Zinc Oxide Nanoparticles Biosynthesized by Eriobotrya japonica Leaf Extract: Characterization, Insecticidal and Antibacterial Properties
Abstract
1. Introduction
2. Results and Discussion
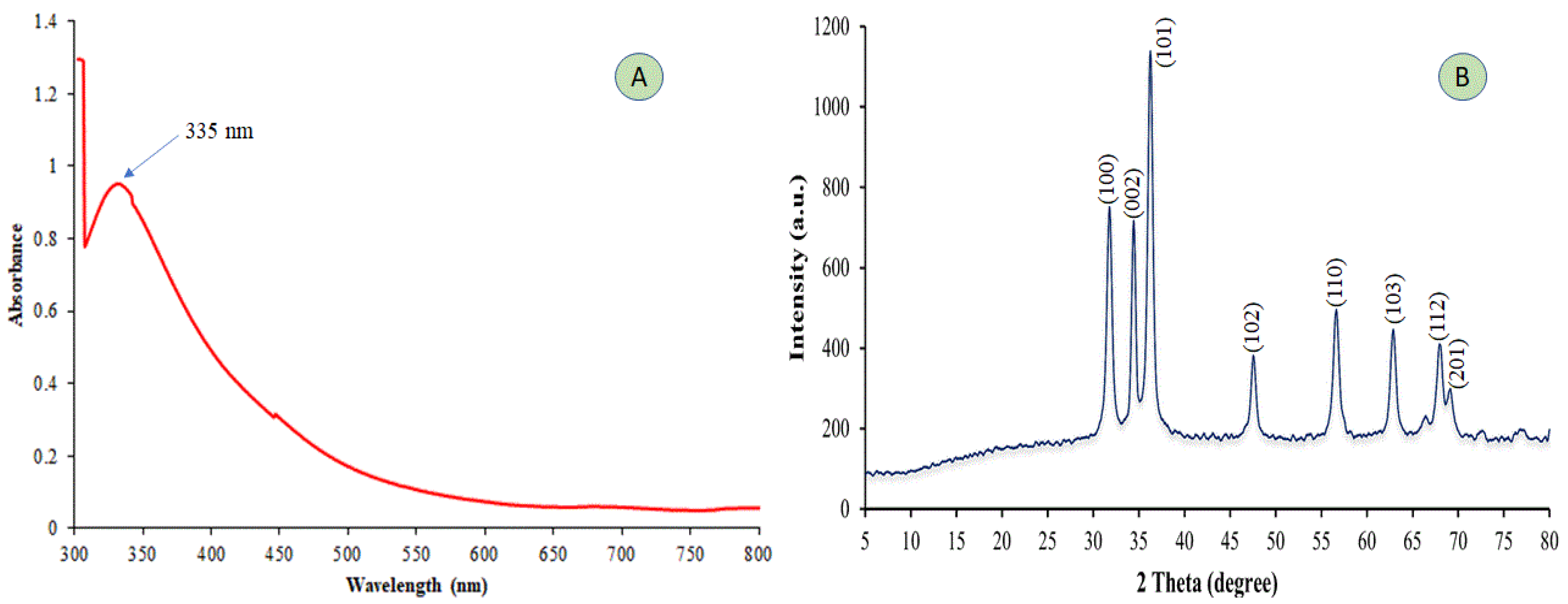
2.1. UV–vis and X-ray Diffraction (XRD) Analysis
2.2. Scanning Electron Microscopy Analysis
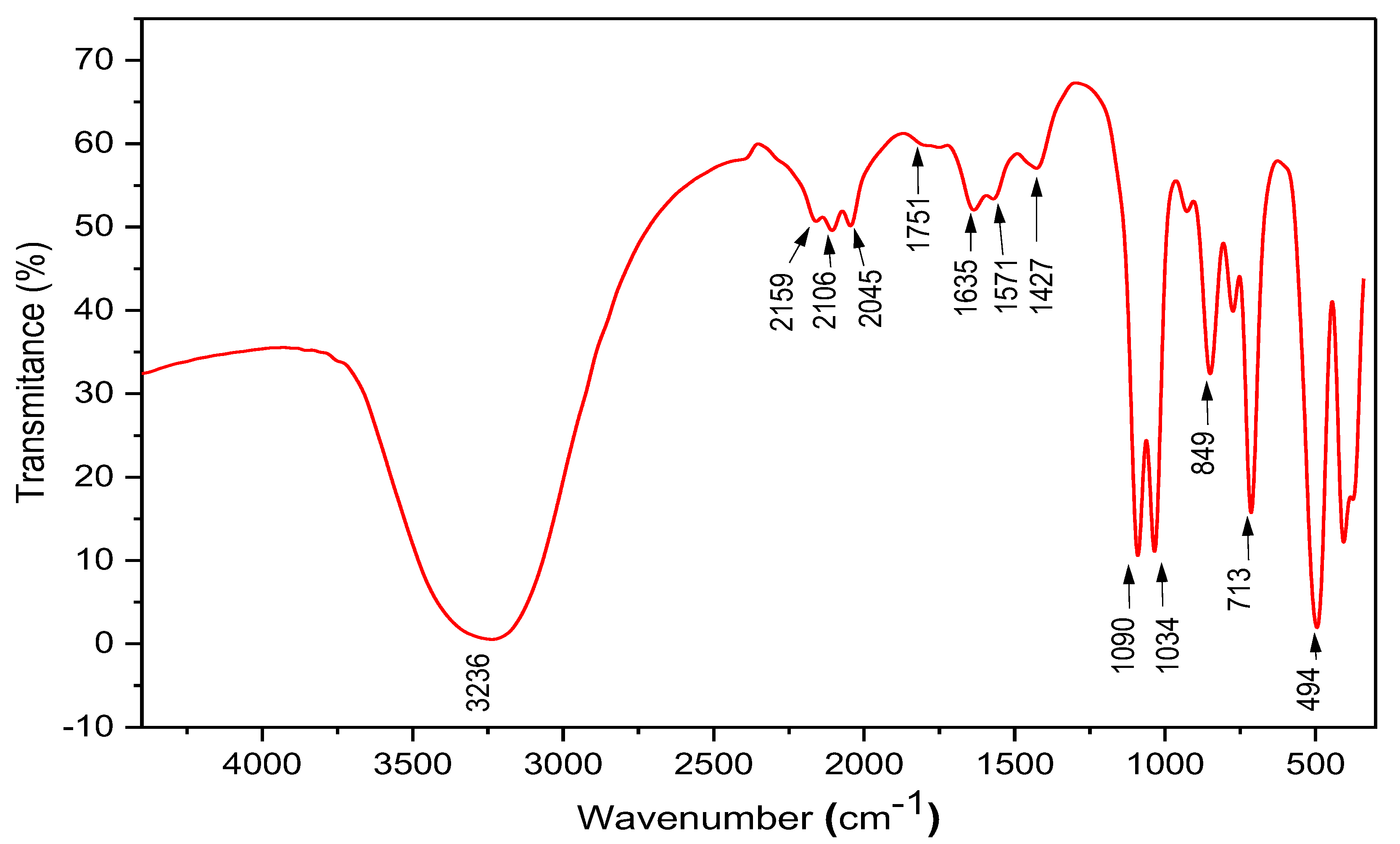
2.3. FTIR Analysis
2.4. Particle Size Distribution and Zeta Potential Evaluation
2.5. Toxicity of Zinc Oxide Nanoparticles
2.6. ZnO-NPs Effect on Bacterial Strains
3. Materials and Methods
3.1. Preparation of ZnO-NPs through Eriobotrya japonica Leaf Extract (EJE)
3.2. Characterization of the Green Synthesized ZnO-NPs
3.3. Insect Rearing Culture
3.3.1. Tribolium castaneum (Herbst)
3.3.2. Sitophilus oryzae (Linnaeus)
3.4. Fumigant Toxicity Bioassay
3.5. Bacterial Cultures and Antibacterial Study
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbass, K.; Qasim, M.Z.; Song, H.; Murshed, M.; Mahmood, H.; Younis, I. A review of the global climate change impacts, adaptation, and sustainable mitigation measures. Environ. Sci. Pollut. Res. 2022, 29, 42539–42559. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Rahim, W.M.; Mostafa, E.M.; Moawad, H. High cell density cultivation of six fungal strains efficient in azo dye bioremediation. Biotechnol. Rep. 2016, 12, 1–5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Godde, C.M.; Mason-D’Croz, D.; Mayberry, D.E.; Thornton, P.K.; Herrero, M. Impacts of climate change on the livestock food supply chain; a review of the evidence. Glob. Food Secur. 2021, 28, 100488. [Google Scholar] [CrossRef] [PubMed]
- Swelam, N.O. Ecological and control studies on Sitophilus oryzae and Oryzaephilus surinamensis with reference to insect store pests at menoufia governorate. Menoufia J. Plant Prot. 2022, 7, 1–7. [Google Scholar] [CrossRef]
- Prakash, B.; Kumar, A.; Singh, P.P.; Das, S.; Dubey, N.K. Prospects of plant products in the management of insect pests of food grains: Current status and future perspectives. In Natural Bioactive Compounds; Academic Press: New York, NY, USA, 2021; pp. 317–335. [Google Scholar]
- Mehta, V.; Kumar, S.; CS, J. Damage potential, effect on germination, and development of Sitophilus oryzae (Coleoptera: Curculionidae) on wheat grains in Northwestern Himalayas. J. Insect Sci. 2021, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Peterson, C.; Zhao, S.; Coats, J.R. Fumigation toxicity of volatile natural and synthetic cyanohydrins to stored-product pests and activity as soil fumigants. Pest Manag. Sci. Former. Pestic. Sci. 2004, 60, 833–838. [Google Scholar] [CrossRef]
- Dars, F.; Rustamani, M.A.; Khuhro, R.D.; Baloch, H.B. Effect of wheat grain moisture on infestation of red flour beetle, Tribolium castaneum (Herbst.). Pakistan J. Zool. 2001, 33, 189–192. [Google Scholar]
- Qurban, A.L.; ul Hasan, M.; Sagheer, M.; Ranjha, M.H.; Shahbaz, M.; Faisal, M. Appraisal of quantitative losses caused by Trogoderma granarium (Everts) and Tribolium castaneum (Herbst) in different genotypes of wheat, rice and maize during storage. J. Appl. Biol. Sci. 2016, 10, 8–14. [Google Scholar]
- Javed, M.; Zeeshan Majeed, M.; Khaliq, A.; Arshad, M.; Ahmad, M.H.; Sufyan, M. Quantitative losses in some advanced genotypes of barley incurred by Tribolium castaneum L.(Herbst.). Int. J. Agron. Agric. Res. 2016, 8, 45–50. [Google Scholar]
- Hassan, M.W.; Jamil, M.; Muzaffar, Z.; Naz, S.; Fakhar, F. Evaluation of different wheat byproducts and varieties for relative population growth and weight loss by Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) and Trogoderma granarium Everts (Coleoptera: Dermestidae). J. Entomol. Zool. Stud. 2017, 5, 804–809. [Google Scholar]
- Mansoor, M.M.; Afzal, M.; Raza, A.B.M.; Akram, Z.; Waqar, A.; Afzal, M.B.S. Post-exposure temperature influence on the toxicity of conventional and new chemistry insecticides to green lacewing Chrysoperla carnea (Stephens)(Neuroptera: Chrysopidae). Saudi J. Biol. Sci. 2015, 22, 317–321. [Google Scholar] [PubMed]
- Behiry, S.I.; Ashmawy, N.A.; Abdelkhalek, A.A.; Younes, H.A.; Khaled, A.E.; Hafez, E.E. Compatible- and incompatible-type interactions related to defense genes in potato elucidation by Pectobacterium carotovorum. J. Plant Dis. Prot. 2018, 125, 197–204. [Google Scholar] [CrossRef]
- Aguilar-Marcelino, L.; Mendoza-de-Gives, P.; Al-Ani, L.K.T.; López-Arellano, M.E.; Gómez-Rodríguez, O.; Villar-Luna, E.; Reyes-Guerrero, D.E. Using molecular techniques applied to beneficial microorganisms as biotechnological tools for controlling agricultural plant pathogens and pest. In Molecular Aspects of Plant Beneficial Microbes in Agriculture; Elsevier: Amsterdam, The Netherlands, 2020; pp. 333–349. [Google Scholar]
- Davidsson, P.R.; Kariola, T.; Niemi, O.; Palva, E.T. Pathogenicity of and plant immunity to soft rot pectobacteria. Front. Plant Sci. 2013, 4, 191. [Google Scholar] [PubMed]
- Khamis, W.M.; Heflish, A.A.; El-Messeiry, S.; Behiry, S.I.; Al-Askar, A.A.; Su, Y.; Abdelkhalek, A.; Gaber, M.K. Swietenia mahagoni Leaves Extract: Antifungal, Insecticidal, and Phytochemical Analysis. Separations 2023, 10, 301. [Google Scholar] [CrossRef]
- Abd El-Rahim, W.M.; Moawad, H.; Hashem, M.M.; Gebreil, G.M.M.; Zakaria, M. Highly efficient fungal pectinase and laccase producers among isolates from flax retting liquor. Biocatal. Agric. Biotechnol. 2020, 25, 101570. [Google Scholar] [CrossRef]
- Eisfeld, C.; Schijven, J.F.; Kastelein, P.; Van Breukelen, B.M.; Medema, G.; Velstra, J.; Teunis, P.F.M.; van der Wolf, J.M. Dose-response relationship of Ralstonia solanacearum and potato in greenhouse and in vitro experiments. Front. Plant Sci. 2022, 13, 1074192. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate change impacts on plant pathogens, food security and paths forward. Nat. Rev. Microbiol. 2023, 1–17. [Google Scholar] [CrossRef]
- Moawad, H.; Abd el-Rahim, W.M.; Abd el-Aleem, D.; Abo Sedera, S.A. Persistence of two Rhizobium etli inoculant strains in clay and silty loam soils. J. Basic Microbiol. An Int. J. Biochem. Physiol. Genet. Morphol. Ecol. Microorg. 2005, 45, 438–446. [Google Scholar]
- Moawad, H.; El-Rahim, W.M.A. Bioremediation of irrigation water contaminated with textile dyes. Fresenius Environ. Bull. 2003, 12, 786–792. [Google Scholar]
- Ngegba, P.M.; Cui, G.; Khalid, M.Z.; Zhong, G. Use of botanical pesticides in agriculture as an alternative to synthetic pesticides. Agriculture 2022, 12, 600. [Google Scholar] [CrossRef]
- Nguyen, T.N.Q.; Hua, Q.C.; Nguyen, T.T. Enhancing insecticide activity of anacardic acid by intercalating it into MgAl layered double hydroxides nanoparticles. J. Vietnam. Environ. 2014, 6, 208–211. [Google Scholar] [CrossRef]
- Baker, S.; Satish, S.; Prasad, N.; Chouhan, R.S. Nano-agromaterials: Influence on plant growth and crop protection. In Industrial Applications of Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 341–363. [Google Scholar]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; ur Rehman, H.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef] [PubMed]
- Aseel, D.G.; Behiry, S.I.; Abdelkhalek, A. Green and Cost-Effective Nanomaterials Synthesis from Desert Plants and Their Applications. In Secondary Metabolites Based Green Synthesis of Nanomaterials and Their Applications; Springer: Singapore, 2023; pp. 327–357. [Google Scholar]
- Bratovcic, A. Different applications of nanomaterials and their impact on the environment. SSRG Int. J. Mater. Sci. Eng. 2019, 5, 1–7. [Google Scholar]
- Abdelkhalek, A.; Yassin, Y.; Abdel-Megeed, A.; Abd-Elsalam, K.A.; Moawad, H.; Behiry, S.I. Rhizobium leguminosarum bv. viciae-Mediated Silver Nanoparticles for Controlling Bean Yellow Mosaic Virus (BYMV) Infection in Faba Bean Plants. Plants 2023, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Jin, Y.; Li, Y.; Tjong, S.C. Interactions of zinc oxide nanostructures with mammalian cells: Cytotoxicity and photocatalytic toxicity. Int. J. Mol. Sci. 2020, 21, 6305. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloys Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Zanet, V.; Vidic, J.; Auger, S.; Vizzini, P.; Lippe, G.; Iacumin, L.; Comi, G.; Manzano, M. Activity evaluation of pure and doped zinc oxide nanoparticles against bacterial pathogens and Saccharomyces cerevisiae. J. Appl. Microbiol. 2019, 127, 1391–1402. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef]
- Karekar, S.E.; Bhanvase, B.A.; Sonawane, S.H.; Deosarkar, M.P.; Pinjari, D.V.; Pandit, A.B. Synthesis of zinc molybdate and zinc phosphomolybdate nanopigments by an ultrasound assisted route: Advantage over conventional method. Chem. Eng. Process. Process Intensif. 2015, 87, 51–59. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef]
- Agarwal, H.; Menon, S.; Kumar, S.V.; Rajeshkumar, S. Mechanistic study on antibacterial action of zinc oxide nanoparticles synthesized using green route. Chem. Biol. Interact. 2018, 286, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Burman, U.; Saini, M.; Kumar, P.-. Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings. Toxicol. Environ. Chem. 2013, 95, 605–612. [Google Scholar] [CrossRef]
- Amuthavalli, P.; Hwang, J.-S.; Dahms, H.-U.; Wang, L.; Anitha, J.; Vasanthakumaran, M.; Gandhi, A.D.; Murugan, K.; Subramaniam, J.; Paulpandi, M. Zinc oxide nanoparticles using plant Lawsonia inermis and their mosquitocidal, antimicrobial, anticancer applications showing moderate side effects. Sci. Rep. 2021, 11, 8837. [Google Scholar] [CrossRef]
- Fadillah, R.; Rati, Y.; Dewi, R.; Farma, R.; Rini, A.S. Optical and structural studies on bio-synthesized ZnO using Citrullus lanatus peel extract. In Proceedings of the Journal of Physics: Conference Series, Mataram, Indonesia, 20–22 November 2020; IOP Publishing: Bristol, UK, 2021; Volume 1816, p. 12019. [Google Scholar]
- Talam, S.; Karumuri, S.R.; Gunnam, N. Synthesis, characterization, and spectroscopic properties of ZnO nanoparticles. Int. Sch. Res. Not. 2012, 2012, 372505. [Google Scholar] [CrossRef]
- Jose, L.M.; Kuriakose, S.; Thomas, S. Fabrication, characterization and in vitro antifungal property evaluation of biocompatible lignin-stabilized zinc oxide nanoparticles against selected pathogenic fungal strains. Bionanoscience 2020, 10, 583–596. [Google Scholar] [CrossRef]
- Arefi, M.R.; Rezaei-Zarchi, S. Synthesis of zinc oxide nanoparticles and their effect on the compressive strength and setting time of self-compacted concrete paste as cementitious composites. Int. J. Mol. Sci. 2012, 13, 4340–4350. [Google Scholar] [CrossRef] [PubMed]
- Nazir, A.; Akbar, A.; Baghdadi, H.B.; ur Rehman, S.; Al-Abbad, E.; Fatima, M.; Iqbal, M.; Tamam, N.; Alwadai, N.; Abbas, M. Zinc oxide nanoparticles fabrication using Eriobotrya japonica leaves extract: Photocatalytic performance and antibacterial activity evaluation. Arab. J. Chem. 2021, 14, 103251. [Google Scholar] [CrossRef]
- Rad, S.S.; Sani, A.M.; Mohseni, S. Biosynthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from leaf extract of Mentha pulegium (L.). Microb. Pathog. 2019, 131, 239–245. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A. Green Synthesized ZnO Nanoparticles Mediated by Mentha Spicata Extract Induce Plant Systemic Resistance against Tobacco Mosaic Virus. Appl. Sci. 2020, 10, 5054. [Google Scholar] [CrossRef]
- Jamdagni, P.; Khatri, P.; Rana, J.-S. Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity. J. King Saud Univ. 2018, 30, 168–175. [Google Scholar] [CrossRef]
- Shabaani, M.; Rahaiee, S.; Zare, M.; Jafari, S.M. Green synthesis of ZnO nanoparticles using loquat seed extract; Biological functions and photocatalytic degradation properties. LWT 2020, 134, 110133. [Google Scholar] [CrossRef]
- Ali, K.; Dwivedi, S.; Azam, A.; Saquib, Q.; Al-Said, M.S.; Alkhedhairy, A.A.; Musarrat, J. Aloe vera extract functionalized zinc oxide nanoparticles as nanoantibiotics against multi-drug resistant clinical bacterial isolates. J. Colloid Interface Sci. 2016, 472, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Stan, M.; Popa, A.; Toloman, D.; Dehelean, A.; Lung, I.; Katona, G. Enhanced photocatalytic degradation properties of zinc oxide nanoparticles synthesized by using plant extracts. Mater. Sci. Semicond. Process. 2015, 39, 23–29. [Google Scholar] [CrossRef]
- Bigdeli, F.; Morsali, A.; Retailleau, P. Syntheses and characterization of different zinc (II) oxide nano-structures from direct thermal decomposition of 1D coordination polymers. Polyhedron 2010, 29, 801–806. [Google Scholar] [CrossRef]
- Kashyout, A.B.; Soliman, H.M.A.; Shokry Hassan, H.; Abousehly, A.M. Fabrication of ZnO and ZnO: Sb nanoparticles for gas sensor applications. J. Nanomater. 2010, 2010, 1–8. [Google Scholar] [CrossRef]
- Abdelmigid, H.M.; Hussien, N.A.; Alyamani, A.A.; Morsi, M.M.; AlSufyani, N.M.; Kadi, H.A. Green Synthesis of Zinc Oxide Nanoparticles Using Pomegranate Fruit Peel and Solid Coffee Grounds vs. Chemical Method of Synthesis, with Their Biocompatibility and Antibacterial Properties Investigation. Molecules 2022, 27, 1236. [Google Scholar] [CrossRef]
- Alamdari, S.; Sasani Ghamsari, M.; Lee, C.; Han, W.; Park, H.-H.; Tafreshi, M.J.; Afarideh, H.; Ara, M.H.M. Preparation and Characterization of Zinc Oxide Nanoparticles Using Leaf Extract of Sambucus ebulus. Appl. Sci. 2020, 10, 3620. [Google Scholar] [CrossRef]
- Degefa, A.; Bekele, B.; Jule, L.T.; Fikadu, B.; Ramaswamy, S.; Dwarampudi, L.P.; Nagaprasad, N.; Ramaswamy, K. Green Synthesis, Characterization of Zinc Oxide Nanoparticles, and Examination of Properties for Dye-Sensitive Solar Cells Using Various Vegetable Extracts. J. Nanomater. 2021, 2021, 3941923. [Google Scholar] [CrossRef]
- Golmohammadi, M.; Honarmand, M.; Ghanbari, S. A green approach to synthesis of ZnO nanoparticles using jujube fruit extract and their application in photocatalytic degradation of organic dyes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 117961. [Google Scholar] [CrossRef]
- Padalia, H.; Chanda, S. Characterization, antifungal and cytotoxic evaluation of green synthesized zinc oxide nanoparticles using Ziziphus nummularia leaf extract. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1751–1761. [Google Scholar] [CrossRef] [PubMed]
- Muhideen Badhusha, M.S.; Joel, C.; Imran Khan, R.; Vijayakumar, N. Green synthesis and characterization of Fe doped ZnO nanoparticles and their interaction with bovine serum albumin. J. Indian Chem. Soc. 2021, 98, 100197. [Google Scholar] [CrossRef]
- Ifeanyichukwu, U.L.; Fayemi, O.E.; Ateba, C.N. Green Synthesis of Zinc Oxide Nanoparticles from Pomegranate (Punica granatum) Extracts and Characterization of Their Antibacterial Activity. Molecules 2020, 25, 4521. [Google Scholar] [CrossRef]
- Oves, M.; Rauf, M.A.; Hussain, A.; Qari, H.A.; Khan, A.A.P.; Muhammad, P.; Rehman, M.T.; Alajmi, M.F.; Ismail, I.I.M. Antibacterial silver nanomaterial synthesis from Mesoflavibacter zeaxanthinifaciens and targeting biofilm formation. Front. Pharmacol. 2019, 10, 801. [Google Scholar] [CrossRef]
- Bhuyan, T.; Mishra, K.; Khanuja, M.; Prasad, R.; Varma, A. Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater. Sci. Semicond. Process. 2015, 32, 55–61. [Google Scholar] [CrossRef]
- Ghidan, A.Y.; Al-Antary, T.M.; Salem, N.M.; Awwad, A.M. Facile green synthetic route to the zinc oxide (ZnONPs) nanoparticles: Effect on green peach aphid and antibacterial activity. J. Agric. Sci. 2017, 9, 131–138. [Google Scholar] [CrossRef]
- Selim, Y.A.; Azb, M.A.; Ragab, I.; Abd El-Azim, M.H.M. Green Synthesis of Zinc Oxide Nanoparticles Using Aqueous Extract of Deverra tortuosa and their Cytotoxic Activities. Sci. Rep. 2020, 10, 3445. [Google Scholar] [CrossRef]
- Sharmila, G.; Thirumarimurugan, M.; Muthukumaran, C. Green synthesis of ZnO nanoparticles using Tecoma castanifolia leaf extract: Characterization and evaluation of its antioxidant, bactericidal and anticancer activities. Microchem. J. 2019, 145, 578–587. [Google Scholar] [CrossRef]
- Faisal, S.; Jan, H.; Shah, S.A.; Shah, S.; Khan, A.; Akbar, M.T.; Rizwan, M.; Jan, F.; Wajidullah; Akhtar, N.; et al. Green Synthesis of Zinc Oxide (ZnO) Nanoparticles Using Aqueous Fruit Extracts of Myristica fragrans: Their Characterizations and Biological and Environmental Applications. ACS Omega 2021, 6, 9709–9722. [Google Scholar] [CrossRef]
- Kordy, M.G.M.; Abdel-Gabbar, M.; Soliman, H.A.; Aljohani, G.; BinSabt, M.; Ahmed, I.A.; Shaban, M. Phyto-Capped Ag Nanoparticles: Green Synthesis, Characterization, and Catalytic and Antioxidant Activities. Nanomaterials 2022, 12, 373. [Google Scholar] [CrossRef]
- Cumberland, S.A.; Lead, J.R. Particle size distributions of silver nanoparticles at environmentally relevant conditions. J. Chromatogr. A 2009, 1216, 9099–9105. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Chowdhury, D.; Kotcherlakota, R.; Patra, S.; Vinothkumar, B.; Bhadra, M.P.; Sreedhar, B.; Patra, C.R. Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system). Theranostics 2014, 4, 316–335. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Tomar, R.S.; Kaushik, S.; Mishra, R.K.; Sharma, D. Effective antimicrobial activity of green ZnO nano particles of Catharanthus roseus. Front. Microbiol. 2018, 9, 2030. [Google Scholar] [CrossRef]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomedicine 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, D.; Bhuvaneshwari, V. Synthesis of zinc oxide nanoparticles (ZnO NPs) using pure bioflavonoid rutin and their biomedical applications: Antibacterial, antioxidant and cytotoxic activities. Res. Chem. Intermed. 2019, 45, 2065–2078. [Google Scholar] [CrossRef]
- Wypij, M.; Jędrzejewski, T.; Trzcińska-Wencel, J.; Ostrowski, M.; Rai, M.; Golińska, P. Green Synthesized Silver Nanoparticles: Antibacterial and Anticancer Activities, Biocompatibility, and Analyses of Surface-Attached Proteins. Front. Microbiol. 2021, 12, 632505. [Google Scholar] [CrossRef]
- Steffy, K.; Shanthi, G.; Maroky, A.S.; Selvakumar, S. Enhanced antibacterial effects of green synthesized ZnO NPs using Aristolochia indica against Multi-drug resistant bacterial pathogens from Diabetic Foot Ulcer. J. Infect. Public Health 2018, 11, 463–471. [Google Scholar] [CrossRef]
- Haroun, S.A.; Elnaggar, M.E.; Zein, D.M.; Gad, R.I. Insecticidal efficiency and safety of zinc oxide and hydrophilic silica nanoparticles against some stored seed insects. J. Plant Prot. Res. 2020, 60, 77–85. [Google Scholar]
- Ibrahim, S.; Elbehery, H.; Samy, A. Insecticidal activity of ZnO NPs synthesized by green method using pomegranate peels extract on stored product insects. Egypt. J. Chem. 2022, 65, 135–145. [Google Scholar] [CrossRef]
- Hilal, S.M.; Mohmed, A.S.; Barry, N.M.; Ibrahim, M.H. Entomotoxicity of TiO2 and ZnO Nanoparticles Against Adults Tribolium Castaneum (Herbest)(Coleoptera: Tenebrionidae). In Proceedings of the IOP Conference Series: Earth and Environmental Science, Babil, Iraq, 4–5 October 2021; IOP Publishing: Bristol, UK, 2021; Volume 910, p. 12088. [Google Scholar]
- Elkobrosy, D.; Al-Askar, A.A.; El-Gendi, H.; Su, Y.; Nabil, R.; Abdelkhalek, A.; Behiry, S. Nematocidal and Bactericidal Activities of Green Synthesized Silver Nanoparticles Mediated by Ficus sycomorus Leaf Extract. Life 2023, 13, 1083. [Google Scholar] [CrossRef]
- Zubair, N.; Akhtar, K. Morphology controlled synthesis of ZnO nanoparticles for in-vitro evaluation of antibacterial activity. Trans. Nonferr. Met. Soc. China 2020, 30, 1605–1614. [Google Scholar] [CrossRef]
- Yusof, N.A.A.; Zain, N.M.; Pauzi, N. Synthesis of ZnO nanoparticles with chitosan as stabilizing agent and their antibacterial properties against Gram-positive and Gram-negative bacteria. Int. J. Biol. Macromol. 2019, 124, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Saqib, S.; Munis, M.F.H.; Zaman, W.; Ullah, F.; Shah, S.N.; Ayaz, A.; Farooq, M.; Bahadur, S. Synthesis, characterization and use of iron oxide nano particles for antibacterial activity. Microsc. Res. Tech. 2019, 82, 415–420. [Google Scholar] [CrossRef]
- Moritz, M.; Geszke-Moritz, M. The newest achievements in synthesis, immobilization and practical applications of antibacterial nanoparticles. Chem. Eng. J. 2013, 228, 596–613. [Google Scholar]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; Sapata, V.d.M.R.; Lopes, P.R.M.; de Moraes, P.B.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef]
- Kasemets, K.; Ivask, A.; Dubourguier, H.-C.; Kahru, A. Toxicity of nanoparticles of ZnO, CuO and TiO2 to yeast Saccharomyces cerevisiae. Toxicol. Vitr. 2009, 23, 1116–1122. [Google Scholar] [CrossRef]
- Li, M.; Zhu, L.; Lin, D. Toxicity of ZnO nanoparticles to Escherichia coli: Mechanism and the influence of medium components. Environ. Sci. Technol. 2011, 45, 1977–1983. [Google Scholar]
- Lipovsky, A.; Nitzan, Y.; Gedanken, A.; Lubart, R. Antifungal activity of ZnO nanoparticles—The role of ROS mediated cell injury. Nanotechnology 2011, 22, 105101. [Google Scholar] [CrossRef]
- Jalal, R.; Goharshadi, E.K.; Abareshi, M.; Moosavi, M.; Yousefi, A.; Nancarrow, P. ZnO nanofluids: Green synthesis, characterization, and antibacterial activity. Mater. Chem. Phys. 2010, 121, 198–201. [Google Scholar]
- Dutta, R.K.; Nenavathu, B.P.; Gangishetty, M.K.; Reddy, A.V.R. Studies on antibacterial activity of ZnO nanoparticles by ROS induced lipid peroxidation. Colloids Surfaces B Biointerfaces 2012, 94, 143–150. [Google Scholar]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.K.; Debanath, M.K.; Paul, B.; Medhi, S.; Saikia, E. Antibacterial and nonlinear dynamical analysis of flower and hexagon-shaped ZnO microstructures. Sci. Rep. 2020, 10, 2598. [Google Scholar] [CrossRef] [PubMed]
- Akbar, A.; Sadiq, M.B.; Ali, I.; Muhammad, N.; Rehman, Z.; Khan, M.N.; Muhammad, J.; Khan, S.A.; Rehman, F.U.; Anal, A.K. Synthesis and antimicrobial activity of zinc oxide nanoparticles against foodborne pathogens Salmonella typhimurium and Staphylococcus aureus. Biocatal. Agric. Biotechnol. 2019, 17, 36–42. [Google Scholar] [CrossRef]
- Puliyankulama, A.; Lanka, S. Weight gain performance of Tribolium castaneum (Herbst)(Coleoptera: Tenebrionidae) larvae and adults in different diets. J. Sci. 2021, 2, 19–24. [Google Scholar]
- Mackled, M.I.; EL-Hefny, M.; Bin-Jumah, M.; Wahba, T.F.; Allam, A.A. Assessment of the toxicity of natural oils from Mentha piperita, Pinus roxburghii, and Rosa spp. against three stored product insects. Processes 2019, 7, 861. [Google Scholar] [CrossRef]
- Germinara, G.S.; De Cristofaro, A.; Rotundo, G. Bioactivity of short-chain aliphatic ketones against adults of the granary weevil, Sitophilus granarius (L.). Pest Manag. Sci. 2012, 68, 371–377. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. econ. Entomol 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
- Wikler, M.A.; Cockerill, F.R.; Craig, W.A. Performance Standards for Antimicrobial Susceptibility Testing: Eighteenth Informational Supplement; Clinical and Laboratory Standards Institute: Wayne, IL, USA, 2008. [Google Scholar]



| Adult Insect Pest | Lethal Concentration (μg/mL) | 95% Fiducial Limits (μg/mL) | Slope ± SE ** | χ2 *** | df | N **** | |
|---|---|---|---|---|---|---|---|
| Sitophilus oryzae | LC50 | 7125.35 | (4443.56–11,425.67) | 2.23 ± 0.03 | 34.26 | 3 | 225 |
| LC90 | 121,824.56 | (9303.46–1,595,237.78) | |||||
| Tribolium castaneum | LC50 | 5642.65 | (3867.93–8231.64) | 2.23 ± 0.03 | 48.44 | 3 | 255 |
| LC90 | 66,825.76 | (10,529.88–424,096.07) | |||||
| ZnO-NPs Concentration (µg/mL) | Bacteria Inhibition Zone (mm) | ||
|---|---|---|---|
| Pectobacterium carotovorum | P. atrosepticum | Ralstonia solanacearum | |
| 1 | 8.00 e | 9.33 d | 13.67 c |
| 2 | 10.33 de | 9.67 d | 11.33 c |
| 3 | 13.67 bc | 14.33 c | 18.00 b |
| 4 | 16.67 ab | 21.00 b | 19.33 b |
| 5 | 17.00 a | 21.67 b | 23.00 a |
| Negative control (sterile distilled water) | 0.00 f | 0.00 e | 0.00 d |
| Augmentin 10 µg/disc | 13.33 cd | 25.00 a | 19.00 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamdy, E.; Al-Askar, A.A.; El-Gendi, H.; Khamis, W.M.; Behiry, S.I.; Valentini, F.; Abd-Elsalam, K.A.; Abdelkhalek, A. Zinc Oxide Nanoparticles Biosynthesized by Eriobotrya japonica Leaf Extract: Characterization, Insecticidal and Antibacterial Properties. Plants 2023, 12, 2826. https://doi.org/10.3390/plants12152826
Hamdy E, Al-Askar AA, El-Gendi H, Khamis WM, Behiry SI, Valentini F, Abd-Elsalam KA, Abdelkhalek A. Zinc Oxide Nanoparticles Biosynthesized by Eriobotrya japonica Leaf Extract: Characterization, Insecticidal and Antibacterial Properties. Plants. 2023; 12(15):2826. https://doi.org/10.3390/plants12152826
Chicago/Turabian StyleHamdy, Esraa, Abdulaziz A. Al-Askar, Hamada El-Gendi, Wael M. Khamis, Said I. Behiry, Franco Valentini, Kamel A. Abd-Elsalam, and Ahmed Abdelkhalek. 2023. "Zinc Oxide Nanoparticles Biosynthesized by Eriobotrya japonica Leaf Extract: Characterization, Insecticidal and Antibacterial Properties" Plants 12, no. 15: 2826. https://doi.org/10.3390/plants12152826
APA StyleHamdy, E., Al-Askar, A. A., El-Gendi, H., Khamis, W. M., Behiry, S. I., Valentini, F., Abd-Elsalam, K. A., & Abdelkhalek, A. (2023). Zinc Oxide Nanoparticles Biosynthesized by Eriobotrya japonica Leaf Extract: Characterization, Insecticidal and Antibacterial Properties. Plants, 12(15), 2826. https://doi.org/10.3390/plants12152826










