Engaging Precision Phenotyping to Scrutinize Vegetative Drought Tolerance and Recovery in Chickpea Plant Genetic Resources
Abstract
1. Introduction
2. Results
2.1. Data Quality and HTP Experiment
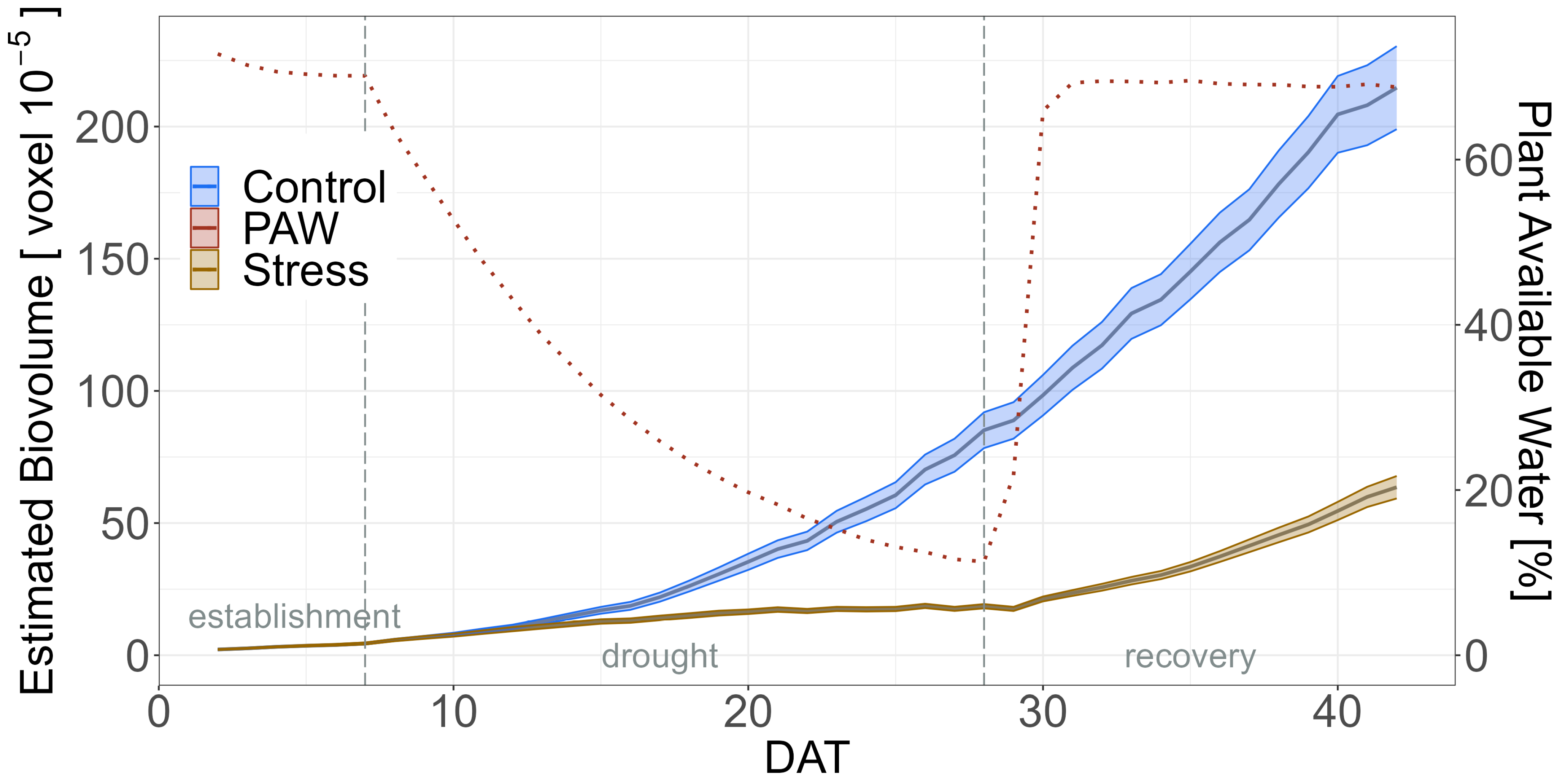
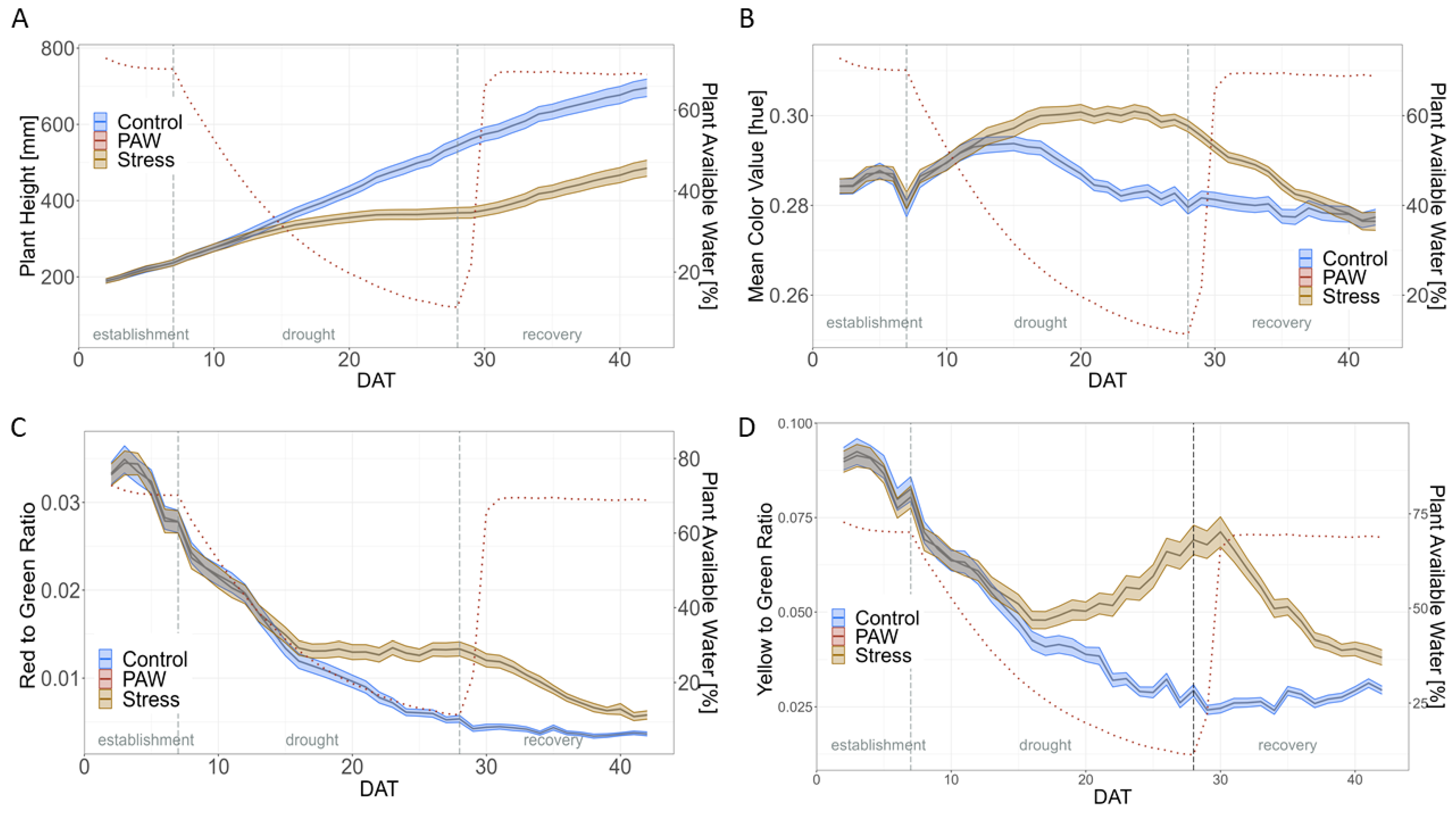
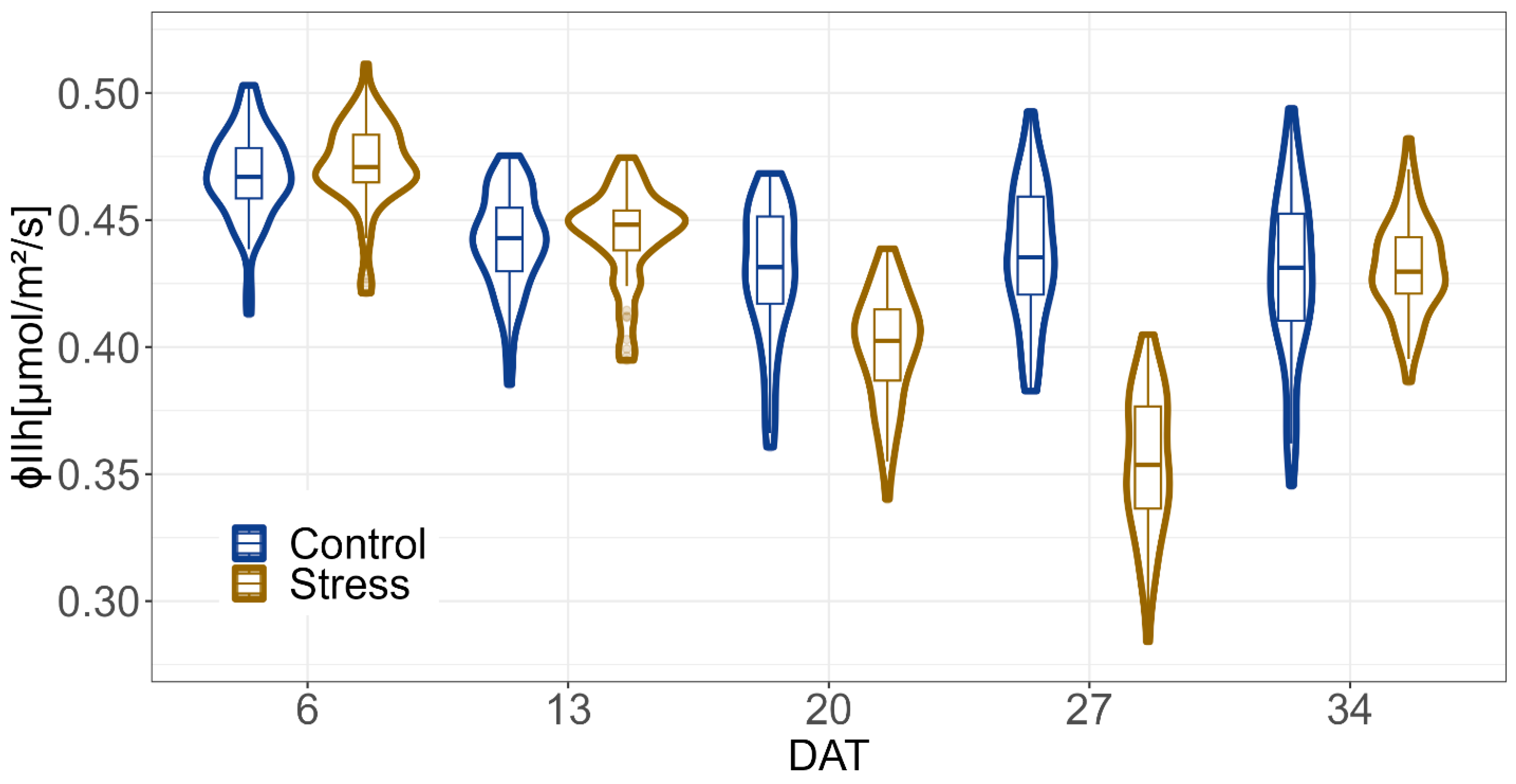
2.2. Impact of Drought
2.3. Correlations between Traits
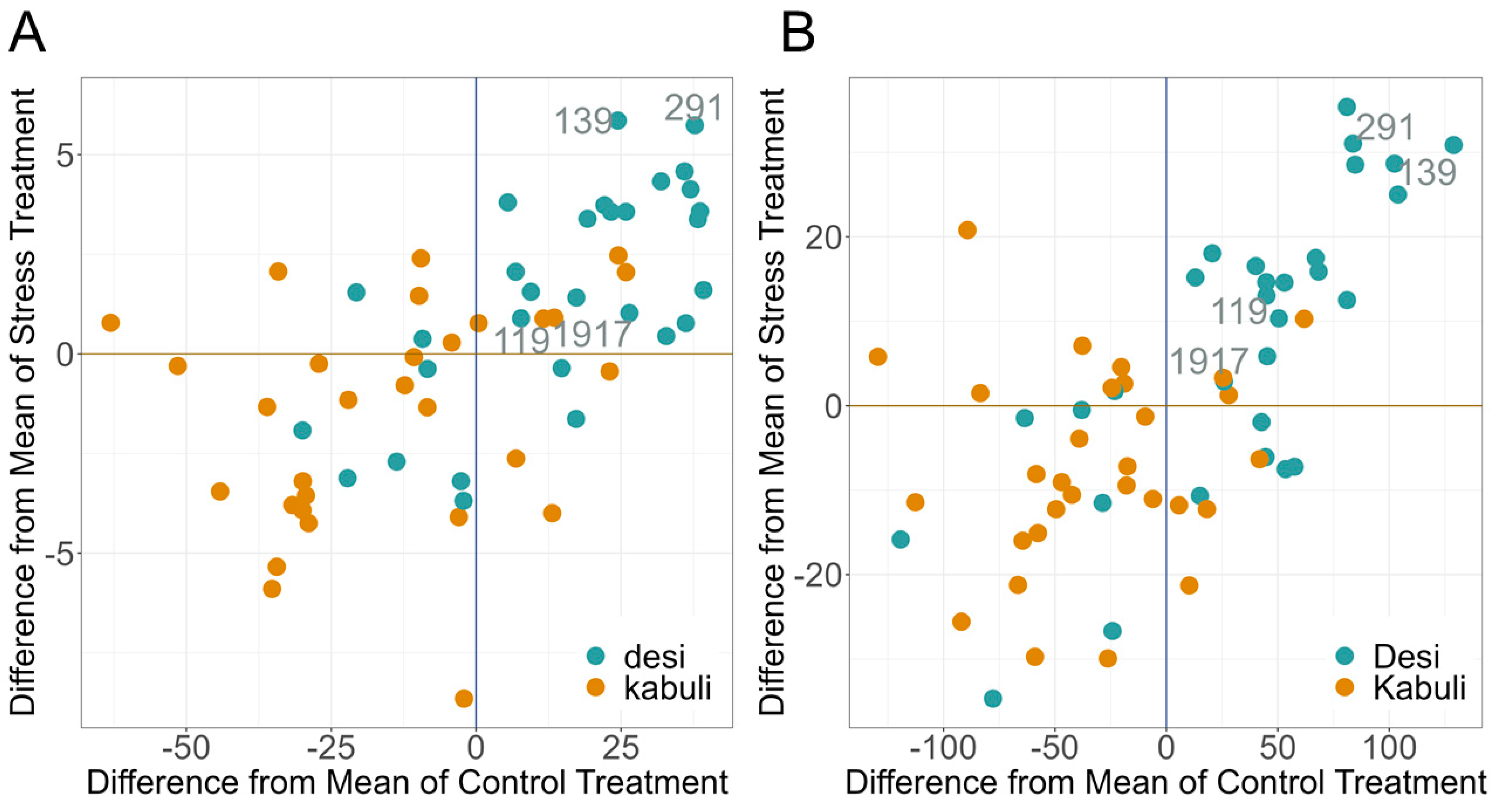
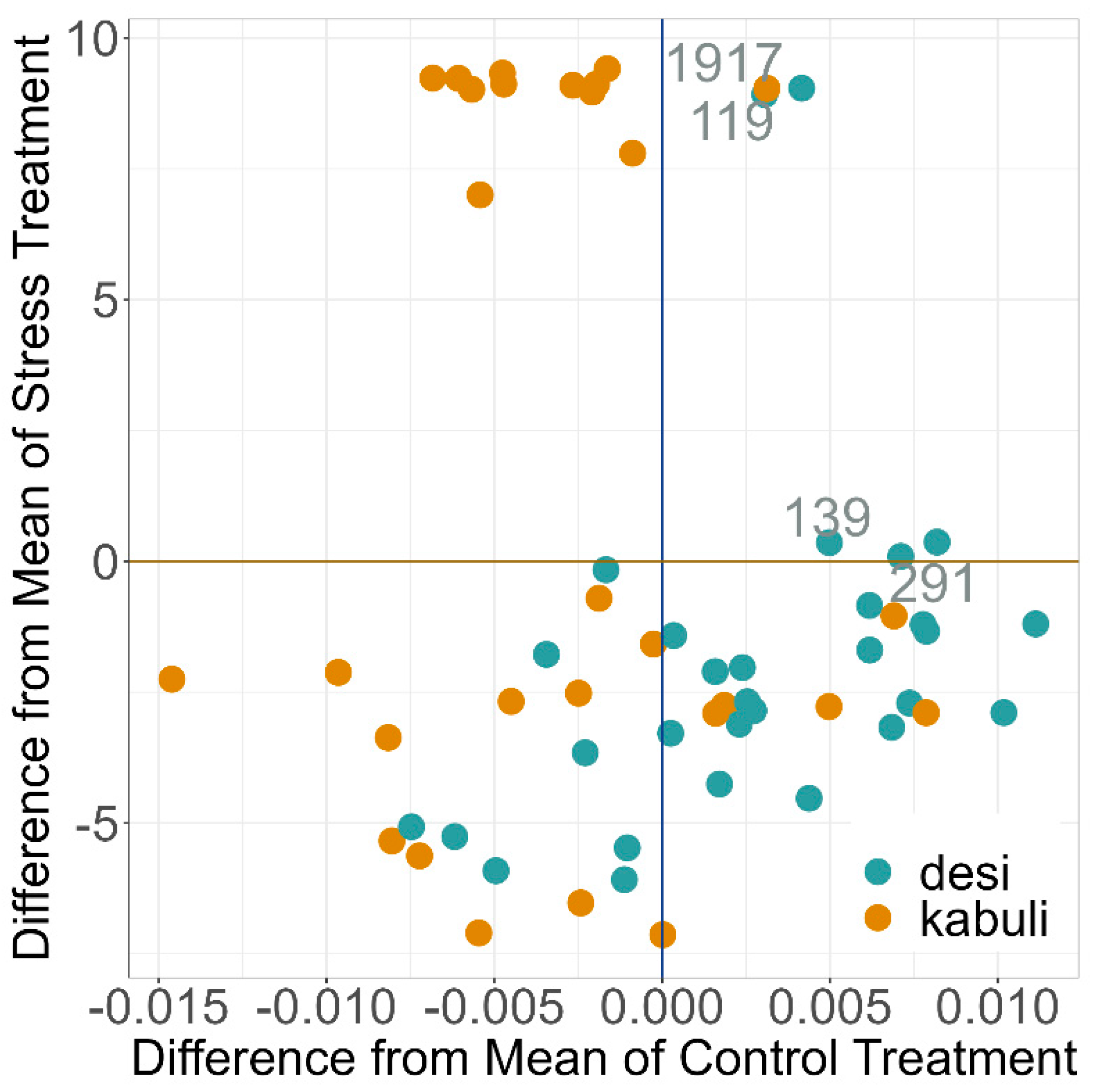
2.4. PGR under Drought Stress
3. Discussion
3.1. Suitability of HTP
3.2. General Effect of Drought Stress during HTP Experiments
3.3. PGR for Drought Stress Tolerance during Vegetative Phase
4. Material and Methods
4.1. Plant Material
4.2. HTP Experiments
4.3. Image-Derived Plant Traits
4.4. Chlorophyll Fluorescence Imaging and Image Analysis
4.5. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beillouin, D.; Schauberger, B.; Bastos, A.; Ciais, P.; Makowski, D. Impact of extreme weather conditions on european crop production in 2018. Philos. Trans. R Soc. Lond. B Biol. Sci. 2020, 375, 20190510. [Google Scholar] [CrossRef] [PubMed]
- Grillakis, M.G. Increase in severe and extreme soil moisture droughts for europe under climate change. Sci. Total Environ. 2019, 660, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2022: Impacts, Adaptation and Vulnerability. Ph.D. Thesis, Cambridge University Press, Cambridge, UK; New York, NY, USA, 2022; pp. 3–33. [Google Scholar]
- Peltonen-Sainio, P.; Jauhiainen, L.; Trnka, M.; Olesen, J.E.; Calanca, P.; Eckersten, H.; Eitzinger, J.; Gobin, A.; Kersebaum, K.C.; Kozyra, J.; et al. Coincidence of variation in yield and climate in europe. Agric. Ecosyst. Environ. 2010, 139, 483–489. [Google Scholar] [CrossRef]
- Cernay, C.; Ben-Ari, T.; Pelzer, E.; Meynard, J.M.; Makowski, D. Estimating variability in grain legume yields across europe and the americas. Sci. Rep. 2015, 5, 11171. [Google Scholar] [CrossRef]
- Reckling, M.; Doring, T.F.; Bergkvist, G.; Stoddard, F.L.; Watson, C.A.; Seddig, S.; Chmielewski, F.M.; Bachinger, J. Grain legume yields are as stable as other spring crops in long-term experiments across northern europe. Agron. Sustain. Dev. 2018, 38, 63. [Google Scholar] [CrossRef]
- Krishnamurthy, L.; Kashiwagi, J.; Gaur, P.M.; Upadhyaya, H.D.; Vadez, V. Sources of tolerance to terminal drought in the chickpea (Cicer arietinum L.) minicore germplasm. Field Crops Res. 2010, 119, 322–330. [Google Scholar] [CrossRef]
- Merchuk-Ovnat, L.; Barak, V.; Fahima, T.; Ordon, F.; Lidzbarsky, G.A.; Krugman, T.; Saranga, Y. Ancestral qtl alleles from wild emmer wheat improve drought resistance and productivity in modern wheat cultivars. Front. Plant Sci. 2016, 7, 452. [Google Scholar] [CrossRef] [PubMed]
- Pham, A.T.; Maurer, A.; Pillen, K.; Brien, C.; Dowling, K.; Berger, B.; Eglinton, J.K.; March, T.J. Genome-wide association of barley plant growth under drought stress using a nested association mapping population. BMC Plant Biol. 2019, 19, 134. [Google Scholar] [CrossRef]
- Berger, J.; Pushpavalli, R.; Ludwig, C.; Parsons, S.; Basdemir, F.; Whisson, K. Wild and domestic differences in plant development and responses to water deficit in cicer. Front. Genet. 2020, 11, 607819. [Google Scholar] [CrossRef]
- Varshney, R.K.; Gaur, P.M.; Chamarthi, S.K.; Krishnamurthy, L.; Tripathi, S.; Kashiwagi, J.; Samineni, S.; Singh, V.K.; Thudi, M.; Jaganathan, D. Fast-track introgression of “qtl-hotspot”for root traits and other drought tolerance traits in jg 11, an elite and leading variety of chickpea. Plant Genome 2013, 9, 1–9. [Google Scholar] [CrossRef]
- Varshney, R.K.; Mohan, S.M.; Gaur, P.M.; Chamarthi, S.K.; Singh, V.K.; Srinivasan, S.; Swapna, N.; Sharma, M.; Pande, S.; Singh, S.; et al. Marker-assisted backcrossing to introgress resistance to fusarium wilt race 1 and ascochyta blight in c 214, an elite cultivar of chickpea. Plant Genome 2014, 7. [Google Scholar] [CrossRef]
- Singh, C.; Kumar, R.; Sehgal, H.; Bhati, S.; Singhal, T.; Gayacharan; Nimmy, M.S.; Yadav, R.; Gupta, S.K.; Abdallah, N.A.; et al. Unclasping potentials of genomics and gene editing in chickpea to fight climate change and global hunger threat. Front. Plant Sci. 2023, 14, 1085024. [Google Scholar] [CrossRef] [PubMed]
- Badhan, S.; Bali, A.S.; Mantri, N. First report of crispr/cas9 mediated DNA-free editing of 4cl and rve7 genes in chickpea protoplasts. Int. J. Mol. Sci. 2021, 22, 396. [Google Scholar] [CrossRef] [PubMed]
- Hari, V.; Rakovec, O.; Markonis, Y.; Hanel, M.; Kumar, R. Increased future occurrences of the exceptional 2018–2019 central european drought under global warming. Sci. Rep. 2020, 10, 12207. [Google Scholar] [CrossRef] [PubMed]
- Elsalahy, H.H.; Reckling, M. Soybean resilience to drought is supported by partial recovery of photosynthetic traits. Front. Plant Sci. 2022, 13, 971893. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Lam, H.M.; Nguyen, H.T.; Siddique, K.H.; Varshney, R.K.; Colmer, T.D.; Cowling, W.; Bramley, H.; Mori, T.A.; Hodgson, J.M.; et al. Neglecting legumes has compromised human health and sustainable food production. Nat. Plants 2016, 2, 16112. [Google Scholar] [CrossRef]
- Rani, A.; Devi, P.; Jha, U.C.; Sharma, K.D.; Siddique, K.H.M.; Nayyar, H. Developing climate-resilient chickpea involving physiological and molecular approaches with a focus on temperature and drought stresses. Front. Plant Sci. 2019, 10, 1759. [Google Scholar] [CrossRef]
- Karges, K.; Bellingrath-Kimura, S.D.; Watson, C.A.; Stoddard, F.L.; Halwani, M.; Reckling, M. Agro-economic prospects for expanding soybean production beyond its current northerly limit in europe. Eur. J. Agron. 2022, 133, 126415. [Google Scholar] [CrossRef]
- Rocchetti, L.; Bellucci, E.; Cortinovis, G.; Di Vittori, V.; Lanzavecchia, G.; Frascarelli, G.; Nanni, L.; Del Gatto, A.; Pieri, S.; Mangoni, L.; et al. The development of a european and mediterranean chickpea association panel (emcap). Agronomy 2020, 10, 1417. [Google Scholar] [CrossRef]
- Bellucci, E.; Mario Aguilar, O.; Alseekh, S.; Bett, K.; Brezeanu, C.; Cook, D.; De la Rosa, L.; Delledonne, M.; Dostatny, D.F.; Ferreira, J.J.; et al. The increase project: Intelligent collections of food-legume genetic resources for european agrofood systems. Plant J. 2021, 108, 646–660. [Google Scholar] [CrossRef]
- Esfahani, M.N.; Sulieman, S.; Schulze, J.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S. Approaches for enhancement of n(2) fixation efficiency of chickpea (Cicer arietinum L.) under limiting nitrogen conditions. Plant Biotechnol. J. 2014, 12, 387–397. [Google Scholar] [CrossRef]
- Hughes, J.; Pearson, E.; Grafenauer, S. Legumes-a comprehensive exploration of global food-based dietary guidelines and consumption. Nutrients 2022, 14, 3080. [Google Scholar] [CrossRef]
- Wallace, T.C.; Murray, R.; Zelman, K.M. The nutritional value and health benefits of chickpeas and hummus. Nutrients 2016, 8, 766. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, R.; Upadhyaya, H.D.; Gaur, P.M.; Gowda, C.L.L.; Krishnamurthy, L. Kabuli and desi chickpeas differ in their requirement for reproductive duration. Field Crops Res. 2014, 163, 24–31. [Google Scholar] [CrossRef]
- Varma Penmetsa, R.; Carrasquilla-Garcia, N.; Bergmann, E.M.; Vance, L.; Castro, B.; Kassa, M.T.; Sarma, B.K.; Datta, S.; Farmer, A.D.; Baek, J.M.; et al. Multiple post-domestication origins of kabuli chickpea through allelic variation in a diversification-associated transcription factor. New Phytol. 2016, 211, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- Nisa, Z.U.; Arif, A.; Waheed, M.Q.; Shah, T.M.; Iqbal, A.; Siddiqui, A.J.; Choudhary, M.I.; El-Seedi, H.R.; Musharraf, S.G. A comparative metabolomic study on desi and kabuli chickpea (Cicer arietinum L.) genotypes under rainfed and irrigated field conditions. Sci. Rep. 2020, 10, 13919. [Google Scholar] [CrossRef]
- Neugschwandtner, R.W.; Wichmann, S.; Gimplinger, D.M.; Wagentristl, H.; Kaul, H.-P. Chickpea performance compared to peam barley and oat in central europe: Growth analysis and yield. Turk. J. Field Crops 2013, 18, 179–184. [Google Scholar]
- Reckling, M.; Bergkvist, G.; Watson, C.A.; Stoddard, F.L.; Zander, P.M.; Walker, R.L.; Pristeri, A.; Toncea, I.; Bachinger, J. Trade-offs between economic and environmental impacts of introducing legumes into cropping systems. Front. Plant Sci. 2016, 7, 669. [Google Scholar] [CrossRef]
- Rubiales, D.; Annicchiarico, P.; Vaz Patto, M.C.; Julier, B. Legume breeding for the agroecological transition of global agri-food systems: A european perspective. Front. Plant Sci. 2021, 12, 782574. [Google Scholar] [CrossRef]
- Fanning, J.; Brand, J.; Munoz Santa, I.; McDonald, L.; Taylor, J.; Hollaway, G. Management of chickpea ascochyta blight using fungicides and cultivar resistance improves grain yield, quality, and grower profitability. Front. Plant Sci. 2022, 13, 942220. [Google Scholar] [CrossRef]
- Pang, J.; Turner, N.C.; Khan, T.; Du, Y.L.; Xiong, J.L.; Colmer, T.D.; Devilla, R.; Stefanova, K.; Siddique, K.H.M. Response of chickpea (Cicer arietinum L.) to terminal drought: Leaf stomatal conductance, pod abscisic acid concentration, and seed set. J. Exp. Bot. 2017, 68, 1973–1985. [Google Scholar] [PubMed]
- Ramamoorthy, P.; Lakshmanan, K.; Upadhyaya, H.D.; Vadez, V.; Varshney, R.K. Shoot traits and their relevance in terminal drought tolerance of chickpea (Cicer arietinum L.). Field Crops Res. 2016, 197, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.K.; Gupta, S.K.; Dwivedi, V.; Chattopadhyay, D. Lignin deposition in chickpea root xylem under drought. Plant Signal. Behav. 2020, 15, 1754621. [Google Scholar] [CrossRef]
- Purushothaman, R.; Krishnamurthy, L.; Upadhyaya, H.D.; Vadez, V.; Varshney, R.K. Genotypic variation in soil water use and root distribution and their implications for drought tolerance in chickpea. Funct. Plant Biol. 2017, 44, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Rurushothaman, R.; Krishnamurthy, L.; Upadhyaya, H.D.; Vadez, V.; Varshney, R.K. Root traits confer grain yield advantages under terminal drought inchickpea (Cicer arietinum L.). Field Crops Res. 2017, 201, 146–161. [Google Scholar]
- Istanbuli, T.; Assar, A.A.; Twakaz, S.; Kumar, T.; Alsamman, A.M.; Hamwieh, A. The interaction between drought stress and nodule formation under multiple environments in chickpea. PLoS ONE 2022, 17, e0276732. [Google Scholar] [CrossRef]
- Esfahani, M.N.; Sulieman, S.; Schulze, J.; Yama-Shinozaki, K.; Shinozaki, K.; Tran, L.-S.P. Mechanisms of physiological adjustment of n2 fixation in Cicer arietinum L. (chickpea) during early stages of water deficit: Single or multi-factor controls. Plant J. 2014, 79, 964–980. [Google Scholar] [CrossRef]
- Mirkovic, T.; Ostroumov, E.E.; Anna, J.M.; van Grondelle, R.; Govindjee; Scholes, G.D. Light absoR.P.tion and energy transfer in the antenna complexes of photosynthetic organisms. Chem. Rev. 2017, 117, 249–293. [Google Scholar] [CrossRef]
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef]
- Macar, T.K.; Ekmekci, Y. Psii photochemistry and antioxidant response of a chickpea variety exposed to drought. Z. Für Naturforschung—Sect. C J. Biosci. 2008, 63, 583–594. [Google Scholar] [CrossRef]
- Saglam, A.; Terzi, R.; Demiralay, M. Effect of polyethylene glycol induced drought stress on photosynthesis in two chickpea genotypes with different drought tolerance. Acta Biol. Hung. 2014, 65, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Tschiersch, H.; Junker, A.; Meyer, R.C.; Altmann, T. Establishment of integrated protocols for automated high throughput kinetic chlorophyll fluorescence analyses. Plant Methods 2017, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Furbank, R.T.; Tester, M. Phenomics—Technologies to relieve the phenotyping bottleneck. Trends Plant Sci. 2011, 16, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, F.; Cabrera-Bosquet, L.; Pridmore, T.; Bennett, M. Plant phenomics, from sensors to knowledge. Curr. Biol. 2017, 27, R770–R783. [Google Scholar] [CrossRef]
- Yang, W.; Feng, H.; Zhang, X.; Zhang, J.; Doonan, J.H.; Batchelor, W.D.; Xiong, L.; Yan, J. Crop phenomics and high-throughput phenotyping: Past decades, current challenges, and future perspectives. Mol. Plant 2020, 13, 187–214. [Google Scholar] [CrossRef]
- Lauterberg, M.; Saranga, Y.; Deblieck, M.; Klukas, C.; Krugman, T.; Perovic, D.; Ordon, F.; Graner, A.; Neumann, K. Precision phenotyping across the life cycle to validate and decipher drought-adaptive qtls of wild emmer wheat (Triticum turgidum ssp. Dicoccoides) introduced into elite wheat varieties. Front. Plant Sci. 2022, 13, 965287. [Google Scholar]
- Atieno, J.; Colmer, T.D.; Taylor, J.; Li, Y.; Quealy, J.; Kotula, L.; Nicol, D.; Nguyen, D.T.; Brien, C.; Langridge, P.; et al. Novel salinity tolerance loci in chickpea identified in glasshouse and field environments. Front. Plant Sci. 2021, 12, 667910. [Google Scholar] [CrossRef]
- Atieno, J.; Li, Y.; Langridge, P.; Dowling, K.; Brien, C.; Berger, B.; Varshney, R.K.; Sutton, T. Exploring genetic variation for salinity tolerance in chickpea using image-based phenotyping. Sci. Rep. 2017, 7, 1300. [Google Scholar] [CrossRef]
- Humplik, J.F.; Lazar, D.; Furst, T.; Husickova, A.; Hybl, M.; Spichal, L. Automated integrative high-throughput phenotyping of plant shoots: A case study of the cold-tolerance of pea (Pisum sativum L.). Plant Methods 2015, 11, 20. [Google Scholar] [CrossRef]
- Awlia, M.; Nigro, A.; Fajkus, J.; Schmoeckel, S.M.; Negrao, S.; Santelia, D.; Trtilek, M.; Tester, M.; Julkowska, M.M.; Panzarova, K. High-throughput non-destructive phenotyping of traits that contribute to salinity tolerance in arabidopsis thaliana. Front. Plant Sci. 2016, 7, 1414. [Google Scholar] [CrossRef]
- Dhanagond, S.; Liu, G.; Zhao, Y.; Chen, D.; Grieco, M.; Reif, J.; Kilian, B.; Graner, A.; Neumann, K. Non-invasive phenotyping reveals genomic regions involved in pre-anthesis drought tolerance and recovery in spring barley. Front. Plant Sci. 2019, 10, 1307. [Google Scholar] [CrossRef] [PubMed]
- Dodig, D.; Bozinovic, S.; Nikolic, A.; Zoric, M.; Vancetovic, J.; Ignjatovic-Micic, D.; Delic, N.; Weigelt-Fischer, K.; Altmann, T.; Junker, A. Dynamics of maize vegetative growth and drought adaptability using image-based phenotyping under controlled conditions. Front. Plant Sci. 2021, 12, 652116. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.L.; Kim, N.; Lee, H.; Lee, E.; Cheon, K.S.; Kim, M.; Baek, J.; Choi, I.; Ji, H.; Yoon, I.S.; et al. High-throughput phenotyping platform for analyzing drought tolerance in rice. Planta 2020, 252, 38. [Google Scholar] [CrossRef]
- Neumann, K.; Klukas, C.; Friedel, S.; Rischbeck, P.; Chen, D.; Entzian, A.; Stein, N.; Graner, A.; Kilian, B. Dissecting spatiotemporal biomass accumulation in barley under different water regimes using high-throughput image analysis. Plant Cell Environ. 2015, 38, 1980–1996. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, Y.; Du, J.; Guo, X.; Wen, W.; Gu, S.; Wang, J.; Fan, J. Crop phenomics: Current status and perspectives. Front. Plant Sci. 2019, 10, 714. [Google Scholar] [CrossRef]
- Condon, A.G.; Richards, R.A.; Rebetzke, G.J.; Farquhar, G.D. Breeding for high water-use efficiency. J. Exp. Bot. 2004, 55, 2447–2460. [Google Scholar] [CrossRef]
- Burridge, J.D.; Grondin, A.; Vadez, V. Optimizing crop water use for drought and climate change adaptation requires a multi-scale approach. Front. Plant Sci. 2022, 13, 824720. [Google Scholar] [CrossRef]
- Oweis, T.; Hachum, A.; Pala, M. Water use efficiency of winter-sown chickpea under supplemental irrigation in a mediterranean environment. Agric. Water Manag. 2004, 66, 163–179. [Google Scholar] [CrossRef]
- Das, A.; Basu, P.S.; Kumar, M.; Ansari, J.; Shukla, A.; Thakur, S.; Singh, P.; Datta, S.; Chaturvedi, S.K.; Sheshshayee, M.S.; et al. Transgenic chickpea (Cicer arietinum L.) harbouring atdreb1a are physiologically better adapted to water deficit. BMC Plant Biol. 2021, 21, 39. [Google Scholar] [CrossRef]
- Mikołajczak, K.; Ogrodowicz, P.; Cwiek-Kupczynska, H.; Weigelt-Fischer, K.; Mothukuri, S.R.; Junker, A.; Altmann, T.; Krystkowiak, K.; Adamski, T.; Surma, M.; et al. Image phenotyping of spring barley (Hordeum vulgare L.) ril population under drought: Selection of traits and biological inteR.P.retation. Front. Plant Sci. 2022, 11, 1–18. [Google Scholar] [CrossRef]
- Amitrano, C.; Junker, A.; D’Agostino, N.; De Pascale, S.; De Micco, V. Integration of high-throughput phenotyping with anatomical traits of leaves to help understanding lettuce acclimation to a changing environment. Planta 2022, 256, 68. [Google Scholar] [CrossRef]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The role of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Steyn, W.J.; Wand, S.J.E.; Holcroft, D.M.; Jacobs, G. Anthocyanins in vegetative tissues: A proposed unified function in photoprotection. New Phytol. 2002, 155, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Farago, D.; Sass, L.; Valkai, I.; Andrasi, N.; Szabados, L. Plantsize offers an affordable, non-destructive method to measure plant size and color in vitro. Front. Plant Sci. 2018, 9, 219. [Google Scholar] [CrossRef]
- Mafakheri, A.; Siosemardeh, A.; Bahramnejad, B.; Struik, P.C.; Sohrabi, Y. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010, 4, 580–585. [Google Scholar]
- Talebi, R.; Ensafi, M.H.; Baghebani, N.; Karami, E.; Mohammadi, K. Physiological responses of chickpea (cicer arietinum) genotypes to drought stress. Environ. Exp. Biol. 2013, 11, 9–15. [Google Scholar]
- Majer, P.; Sass, L.; Horvath, G.V.; Hideg, E. Leaf hue measurements offer a fast, high-throughput initial screening of photosynthesis in leaves. J. Plant Physiol. 2010, 167, 74–76. [Google Scholar] [CrossRef]
- Liang, Y.; Urano, D.; Liao, K.L.; Hedrick, T.L.; Gao, Y.; Jones, A.M. A nondestructive method to estimate the chlorophyll content of arabidopsis seedlings. Plant Methods 2017, 13, 26. [Google Scholar] [CrossRef]
- Dong, S.; Jiang, Y.; Dong, Y.; Wang, L.; Wang, W.; Ma, Z.; Yan, C.; Ma, C.; Liu, L. A study on soybean responses to drought stress and rehydration. Saudi J. Biol. Sci. 2019, 26, 2006–2017. [Google Scholar] [CrossRef]
- Zendonadi Dos Santos, N.; Piepho, H.P.; Condorelli, G.E.; Licieri Groli, E.; Newcomb, M.; Ward, R.; Tuberosa, R.; Maccaferri, M.; Fiorani, F.; Rascher, U.; et al. High-throughput field phenotyping reveals genetic variation in photosynthetic traits in durum wheat under drought. Plant Cell Environ. 2021, 44, 2858–2878. [Google Scholar] [CrossRef]
- Dodig, D.; Bozinovic, S.; Nikolic, A.; Zoric, M.; Vancetovic, J.; Ignjatovic-Micic, D.; Delic, N.; Weigelt-Fischer, K.; Junker, A.; Altmann, T. Image-derived traits related to mid-season growth performance of maize under nitrogen and water stress. Front. Plant Sci. 2019, 10, 814. [Google Scholar] [CrossRef] [PubMed]
- Araus, J.L.; Cairns, J.E. Field high-throughput phenotyping: The new crop breeding frontier. Trends Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Ullah, A.; Lee, D.-L.; Alghamdi, S.S.; Siddique, K.H.M. Desi chickpea genotypes tolerate drought stress better than kabuli types by modulating germination metabolism, trehalose accumulation, and carbon assimilation. Plant Physiol. Biochem. 2018, 126, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Thudi, M.; Chitikineni, A.; Liu, X.; He, W.; Roorkiwal, M.; Yang, W.; Jian, J.; Doddamani, D.; Gaur, P.M.; Rathore, A.; et al. Recent breeding programs enhanced genetic diversity in both desi and kabuli varieties of chickpea (Cicer arietinum L.). Sci. Rep. 2016, 6, 38636. [Google Scholar] [CrossRef]
- Rocchetti, L.; Gioia, T.; Logozzo, G.; Brezeanu, C.; Pereira, L.G.; la Rosa, L.; Marzario, S.; Pieri, A.; Fernie, A.R.; Alseekh, S.; et al. Towards the development, maintenance and standardized phenotypic characterization of single-seed-descent genetic resources for chickpea. Curr. Protoc. 2022, 2, e371. [Google Scholar] [CrossRef]
- Klukas, C.; Chen, D.; Pape, J.M. Integrated analysis platform: An open-source information system for high-throughput plant phenotyping. Plant Physiol. 2014, 165, 506–518. [Google Scholar] [CrossRef]



| DAS | DAT | Action |
|---|---|---|
| 0 | Sowing and pre-cultivation in greenhouse | |
| 14 | 0 | Transferring to HTP system and set watering to 70% PAW |
| 15 | 1 | First image |
| 20 | 6 | Chlorophyll fluorescence measurement |
| 22 | 8 | Initiation drought stress: 10% PAW |
| 27 | 13 | Chlorophyll fluorescence measurement |
| 34 | 20 | Chlorophyll fluorescence measurement |
| 41 | 27 | Chlorophyll fluorescence measurement |
| 43 | 29 | First step of recovery: + 300 mL |
| 44 | 30 | Second step of recovery: 70% PAW |
| 48 | 34 | Chlorophyll fluorescence measurement |
| 55 | 41 | Last imaging on HTP system |
| 56 | 42 | Harvest |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauterberg, M.; Tschiersch, H.; Papa, R.; Bitocchi, E.; Neumann, K. Engaging Precision Phenotyping to Scrutinize Vegetative Drought Tolerance and Recovery in Chickpea Plant Genetic Resources. Plants 2023, 12, 2866. https://doi.org/10.3390/plants12152866
Lauterberg M, Tschiersch H, Papa R, Bitocchi E, Neumann K. Engaging Precision Phenotyping to Scrutinize Vegetative Drought Tolerance and Recovery in Chickpea Plant Genetic Resources. Plants. 2023; 12(15):2866. https://doi.org/10.3390/plants12152866
Chicago/Turabian StyleLauterberg, Madita, Henning Tschiersch, Roberto Papa, Elena Bitocchi, and Kerstin Neumann. 2023. "Engaging Precision Phenotyping to Scrutinize Vegetative Drought Tolerance and Recovery in Chickpea Plant Genetic Resources" Plants 12, no. 15: 2866. https://doi.org/10.3390/plants12152866
APA StyleLauterberg, M., Tschiersch, H., Papa, R., Bitocchi, E., & Neumann, K. (2023). Engaging Precision Phenotyping to Scrutinize Vegetative Drought Tolerance and Recovery in Chickpea Plant Genetic Resources. Plants, 12(15), 2866. https://doi.org/10.3390/plants12152866









