Abstract
Tropane alkaloids (TAs) are large secondary metabolite alkaloids that find extensive applications in the synthesis of antidotes, anesthetics, antiemetics, motion sickness drugs, and antispasmodics. The current production method primarily depends on extraction from medicinal plants of the Solanaceae family. Elicitation, as a highly effective biotechnological approach, offers significant advantages in augmenting the synthesis of secondary metabolites. The advantages include its simplicity of operation, low cost, and reduced risk of contamination. This review focuses on the impact of elicitation on the biosynthesis of TAs from three aspects: single-elicitor treatment, multiple-elicitor treatment, and the combination of elicitation strategy with other strategies. Some potential reasons are also proposed. Plant hormones and growth regulators, such as jasmonic acid (JA), salicylic acid (SA), and their derivatives, have been extensively employed in the separate elicitation processes. In recent years, novel elicitors represented by magnetic nanoparticles have emerged as significant factors in the investigation of yield enhancement in TAs. This approach shows promising potential for further development. The current utilization of multi-elicitor treatment is constrained, primarily relying on the combination of only two elicitors for induction. Some of these combinations have been found to exhibit synergistic amplification effects. However, the underlying molecular mechanism responsible for this phenomenon remains largely unknown. The literature concerning the integration of elicitation strategy with other strategies is limited, and several research gaps require further investigation. In conclusion, the impact of various elicitors on the accumulation of TAs is well-documented. However, further research is necessary to effectively implement elicitation strategies in commercial production. This includes the development of stable bioreactors, the elucidation of regulatory mechanisms, and the identification of more potent elicitors.
1. Introduction
Tropane alkaloids (TAs) are a distinct class of alkaloids characterized by the presence of a tropane skeleton, which comprises a pyrrole ring and a piperidine ring. More than three hundred of them have been isolated and identified from plants of Solanaceae, Convolvulaceae, Proteaceae, Rhizophoraceae, etc., families [1,2]. The representative compounds hyoscyamine and scopolamine are of great interest as anticholinergic factors in the human parasympathetic nervous system. They have been used for the treatment of motion sickness, pesticide poisoning, Parkinson’s disease, anesthesia, analgesia, cough, and asthma relief [3]. The availability of hyoscyamine and scopolamine is still dependent on the isolation from a few Solanaceae family. However, the concentration of them in these plants is relatively low, with 0.2% dry weight (DW) and 0.02% DW in Atropa belladonna, an important medicinal plant for TAs [4]. This leads to many problems, such as a shortage of natural drug resources and high drug costs. Currently, the main strategies are chemical total synthesis or plant genetic engineering to enhance the content of natural products in medicinal plants, both of which rely on the complete resolution of the biosynthetic pathway of TAs [2,5] (Figure 1). Nevertheless, the total chemical synthesis of TAs has been hampered as a result of long synthetic routes, high-yield by-products, low yields, and high costs, making this approach less valuable for contemporary commercial applications. Due to the current statutory restrictions on the application of transgenic technologies, the direct commercialization of high-yield TAs transgenic plants produced through plant genetic engineering approaches is frequently challenging.
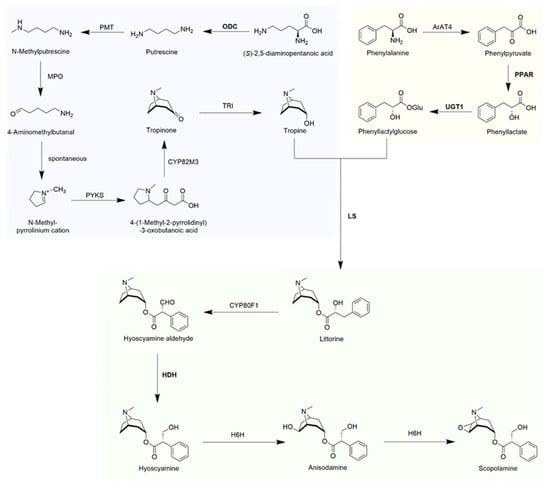
Figure 1.
Synthetic pathways of medicinal tropane alkaloids in Solanaceae.
There are 13 TA biosynthetic genes in the synthetic pathway: ODC, ornithine decarboxylase; PMT, putrescine N-methyltransferase; MPO, N-methyl-putrescine oxidase; PYKS, pyrrolidine ketide synthase; CYP82M3, tropinone synthase; TRI, tropinone reductase I; ArAT4, aromatic amino acid aminotransferase; PPAR, phenylpyruvic acid reductase; UGT1, phenyllactate UDP-glycosyltransferase; LS, littorine synthase; CYP80F1, littorine mutase; HDH, hyoscyamine dehydrogenase; H6H, hyoscyamine 6β-hydroxylase.
Elicitation strategies are a technology that involves external interventions to directly stimulate the expression of target genes in cells or tissues, resulting in a significant enhancement of secondary metabolite production. This approach offers several advantages, including high efficiency, environmental friendliness, and low cost [6]. Elicitors that induce the immune response in plants do not need to participate in metabolic pathways. Instead, they function as signaling molecules that influence the biosynthesis and accumulation of secondary metabolites in plants. Numerous experiments have demonstrated that elicitation strategy can significantly enhance the production or accumulation of secondary metabolites in a variety of medicinal plant species. With further research on secondary metabolites and optimization of the elicitation strategy, this strategy holds promise for broader application and prospects.
We reviewed the effects of elicitors derived from different sources on the synthesis, accumulation, and release of TAs (especially hyoscyamine and scopolamine) in different Solanaceae plant species. Our analysis was based on an extensive review of the relevant literature. Additionally, we identified several current challenges and proposed future research directions for this strategy. It is imperative for researchers to comprehensively understand the recent advancements in the elicitation strategy for TAs synthesis to design and realize commercial applications. To our knowledge, this is the first material to provide a detailed review of the effects of various elicitors on the biosynthesis of TAs in medicinal plants.
2. Elicitors
Elicitors are molecules that are typically generated by pathogens, insects, or other organisms. These molecules are recognized by specific receptors in plants, leading to the activation of plant immune responses against these external threats [7]. From a pathogenesis perspective, most elicitors serve as non-toxic factors that contribute to the development of genetic pseudo-resistance in plant innate immunity. These elicitors are recognized by R proteins or plant receptors localized at the cytoplasm or plasma membrane to initiate signaling pathways, leading to immune responses and the biosynthesis of secondary metabolites [8]. The signal transduction mechanism can be summarized as follows: the specific assembly of the elicitor with the receptor causes the conformational change of the receptor or the activation of receptor kinase. This, in turn, indirectly activates its corresponding effectors, such as G proteins, ion channels, and lipases, which then transduce the signal further downstream to initiate an immune response. The precise intricacies of the mechanism remain uncertain. Plant-specific recognition of elicitors provides a comprehensive explanation for the selective ability of certain elicitors to induce the accumulation of phytochemicals or secondary metabolic compounds in specific plant species [9]. The elicitor signal is a multi-component network with a variety of responses in succession, consisting of multiple parallel or cross-linked signaling pathways that lead to different targeting responses and may change with the recognition of different elicitor signals [10]. Elicitors include biotic elicitors, such as bacteria, fungi, viruses, and plant cell wall components, as well as abiotic elicitors, including environmental factors, metal ions, and hormones [8] (Figure 2). In addition to being classified based on their source, elicitors can also be classified based on their chemical properties, structure, and other factors. It is worth noting that certain elicitors may fall into multiple categories simultaneously.
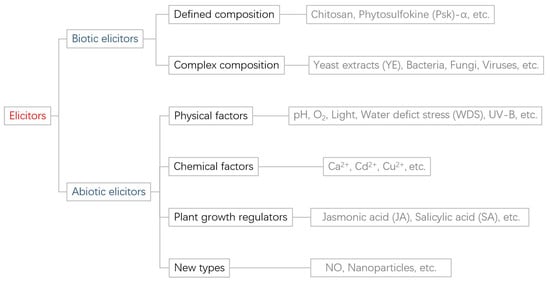
Figure 2.
Elicitor classification diagram.
Many factors can influence the elicitation effect, such as the choice of the elicitor and elicited plants, the concentration, duration, and site of the elicitation, and the composition of the medium [11]. Elicitation can be carried out in vitro or in vivo in plants. In vitro, elicitation mainly occurs in cultured cells, organs, or tissues. In vitro elicitation enables faster product synthesis compared to whole-plant culture [12]. Although studies have shown that the elicitation of plant cell cultures can effectively enhance the production and accumulation of valuable secondary metabolites [13], the instability and variability of this method make it difficult to apply in production practice [14]. By way of contrast, hairy roots are considered to be the material for elicitation studies due to their rapid growth, suitability for large-scale culture, genetic and biochemical stability, and ability to produce similar or higher levels of alkaloids compared to intact plants [15,16]. When plant tissue is infected with Agrobacterium rhizogenes carrying the Ri plasmid, the infected areas will grow adventitious roots. These roots can then be cut off and grown as individual clonal lines in hormone-free solid or liquid media that contain essential nutrients. This results in the formation of hairy root systems [17,18]. Additionally, in vivo induction experiments are also conducted using foliar spraying, root watering, or seed soaking of plant bodies. The elicitation strategy has been widely used to promote the accumulation of secondary metabolites, showing promising applications. In addition to its use in plants that produce TAs, it has also been utilized in a range of medicinal plants, including Panax ginseng, Calendula officinalis, and Digitalis purpurea [19,20,21]. By promoting the accumulation and yield of secondary metabolites, the elicitation strategy can effectively reduce the production cost of related drugs. This is valuable for addressing the issue of a shortage of natural medicine resources.
3. Effects of Biotic Elicitors on TAs
3.1. Sugars, Proteins, and Their Precursors and Derivatives
According to Rothe et al., sugar is not only a carbon source but also a signaling compound for root cultures [22]. Chitosan is a non-antigenic, non-toxic, and biocompatible polysaccharide polymer derived from chitin [23]. In recent years, it has been shown that chitosan has been widely used as an elicitor due to its favorable physicochemical properties and multidirectional biological activity [24,25]. The induction of TAs biosynthesis by chitosan varies among species. At pH 5.5 and certain concentrations, chitosan positively affects the accumulation and release of hyoscyamine and scopolamine from Brugmansia candida hairy root cultures (optimum concentration for accumulation is 10 mg/L and for release is 1000 mg/L) [26]. However, chitosan did not affect the accumulation and release of alkaloids in Atropa belladonna [27,28] and was even lethal to Hyoscyamus niger [29]. Oligogalacturonides, which belong to a group of oligosaccharides, are pectin fragments derived from the partial degradation of high galacturonic acid in plant cell walls [30]. It has been shown to be the most effective elicitor of plant responses in smaller pectin fragments [31]. Oligogalacturonides promoted the yield of TAs in the hairy roots of Datura stramonium, and a significant increase in the amount of tropine was found by testing precursor concentrations [32]. Although sucrose is not explicitly categorized as an elicitor in some literature, it is also capable of activating immune responses in defense [33,34,35]. Therefore, it is discussed here as an elicitor. Noteworthily, while the accumulation of biomass is often thought to favor the yield of the corresponding secondary metabolites, there is an exception in the production of elicitors. The relationship between biomass and alkaloid content may be modified in some way by sucrose [36]. In addition, hairy root clones from the same plant infected with different Agrobacterium species respond differently to the same elicitor. One established hairy root clone of Hyoscyamus muticus, Cairo LBA1S, grew poorly at lower sucrose concentrations but had twice the hyoscyamine content of another clone, C58A [37]. We need to explore optimal elicitation conditions for the different clones. As far as we know, the physiological and biochemical status of hairy root clones can vary, even when obtained from the same Agrobacterium infestation. Therefore, it is often necessary to test the selection of high-performance root systems before conducting elicitation experiments.
Protein is an important macromolecular substance in plants, and its precursors and derivatives play a key role in growth and development. Phytosulfokine (Psk)-α, a sulfated pentapeptide isolated from Asparagus officinalis cultures, has been identified as a promoter of cell proliferation [38,39]. Stimulation of Atropa belladonna hairy roots with 10−4 and 10−5 mM of Psk-α promotes the production of TAs in the roots, specifically hyoscyamine [40]. Casein hydrolysate can provide cultures with a mixture of organic nitrogen, phosphate, trace elements, vitamins, and amino acids. However, its addition may be unnecessary for growth and production when nutrients are already available in the medium. For instance, a study found that adding casein hydrolysate to Hyoscyamus niger root cultures grown under optimal conditions did not have a significant effect on their TAs production [29]. Pectinase is a general term for a category of enzymes that break down pectin. Its main sources are microorganisms, and it has also been studied as an elicitor in some experiments [41,42]. Pitta Alvarez et al. found that pectinase increased the hyoscyamine content in Brugmansia candida hairy root cells and promoted the release of both hyoscyamine and scopolamine. However, the acetate buffer was found to be superior in comparison [43]. An enzyme called hemicellulase was found to increase the levels of hyoscyamine and scopolamine in Brugmansia candida hairy roots at 24 and 48 h after addition, and it also facilitated the release of scopolamine [44]. Both hemicellulases and pectinases break down the components of the plant cell wall, thereby altering its mechanical strength. It is speculated that their mechanisms of action may be related to this [45].
3.2. Yeast Extract
Yeast extract (YE) is a complex product containing different types of macromolecules and small molecules with diverse nutrients [46]. Studies have shown that YE can accelerate the synthesis and increase the accumulation of metabolites by activating the relevant enzymes in the metabolic pathway [47,48]. YE is also applied in the production of TAs. Guo et al. found that the addition of YE increased the content of metabolic precursor amino acids (ornithine and arginine) in Atropa belladonna. It also led to an upregulation in the expression of the rate-limiting enzyme genes PMT, TRI, and H6H in the synthetic pathway, thereby enhancing secondary metabolism and ultimately increasing the yield of hyoscyamine and scopolamine [49]. Hedayati et al. used different concentrations of YE (0, 0.5, 1, and 1.5 mg/L) as an elicitor to treat the hairy roots of Atropa belladonna. The results showed that the highest level of scopolamine and atropine yield was achieved under the treatment of 1 mg/L and 1.5 mg/L of YE for 48 h, respectively [50]. YE also increased the levels of hyoscyamine and scopolamine in Brugmansia candida hairy root cells, and more importantly, the ratio of scopolamine content to hyoscyamine content (St/Ht) was significantly increased [51]. Scopolamine has a higher value than hyoscyamine, the precursor of scopolamine, due to its fewer side effects, better efficacy in medicinal applications, and low abundance in many species [52,53]. The increase in this ratio is partly an indication of the value of YE in scopolamine production.
3.3. Fungi
Fungi are composed of a variety of complex compounds, including chitin, dextran, NEP1-like protein (NLP), Harpin protein, and many other proteins, as well as various secreted proteins, all of which can be used individually as an elicitor [54]. Current research primarily focuses on the antimicrobial activity of TAs and the changes in substances following the feeding of TAs [55,56,57]. In fact, fungi also have an influence on the synthesis and accumulation of TAs. Adding cell wall fragments of Phytophthora megasperma (Pmg) to Datura stramonium cell cultures increased the final production of TAs five-fold, as reported by Ballica et al. [58]. Three fungi (Aspergillus niger, Alternaria sp., and Fusarium monoliforme) effectively promoted the accumulation of hyoscyamine and scopolamine despite inhibiting the growth of Datura metel hairy roots. Among these fungi, Aspergillus niger was found to be the most effective elicitor [59]. Pitta Alvarez et al. cultured the hormone-like fungus Hormonema ssp. isolated from Brugmansia candida in two different substrates, Sabouraud-dextrose and MSRT, and treated the hairy roots of Brugmansia candida separately with the fungal homogenate and medium. The accumulation and release of hyoscyamine and scopolamine varied significantly with treatment time. The authors speculate that due to the complex composition of the fungal homogenate and the spent medium, multiple elicitors may be induced to act together on the accumulation and release of TAs [43].
3.4. Bacteria
Compared to fungi, bacterial elicitors have good aspects, such as shorter culture times and simpler preparation [60]. One study used two Gram-positive strains (Staphylococcus aureus KCTC 1916 and Pseudomonas aeruginosa KCTC 1750) and one Gram-negative strain (Bacillus cereus KCTC 1012) to induce Scopolia parviflora hairy roots. The first two were found to be more effective than the latter in the production of scopolamine, and they all increased the St/Ht ratio in the roots, especially Staphylococcus aureus KCTC 1916. This would also imply that the three strains may have increased the conversion of hyoscyamine to scopolamine. However, paradoxically, the expression of H6H was reduced, suggesting that other unknown regulatory pathways may exist [61]. Moussous et al. explored the influence of four Pseudomonas strains (Pseudomonas putida PP01 and Pseudomonas fluorescens P64, P66, and C7R12) on the levels of TAs in three Datura transgenic root lines (Datura stramonium, Datura tatula, and Datura innoxia). The results indicated that several of the Pseudomonas lines studied could positively and significantly affect hyoscyamine and scopolamine. The highest levels of hyoscyamine were observed in Datura tatula lines exposed to C7R12 for 5 days, while scopolamine levels were highest in Datura innoxia lines exposed to P64 for 5 days, and P66 for 5 days and 10 days [62].
3.5. Viruses
Viruses, as foreign invaders, interact with the plant body and cause corresponding changes in secondary metabolism [63]. Different classes of secondary metabolites have also been shown to be resistant to pathogens [64]. Examples of viral infections to increase specific secondary metabolites are rare, likely because their mechanism of action is more intricate compared to standard biotic or abiotic elicitors. Mihálik et al. used three tobacco mosaic viruses (PMMoV, TMV, and ToMV) to artificially infect Datura stramonium through in vivo elicitation. They discovered that the hyoscyamine content was significantly higher compared to in vitro induction or non-elicited capillary root cultures, which illustrates the potential value of viral elicitation [65]. In order to clearly demonstrate the role of each biotic elicitor in TAs biosynthesis, a table (Table 1) is provided, which includes all the relevant elements. Some of these elements are not described in detail in the main text. The rising arrow in the table represents a positive impact, and the falling arrow represents a negative impact.

Table 1.
Effects of biotic elicitors on TAs.
4. Effects of Abiotic Elicitors on TAs
4.1. Physical Elicitors
Physical elicitors include factors related to the environment. Several studies have demonstrated that the release of TAs is facilitated by an appropriate reduction in the pH of the medium. An elevated release of hyoscyamine and scopolamine was observed in Brugmansia candida hairy roots when subjected to specific concentrations of citric acid and acetic acid at different growth stages [26]. According to the alkaloid “ion trapping” mechanism, alkaloids tend to be mobile and stored in media with low pH [68]. Acetic acid molecules have the potential to enter the TAs synthesis pathway through conversion to acetoacetyl-CoA, and citric acid may act by influencing the TCA cycle [26,43]. Acids can also change the properties of cell walls and cell membranes, thereby affecting the release of alkaloids [69,70]. In conclusion, there are a number of reasons that could be used to explain this phenomenon. Oxygen is necessary for both plant growth and the biosynthesis of TAs [71]. Under conditions of pure oxygen, the root cultures of Duboisia myoporoides exhibited elevated levels of H6H and tropine. This led to the activation of the scopolamine synthesis pathway while simultaneously inhibiting the synthesis pathway of nicotine and other tropine derivatives. Consequently, the production of scopolamine was facilitated [72]. In the conventional hairy root bioreactor, little vigorous mixing is employed to prevent harm to the root system, which, however, leads to inadequate oxygen provision [73]. The objective of reactor development is to achieve a reasonable and effective supply of oxygen. Light makes a significant impact on plant growth and production of TAs. Hyoscyamus albus root cultures showed an increase in alkaloid content under light, especially scopolamine [74]. However, for the roots of Atropa belladonna, the effect of light on scopolamine and calystegine contents was almost the same, and scopolamine was only present in trace amounts [22]. Plants exposed to stressful growth conditions are likely to slow down their metabolism in favor of accelerated synthesis of non-toxic alkaloids, potentially serving as a form of nitrogen storage [75]. Drought, one of the major stressors in physical conditions, causes a slowdown in growth but can mostly positively affect the synthesis of active compounds in medicinal plants. Hyoscyamus muticus hairy roots were subjected to osmotic stress treatment using mannitol to simulate water stress. The results revealed a decrease in the biomass of the hairy roots, while the total production of hyoscyamine increased twofold [76]. The content of hyoscyamine and scopolamine in Atropa belladonna hairy roots increased significantly under high water deficit stress (WDS) and high nitrogen fertilization [75]. UV-B radiation, as one of the stresses, was able to stimulate the synthesis of secondary metabolites in different organisms [77]. UV-B stress exerted on hairy root cultures of Anisodus luridus demonstrated significant up-regulation of four genes, PMT, TRI, CYP80F1, and H6H. Additionally, it was observed that the levels of hyoscyamine decreased while scopolamine levels increased due to the facilitated conversion of hyoscyamine to scopolamine, with no effect of UV-B on the release of either [78].
4.2. Chemical Elicitors
Chemical elicitors are commonly linked to a range of ions. Ca2+ is a well-established second messenger that produces a marked effect on signal transduction and cellular regulation [79]. It can also induce defense responses, which is similar to the mechanism of action exhibited by elicitors in general [80]. It has been shown that Ca2+ activates the expression of PMT in the synthetic pathway, thereby enhancing the production of TAs [81]. Gontier et al. discovered that adding 10 mM CaCl2 to suspension cell cultures of Datura innoxia led to an approximately tenfold increase in the amount of hyoscyamine and scopolamine in the cells [82,83]. The study conducted by Boualem found that the treatment of approximately 9 mM CaCl2 for 24 h resulted in the highest yield of TAs in Datura innoxia hairy roots [84]. For the hairy roots of Datura stramonium, higher concentrations of Ca2+ (~18 mM) significantly increased hyoscyamine content, while lower concentrations of Ca2+ (less than 1 mM) inhibited PMT activity, resulting in a decrease in hyoscyamine content [84,85]. Al is a silvery-white light metal like Ca monomers. Micromolar levels of Al-induced in micropropagated plants of Datura innoxia were found to promote the activity of the antioxidant enzyme, scavenging of ROS, prevention of oxidative damage, and an increase in the content of TAs [86]. Heavy metal ions will disrupt the structure of the cytoplasmic membrane and increase the permeability of substances, which are detrimental to the integrity and viability of plant tissues [87]. CdCl2 and CuCl2 have adverse effects on the growth of Atropa belladonna, Brugmansia candida, and Datura stramonium hairy roots while promoting the release of hyoscyamine and scopolamine [27,51,88]. Certain heavy metal ions have the potential to induce the synthesis and accumulation of TAs. The presence of the Ag+ in Anisodus acutangulus resulted in an elevation of putrescine levels and the expression of AaPMT1, with a trend of increasing, followed by decreasing, and then increasing production of TAs up to 96 h compared to the control [89]. In vitro propagated Atropa belladonna plants elicited with chromium revealed increased levels of H6H transcripts and elevated levels of hyoscyamine and scopolamine [90].
4.3. Plant Hormones and Growth Regulators
The main plant hormones include auxin (IAA), gibberellin (GA), jasmonic acid (JA), cytokinin (CTK), ethylene(ETH), abscisic acid (ABA), brassinosteroids (BR), salicylic acid (SA), and strigolactone (SL) [91]. In addition to these, there are many other plant growth regulators and their derivatives with similar effects. Among them, the JA and SA analogs are most studied for their ability to induce TAs. The following section highlights the actions of these two substances.
JA is an important signal for the biosynthesis of many plant secondary metabolites, and JA signaling is a significant pathway that regulates the induced systemic resistance (ISR) mediated by inter-rhizosphere bacteria [92]. Methyl jasmonate (MeJA), the methyl ester of JA, is widely recognized as a potent elicitor for inducing the accumulation of TAs in Solanaceae. It has been shown to promote the accumulation of TAs in the hairy roots of Anisodus acutangulus, Atropa baetica, Scopolia parviflora, Hyoscyamus niger, and others. The genes involved in the biosynthesis pathways of TAs in various species were found to be more responsive to MeJA. Analysis of gene expression profiles revealed that TRI expression was increased in Anisodus acutangulus, PMT and H6H expression was increased in Atropa belladonna, MeJA may transiently regulate PMT and H6H expression in Scopolia parviflora under MeJA treatment [89,93,94]. MeJA can effectively enhance the activity of endogenous H6H in Hyoscyamus niger, thereby facilitating the conversion of hyoscyamine to scopolamine and significantly augmenting the economic value [95]. In contrast, a separate elicitation experiment conducted on the Atropa belladonna hairy roots yielded contrasting findings. As the concentration of MeJA increased, there was an observed enhancement in H6H expression, but accompanied by an increase in hyoscyamine content and a decrease in scopolamine content. This outcome can be attributed to the increase in the substrate hyoscyamine but a limited amount of H6H and inhibition of H6H enzyme activity [93]. Similarly, the induction of Hyoscyamus muticus root cultures by JA resulted in the accumulation of synthetic precursors (putrescine, methyl putrescine), but the production of hyoscyamine and scopolamine was not effectively induced [96]. This also suggests that increasing precursor mass does not necessarily result in an appreciable increase in end-product synthesis, which may involve extremely complex regulatory mechanisms. In addition to stimulating the synthesis of TAs in root cultures, MeJA can also act as an osmotic agent to facilitate the accumulation of substances in the medium [96]. Jaremicz et al. treated hairy roots of Hyoscyamus niger with 0.1 mM and 1 mM of MeJA, respectively, and found that the medium hyoscyamine and scopolamine content was higher than the control at the time of the assay, especially at the 1 mM treatment [53]. Similar results were obtained for the elicitation of Datura stramonium hairy roots using MeJA, which is highly advantageous due to the relatively easier collection of extracellular alkaloids [97].
SA mainly mediates systemic acquired resistance (SAR), and its increased levels are often seen as a marker of SAR [98]. There is a strong correlation between increased levels of SA and its conjugates in infected plants and the development of disease resistance [99]. This also suggests that SA might be a preferable elicitor. The effect of SA and its derivative acetylsalicylic acid (ASA) on secondary metabolites exhibits variability across different species. SA increases scopolamine levels in Scopolia parviflora adventitious root cultures by inducing the expression of H6H [94]. Harfi et al. elicited three Datura species (Datura stramonium, Datura tatula, and Datura innoxia) with SA and ASA, then found that 0.1 mM was the optimum treatment concentration for all three Datura species, with the highest hyoscyamine yield of all treatments obtained at 0.1 mM ASA for Datura tatula [100]. Anisodus luridus hairy root cultures were induced with three different concentrations (0.01 mM, 0.1 mM, and 1 mM) of ASA. The results revealed that 1 mM ASA resulted in the highest expression levels of PMT, TRI, CYP80F1, and H6H, corresponding to the strongest TAs synthesis and significantly induced the release of scopolamine [78]. In contrast, the induction of Anisodus acutangulus hairy roots with SA dissolved in ethanol revealed a decrease in the average production of hyoscyamine and scopolamine but a consistent increase in the production of anisodine. Interestingly, when treated with ethanol alone, it was observed that the expression of H6H increased, the competition response was inhibited, and the yield of TAs effectively increased. This suggests that SA may strongly inhibit the effect of ethanol during the synthesis of TAs in Anisodus acutangulus [89]. In a study conducted, SA (0.2–2 mM) had no significant effect or even a negative effect on TAs accumulation in Atropa belladonna hairy roots, but the release ratio of TAs increased as the concentration of SA exceeded 0.5 mM. It showed that SA possesses a strong capability in facilitating the release of TAs [101].
In addition to the aforementioned commonly used hormones, researchers have explored the impacts of various other prevalent hormones. IAA and its analogs are usually detrimental to the synthesis of TAs in hairy roots. Probably because the T-DNA of Agrobacterium tumefaciens contains the gene responsible for IAA synthesis, the addition of exogenous IAA-like substances causes excessive inhibition [102]. This seems to be confirmed by the decreased production of TAs in Hyoscyamus niger and Datura stramonium when the external concentration of IAA analogs is increased [103,104,105]. However, there are exceptional cases, as observed in Hyoscyamus muticus, where the authors postulate the possibility of a deficiency in endogenous IAA within the cultured root system [102]. GA and ABA are among the main internal signals for plant survival and growth in stressful environments [106]. There are at least 37 known species of GA. GA7 can positively affect the accumulation and transformation of TAs in Brugmansia candida at effective concentrations of 10−4, 10−1, 1 mg/L, and 10−1 mg/L for two different clones [107]. Both GA3 and ABA strongly inhibited the production of scopolamine in the hairy roots of Hyoscyamus muticus but had no significant effect on root morphology [102]. ABA did not alter the content of TAs in Datura stramonium root cultures, but in another study, ABA promoted alkaloid accumulation in leaves, suggesting the conjecture that ABA-related receptors may be in the leaves [105]. However, this does not explain well the results obtained in the former plant. Different effects of ABA on the TAs content of leaves and roots were indeed also found in studies on the effect of ABA on TAs content in Anisodus acutangulus plants. In the 24 h period, the roots exhibited an increase in hyoscyamine and anisodine content, while scopolamine content decreased. Meanwhile, the leaves showed an increase in scopolamine and anisodine content, with a decrease in hyoscyamine content. Surprisingly, none of the genes in the synthesis pathway were significantly induced, The reason has not been clearly explained [108].
In recent years, new plant growth regulators have been discovered and applied in the production of TAs as elicitors. Glyphosate belongs to the organophosphorus herbicide class and functions as a plant growth regulator [109]. It reduced the content of phenylalanine in jimsonweed (Datura stramonium) seedlings, reduced the content of tropinone and tropine at 10−7 and 10−6 M, as well as the expression of PMT mRNA transcripts in roots at 10−6 M. Although no direct influence on the content of hyoscyamine and scopolamine was revealed, a significant inhibitory effect can be inferred [110]. Coronarin (COR) is a bacterial toxin produced by Pseudomonas syringae [111]. The mechanism of action of COR is to mimic a bioactive JA coupling (JA-Ile), which subsequently targets the JA receptor for additional modulation [112]. Its effect on the synthesis of TAs is more complex. The inhibitory effect of COR at 0.5 uM on hyoscyamine production in the hairy roots of Atropa acuminata increased with time, but scopolamine levels were found to be fivefold higher than the control at one week after treatment [113].
4.4. New Types
Nanoparticles are widely used in medicine and immunology-related fields and, in recent years, have emerged as a novel inducing material capable of eliciting metabolic and physiological responses [114]. They can increase the activity of nitrate reductase and glutamate dehydrogenase to affect nitrogen metabolism in plants, thus increasing protein levels, stimulating gene expression, and inducing the biosynthesis of secondary metabolites [115]. Different researchers have made attempts to apply nanoparticles in the production of TAs. Moharrami et al. treated the hairy roots of Hyoscyamus reticulatus with different concentrations of iron oxide nanoparticles and found that the maximum levels of hyoscyamine and scopolamine were reached at 900 mg/L for 24 h and 450 mg/L for 48 h, respectively. The genetic DNA experienced toxicity as a result of higher concentrations and longer treatment times, leading to a decrease in product content [116,117]. The nanoparticles could provide abundant Fe2+ for the enzymatic reaction, and further analysis showed that this induction method had an effect on the activity and expression of both PMT and H6H, thus increasing the yield of the corresponding TAs [118]. Iron oxide nanoparticles could also stimulatingly affect the expression of H6H in Atropa belladonna hairy roots, leading to the accumulation of scopolamine [119]. Similarly, the application of different concentrations of zinc oxide nanoparticles (50, 100, 200, and 24 mg/L) to Hyoscyamus reticulatus hairy roots led to the highest levels of hyoscyamine and scopolamine content at 100 mg/L, 48 h, and 100 mg/L, 72 h, respectively. The analysis using RT-PCR demonstrated that zinc oxide increased the expression level of the H6H transcript, and scopolamine accumulation was positively correlated with H6H expression [115]. 100 mg/L of silica nanoparticles treated with Hyoscyamus reticulatus for 24 h revealed that the highest levels of hyoscyamine and scopolamine were achieved through the increase of PMT and H6H expression levels [120].
Sodium nitroprusside (SNP) is a nitric oxide donor that releases NO, which is involved in disease and stress resistance responses in plants as a cellular and intercellular signal molecule [121]. NO can interact with JA, MeJA, and SA signals to mediate the biosynthesis of secondary metabolites [122]. Treatment of Hyoscyamus reticulatus hairy root cultures with various concentrations of SNP resulted in significant alterations in the activities of antioxidant enzymes, including ascorbate peroxidase (APX), catalase (CAT), and peroxidase (POD). Additionally, the production of hyoscyamine and scopolamine reached a maximum at 50 μM, 48 h and 100 μM, 24 h, respectively [123]. The effects of abiotic elicitors on TAs are presented in Table 2, including some that are not extensively discussed in the main text.

Table 2.
Effects of abiotic elicitors on TAs.
5. Effects of Combined Elicitation on TAs
It is a common practice to employ multiple elicitors to investigate their impact on the yield of TAs. Since several elicitors have a positive effect on TAs biosynthesis, it is worthwhile to explore the potential synergistic effects that may arise from their combined application. Additionally, if one elicitor produces a negative impact, we also need to assess the potential of other elicitors to counteract or alleviate adverse effects, particularly in relation to physical stress responses.
We have previously described the respective effects of different elicitors. In the following section, we will describe the combined effects of various elicitors. Cyclodextrins (CDs) are a class of cyclic oligosaccharides produced by Bacillus that have the ability to induce immune responses and promote the accumulation of secondary metabolites in plants [129,130]. Co-treatment of Atropa acuminata hairy roots with 50 mM methyl-β-cyclodextrin (β-CD) and 0.5 uM of COR was found to positively affect both scopolamine production and hyoscyamine release. However, the same treatment negatively affected both hyoscyamine and scopolamine production from Atropa belladonna hairy roots [113]. Ghorbanpor et al. discovered that the combination of biotic elicitors (plant growth promoting rhizobacteria (PGPR) strains) and abiotic elicitors (WDS) on Hyoscyamus niger plants was very beneficial. At low WDS levels (30% depletion in field water holding), Pseudomonas putida (PP) is considered effective, while at moderate and heavy levels (60% depletion and 90% depletion in field water holding), Pseudomonas fluorescens (PF) is considered more effective [131]. Studies have shown that PGPR can improve the activity of related enzymes in plants through the release of plant growth regulators, and WDS also induces the yield of IAA in plants, with a clear synergistic amplification between the two in this respect [132]. Khanam et al. discovered that a combination of two growth factors and CTK(10 mM benzyladenine (BA) + 1 mM napthaleneacetic acid (NAA) and 10 mM BA + 0.1 mM indolyl-3-butyric acid (IBA)) in cultured Duboisia myoporoides rootless shoots also produced TAs [133], in contrast to some species without TAs in rootless shoots [134,135]. This greatly breaks our existing understanding, as to our knowledge, TAs are primarily produced in the roots, and this culture provides us with a novel avenue for further investigation. The authors also show that a further search for the optimal combination of plant growth regulators on this basis would facilitate the production of more TAs in rootless shoots.
Understanding the mechanisms of interaction between multiple elicitors is important when designing to enhance induction, as it allows for better regulation. For instance, the much-studied signaling pathway between SA and JA/ETH may respond differently under different conditions [136]. Unfortunately, there is little literature available to elucidate the mechanisms underlying the interactions between different elicitors during the biosynthesis of TAs. The effects of combined elicitors on TAs are presented in Table 3. It should be noted that certain effects are not extensively discussed in the main text.

Table 3.
Effect of combined elicitation on TAs.
6. Combination of Elicitors with Other Strategies to Increase TAs
In addition to elicitation, strategies such as gene overexpression, transcription factor regulation, plant polyploidization, and precursor feeding are also effective methods to improve the synthesis of secondary metabolites in medicinal plants [5,138,139,140]. The combination of multiple strategies tends to improve the content and/or yield of plant secondary metabolites more effectively compared to the single strategy. The literature has documented the utilization of a combination of three strategies along with elicitors (Table 4).

Table 4.
Effect of elicitation strategies in combination with other strategies on TAs.
Overexpressing key genes involved in the synthetic pathway of TAs in plants/hairy roots is a common strategy to enhance the yield of TAs. In a scientific experiment, transgenic Atropa baetica hairy roots that overexpressed the H6H gene were induced with SA, MeJA, and ASA. It was observed that MeJA (0.1 mM, 4 h) had the most pronounced impact on the accumulation of scopolamine, with a 25-fold improvement in H6H gene expression [107]. Overexpression of PMT in Hyoscyamus niger hairy roots caused an increase in PMT activity and an increase in methylputrescine content but was unable to significantly increase TAs content. In contrast, PMT and H6H activity was significantly enhanced, and scopolamine content increased after MeJA treatment. Once again, this indicated the strong induced effect of MeJA on the synthesis pathway of TAs [99]. For medicinal plants, polyploids are often more valuable for the higher biomass and bioactive compounds in comparison to haploid plants [143]. Belabbassi et al. induced Datura stramonium hairy roots with different concentrations of colchicine to obtain tetraploid hairy root systems. After SA and ASA treatments, a notable increase in the yield of scopolamine was observed in comparison to the other treatment groups and control groups. This finding suggests the favorable combined effect of polyploidization and elicitation on the synthesis of scopolamine [141]. The precursor feeding strategy is an efficacious approach to enhancing the yield of secondary metabolites in plants, as the endogenous level of biological precursors is usually a major limiting factor for biosynthesis. Boitel et al. discovered that the addition of the surfactant Tween 20 and the precursors (L-phenylalanine or DL-β-phenyllactic acid (0.5 mM)) at the late growth stage of Datura innoxia hairy roots greatly increased the total hyoscyamine content, whereas feeding the precursors alone had no effect on the synthesis of TAs. Tween 20 was characterized as a chemical elicitor that potentially possesses the capability to induce the synthesis pathway and the release of TAs [142]. This provides visual experimental evidence for combining elicitation with a precursor feeding strategy to enhance TAs production.
In addition to the above-combined strategies, the combination of immobilization of cultured cells or in situ, product removal with elicitation treatment has not been reported to improve the metabolic yield of TAs. However, these approaches have been applied in Plumbago indica, Tripterygium wilfordii, and Lithospermum erythrorhiz, resulting in increased production of secondary metabolites [144,145,146]. In the future, the combination of these two strategies can also be applied to medicinal plants of the Solanaceae family in order to offer novel techniques for enhancing the synthesis of TAs.
7. Prosperity
Previous studies have shown that the utilization of various elicitors, either individually or in combination, along with other strategies, effectively regulates the content change of TAs in medicinal plants. With the change in elicitation concentration and experimental duration, the regulatory effect may be complex and variable. Simultaneously, achieving identical outcomes by using the same concentration of elicitors in the same plant is challenging due to variations in external conditions and the diverse physiological and biochemical states of plant lines. Therefore, we emphasize that the existing data should only be used for reference, and it is necessary to further optimize the elicitation conditions based on the actual situation. In the elicitation studies of TAs, hairy roots are the predominant site for elicitation. Hairy root elicitation is an optimal model for fundamental research in the commercial production of TAs. There are currently very limited examples of this strategy in industrial practice, hampered by the immaturity of large-scale cultivation techniques for hairy roots. We believe that the utilization of automated technology to regulate multiple parameters within the hairy root bioreactor and complete the extraction of TAs can effectively overcome this constraint and expedite the commercialization process.
In the exploration of the elicitation mechanism, due to the underdeveloped state of technology development and the ambiguity of genes related to TAs synthesis, the previous experiments were rarely able to show the comprehensive gene expression and transcriptional regulation change during induction. With the complete elucidation of the TAs biosynthesis pathway, an increasing number of researchers have attempted to explain the elicited changes at the molecular level with some success. However, the signal transduction pathway involved in the elicitor treatment is not a monolithic and linear process. Instead, it comprises a tightly linked network of numerous genes and transcription factors, which also makes it extremely difficult to completely elucidate the mechanism of action of the elicitor at the molecular level. In addition to employing conventional methods for transcriptional regulation analysis, researchers have endeavored to use mathematical modeling and algorithmic prediction to address pertinent issues. As a result, substantial advancements have been achieved in many research domains, including disease intervention and organ development. We possess grounds to assert that information technology will also play an increasingly important role in the study of elicitors. At the same time, relevant procedures need to be further developed and improved to achieve the goal of accurate prediction. Based on an analysis of the relevant mechanisms, the combination of joint elicitation and multi-strategy approaches will further stimulate the production potential. Additionally, the precise regulation of metabolic pathways will offer greater opportunities for the identification of novel elicitors, thereby facilitating the synthesis of TAs.
Author Contributions
Conceptualization, Y.W. and Z.L.; methodology, Y.W. and Y.L.; investigation, Y.W., H.Z. and Y.T.; writing—original draft preparation, Y.W. and Y.L.; writing—review and editing, Z.L., J.Z. and Y.T.; visualization, Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers: U1902212, 31770335, 81803660, and 32270276); the National Transgenic Major Project of China (grant number: 2019ZX08010-004); the Fourth National Survey of Traditional Chinese Medicine Resources, Chinese or Tibet Medicinal Resources Investigation in Tibet Autonomous Region (State Administration of Chinese Traditional Medicine, grant number: 20191217-540124, 20191223-540126 and 20200501-542329); Key project at central government level: The ability establishment of sustainable use for valuable Chinese medicine resources (2060302).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- O’Hagan, D. Pyrrole, Pyrrolidine, Pyridine, Piperidine and Tropane Alkaloids. Nat. Prod. Rep. 2000, 17, 435–446. [Google Scholar] [CrossRef]
- Huang, J.P.; Wang, Y.J.; Tian, T.; Wang, L.; Yan, Y.; Huang, S.X. Tropane Alkaloid Biosynthesis: A Centennial Review. Nat. Prod. Rep. 2021, 38, 1634–1658. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.; Liu, X.; Zhang, Q.; Qiang, W.; Guo, J.; Lan, X.; Chen, M.; Liao, Z. Promoting Scopolamine Biosynthesis in Transgenic Atropa belladonna Plants with Pmt and H6h Overexpression under Field Conditions. Plant Physiol. Biochem. 2016, 106, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kai, G.Y.; Lu, B.B.; Zhang, H.M.; Tang, K.X.; Jiang, J.H.; Chen, W.S. Metabolic Engineering of Tropane Alkaloid Biosynthesis in Plants. J. Integr. Plant Biol. 2005, 47, 136–143. [Google Scholar] [CrossRef]
- Ramachandra Rao, S.; Ravishankar, G.A. Plant Cell Cultures: Chemical Factories of Secondary Metabolites. Biotechnol. Adv. 2002, 20, 101–153. [Google Scholar] [CrossRef] [PubMed]
- Boller, T.; Felix, G. A Renaissance of Elicitors: Perception of Microbe-Associated Molecular Patterns and Danger Signals by Pattern-Recognition Receptors. Annu. Rev. Plant Biol. 2009, 60, 379–406. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor Signal Transduction Leading to Production of Plant Secondary Metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef]
- Hammerschmidt, R. PHYTOALEXINS: What Have We Learned After 60 Years? Annu. Rev. Phytopathol. 1999, 37, 285–306. [Google Scholar] [CrossRef]
- Boller, T. Chemoperception of Microbial Signals in Plant Cells. Annu. Rev. Plant Physiol. Mol. Biol. 1995, 46, 189–214. [Google Scholar] [CrossRef]
- Halder, M.; Sarkar, S.; Jha, S. Elicitation: A Biotechnological Tool for Enhanced Production of Secondary Metabolites in Hairy Root Cultures. Eng. Life Sci. 2019, 19, 880–895. [Google Scholar] [CrossRef] [PubMed]
- Guru, A.; Dwivedi, P.; Kaur, P.; Pandey, D.K. Exploring the Role of Elicitors in Enhancing Medicinal Values of Plants under in Vitro Condition. S. Afr. J. Bot. 2022, 149, 1029–1043. [Google Scholar] [CrossRef]
- Chen, H.; Chena, F.; Chiu, F.C.K.; Lo, C.M.Y. The Effect of Yeast Elicitor on the Growth and Secondary Metabolism of Hairy Root Cultures of Salvia miltiorrhiza. Enzym. Microb. Technol. 2001, 28, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Chattopadhyay, T.; Thakur, D.; Kumar, N.; Kumar, T.; Singh, P.K. Hairy Root Culture for In Vitro Production of Secondary Metabolites: A Promising Biotechnological Approach. In Biotechnological Approaches for Medicinal and Aromatic Plants: Conservation, Genetic Improvement and Utilization; Kumar, N., Ed.; Springer: Singapore, 2018; pp. 235–250. ISBN 9789811305351. [Google Scholar]
- Georgiev, M.I.; Agostini, E.; Ludwig-Müller, J.; Xu, J. Genetically Transformed Roots: From Plant Disease to Biotechnological Resource. Trends Biotechnol. 2012, 30, 528–537. [Google Scholar] [CrossRef]
- Halder, T.; Ghosh, B. Hairy Root Cultures of Physalis minima L.—An Alternative Source of Withaferin A Production. Plant Cell Tissue Organ Cult. 2023, 152, 31–44. [Google Scholar] [CrossRef]
- Frankfater, C.R.; Dowd, M.K.; Triplett, B.A. Effect of Elicitors on the Production of Gossypol and Methylated Gossypol in Cotton Hairy Roots. Plant Cell Tissue Organ Cult. 2009, 98, 341–349. [Google Scholar] [CrossRef]
- Häkkinen, S.T.; Moyano, E.; Cusidó, R.M.; Oksman-Caldentey, K.M. Exploring the Metabolic Stability of Engineered Hairy Roots after 16 Years Maintenance. Front. Plant Sci. 2016, 7, 1486. [Google Scholar] [CrossRef]
- Jeong, G.T.; Park, D.H.; Ryu, H.W.; Hwang, B.; Woo, J.C.; Kim, D.; Kim, S.W. Production of Antioxidant Compounds by Culture of Panax ginseng C.A. Meyer Hairy Roots. In Twenty-Sixth Symposium on Biotechnology for Fuels and Chemicals; Davison, B.H., Evans, B.R., Finkelstein, M., McMillan, J.D., Eds.; Humana Press: Totowa, NJ, USA, 2005; pp. 1147–1157. ISBN 978-1-59259-991-2. [Google Scholar]
- Wiktorowska, E.; Długosz, M.; Janiszowska, W. Significant Enhancement of Oleanolic Acid Accumulation by Biotic Elicitors in Cell Suspension Cultures of Calendula officinalis L. Enzym. Microb. Technol. 2010, 46, 14–20. [Google Scholar] [CrossRef]
- Patil, J.G.; Ahire, M.L.; Nitnaware, K.M.; Panda, S.; Bhatt, V.P.; Kishor, P.B.K.; Nikam, T.D. In Vitro Propagation and Production of Cardiotonic Glycosides in Shoot Cultures of Digitalis purpurea L. by Elicitation and Precursor Feeding. Appl. Microbiol. Biot. 2013, 97, 2379–2393. [Google Scholar] [CrossRef]
- Rothe, G.; Dräger, B. Tropane Alkaloids—Metabolic Response to Carbohydrate Signal in Root Cultures of Atropa belladonna. Plant Sci. 2002, 163, 979–985. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and Antioxidant Properties of Chitosan and Its Derivatives and Their Applications: A Review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef] [PubMed]
- Stasińska-Jakubas, M.; Hawrylak-Nowak, B. Protective, Biostimulating, and Eliciting Effects of Chitosan and Its Derivatives on Crop Plants. Molecules 2022, 27, 2801. [Google Scholar] [CrossRef] [PubMed]
- El Hadrami, A.; Adam, L.R.; El Hadrami, I.; Daayf, F. Chitosan in Plant Protection. Mar. Drugs 2010, 8, 968–987. [Google Scholar] [CrossRef] [PubMed]
- Pitta-Alvarez, S.I.; Giulietti, A.M. Influence of Chitosan, Acetic Acid and Citric Acid on Growth and Tropane Alkaloid Production in Transformed Roots of Brugmansia candida Effect of Medium PH and Growth Phase. Plant Cell Tissue Organ Cult. 1999, 59, 31–38. [Google Scholar] [CrossRef]
- Lee, K.T.; Yamakawa, T.; Kodama, T.; Shimomura, K. Effects of Chemicals on Alkaloid Production by Transformed Roots of Belladonna. Phytochemistry 1998, 49, 2343–2347. [Google Scholar] [CrossRef]
- Rothe, G.; Garske, U.; Dräger, B. Calystegines in Root Cultures of Atropa belladonna Respond to Sucrose, Not to Elicitation. Plant Sci. 2001, 160, 1043–1053. [Google Scholar] [CrossRef]
- Hong, M.L.K.; Bhatt, A.; Ping, N.S.; Keng, C.L. Detection of Elicitation Effect on Hyoscyamus niger L. Root Cultures for the Root Growth and Production of Tropane Alkaloids. Rom. Biotechnol. Lett. 2012, 17, 7340–7351. [Google Scholar]
- Benedetti, M.; Mattei, B.; Pontiggia, D.; Salvi, G.; Savatin, D.V.; Ferrari, S. Methods of Isolation and Characterization of Oligogalacturonide Elicitors. In Plant Pattern Recognition Receptors: Methods and Protocols; Shan, L., He, P., Eds.; Springer: New York, NY, USA, 2017; pp. 25–38. ISBN 978-1-4939-6859-6. [Google Scholar]
- Cabrera, J.C.; Boland, A.; Messiaen, J.; Cambier, P.; Van Cutsem, P. Egg Box Conformation of Oligogalacturonides: The Time-Dependent Stabilization of the Elicitor-Active Conformation Increases Its Biological Activity. Glycobiology 2008, 18, 473–482. [Google Scholar] [CrossRef]
- Zabetakis, I.; Edwards, R.; O’Hagan, D. Elicitation of Tropane Alkaloid Biosynthesis in Transformed Root Cultures of Datura stramonium. Phytochemistry 1999, 50, 53–56. [Google Scholar] [CrossRef]
- Tauzin, A.S.; Giardina, T. Sucrose and Invertases, a Part of the Plant Defense Response to the Biotic Stresses. Front. Plant Sci. 2014, 5, 293. [Google Scholar] [CrossRef]
- Tun, W.; Yoon, J.; Vo, K.T.X.; Cho, L.H.; Hoang, T.V.; Peng, X.; Kim, E.J.; Win, K.T.Y.S.; Lee, S.W.; Jung, K.H.; et al. Sucrose Preferentially Promotes Expression of OsWRKY7 and OsPR10a to Enhance Defense Response to Blast Fungus in Rice. Front. Plant Sci. 2023, 14, 1117023. [Google Scholar] [CrossRef]
- Morkunas, I.; Marczak, Ł.; Stachowiak, J.; Stobiecki, M. Sucrose-Induced Lupine Defense against Fusarium oxysporum. Sucrose-Stimulated Accumulation of Isoflavonoids as a Defense Response of Lupine to Fusarium oxysporum. Plant Physiol. Biochem. 2005, 43, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmson, A.; Häkkinen, S.T.; Kallio, P.T.; Oksman-Caldentey, K.M.; Nuutila, A.M. Heterologous Expression of Vitreoscilla Hemoglobin (VHb) and Cultivation Conditions Affect the Alkaloid Profile of Hyoscyamus muticus Hairy Roots. Biotechnol. Prog. 2006, 22, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Oksman-Caldentey, K.M.; Sevón, N.; Vanhala, L.; Hiltunen, R. Effect of Nitrogen and Sucrose on the Primary and Secondary Metabolism of Transformed Root Cultures of Hyoscyamus muticus. Plant Cell Tissue Organ Cult. 1994, 38, 263–272. [Google Scholar] [CrossRef]
- Matsubayashi, Y.; Takagi, L.; Sakagami, Y. Phytosulfokine-α, a Sulfated Pentapeptide, Stimulates the Proliferation of Rice Cells by Means of Specific High- and Low-Affinity Binding Sites. Proc. Natl. Acad. Sci. USA 1997, 94, 13357–13362. [Google Scholar] [CrossRef]
- Yu, L.; Di, Q.; Zhang, D.; Liu, Y.; Li, X.; Mysore, K.S.; Wen, J.; Yan, J.; Luo, L. A Legume-Specific Novel Type of Phytosulfokine, PSK-δ, Promotes Nodulation by Enhancing Nodule Organogenesis. J. Exp. Bot. 2022, 73, 2698–2713. [Google Scholar] [CrossRef]
- Sasaki, K.; Ishise, T.; Shimomura, K.; Kobayashi, T.; Matsubayashi, Y.; Sakagami, Y.; Umetsu, H.; Kamada, H. Effects of Phytosulfokine-α on Growth and Tropane Alkaloid Production in Transformed Roots of Atropa belladonna. Plant Growth Regul. 2002, 36, 87–90. [Google Scholar] [CrossRef]
- Haile, S.; Ayele, A. Pectinase from Microorganisms and Its Industrial Applications. Sci. World J. 2022, 2022, e1881305. [Google Scholar] [CrossRef]
- Płażek, A.; Hura, K.; Żur, I. Reaction of Winter Oilseed Rape Callus to Different Concentrations of Elicitors: Pectinase or Chitosan. Acta Physiol. Plant. 2003, 25, 83–89. [Google Scholar] [CrossRef]
- Pitta Alvarez, S.; Marconi, P.L.; Giulietti, A. Comparison of the Influence of Different Elicitors on Hyoscyamine and Scopolamine Content in Hairy Root Cultures of Brugmansia candida. In Vitro Cell. Dev. Biol.-Plant 2003, 39, 640–644. [Google Scholar] [CrossRef]
- Pitta-Alvarez, S.I.; Spollansky, T.C.; Giulietti, A.M. Scopolamine and Hyoscyamine Production by Hairy Root Cultures of Brugmansia candida: Influence of Calcium Chloride, Hemicellulase and Theophylline. Biotechnol. Lett. 2000, 22, 1653–1656. [Google Scholar] [CrossRef]
- Kozioł, A.; Cybulska, J.; Pieczywek, P.M.; Zdunek, A. Changes of Pectin Nanostructure and Cell Wall Stiffness Induced in Vitro by Pectinase. Carbohyd. Polym. 2017, 161, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Yuan, H.; Liu, M.; Liu, Q.; Zhang, S.; Liu, H.; Jiang, Y.; Huang, D.; Wang, T. Yeast Extract: Characteristics, Production, Applications and Future Perspectives. J. Microbiol. Biotechnol. 2023, 33, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, M.; Abdul, M. Yeast Extract Elicitation Increases Vinblastine and Vincristine Yield in Protoplast Derived Tissues and Plantlets in Catharanthus roseus. Rev. Bras. Farm. 2017, 27, 549–556. [Google Scholar] [CrossRef]
- Chen, H.; Chen, F. Effect of Yeast Elicitor on the Secondary Metabolism of Ti-Transformed Salvia miltiorrhiza Cell Suspension Cultures. Plant Cell Rep. 2000, 19, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Yang, Y.; Liu, X.; Wu, N.B. Mechanism exploration on nitrogen metabolism and secondary metabolism in Atropa belladonna hairy roots treated with yeast extract. China J. Chin. Mater. Medica 2018, 43, 1610–1617. (In Chinese) [Google Scholar] [CrossRef]
- Hedayati, A.; Hemmaty, S.; Nourozi, E.; Amirsadeghi, A. Effect of Yeast Extract on H6h Gene Expression and Tropane Alkaloids Production in Atropa belladonna L. Hairy Roots. Russ. J. Plant Physiol. 2021, 68, 102–109. [Google Scholar] [CrossRef]
- Pitta–Alvarez, S.I.; Spollansky, T.C.; Giulietti, A.M. The Influence of Different Biotic and Abiotic Elicitors on the Production and Profile of Tropane Alkaloids in Hairy Root Cultures of Brugmansia candida. Enzym. Microb. Technol. 2000, 26, 252–258. [Google Scholar] [CrossRef]
- Cardillo, A.B.; Perassolo, M.; Sartuqui, M.; Rodríguez Talou, J.; Giulietti, A.M. Production of Tropane Alkaloids by Biotransformation Using Recombinant Escherichia Coli Whole Cells. Biochem. Eng. J. 2017, 125, 180–189. [Google Scholar] [CrossRef]
- Jaremicz, Z.; Luczkiewicz, M.; Kokotkiewicz, A.; Krolicka, A.; Sowinski, P. Production of Tropane Alkaloids in Hyoscyamus niger (Black Henbane) Hairy Roots Grown in Bubble-Column and Spray Bioreactors. Biotechnol. Lett. 2014, 36, 843–853. [Google Scholar] [CrossRef]
- Guo, J.; Cheng, Y. Advances in Fungal Elicitor-Triggered Plant Immunity. Int. J. Mol. Sci. 2022, 23, 12003. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Motaal, F.F.; Nassar, M.S.M.; El-Zayat, S.A.; El-Sayed, M.A.; Ito, S. Responses of Fungi to Tropane Alkaloids Produced by a Medicinal Plant Hyoscyamus muticus (Egyptian Henbane). Folia Microbiol. 2009, 54, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Dabur, R.; Singh, H.; Chhillar, A.K.; Ali, M.; Sharma, G.L. Antifungal Potential of Indian Medicinal Plants. Fitoterapia 2004, 75, 389–391. [Google Scholar] [CrossRef]
- Shafique, S.; Shafique, S. Antifungal Activity of N-Hexane Extracts of Datura metel against Ascochyta rabiei. Mycopath 2008, 6, 31–35. [Google Scholar]
- Ballica, R.; Ryu, D.D.Y.; Kado, C.I. Tropane Alkaloid Production in Datura stramonium Suspension Cultures: Elicitor and Precursor Effects. Biotechnol. Bioeng. 1993, 41, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Ajungla, L.; Patil, P.P.; Barmukh, R.B.; Nikam, T.D. Influence of Biotic and Abiotic Elicitors on Accumulation of Hyoscyamine and Scopolamine in Root Cultures of Datura metel L. Indian J. Biotechnol. 2009, 8, 317–322. [Google Scholar]
- Kang, S.M.; Min, J.Y.; Kim, Y.D.; Karigar, C.S.; Kim, S.W.; Goo, G.H.; Choi, M.S. Effect of Biotic Elicitors on the Accumulation of Bilobalide and Ginkgolides in Ginkgo biloba Cell Cultures. J. Biotechnol. 2009, 139, 84–88. [Google Scholar] [CrossRef]
- Jung, H.Y.; Kang, S.M.; Kang, Y.M.; Kang, M.J.; Yun, D.J.; Bahk, J.D.; Yang, J.K.; Choi, M.S. Enhanced Production of Scopolamine by Bacterial Elicitors in Adventitious Hairy Root Cultures of Scopolia parviflora. Enzym. Microb. Technol. 2003, 33, 987–990. [Google Scholar] [CrossRef]
- Moussous, A.; Paris, C.; Khelifi-Slaoui, M.; Bekhouche, M.; Zaoui, D.; Rosloski, S.M.; Makhzoum, A.; Desobry, S.; Khelifi, L. Pseudomonas spp. Increases Root Biomass and Tropane Alkaloid Yields in Transgenic Hairy Roots of Datura spp. In Vitro Cell. Dev. Biol.-Plant 2018, 54, 117–126. [Google Scholar] [CrossRef]
- Lan, H.; Lai, B.; Zhao, P.; Dong, X.; Wei, W.; Ye, Y.; Wu, Z. Cucumber Mosaic Virus Infection Modulated the Phytochemical Contents of Passiflora edulis. Microb. Pathog. 2020, 138, 103828. [Google Scholar] [CrossRef]
- Wink, M. Plant Breeding: Importance of Plant Secondary Metabolites for Protection against Pathogens and Herbivores. Theoret. Appl. Genet. 1988, 75, 225–233. [Google Scholar] [CrossRef]
- Mihálik, D.; Hančinský, R.; Kaňuková, Š.; Mrkvová, M.; Kraic, J. Elicitation of Hyoscyamine Production in Datura stramonium L. Plants Using Tobamoviruses. Plants 2022, 11, 3319. [Google Scholar] [CrossRef] [PubMed]
- Min, J.Y.; Jung, H.Y.; Kang, S.M.; Kim, Y.D.; Kang, Y.M.; Park, D.J.; Prasad, D.T.; Choi, M.S. Production of Tropane Alkaloids by Small-Scale Bubble Column Bioreactor Cultures of Scopolia parviflora Adventitious Roots. Bioresour. Technol. 2007, 98, 1748–1753. [Google Scholar] [CrossRef]
- Shakeran, Z.; Keyhanfar, M.; Ghanadian, M. Biotic Elicitation for Scopolamine Production by Hairy Root Cultures of Datura metel. Mol. Biol. Res. Commun. 2017, 6, 169–179. [Google Scholar] [CrossRef]
- Nowak, M.; Selmar, D. Cellular Distribution of Alkaloids and Their Translocation via Phloem and Xylem: The Importance of Compartment PH. Plant Biol. 2016, 18, 879–882. [Google Scholar] [CrossRef]
- Phyo, P.; Gu, Y.; Hong, M. Impact of Acidic PH on Plant Cell Wall Polysaccharide Structure and Dynamics: Insights into the Mechanism of Acid Growth in Plants from Solid-State NMR. Cellulose 2019, 26, 291–304. [Google Scholar] [CrossRef]
- Angelova, M.I.; Bitbol, A.F.; Seigneuret, M.; Staneva, G.; Kodama, A.; Sakuma, Y.; Kawakatsu, T.; Imai, M.; Puff, N. PH Sensing by Lipids in Membranes: The Fundamentals of PH-Driven Migration, Polarization and Deformations of Lipid Bilayer Assemblies. Biochim. Biophys. Acta-Biomembr. 2018, 1860, 2042–2063. [Google Scholar] [CrossRef] [PubMed]
- Kohnen-Johannsen, K.L.; Kayser, O. Tropane Alkaloids: Chemistry, Pharmacology, Biosynthesis and Production. Molecules 2019, 24, 796. [Google Scholar] [CrossRef]
- Yukimune, Y.; Yamagata, H.; Hara, Y.; Yamada, Y. Effects of Oxygen on Nicotine and Tropane Alkaloid Production in Cultured Roots of Duboisia myoporoides. Biosci. Biotechnol. Biochem. 1994, 58, 1824–1827. [Google Scholar] [CrossRef]
- Williams, G.R.C.; Doran, P.M. Hairy Root Culture in a Liquid-Dispersed Bioreactor: Characterization of Spatial Heterogeneity. Biotechnol. Prog. 2000, 16, 391–401. [Google Scholar] [CrossRef]
- Sauerwein, M.; Wink, M.; Shimomura, K. Influence of Light and Phytohormones on Alkaloid Production in Transformed Root Cultures of Hyoscyamus albus. J. Plant Physiol. 1992, 140, 147–152. [Google Scholar] [CrossRef]
- Baricevic, D.; Umek, A.; Kreft, S.; Maticic, B.; Zupancic, A. Effect of Water Stress and Nitrogen Fertilization on the Content of Hyoscyamine and Scopolamine in the Roots of Deadly Nightshade (Atropa belladonna). Environ. Exp. Bot. 1999, 42, 17–24. [Google Scholar] [CrossRef]
- Halperin, S.J.; Flores, H.E. Hyoscyamine and Proline Accumulation in Water-Stressed Hyoscyamus muticus ‘Hairy Root’ Cultures. In Vitro Cell. Dev. Biol.-Plant 1997, 33, 240–244. [Google Scholar] [CrossRef]
- Zhang, W.J.; Björn, L.O. The Effect of Ultraviolet Radiation on the Accumulation of Medicinal Compounds in Plants. Fitoterapia 2009, 80, 207–218. [Google Scholar] [CrossRef]
- Qin, B.; Ma, L.; Wang, Y.; Chen, M.; Lan, X.; Wu, N.; Liao, Z. Effects of Acetylsalicylic Acid and UV-B on Gene Expression and Tropane Alkaloid Biosynthesis in Hairy Root Cultures of Anisodus luridus. Plant Cell Tissue Organ Cult. 2014, 117, 483–490. [Google Scholar] [CrossRef]
- Petersen, O.H.; Michalak, M.; Verkhratsky, A. Calcium Signalling: Past, Present and Future. Cell Calcium 2005, 38, 161–169. [Google Scholar] [CrossRef]
- Kadota, Y.; Kuchitsu, K. Regulation of Elicitor-Induced Defense Responses by Ca2+ Channels and the Cell Cycle in Tobacco BY-2 Cells. In Tobacco BY-2 Cells: From Cellular Dynamics to Omics; Nagata, T., Matsuoka, K., Inzé, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 207–221. ISBN 978-3-540-32674-8. [Google Scholar]
- Amdoun, R.; Khelifi, L.; Khelifi-Slaoui, M.; Amroune, S.; Asch, M.; Assaf-Ducrocq, C.; Gontier, E. Optimization of the Culture Medium Composition to Improve the Production of Hyoscyamine in Elicited Datura stramonium L. Hairy Roots Using the Response Surface Methodology (RSM). Int. J. Mol. Sci. 2010, 11, 4726–4740. [Google Scholar] [CrossRef]
- Gontier, E.; Sangwan, B.S.; Barbotin, J.N. Effects of Calcium, Alginate, and Calcium-Alginate Immobilization on Growth and Tropane Alkaloid Levels of a Stable Suspension Cell Line of Datura innoxia Mill. Plant Cell Rep. 1994, 13, 533–536. [Google Scholar] [CrossRef]
- Murthy, H.N.; Dandin, V.S.; Zhong, J.J.; Paek, K.Y. Strategies for Enhanced Production of Plant Secondary Metabolites from Cell and Organ Cultures. In Production of Biomass and Bioactive Compounds Using Bioreactor Technology; Paek, K.Y., Murthy, H.N., Zhong, J.J., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 471–508. ISBN 978-94-017-9223-3. [Google Scholar]
- Harfi, B.; Khelifi-Slaoui, M.; Bekhouche, M.; Benyammi, R.; Hefferon, K.; Makhzoum, A.; Khelifi, L. Hyoscyamine Production in Hairy Roots of Three Datura Species Exposed to High-Salt Medium. In Vitro Cell. Dev. Biol.-Plant 2016, 52, 92–98. [Google Scholar] [CrossRef]
- Piñol, M.T.; Palazón, J.; Cusidó, R.M.; Ribó, M. Influence of Calcium Ion-Concentration in the Medium on Tropane Alkaloid Accumulation in Datura stramonium Hairy Roots. Plant Sci. 1999, 141, 41–49. [Google Scholar] [CrossRef]
- Karimi, F.; Khataee, E. Aluminum Elicits Tropane Alkaloid Production and Antioxidant System Activity in Micropropagated Datura innoxia Plantlets. Acta Physiol. Plant. 2012, 34, 1035–1041. [Google Scholar] [CrossRef]
- Hall, J.L. Cellular Mechanisms for Heavy Metal Detoxification and Tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Furze, J.M.; Rhodes, M.J.C.; Parr, A.J.; Robins, R.J.; Withehead, I.M.; Threlfall, D.R. Abiotic Factors Elicit Sesquiterpenoid Phytoalexin Production but Not Alkaloid Production in Transformed Root Cultures of Datura stramonium. Plant Cell Rep. 1991, 10, 111–114. [Google Scholar] [CrossRef]
- Kai, G.; Yang, S.; Zhang, Y.; Luo, X.; Fu, X.; Zhang, A.; Xiao, J. Effects of Different Elicitors on Yield of Tropane Alkaloids in Hairy Roots of Anisodus acutangulus. Mol. Biol. Rep. 2012, 39, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Vakili, B.; Karimi, F.; Sharifi, M.; Behmanesh, M. Chromium-Induced Tropane Alkaloid Production and H6H Gene Expression in Atropa belladonna L. (Solanaceae) in Vitro-Propagated Plantlets. Plant Physiol. Biochnol. 2012, 52, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant Hormone-Mediated Regulation of Stress Responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Mou, W.; Kao, Y.T.; Michard, E.; Simon, A.A.; Li, D.; Wudick, M.M.; Lizzio, M.A.; Feijó, J.A.; Chang, C. Ethylene-Independent Signaling by the Ethylene Precursor ACC in Arabidopsis Ovular Pollen Tube Attraction. Nat. Commun. 2020, 11, 4082. [Google Scholar] [CrossRef]
- Moradi, A.; Sharifi, M.; Mousavi, A. Induced Production of Tropane Alkaloids, and Expression of Hyoscyamine 6β-Hydroxylase (h6h) and Putrescine N-Methyl Transferase (pmt2) Genes in Hairy Roots and Propagated Plantlets of Atropa belladonna L. Elicited by Methyl Jasmonate. S. Afr. J. Bot. 2020, 131, 328–334. [Google Scholar] [CrossRef]
- Kang, S.M.; Jung, H.Y.; Kang, Y.M.; Yun, D.J.; Bahk, J.D.; Yang, J.; Choi, M.S. Effects of Methyl Jasmonate and Salicylic Acid on the Production of Tropane Alkaloids and the Expression of PMT and H6H in Adventitious Root Cultures of Scopolia parviflora. Plant Sci. 2004, 166, 745–751. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, B.; Lu, B.; Kai, G.; Wang, Z.; Xia, Y.; Ding, R.; Zhang, H.; Sun, X.; Chen, W.; et al. Tropane Alkaloids Production in Transgenic Hyoscyamus niger Hairy Root Cultures Over-Expressing Putrescine N-Methyltransferase Is Methyl Jasmonate-Dependent. Planta 2007, 225, 887–896. [Google Scholar] [CrossRef]
- Biondi, S.; Fornalé, S.; Oksman-Caldentey, K.M.; Eeva, M.; Agostani, S.; Bagni, N. Jasmonates Induce Over-Accumulation of Methylputrescine and Conjugated Polyamines in Hyoscyamus muticus L. Root Cultures. Plant Cell Rep. 2000, 19, 691–697. [Google Scholar] [CrossRef]
- Sun, J.W.; Zhang, H.; Wang, F.Y.; Sun, Y.M.; Sun, M. Effects of methyl jasmonate on accumulation and release of main tropane alkaloids in liquid cultures of Datura stramonium hairy root. China J. Chin. Mater. Medica 2013, 38, 1712–1718. (In Chinese) [Google Scholar]
- Zehra, A.; Raytekar, N.A.; Meena, M.; Swapnil, P. Efficiency of Microbial Bio-Agents as Elicitors in Plant Defense Mechanism under Biotic Stress: A Review. Curr. Res. Microb. Sci. 2021, 2, 100054. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Kachroo, P.; Klessig, D.F. The Arabidopsis ssi1 Mutation Restores Pathogenesis-Related Gene Expression in npr1 Plants and Renders Defensin Gene Expression Salicylic Acid Dependent. Plant Cell 1999, 11, 191–206. [Google Scholar] [CrossRef]
- Harfi, B.; Khelifi, L.; Khelifi-Slaoui, M.; Assaf-Ducrocq, C.; Gontier, E. Tropane Alkaloids GC/MS Analysis and Low Dose Elicitors’ Effects on Hyoscyamine Biosynthetic Pathway in Hairy Roots of Algerian Datura Species. Sci. Rep. 2018, 8, 17951. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Hirano, H.; Yamakawa, T.; Kodama, T.; Igarashi, Y.; Shimomura, K. Responses of Transformed Root Culture of Atropa belladonna to Salicylic Acid Stress. J. Biosci. Bioeng. 2001, 91, 586–589. [Google Scholar] [CrossRef]
- Vanhala, L.; Eeva, M.; Lapinjoki, S.; Hiltunen, R.; Oksman-Caldentey, K.M. Effect of Growth Regulators on Transformed Root Cultures of Hyoscyamus muticus. J. Plant Physiol. 1998, 153, 475–481. [Google Scholar] [CrossRef]
- Hashimoto, T.; Yukimune, Y.; Yamada, Y. Tropane Alkaloid Production in Hyoscyamus Root Cultures. J. Plant Physiol. 1986, 124, 61–75. [Google Scholar] [CrossRef]
- Hashimoto, T.; Yamada, Y. Effects of Culture Conditions on Tropane Alkaloid Formation in Hyoscyamus niger Suspension Cultures. Agric. Biol. Chem. 1987, 51, 2769–2774. [Google Scholar] [CrossRef]
- Sáenz-Carbonell, L.; Loyola-Vargas, V.M. Datura stramonium Hairy Roots Tropane Alkaloid Content as a Response to Changes in Gamborg’s B5 Medium. Appl. Biochem. Biotechnol. 1997, 61, 321–337. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Bano, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.A.; Khan, F.; Chen, Y.; Wu, C.; et al. Potential Role of Phytohormones and Plant Growth-Promoting Rhizobacteria in Abiotic Stresses: Consequences for Changing Environment. Environ. Sci. Pollut. Res. 2015, 22, 4907–4921. [Google Scholar] [CrossRef] [PubMed]
- Pitta-Alvarez, S.I.; Giulietti, A.M. Effects of Gibberellin GA7 on Kinetics of Growth and Tropane Alkaloid Accumulation in Hairy Roots of Brugmansia candida. In Vitro Cell. Dev. Biol.-Plant 1997, 33, 147–153. [Google Scholar] [CrossRef]
- Luo, X.; Weng, S.; Ni, X.; Wang, X.; Fu, X.; Xiao, J.; Kai, G. The Effects of Elicitation on the Expression of Key Enzyme Genes and on Production of Tropane Alkaloids in Anisodus acutangulus Plant. Biologia 2012, 67, 352–359. [Google Scholar] [CrossRef]
- Dinalli, R.P.; Buzetti, S.; Gazola, R.d.N.; de Castilho, R.M.M.; Jalal, A.; Galindo, F.S.; Teixeira Filho, M.C.M. Nitrogen Fertilization and Glyphosate as a Growth Regulator: Effects on the Nutritional Efficiency and Nutrient Balance in Emerald Grass. Agronomy 2022, 12, 2473. [Google Scholar] [CrossRef]
- Deng, F. Effects of Glyphosate, Chlorsulfuron, and Methyl Jasmonate on Growth and Alkaloid Biosynthesis of Jimsonweed (Datura stramonium L.). Pestic. Biochem. Phys. 2005, 82, 16–26. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, Y.; Xu, Z.; Chen, Z.; Duan, L. Physiological and Transcriptome Profiling Analyses Reveal Important Roles of Coronatine in Improving Drought Tolerance of Tobacco. J. Plant Growth Regul. 2020, 39, 1346–1358. [Google Scholar] [CrossRef]
- Alcalde, M.A.; Perez-Matas, E.; Escrich, A.; Cusido, R.M.; Palazon, J.; Bonfill, M. Biotic Elicitors in Adventitious and Hairy Root Cultures: A Review from 2010 to 2022. Molecules 2022, 27, 5253. [Google Scholar] [CrossRef]
- Fattahi, F.; Shojaeiyan, A.; Palazon, J.; Moyano, E.; Torras-Claveria, L. Methyl-β-Cyclodextrin and Coronatine as New Elicitors of Tropane Alkaloid Biosynthesis in Atropa acuminata and Atropa belladonna Hairy Root Cultures. Physiol. Plant. 2021, 172, 2098–2111. [Google Scholar] [CrossRef]
- Jiao, Q.; Li, L.; Mu, Q.; Zhang, Q. Immunomodulation of Nanoparticles in Nanomedicine Applications. Biomed Res. Int. 2014, 2014, e426028. [Google Scholar] [CrossRef]
- Asl, K.R.; Hosseini, B.; Sharafi, A.; Palazon, J. Influence of Nano-Zinc Oxide on Tropane Alkaloid Production, H6h Gene Transcription and Antioxidant Enzyme Activity in Hyoscyamus reticulatus L. Hairy Roots. Eng. Life Sci. 2019, 19, 73–89. [Google Scholar] [CrossRef]
- Dev, A.; Srivastava, A.K.; Karmakar, S. Nanomaterial Toxicity for Plants. Environ. Chem. Lett. 2018, 16, 85–100. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Shweta; Singh, S.; Singh, S.; Pandey, R.; Singh, V.P.; Sharma, N.C.; Prasad, S.M.; Dubey, N.K.; Chauhan, D.K. An Overview on Manufactured Nanoparticles in Plants: Uptake, Translocation, Accumulation and Phytotoxicity. Plant Physiol. Biochnol. 2017, 110, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Moharrami, F.; Hosseini, B.; Sharafi, A.; Farjaminezhad, M. Enhanced Production of Hyoscyamine and Scopolamine from Genetically Transformed Root Culture of Hyoscyamus reticulatus L. Elicited by Iron Oxide Nanoparticles. In Vitro Cell. Dev. Biol.-Plant 2017, 53, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Sepehri, E.; Hosseini, B.; Hedayati, A. The Effect of Iron Oxide Nano-Particles on the Production of Tropane Alkaloids, h6h Gene Expression and Antioxidant Enzyme Activity in Atropa belladonna Hairy Roots. Russ. J. Plant Physiol. 2022, 69, 122. [Google Scholar] [CrossRef]
- Hedayati, A.; Hosseini, B.; Palazon, J.; Maleki, R. Improved Tropane Alkaloid Production and Changes in Gene Expression in Hairy Root Cultures of Two Hyoscyamus Species Elicited by Silicon Dioxide Nanoparticles. Plant Physiol. Biochnol. 2020, 155, 416–428. [Google Scholar] [CrossRef]
- Lamattina, L.; García-Mata, C.; Graziano, M.; Pagnussat, G. Nitric Oxide: The Versatility of an Extensive Signal Molecule. Annu. Rev. Plant Biol. 2003, 54, 109–136. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, L.P.; Wang, J.W. Nitric Oxide Elicitation for Secondary Metabolite Production in Cultured Plant Cells. Appl. Microbiol. Biotechnol. 2012, 93, 455–466. [Google Scholar] [CrossRef]
- Khezerluo, M.; Hosseini, B.; Amiri, J. Sodium Nitroprusside Stimulated Production of Tropane Alkaloids and Antioxidant Enzymes Activity in Hairy Root Culture of Hyoscyamus reticulatus L. Acta Biol. Hung. 2018, 69, 437–448. [Google Scholar] [CrossRef]
- Endo, T.; Yamada, Y. Alkaloid Production in Cultured Roots of Three Species of Duboisia. Phytochemistry 1985, 24, 1233–1236. [Google Scholar] [CrossRef]
- Flores, H.E.; Dai, Y.; Cuello, J.L.; Maldonado-Mendoza, I.E.; Loyola-Vargas, V.M. Green Roots: Photosynthesis and Photoautotrophy in an Underground Plant Organ. Plant Physiol. 1993, 101, 363–371. [Google Scholar] [CrossRef]
- Zeynali, Z.; Hosseini, B.; Rezaei, E. Effect of Elicitation on Antioxidant Activity and Production of Tropane Alkaloids in Hyoscyamus reticulatus Hairy Root Cultures. J. Pharmacogn. 2016, 3, 43–53. [Google Scholar]
- el Jaber-Vazdekis, N.; Barres, M.L.; Ravelo, Á.G.; Zárate, R. Effects of Elicitors on Tropane Alkaloids and Gene Expression in Atropa baetica Transgenic Hairy Roots. J. Nat. Prod. 2008, 71, 2026–2031. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanpour, M.; Hatami, M.; Hatami, M. Activating Antioxidant Enzymes, Hyoscyamine and Scopolamine Biosynthesis of Hyoscyamus niger L. Plants with Nano-Sized Titanium Dioxide and Bulk Application. Acta Agric. Slov. 2015, 105, 23–32. [Google Scholar] [CrossRef]
- Szejtli, J. Past, Present and Futute of Cyclodextrin Research. Pure Appl. Chem. 2004, 76, 1825–1845. [Google Scholar] [CrossRef]
- Sabater-Jara, A.B.; Onrubia, M.; Moyano, E.; Bonfill, M.; Palazón, J.; Pedreño, M.A.; Cusidó, R.M. Synergistic Effect of Cyclodextrins and Methyl Jasmonate on Taxane Production in Taxus x Media Cell Cultures. Plant Biotechnol. J. 2014, 12, 1075–1084. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Hatami, M.; Khavazi, K. Role of Plant Growth Promoting Rhizobacteria on Antioxidant Enzyme Activities and Tropane Alkaloid Production of Hyoscyamus niger under Water Deficit Stress. Turk. J. Biol. 2013, 37, 350–360. [Google Scholar] [CrossRef]
- Yuwono, T.; Handayani, D.; Soedarsono, J.; Yuwono, T.; Handayani, D.; Soedarsono, J. The Role of Osmotolerant Rhizobacteria in Rice Growth under Different Drought Conditions. Aust. J. Agric. Res. 2005, 56, 715–721. [Google Scholar] [CrossRef]
- Khanam, N.; Khoo, C.; Khan, A.G. Effects of Cytokinin/Auxin Combinations on Organogenesis, Shoot Regeneration and Tropane Alkaloid Production in Duboisia myoporoides. Plant Cell Tissue Organ Cult. 2000, 62, 125–133. [Google Scholar] [CrossRef]
- Lin, G.D.; Griffin, W.J. Scopolamine Content of a Duboisia Hybrid in Callus Cultures. Phytochemistry 1992, 31, 4151–4153. [Google Scholar] [CrossRef]
- Saito, K.; Yamazaki, M.; Kawaguchi, A.; Murakoshi, I. Metabolism of Solanaceous Alkaloids in Transgenic Plant Teratomas Integrated with Genetically Engineered Genes. Tetrahedron 1991, 47, 5955–5968. [Google Scholar] [CrossRef]
- Koo, Y.M.; Heo, A.Y.; Choi, H.W. Salicylic Acid as a Safe Plant Protector and Growth Regulator. Plant Pathol. J. 2020, 36, 1–10. [Google Scholar] [CrossRef]
- Amdoun, R.; Khelifi, L.; Khelifi-Slaoui, M.; Amroune, S.; Benyoussef, E.H.; Thi, D.V.; Assaf-Ducrocq, C.; Gontier, E. Influence of Minerals and Elicitation on Datura stramonium L. Tropane Alkaloid Production: Modelization of the in Vitro Biochemical Response. Plant Sci. 2009, 177, 81–87. [Google Scholar] [CrossRef]
- Yang, C.Q.; Fang, X.; Wu, X.M.; Mao, Y.B.; Wang, L.J.; Chen, X.Y. Transcriptional Regulation of Plant Secondary MetabolismF. J. Integr. Plant Biol. 2012, 54, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Madani, H.; Escrich, A.; Hosseini, B.; Sanchez-Muñoz, R.; Khojasteh, A.; Palazon, J. Effect of Polyploidy Induction on Natural Metabolite Production in Medicinal Plants. Biomolecules 2021, 11, 899. [Google Scholar] [CrossRef] [PubMed]
- Murthy, H.N.; Lee, E.J.; Paek, K.Y. Production of Secondary Metabolites from Cell and Organ Cultures: Strategies and Approaches for Biomass Improvement and Metabolite Accumulation. Plant Cell Tissue Organ Cult. 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Belabbassi, O.; Khelifi-Slaoui, M.; Zaoui, D.; Benyammi, R.; Khalfallah, N.; Malik, S.; Makhzoum, A.; Khelifi, L. Synergistic Effects of Polyploidization and Elicitation on Biomass and Hyoscyamine Content in Hairy Roots of Datura stramonium. Biotechnol. Agron. Soc. Environ. 2016, 20, 408–416. [Google Scholar] [CrossRef]
- Boitel-Conti, M.; Laberche, J.C.; Lanoue, A.; Ducrocq, C.; Sangwan-Norreel, B.S. Influence of Feeding Precursors on Tropane Alkaloid Production during an Abiotic Stress in Datura innoxia Transformed Roots. Plant Cell Tissue Organ Cult. 2000, 60, 131–137. [Google Scholar] [CrossRef]
- Gao, S.L.; Zhu, D.N.; Cai, Z.H.; Xu, D.R. Autotetraploid Plants from Colchicine-Treated Bud Culture of Salvia miltiorrhiza Bge. Plant Cell Tissue Organ Cult. 1996, 47, 73–77. [Google Scholar] [CrossRef]
- Komaraiah, P.; Ramakrishna, S.V.; Reddanna, P.; Kavi Kishor, P.B. Enhanced Production of Plumbagin in Immobilized Cells of Plumbago rosea by Elicitation and in Situ Adsorption. J. Biotechnol. 2003, 101, 181–187. [Google Scholar] [CrossRef]
- Miao, G.P.; Zhu, C.S.; Yang, Y.Q.; Feng, M.X.; Ma, Z.Q.; Feng, J.T.; Zhang, X. Elicitation and in Situ Adsorption Enhanced Secondary Metabolites Production of Tripterygium wilfordii Hook. f. Adventitious Root Fragment Liquid Cultures in Shake Flask and a Modified Bubble Column Bioreactor. Bioproc. Biosyst. Eng. 2014, 37, 641–650. [Google Scholar] [CrossRef]
- Kim, D.J.; Chang, H.N. Increased Shikonin Production in Lithospermum erythrorhizon Suspension Cultures within Situ Extraction and Fungal Cell Treatment (Elicitor). Biotechnol. Lett. 1990, 12, 443–446. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).


