Functional Characterisation of the Transcription Factor GsWRKY23 Gene from Glycine soja in Overexpressed Soybean Composite Plants and Arabidopsis under Salt Stress
Abstract
:1. Introduction
2. Results
2.1. Discovery of the GsWRKY23 Gene and Amino Acid Sequence Analysis of Its Encoding Protein
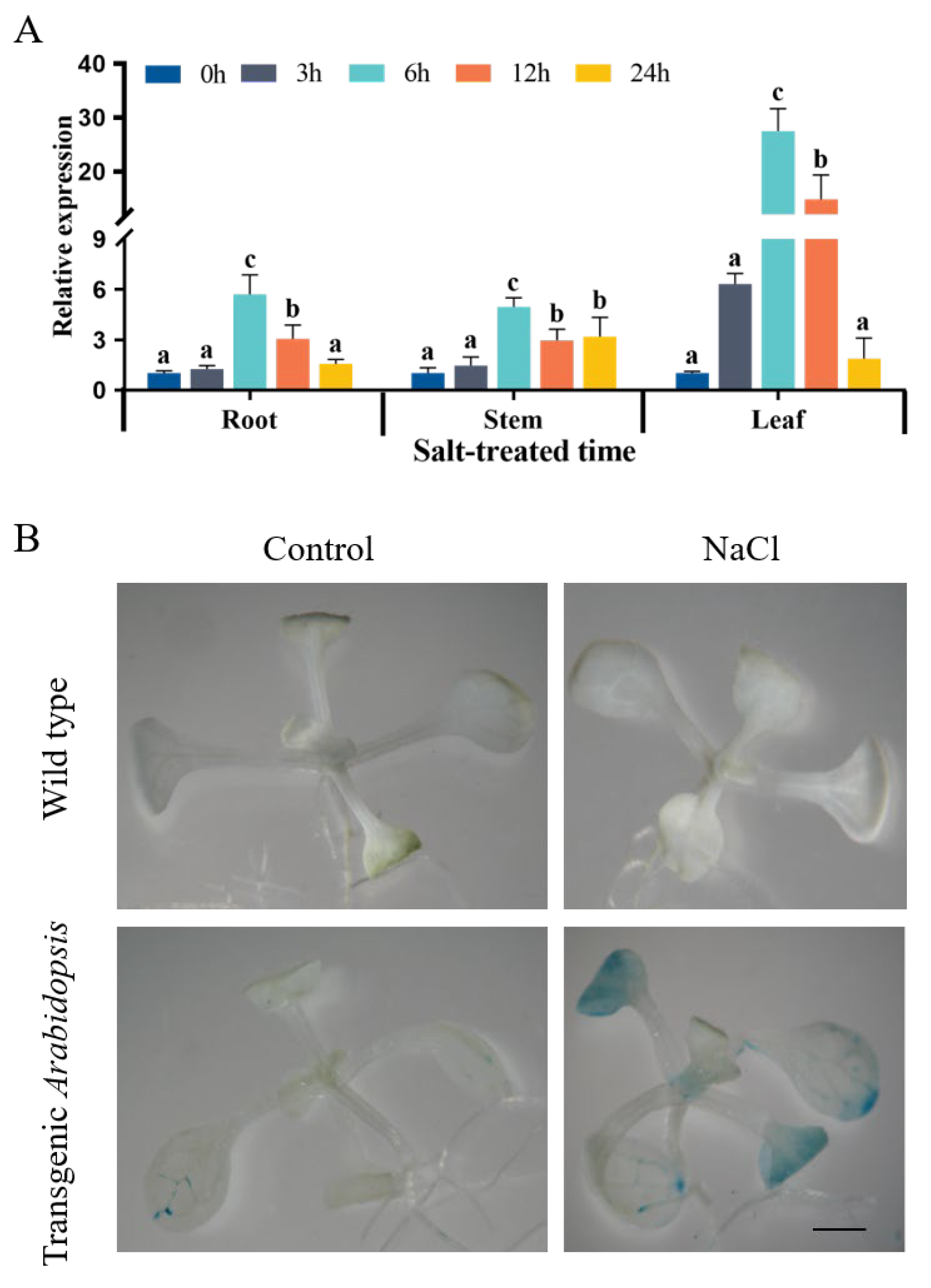
2.2. Response of GsWRKY23 and Its Promoter to Salt Stress
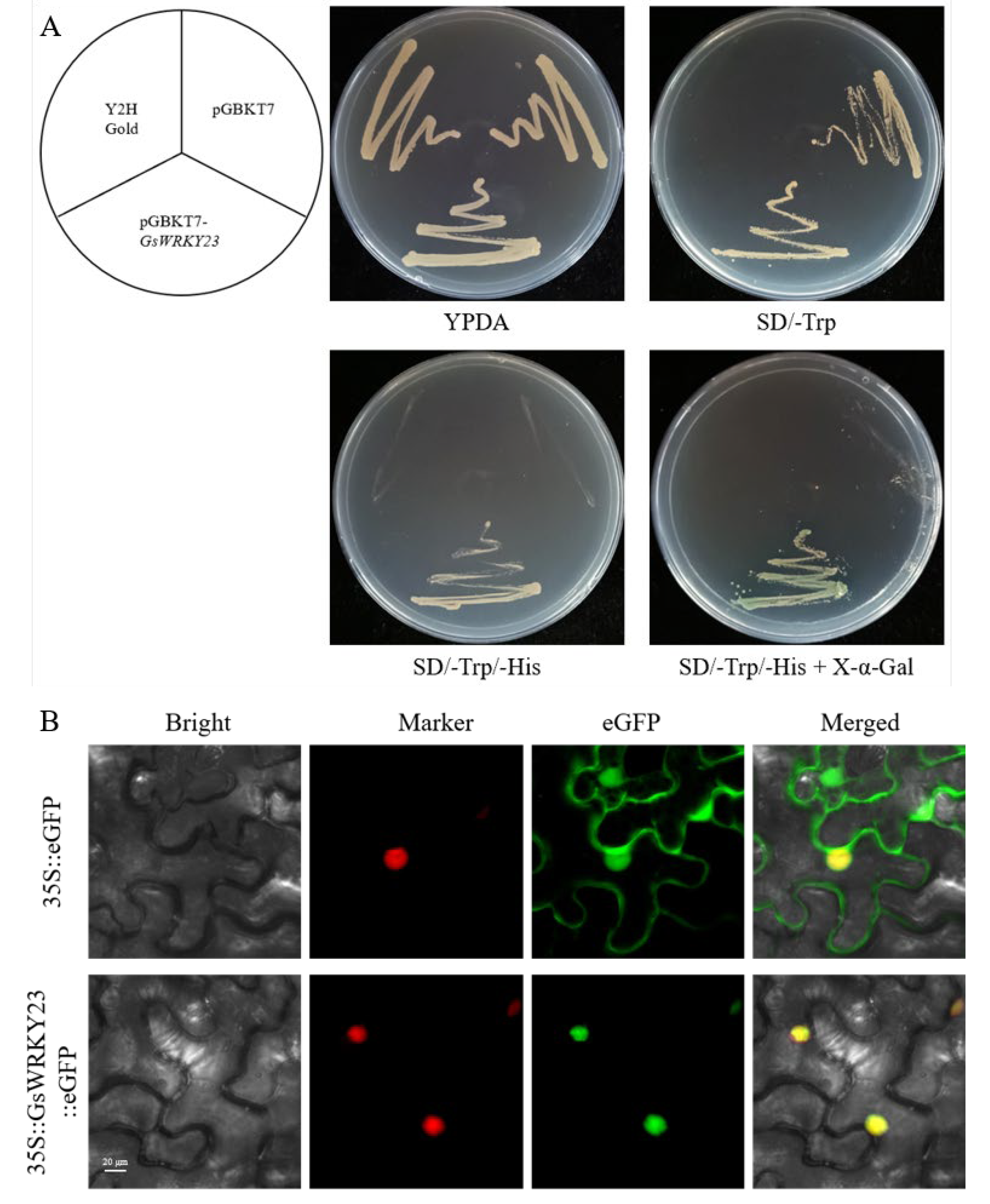
2.3. Transcriptional Activation Ability and Subcellular Localisation of the GsWRKY23 Protein
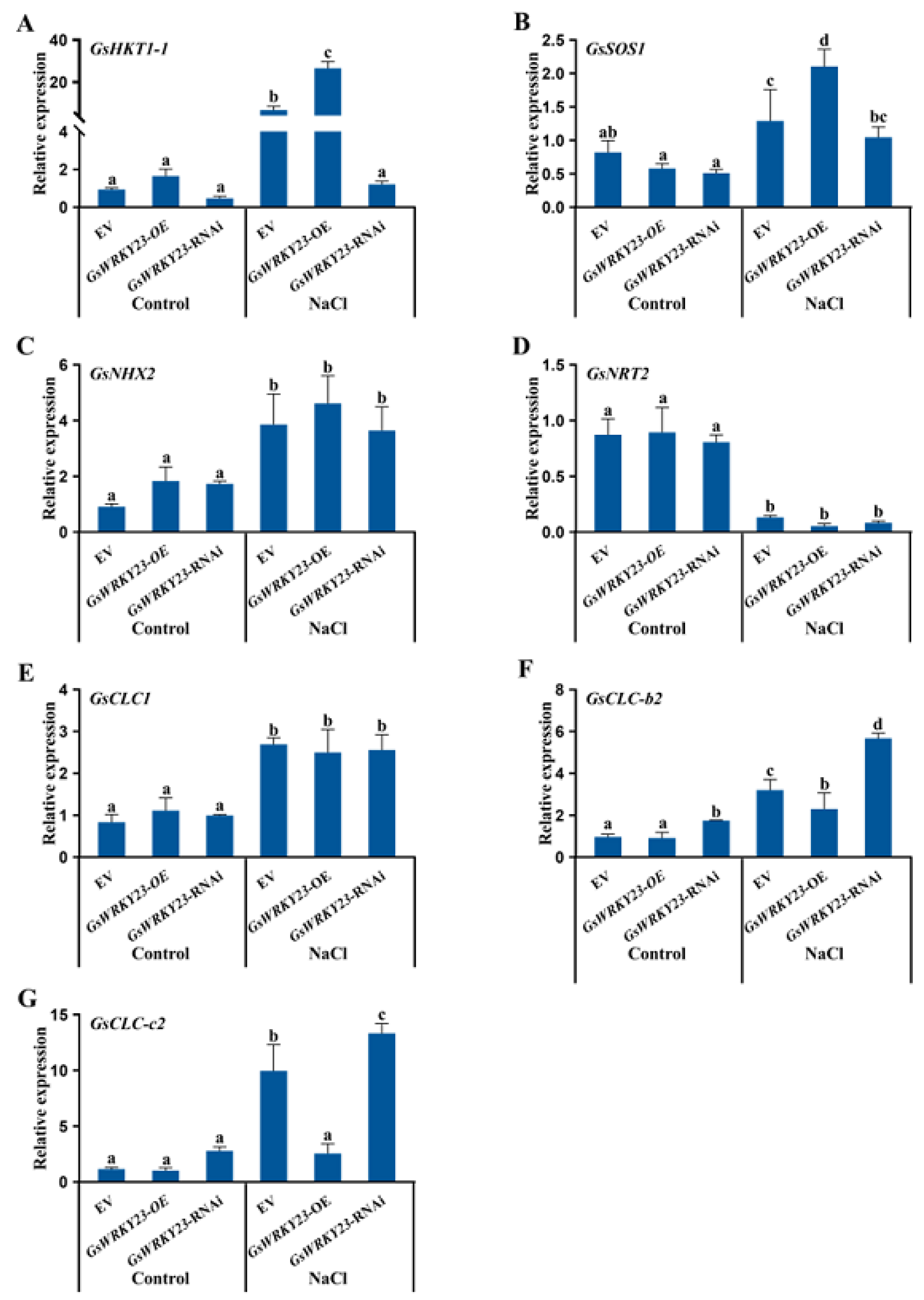
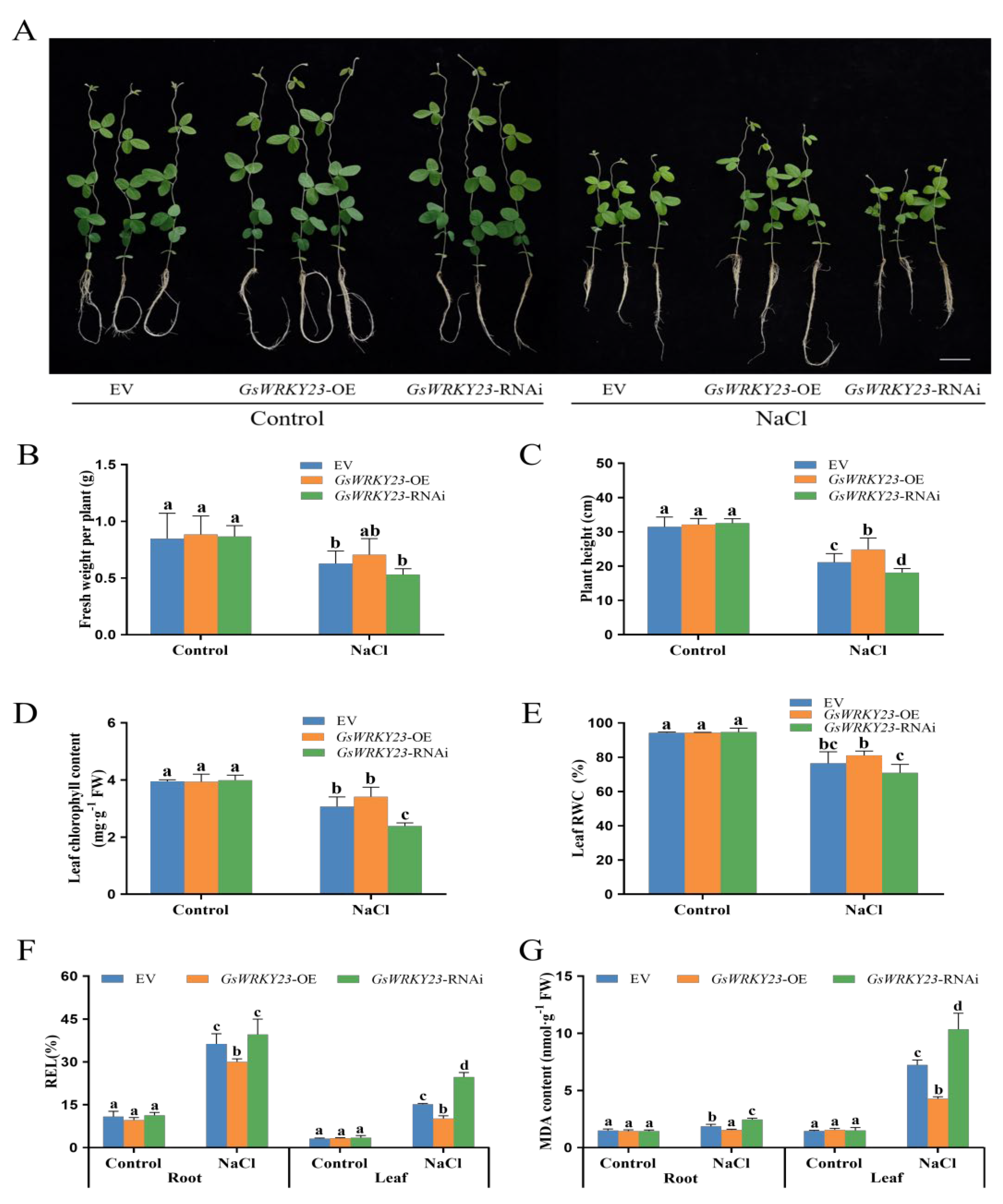
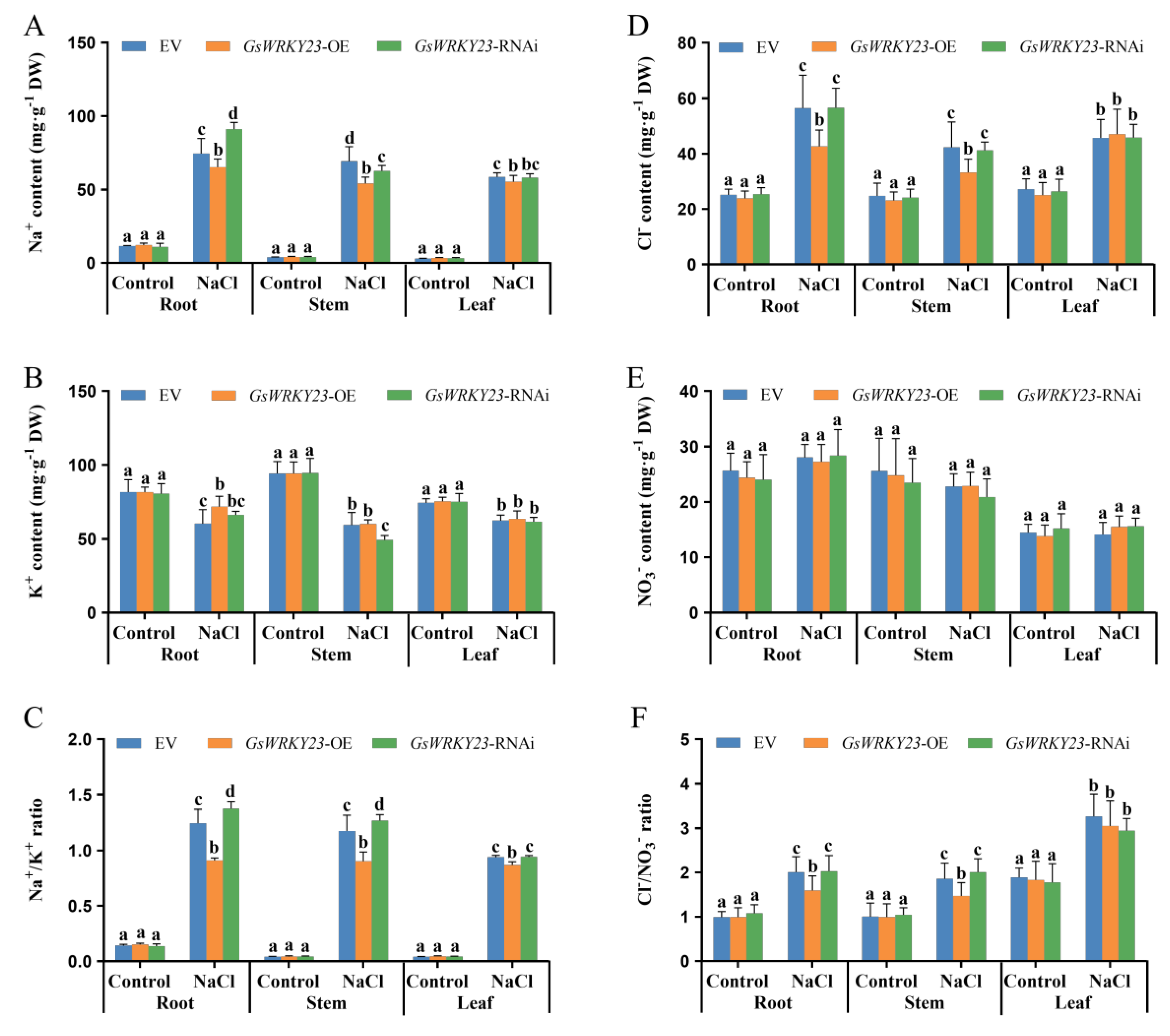
2.4. Analysis of Salt Tolerance in GsWRKY23-Overexpressing and GsWRKY23-RNAi Wild Soybean Hairy-Root Composite Plants
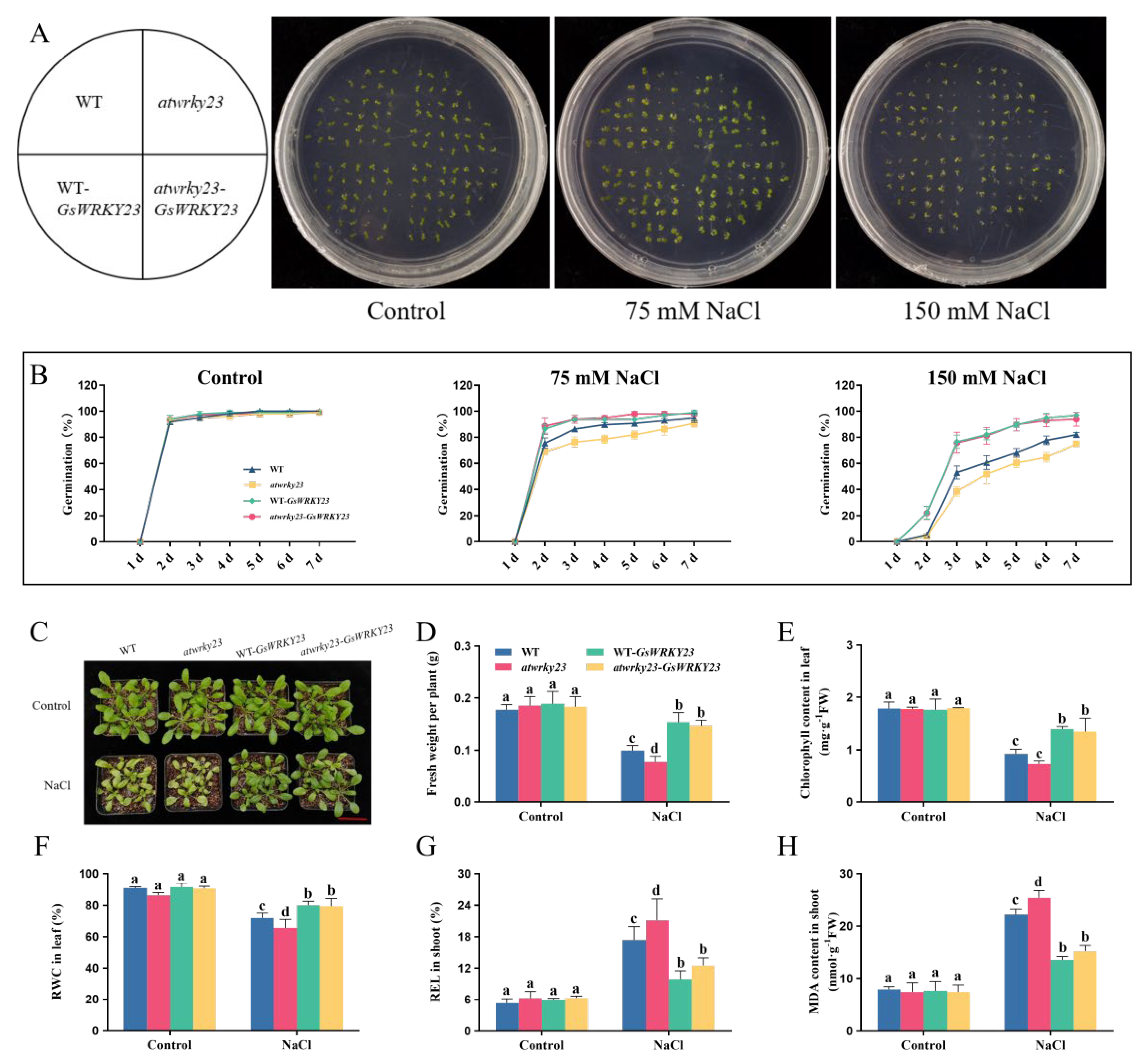
2.5. GsWRKY23-Transgenic Arabidopsis Exhibits Enhanced Salt Tolerance
3. Discussion
3.1. Identification and Characteristics of the Transcription Factor GsWRKY23 Gene from the Salt-Tolerant G. soja Accession BB52
3.2. Mechanisms of the GsWRKY23 Gene Conferred Salt Tolerance to Overexpressed Soybean Composite Plants and Arabidopsis
4. Materials and Methods
4.1. Plant Materials, Bacterial Strains, and Plasmids
4.2. Phylogenetic Analysis and Multiple Alignment of Amino Acid Sequences of the WRKY Family
4.3. Soybean Plant Culture, Salt Treatment, RNA Extraction, and qRT–PCR Analysis
4.4. Expression Vector Construction
4.5. GUS Staining of Transgenic Arabidopsis
4.6. Transcriptional Activation Assay of GsWRKY23 in Yeast
4.7. Subcellular Localisation Analysis of GsWRKY23
4.8. Salt-Tolerance Assays of GsWRKY23-OE and -RNAi Soybean Hairy-Root Composite Plants
4.9. Salt-Tolerance Analysis of GsWRKY23-Transgenic Arabidopsis WT and Mutant Plants
4.10. Data Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, J.; Zhao, J.; Zhu, G.; Wen, Y.; Wang, Y.; Liu, J.; Yang, Z. Effects of ecological water conveyance on soil salinization in the Shiyang river basin’s terminal lake-Qingtu lake-area. Sustainability 2022, 14, 10311. [Google Scholar] [CrossRef]
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.K.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef] [PubMed]
- Kronzucker, H.J.; Coskun, D.; Schulze, L.M.; Wong, J.R.; Britto, D.T. Sodium as nutrient and toxicant. Plant Soil 2013, 369, 1–23. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, X.; Giraldo, J.P.; Shabala, S. It is not all about sodium: Revealing tissue specificity and signalling roles of potassium in plant responses to salt stress. Plant Soil 2018, 431, 1–17. [Google Scholar] [CrossRef]
- Bazihizina, N.; Colmer, T.D.; Cuin, T.A.; Mancuso, S.; Shabala, S. Friend or foe? Chloride patterning in halophytes. Trends Plant Sci. 2019, 24, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Spitz, F.; Furlong, E.E.M. Transcription factors: From enhancer binding to developmental control. Nat. Rev. Genet. 2012, 13, 613–626. [Google Scholar] [CrossRef]
- Strader, L.; Weijers, D.; Wagner, D. Plant transcription factors—Being in the right place with the right company. Curr. Opin. Plant Biol. 2022, 65, 102136. [Google Scholar] [CrossRef]
- Joshi, R.; Wani, S.H.; Singh, B.; Bohra, A.; Dar, Z.A.; Lone, A.A.; Pareek, A.; Singla-Pareek, S.L. Transcription factors and plants response to drought stress: Current understanding and future directions. Front. Plant Sci. 2016, 7, 1029. [Google Scholar] [CrossRef]
- He, F.; Li, H.G.; Wang, J.J.; Su, Y.; Wang, H.L.; Feng, C.H.; Yang, Y.; Niu, M.X.; Liu, C.; Yin, W.; et al. PeSTZ1, a C2H2-type zinc finger transcription factor from Populus euphratica, enhances freezing tolerance through modulation of ROS scavenging by directly regulating PeAPX2. Plant Biotechnol. J. 2019, 17, 2169–2183. [Google Scholar] [CrossRef] [PubMed]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY transcription factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, S.; Nakamura, K. Characterization of a cDNA encoding a novel DNA.binding protein, SPF1, that recognizes SP8 sequences in the 5’ upstream regions of genes coding for sporamin and β-amylase from sweet potato. Mol. Gen. Genet. 1994, 244, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, H.; Yang, Y.; Wang, Y.; Mo, Y.; Zhang, R.; Zhang, Y.; Ma, J.; Wei, C.; Zhang, X. Identification and expression analyses of WRKY genes reveal their involvement in growth and abiotic stress response in watermelon (Citrullus lanatus). PLoS ONE 2018, 13, e191308. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Hu, L.; Zhang, S.; Zhang, M.; Jiang, W.; Wu, T.; Du, X. Rice OsWRKY50 mediates ABA-dependent seed germination and seedling growth, and ABA-independent salt stress tolerance. Int. J. Mol. Sci. 2021, 22, 8625. [Google Scholar] [CrossRef] [PubMed]
- Rinerson, C.I.; Scully, E.D.; Palmer, N.A.; Donze-Reiner, T.; Rabara, R.C.; Tripathi, P.; Shen, Q.J.; Sattler, S.E.; Rohila, J.S.; Sarath, G.; et al. The WRKY transcription factor family and senescence in switchgrass. BMC Genom. 2015, 16, 912. [Google Scholar] [CrossRef]
- Schluttenhofer, C.; Yuan, L. Regulation of specialized metabolism by WRKY transcription factors. Plant Physiol. 2015, 167, 295–306. [Google Scholar] [CrossRef]
- Ding, Z.J.; Yan, J.Y.; Xu, X.Y.; Yu, D.Q.; Li, G.X.; Zhang, S.Q.; Zheng, S.J. Transcription factor WRKY46 regulates osmotic stress responses and stomatal movement independently in Arabidopsis. Plant J. 2014, 79, 13–27. [Google Scholar] [CrossRef]
- Grunewald, W.; De Smet, I.; Lewis, D.R.; Löfke, C.; Jansen, L.; Goeminne, G.; Vanden Bossche, R.; Karimi, M.; De Rybel, B.; Vanholme, B.; et al. Transcription factor WRKY23 assists auxin distribution patterns during Arabidopsis root development through local control on flavonol biosynthesis. Proc. Natl. Acad. Sci. USA 2012, 109, 1554–1559. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, G.; Chen, J.; Liu, X.; Lu, X.; Chen, H.; Tian, Y. Integrated metabolome and transcriptome analysis unveils novel pathway involved in the formation of yellow peel in Cucumber. Int. J. Mol. Sci. 2021, 22, 1494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, S.; Wang, Z.; Yuan, Y.; Zhang, Z.; Liang, Q.; Yang, X.; Duan, Z.; Liu, Y.; Kong, F.; et al. Progress in soybean functional genomics over the past decade. Plant Biotechnol. J. 2022, 20, 256–282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.K.; Zhou, Q.H.; Cao, J.H.; Yu, B.J. Differential Cl−/salt tolerance and NaCl-induced alternations of tissue and cellular ion fluxes in Glycine max, Glycine soja and their hybrid seedlings. J. Agron. Crop Sci. 2011, 197, 329–339. [Google Scholar] [CrossRef]
- Liu, X.; Liu, F.; Zhang, L.; Cheng, C.; Wei, P.; Yu, B. GsCLC-c2 from wild soybean confers chloride/salt tolerance to transgenic Arabidopsis and soybean composite plants by regulating anion homeostasis. Physiol. Plant. 2021, 172, 1867–1879. [Google Scholar] [CrossRef] [PubMed]
- Pi, B.; Liu, X.; Huang, Q.; Zhang, T.; Yu, B. Comparative transcriptomic analysis of Glycine soja and G. max and functional identification of GsCNGC20-d interacted with GsCDPK29 under salt stress. Environ. Exp. Bot. 2023, 206, 105185. [Google Scholar] [CrossRef]
- Liu, Y.; Du, H.; Li, P.; Shen, Y.; Peng, H.; Liu, S.; Zhou, G.; Zhang, H.; Liu, Z.; Shi, M.; et al. Pan-genome of wild and cultivated soybeans. Cell 2020, 182, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Li, M.W.; Xie, M.; Liu, X.; Ni, M.; Shao, G.; Song, C.; Kay-Yuen, Y.A.; Tao, Y.; Wong, F.L.; et al. Identification of a novel salt tolerance gene in wild soybean by whole-genome sequencing. Nat. Commun. 2014, 5, 4340. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, Z. The importance of Cl− exclusion and vacuolar Cl− sequestration: Revisiting the role of Cl− transport in plant salt tolerance. Front. Plant Sci. 2019, 10, 1418. [Google Scholar] [CrossRef]
- Wei, P.; Che, B.; Shen, L.; Cui, Y.; Wu, S.; Cheng, C.; Liu, F.; Li, M.; Yu, B.; Lam, H. Identification and functional characterization of the chloride channel gene, GsCLC-c2 from wild soybean. BMC Plant Biol. 2019, 19, 1–15. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, Q.; Yang, J.; Xie, G.; Liu, J. PtrCDPK10 of Poncirus trifoliata functions in dehydration and drought tolerance by reducing ROS accumulation via phosphorylating PtrAPX. Plant Sci. 2020, 291, 110320. [Google Scholar] [CrossRef]
- Scarpeci, T.E.; Zanor, M.I.; Mueller-Roeber, B.; Valle, E.M. Overexpression of AtWRKY30 enhances abiotic stress tolerance during early growth stages in Arabidopsis thaliana. Plant Mol. Biol. 2013, 83, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.E.; Kumar, C.; Patel, H.K.; Sonti, R.V. Overexpression of a cell wall damage induced transcription factor, OsWRKY42, leads to enhanced callose deposition and tolerance to salt stress but does not enhance tolerance to bacterial infection. BMC Plant Biol. 2018, 18, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, X.; Ruan, H.; Zhang, J.; Xie, F.; Gai, J.; Yang, S. GmWRKY45 enhances tolerance to phosphate starvation and salt stress, and changes fertility in transgenic Arabidopsis. Front. Plant Sci. 2020, 10, 1714. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.Y.; Tian, A.G.; Zou, H.F.; Xie, Z.M.; Lei, G.; Huang, J.; Wang, C.M.; Wang, H.W.; Zhang, J.S.; Chen, S.Y. Soybean WRKY-type transcription factor genes, GmWRKY13, GmWRKY21, and GmWRKY54, confer differential tolerance to abiotic stresses in transgenic Arabidopsis plants. Plant Biotechnol. J. 2008, 6, 486–503. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Wang, X.; Chen, M.; Chai, T.; Wang, H. A WRKY transcription factor, PcWRKY33, from Polygonum cuspidatum reduces salt tolerance in transgenic Arabidopsis thaliana. Plant Cell Rep. 2018, 37, 1033–1048. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, J.; Sui, Y.; Han, G.; Zhang, Y.; Guo, S.; Sui, N. The sweet sorghum SbWRKY50 is negatively involved in salt response by regulating ion homeostasis. Plant Mol. Biol. 2020, 102, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Li, W.; Jiang, H.; Wang, Y.; Gao, H.; Liu, M.; Chen, Q.; Lai, Y.; He, C. Differential expression of a WRKY gene between wild and cultivated soybeans correlates to seed size. J. Exp. Bot. 2017, 68, 2717–2729. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Bai, X.; Sun, X.; Zhu, D.; Liu, B.; Ji, W.; Cai, H.; Cao, L.; Wu, J.; Hu, M.; et al. Expression of wild soybean WRKY20 in Arabidopsis enhances drought tolerance and regulates ABA signalling. J. Exp. Bot. 2013, 64, 2155–2169. [Google Scholar] [CrossRef]
- Tang, L.; Cai, H.; Zhai, H.; Luo, X.; Wang, Z.; Cui, L.; Bai, X. Overexpression of Glycine soja WRKY20 enhances both drought and salt tolerance in transgenic alfalfa (Medicago sativa L.). Plant Cell Tissue Organ Cutl. 2014, 118, 77–86. [Google Scholar] [CrossRef]
- Ning, W.; Zhai, H.; Yu, J.; Liang, S.; Yang, X.; Xing, X.; Huo, J.; Pang, T.; Yang, Y.; Bai, X. Overexpression of Glycine soja WRKY20 enhances drought tolerance and improves plant yields under drought stress in transgenic soybean. Mol. Breed. 2017, 37, 1–10. [Google Scholar] [CrossRef]
- Du, C.; Zhao, P.; Zhang, H.; Li, N.; Zheng, L.; Wang, Y. The Reaumuria trigyna transcription factor RtWRKY1 confers tolerance to salt stress in transgenic Arabidopsis. J. Plant Physiol. 2017, 215, 48–58. [Google Scholar] [CrossRef]
- Alabd, A.; Cheng, H.; Ahmad, M.; Wu, X.; Peng, L.; Wang, L.; Yang, S.; Bai, S.; Ni, J.; Teng, Y. ABRE-BINDING FACTOR3-WRKY DNA-BINDING PROTEIN44 module promotes salinity-induced malate accumulation in pear. Plant Physiol. 2023, 192, 1982–1996. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, R.A.; Kavanagh, T.A.; Bevan, M.W. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987, 6, 3901–3907. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Pi, B.; Du, Z.; Yang, T.; Gu, M.; Sun, S.; Yu, B. The transcription factor GmbHLH3 confers Cl−/salt tolerance to soybean by upregulating GmCLC1 expression for maintenance of anion homeostasis. Environ. Exp. Bot. 2022, 194, 104755. [Google Scholar] [CrossRef]
- Wei, P.; Wang, L.; Liu, A.; Yu, B.; Lam, H. GmCLC1 confers enhanced salt tolerance through regulating chloride accumulation in soybean. Front. Plant Sci. 2016, 7, 1082. [Google Scholar] [CrossRef]
- Cheng, C.; Liu, Y.; Liu, X.; An, J.; Jiang, L.; Yu, B. Recretohalophyte Tamarix TrSOS1 confers higher salt tolerance to transgenic plants and yeast than glycophyte soybean GmSOS1. Environ. Exp. Bot. 2019, 165, 196–207. [Google Scholar] [CrossRef]
- Zhou, Q.; Yu, B.J. Accumulation of inorganic and organic osmolytes and their role in osmotic adjustment in NaCl-stressed vetiver grass seedlings. Russ. J. Plant Physiol. 2009, 56, 678–685. [Google Scholar] [CrossRef]
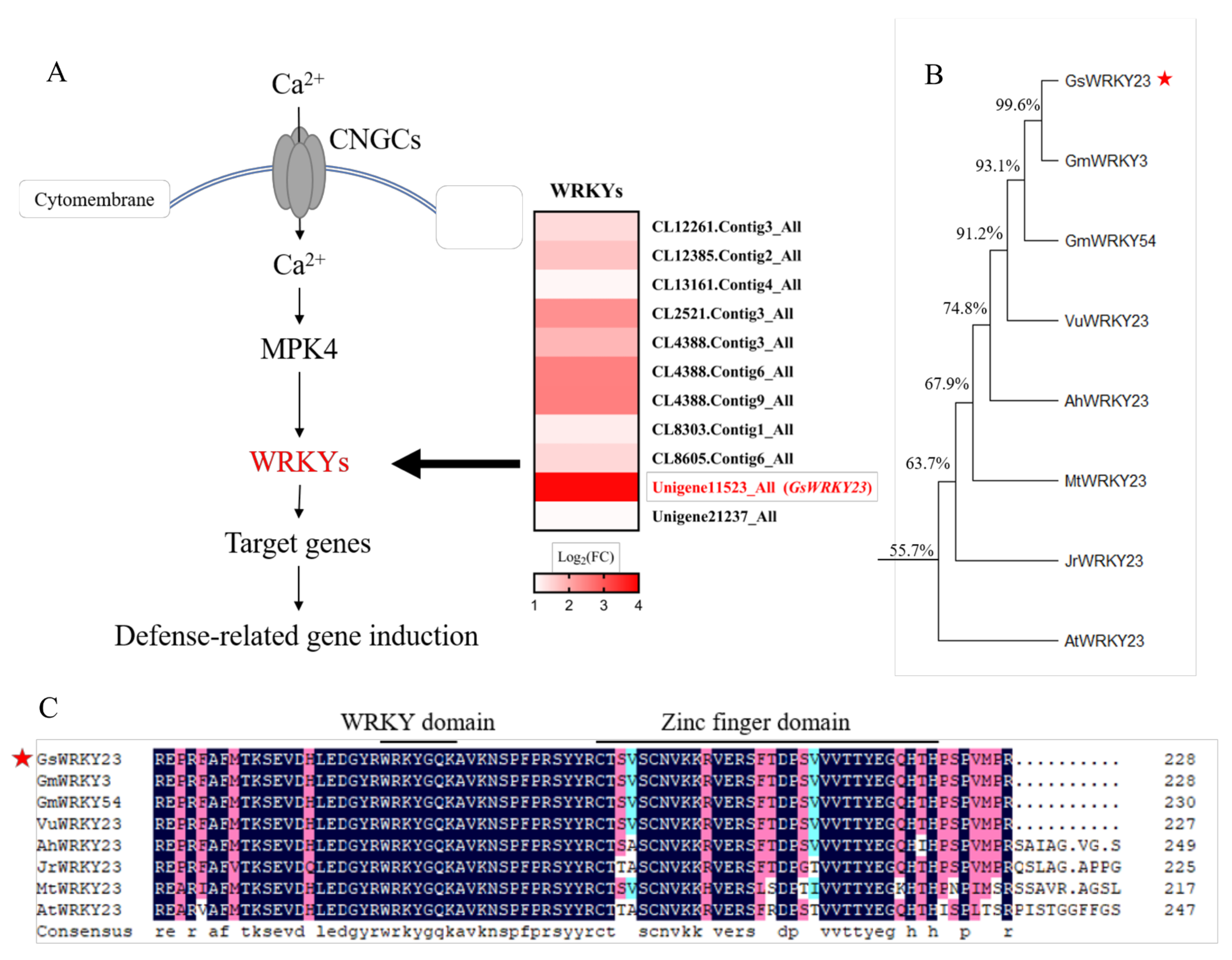

| Gene ID in the Transcriptome Data | Gene ID in the G. soja Database | Gene Names | Log2(Na-BB52/C-BB52) |
|---|---|---|---|
| CL12261.Contig3_All | Glysoja.06G016288 | GsWRKY6 | 1.423857 |
| CL12385.Contig2_All | Glysoja.01G000427 | GsWRKY72 | 1.704426 |
| CL13161.Contig4_All | Glysoja.05G011123 | GsWRKY72 | 1.102701 |
| CL2521.Contig3_All | Glysoja.15G040587 | GsWRKY6 | 2.294813 |
| CL4388.Contig3_All | Glysoja.03G007935 | GsWRKY42 | 1.850349 |
| CL4388.Contig6_All | Glysoja.19G052277 | GsWRKY42 | 2.485088 |
| CL4388.Contig9_All | Glysoja.03G007935 | GsWRKY42 | 2.497004 |
| CL8303.Contig1_All | Glysoja.08G022304 | GsWRKY6 | 1.212138 |
| CL8605.Contig6_All | Glysoja.18G047646 | GsWRKY33 | 1.460307 |
| Unigene11523_All | Glysoja.02G002639 | GsWRKY23 | 3.917525 |
| Unigene21237_All | Glysoja.08G020557 | GsWRKY13 | 1.053174 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Liu, X.; Zhang, T.; Yang, H.; Yu, B. Functional Characterisation of the Transcription Factor GsWRKY23 Gene from Glycine soja in Overexpressed Soybean Composite Plants and Arabidopsis under Salt Stress. Plants 2023, 12, 3030. https://doi.org/10.3390/plants12173030
Sun S, Liu X, Zhang T, Yang H, Yu B. Functional Characterisation of the Transcription Factor GsWRKY23 Gene from Glycine soja in Overexpressed Soybean Composite Plants and Arabidopsis under Salt Stress. Plants. 2023; 12(17):3030. https://doi.org/10.3390/plants12173030
Chicago/Turabian StyleSun, Shile, Xun Liu, Tianlei Zhang, Hao Yang, and Bingjun Yu. 2023. "Functional Characterisation of the Transcription Factor GsWRKY23 Gene from Glycine soja in Overexpressed Soybean Composite Plants and Arabidopsis under Salt Stress" Plants 12, no. 17: 3030. https://doi.org/10.3390/plants12173030
APA StyleSun, S., Liu, X., Zhang, T., Yang, H., & Yu, B. (2023). Functional Characterisation of the Transcription Factor GsWRKY23 Gene from Glycine soja in Overexpressed Soybean Composite Plants and Arabidopsis under Salt Stress. Plants, 12(17), 3030. https://doi.org/10.3390/plants12173030





