the plant hormone jasmonate: A sentinel for tissue damage and master regulator of stress response. Phytochem. Rev. 2017, 17, 51–80. [Google Scholar]
- Diezel, C.; von Dahl, C.C.; Gaquerel, E.; Baldwin, I.T. Different lepidopteran elicitors account for cross-talk in herbivory-induced phytohormone signaling. Plant Physiol. 2009, 150, 1576–1586. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chang, P.; Liu, D.; Narasimhan, M.L.; Raghothama, K.G.; Hasegawa, P.M.; Bressan, R.A. Plant Defense Genes Are Synergistically Induced by Ethylene and Methyl Jasmonate. Plant Cell 1994, 6, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Penninckx, I.A.; Thomma, B.P.; Buchala, A.; Métraux, J.P.; Broekaert, W.F. Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell 1998, 10, 2103–2113. [Google Scholar] [CrossRef] [PubMed]
- Onkokesung, N.; Baldwin, I.T.; Gális, I. The role of jasmonic acid and ethylene crosstalk in direct defense of Nicotiana attenuata plants against chewing herbivores. Plant Signal. Behav. 2010, 5, 1305–1307. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, O.; Piqueras, R.; Sánchez-Serrano, J.J.; Solano, R. ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell 2003, 15, 165–178. [Google Scholar] [CrossRef]
- Zhu, Z.; Lee, B. Friends or foes: New insights in jasmonate and ethylene co-actions. Plant Cell Physiol. 2015, 56, 414–420. [Google Scholar] [CrossRef]
- Zhang, X.N.; Liu, J.; Liu, Y.; Wang, Y.; Abozeid, A.; Yu, Z.G.; Tang, Z.H. Metabolomics Analysis Reveals that Ethylene and Methyl Jasmonate Regulate Different Branch Pathways to Promote the Accumulation of Terpenoid Indole Alkaloids in Catharanthus roseus. J. Nat. Prod. 2018, 81, 335–342. [Google Scholar] [CrossRef]
- O’Donnell, P.J.; Calvert, C.; Atzorn, R.; Wasternack, C.; Leyser HM, O.; Bowles, D.J. Ethylene as a Signal Mediating the Wound Response of Tomato Plants. Science 1996, 274, 1914–1917. [Google Scholar] [CrossRef]
- Ng, D.W.; Abeysinghe, J.K.; Kamali, M. Regulating the Regulators: The Control of Transcription Factors in Plant Defense Signaling. Int. J. Mol. Sci. 2018, 19, 3737. [Google Scholar] [CrossRef]
- Chen, M.; Ji, M.; Wen, B.; Liu, L.; Li, S.; Chen, X.; Gao, D.; Li, L. GOLDEN 2-LIKE Transcription Factors of Plants. Front. Plant Sci. 2016, 7, 1509. [Google Scholar] [CrossRef]
- Bonaventure, G. The Nicotiana attenuata LECTIN RECEPTOR KINASE 1 is involved in the perception of insect feeding. Plant Signal. Behav. 2011, 6, 2060–2063. [Google Scholar] [CrossRef]
- De Vos, M.; Van Zaanen, W.; Koornneef, A.; Korzelius, J.P.; Dicke, M.; Van Loon, L.C.; Pieterse, C.M. Herbivore-induced resistance against microbial pathogens in Arabidopsis. Plant Physiol. 2006, 142, 352–363. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]








| Category | AO | GOX | HPPD | GAOX | ND | COX | RBOH | SOD | FIN4 | QSOX | ACX | AAO | ERO | FMO | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CK3-vs-M3 | up | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| dw | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| CK48-vs-M48 | up | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 9 |
| dw | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 4 | |
| CK96-vs-M96 | up | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 4 | 1 | 0 | 0 | 8 |
| dw | 4 | 0 | 1 | 3 | 0 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 12 | |
| M/CK Total | up | 1 | 0 | 0 | 3 | 1 | 1 | 2 | 1 | 1 | 0 | 4 | 1 | 2 | 0 | 17 |
| dw | 4 | 1 | 1 | 4 | 0 | 2 | 2 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 16 | |
| CK3-vs-P3 | up | 0 | 0 | 0 | 2 | 4 | 0 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 1 | 11 |
| dw | 4 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 10 | |
| CK48-vs-P48 | up | 3 | 0 | 0 | 1 | 0 | 0 | 3 | 1 | 0 | 0 | 2 | 1 | 0 | 1 | 12 |
| dw | 2 | 0 | 1 | 4 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 13 | |
| CK96-vs-P96 | up | 1 | 0 | 1 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 5 | 2 | 0 | 0 | 13 |
| dw | 3 | 0 | 1 | 3 | 1 | 2 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 14 | |
| P/CK Total | up | 4 | 0 | 1 | 6 | 4 | 0 | 4 | 2 | 0 | 2 | 7 | 4 | 0 | 2 | 36 |
| dw | 9 | 1 | 3 | 8 | 2 | 4 | 6 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 37 | |
| M3-vs-P3 | up | 1 | 1 | 0 | 4 | 3 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 2 | 16 |
| dw | 7 | 1 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 14 | |
| M48-vs-P48 | up | 5 | 1 | 0 | 1 | 2 | 0 | 4 | 3 | 0 | 0 | 0 | 1 | 0 | 1 | 18 |
| dw | 5 | 0 | 1 | 3 | 1 | 5 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 20 | |
| M96-vs-P96 | up | 4 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 8 |
| dw | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | |
| P/M Total | up | 10 | 2 | 0 | 7 | 5 | 0 | 4 | 4 | 1 | 1 | 2 | 3 | 0 | 3 | 42 |
| dw | 13 | 1 | 2 | 3 | 2 | 5 | 3 | 2 | 1 | 0 | 0 | 1 | 1 | 2 | 36 | |
| Category | CAT | APX | POD | Fd | TRX | GRX | GST | MDHAR | DHAR | GPX | PrxR | NRX | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CK3-vs-M3 | up | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| dw | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| CK48-vs-M48 | up | 0 | 1 | 3 | 3 | 1 | 0 | 5 | 0 | 1 | 0 | 0 | 0 | 14 |
| dw | 0 | 1 | 2 | 0 | 2 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 10 | |
| CK96-vs-M96 | up | 1 | 0 | 6 | 1 | 5 | 6 | 0 | 0 | 0 | 1 | 0 | 0 | 20 |
| dw | 1 | 3 | 5 | 3 | 10 | 4 | 10 | 0 | 0 | 1 | 1 | 1 | 39 | |
| M/CK Total | up | 1 | 1 | 9 | 4 | 6 | 6 | 5 | 0 | 1 | 1 | 0 | 0 | 34 |
| dw | 1 | 4 | 7 | 3 | 12 | 7 | 12 | 0 | 0 | 1 | 1 | 1 | 49 | |
| CK3-vs-P3 | up | 0 | 0 | 3 | 1 | 4 | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 13 |
| dw | 0 | 3 | 1 | 2 | 2 | 8 | 6 | 0 | 0 | 0 | 0 | 0 | 22 | |
| CK48-vs-P48 | up | 0 | 1 | 22 | 4 | 2 | 15 | 0 | 1 | 1 | 1 | 4 | 0 | 51 |
| dw | 1 | 2 | 1 | 3 | 4 | 5 | 10 | 0 | 0 | 0 | 0 | 1 | 27 | |
| CK96-vs-P96 | up | 1 | 0 | 22 | 1 | 5 | 14 | 1 | 0 | 0 | 1 | 1 | 0 | 46 |
| dw | 1 | 1 | 5 | 2 | 8 | 2 | 7 | 0 | 0 | 0 | 1 | 0 | 27 | |
| P/CK Total | up | 1 | 1 | 47 | 6 | 11 | 29 | 3 | 1 | 3 | 2 | 6 | 0 | 110 |
| dw | 2 | 6 | 7 | 7 | 14 | 15 | 23 | 0 | 0 | 0 | 1 | 1 | 76 | |
| M3-vs-P3 | up | 0 | 0 | 3 | 3 | 5 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 15 |
| dw | 1 | 2 | 9 | 3 | 10 | 14 | 24 | 0 | 0 | 0 | 0 | 1 | 64 | |
| M48-vs-P48 | up | 1 | 3 | 24 | 3 | 3 | 11 | 2 | 1 | 1 | 0 | 5 | 0 | 54 |
| dw | 1 | 2 | 2 | 3 | 8 | 4 | 17 | 0 | 0 | 0 | 0 | 1 | 38 | |
| M96-vs-P96 | up | 0 | 0 | 15 | 3 | 2 | 9 | 2 | 0 | 1 | 0 | 0 | 0 | 32 |
| dw | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | |
| P/M Total | up | 1 | 3 | 42 | 9 | 10 | 20 | 6 | 1 | 3 | 0 | 6 | 0 | 101 |
| dw | 2 | 4 | 11 | 6 | 18 | 21 | 41 | 0 | 0 | 0 | 0 | 2 | 105 | |
| Category | Terpenoids | Flavonoids | Alkaloids | Steroids | Quinone | Total | |
|---|---|---|---|---|---|---|---|
| CK3-vs-M3 | up | 2 | 0 | 0 | 0 | 0 | 2 |
| dw | 0 | 0 | 0 | 0 | 0 | 0 | |
| CK48-vs-M48 | up | 5 | 5 | 0 | 0 | 1 | 11 |
| dw | 10 | 8 | 2 | 0 | 2 | 22 | |
| CK96-vs-M96 | up | 12 | 2 | 7 | 7 | 2 | 30 |
| dw | 28 | 23 | 16 | 8 | 10 | 85 | |
| CK3-vs-P3 | up | 9 | 2 | 6 | 5 | 2 | 24 |
| dw | 18 | 13 | 11 | 3 | 5 | 50 | |
| CK48-vs-P48 | up | 12 | 6 | 15 | 4 | 3 | 40 |
| dw | 24 | 16 | 9 | 4 | 6 | 59 | |
| CK96-vs-P96 | up | 24 | 6 | 12 | 9 | 4 | 55 |
| dw | 23 | 21 | 13 | 5 | 10 | 72 | |
| M3-vs-P3 | up | 15 | 7 | 6 | 9 | 2 | 39 |
| dw | 27 | 24 | 18 | 4 | 6 | 79 | |
| M48-vs-P48 | up | 16 | 8 | 20 | 7 | 5 | 56 |
| dw | 24 | 11 | 8 | 3 | 7 | 53 | |
| M96-vs-P96 | up | 12 | 12 | 8 | 6 | 4 | 42 |
| dw | 0 | 0 | 0 | 0 | 0 | 0 | |
| Category | M3/CK3 | M48/CK48 | M96/CK96 | P3/CK3 | P48/CK48 | P96/CK96 | P3/M3 | P48/M48 | P96/M96 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PIs | up | 0 | 9 | 1 | 5 | 8 | 7 | 10 | 4 | 6 | 50 |
| dw | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 3 | 0 | 6 | |
| Chitinases | up | 0 | 1 | 3 | 1 | 2 | 6 | 2 | 7 | 4 | 26 |
| dw | 0 | 2 | 5 | 7 | 3 | 1 | 11 | 4 | 0 | 33 | |
| MAPK | up | 0 | 0 | 6 | 1 | 1 | 6 | 2 | 2 | 1 | 19 |
| dw | 0 | 0 | 6 | 0 | 4 | 6 | 2 | 4 | 1 | 23 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Yi, X.; Yang, M.; Liu, Y.; Yao, Y.; Zi, S.; Chen, B.; Xiao, G. Comparative Transcriptome Analysis of Defense Response of Potato to Phthorimaea operculella Infestation. Plants 2023, 12, 3092. https://doi.org/10.3390/plants12173092
Zhu C, Yi X, Yang M, Liu Y, Yao Y, Zi S, Chen B, Xiao G. Comparative Transcriptome Analysis of Defense Response of Potato to Phthorimaea operculella Infestation. Plants. 2023; 12(17):3092. https://doi.org/10.3390/plants12173092
Chicago/Turabian StyleZhu, Chunyue, Xiaocui Yi, Miao Yang, Yiyi Liu, Yao Yao, Shengjiang Zi, Bin Chen, and Guanli Xiao. 2023. "Comparative Transcriptome Analysis of Defense Response of Potato to Phthorimaea operculella Infestation" Plants 12, no. 17: 3092. https://doi.org/10.3390/plants12173092
APA StyleZhu, C., Yi, X., Yang, M., Liu, Y., Yao, Y., Zi, S., Chen, B., & Xiao, G. (2023). Comparative Transcriptome Analysis of Defense Response of Potato to Phthorimaea operculella Infestation. Plants, 12(17), 3092. https://doi.org/10.3390/plants12173092





