A Genome-Wide Association Study Reveals Region Associated with Seed Protein Content in Cowpea
Abstract
1. Introduction
2. Results
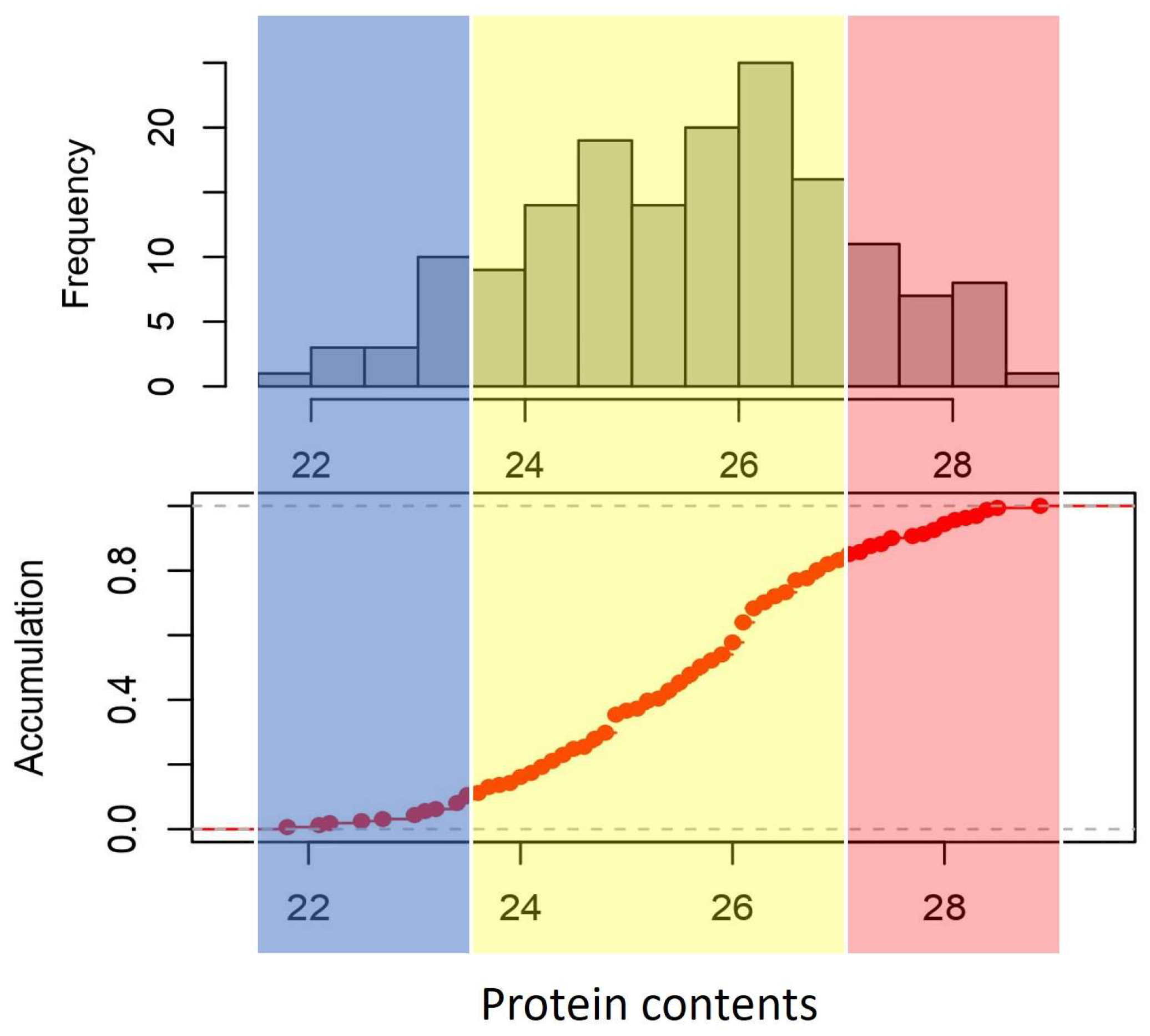
2.1. Phenotypes
2.2. SNP Profile
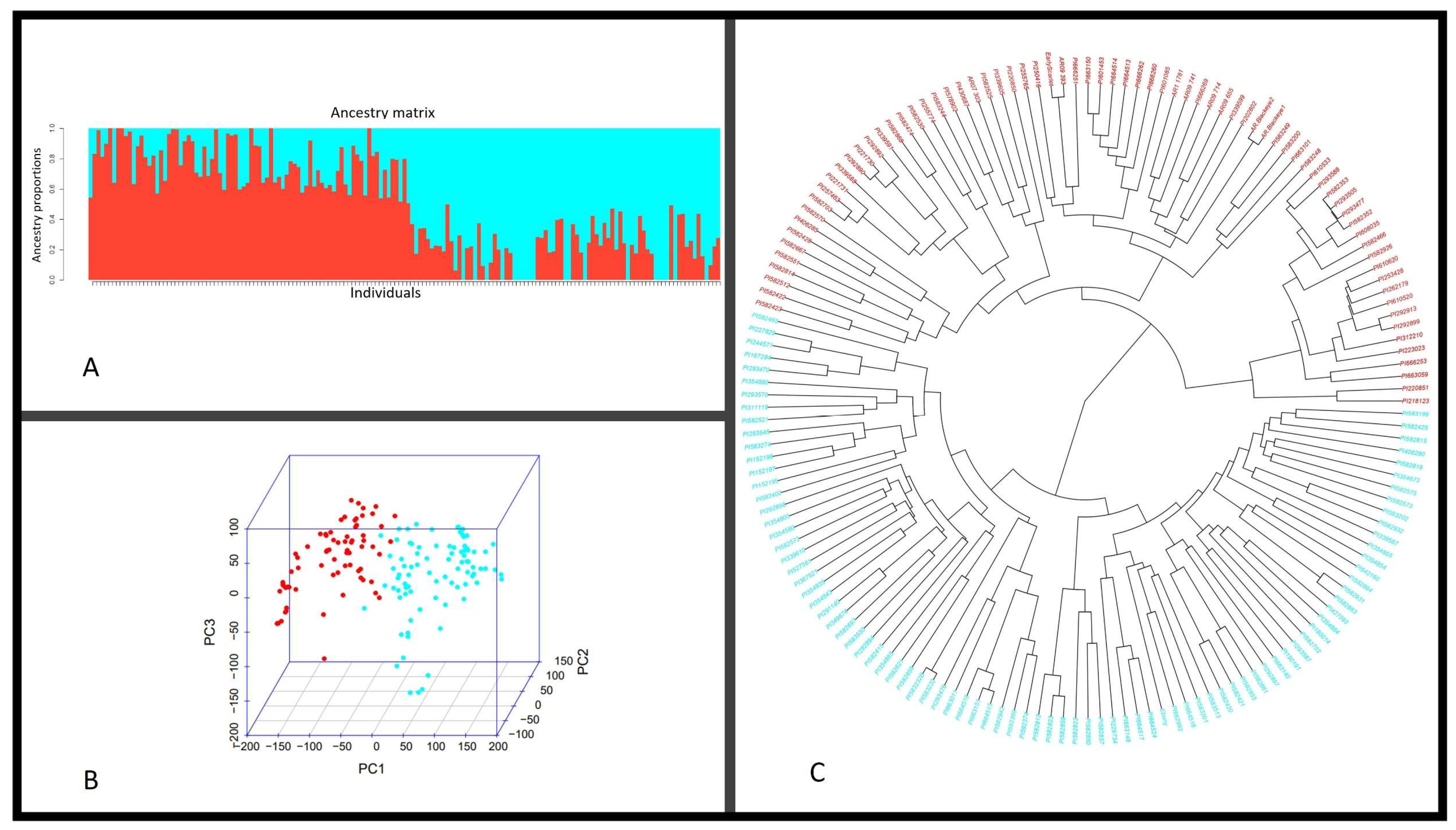
2.3. Population Structure Analysis
2.4. GWAS Analysis and Candidate Gene
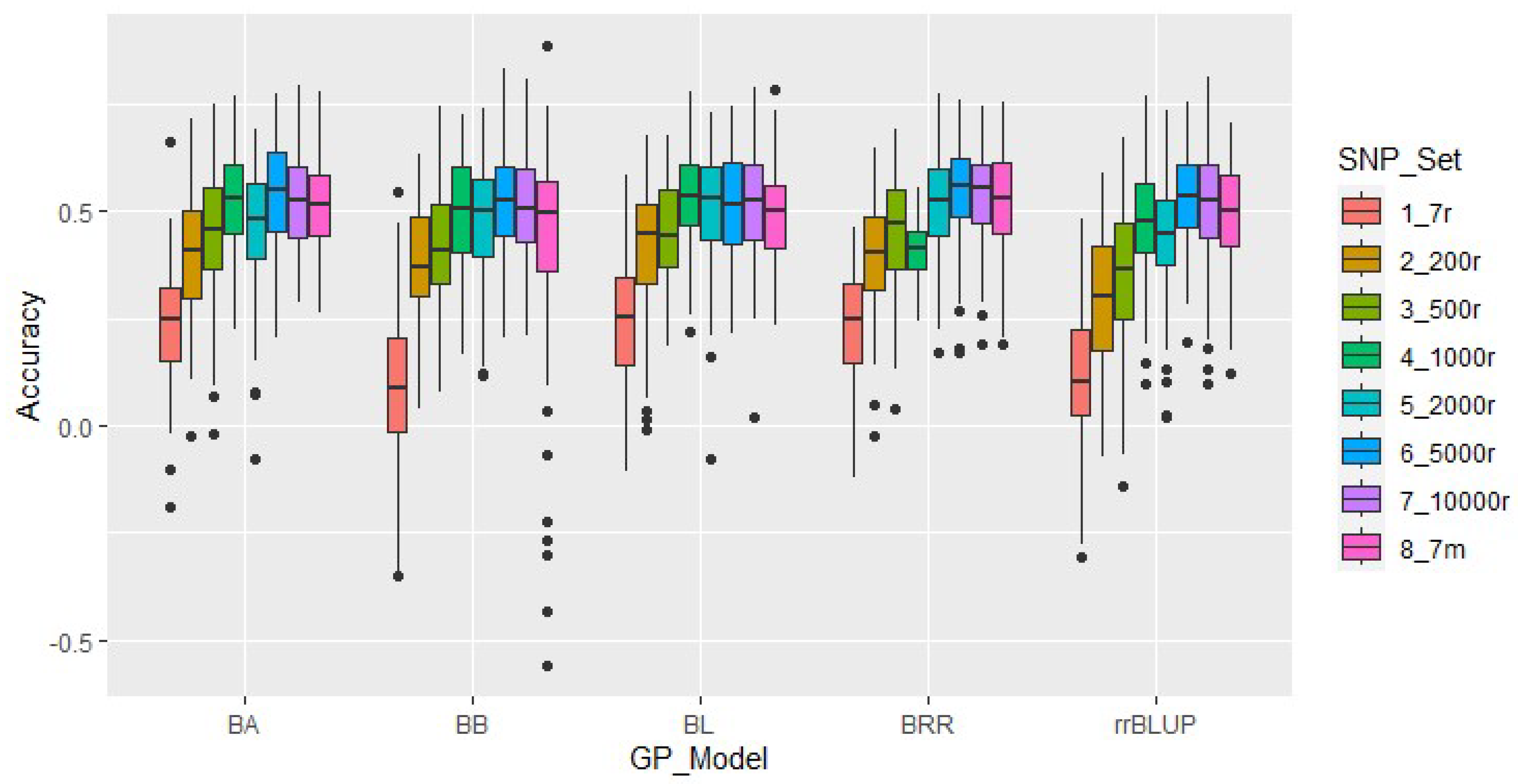
2.5. Genomic Prediction Analysis
3. Discussion
3.1. Population and Phenotyping
3.2. The Models of GWASs
3.3. The SNPs Associated with the Seed Protein Content
3.4. The Candidate Genes
3.5. The Genomic Prediction
4. Materials and Methods
4.1. Plant Materials and Field Experiment
4.2. Seed Protein-Content Assessment
4.3. DNA Extraction and Construction of Gene Library and GBS
4.4. Population Structure and Genetic Diversity
4.5. GWAS and Candidate Gene
4.6. Genomic Prediction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gondwe, T.M.; Alamu, E.O.; Mdziniso, P.; Maziya-Dixon, B. Cowpea (Vigna unguiculata (L.) Walp) for Food Security: An Evaluation of End-User Traits of Improved Varieties in Swaziland. Sci. Rep. 2019, 9, 15991. [Google Scholar] [CrossRef] [PubMed]
- Jayathilake, C.; Visvanathan, R.; Deen, A.; Bangamuwage, R.; Jayawardana, B.C.; Nammi, S.; Liyanage, R. Cowpea: An Overview on Its Nutritional Facts and Health Benefits. J. Sci. Food Agric. 2018, 98, 4793–4806. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.D.; Hall, A.E. Cowpea (Vigna unguiculata L. Walp.). Field Crops Res. 1997, 53, 187–204. [Google Scholar] [CrossRef]
- Jadhav, A.A.; Rayate, S.J.; Mhase, L.B.; Thudi, M.; Chitikineni, A.; Harer, P.N.; Jadhav, A.S.; Varshney, R.K.; Kulwal, P.L. Marker-Trait Association Study for Protein Content in Chickpea (Cicer arietinum L.). J. Genet. 2015, 94, 279–286. [Google Scholar] [CrossRef]
- Seidu, K.T.; Osundahunsi, O.F.; Olaleye, M.T.; Oluwalana, I.B. Amino Acid Composition, Mineral Contents and Protein Solubility of Some Lima Bean (Phaseolus lunatus L. Walp) Seeds Coat. Food Res. Int. 2015, 73, 130–134. [Google Scholar] [CrossRef]
- Mekonnen, T.W.; Gerrano, A.S.; Mbuma, N.W.; Labuschagne, M.T. Breeding of Vegetable Cowpea for Nutrition and Climate Resilience in Sub-Saharan Africa: Progress, Opportunities, and Challenges. Plants 2022, 11, 1583. [Google Scholar] [CrossRef]
- Penchalaraju, M.; John Don Bosco, S. Legume Protein Concentrates from Green Gram, Cowpea, and Horse Gram. J. Food Process Preserv. 2022, 46, e16477. [Google Scholar] [CrossRef]
- Prinyawiwatkul, W.; McWatters, K.H.; Beuchat, L.R.; Phillips, R.D.; Uebersak, M.A. Cowpea Flour: A Potential Ingredient in Food Products. Crit. Rev. Food Sci. Nutr. 1996, 36, 413–436. [Google Scholar] [CrossRef]
- Maleki, G.; Shadordizadeh, T.; Mozafari, M.R.; Attar, F.R.; Hesarinejad, M.A. Physicochemical and Nutritional Characteristics of Nutrition Bar Fortified with Cowpea Protein. J. Food Meas. Charact. 2023, 17, 2010–2015. [Google Scholar] [CrossRef]
- Owade, J.O.; Abong’, G.; Okoth, M.; Mwang’ombe, A.W. A Review of the Contribution of Cowpea Leaves to Food and Nutrition Security in East Africa. Food Sci. Nutr. 2020, 8, 36–47. [Google Scholar] [CrossRef]
- Ddamulira, G.; Santos, C.A.F.; Obuo, P.; Alanyo, M.; Lwanga, C.K. Grain Yield and Protein Content of Brazilian Cowpea Genotypes under Diverse Ugandan Environments. Am. J. Plant Sci. 2015, 6, 2074–2084. [Google Scholar] [CrossRef]
- Weng, Y.; Shi, A.; Ravelombola, W.S.; Yang, W.; Qin, J.; Motes, D.; Moseley, D.O.; Chen, P. A Rapid Method for Measuring Seed Protein Content in Cowpea (Vigna unguiculata (L.) Walp). Am. J. Plant Sci. 2017, 08, 2387–2396. [Google Scholar] [CrossRef]
- Boukar, O.; Massawe, F.; Muranaka, S.; Franco, J.; Maziya-Dixon, B.; Singh, B.; Fatokun, C. Evaluation of Cowpea Germplasm Lines for Protein and Mineral Concentrations in Grains. Plant Genet. Resour. 2011, 9, 515–522. [Google Scholar] [CrossRef]
- Singh, B.; Ehlers, J.; Sharma, B.; Freire Filho, F. Recent Progress in Cowpea Breeding. In Challenges and Opportunities for Enhancing Sustainable Cowpea Production; Fatokun, C., Tarawali, S., Singh, B., Kormawa, P., Eds.; ITAA: Ibadan, Nigeria, 2002; pp. 22–40. [Google Scholar]
- Raina, A.; Laskar, R.A.; Tantray, Y.R.; Khursheed, S.; Wani, M.R.; Khan, S. Characterization of Induced High Yielding Cowpea Mutant Lines Using Physiological, Biochemical and Molecular Markers. Sci. Rep. 2020, 10, 3687. [Google Scholar] [CrossRef]
- Horn, L.N.; Shimelis, H. Production Constraints and Breeding Approaches for Cowpea Improvement for Drought Prone Agro-Ecologies in Sub-Saharan Africa. Ann. Agric. Sci. 2020, 65, 83–91. [Google Scholar] [CrossRef]
- Kongjaimun, A.; Kaga, A.; Tomooka, N.; Somta, P.; Vaughan, D.A.; Srinives, P. The Genetics of Domestication of Yardlong Bean, Vigna unguiculata (L.) Walp. Ssp. Unguiculata Cv.-Gr. Sesquipedalis. Ann. Bot. 2012, 109, 1185. [Google Scholar] [CrossRef]
- Andargie, M.; Pasquet, R.S.; Gowda, B.S.; Muluvi, G.M.; Timko, M.P. Construction of a SSR-Based Genetic Map and Identification of QTL for Domestication Traits Using Recombinant Inbred Lines from a Cross between Wild and Cultivated Cowpea (V. unguiculata (L.) Walp.). Mol. Breed. 2011, 28, 413–420. [Google Scholar] [CrossRef]
- Fatokun, C.A.; Menancio-Hautea, D.I.; Danesh, D.; Young, N.D. Evidence for Orthologous Seed Weight Genes in Cowpea and Mung Bean Based on RFLP Mapping. Genetics 1992, 132, 841–846. [Google Scholar] [CrossRef]
- Muchero, W.; Ehlers, J.D.; Roberts, P.A. Seedling Stage Drought-Induced Phenotypes and Drought-Responsive Genes in Diverse Cowpea Genotypes. Crop Sci. 2008, 48, 541–552. [Google Scholar] [CrossRef]
- Uffelmann, E.; Huang, Q.Q.; Munung, N.S.; de Vries, J.; Okada, Y.; Martin, A.R.; Martin, H.C.; Lappalainen, T.; Posthuma, D. Genome-Wide Association Studies. Nat. Rev. Methods Primers 2021, 1, 59. [Google Scholar] [CrossRef]
- Huynh, B.L.; Ehlers, J.D.; Huang, B.E.; Muñoz-Amatriaín, M.; Lonardi, S.; Santos, J.R.P.; Ndeve, A.; Batieno, B.J.; Boukar, O.; Cisse, N.; et al. A Multi-Parent Advanced Generation Inter-Cross (MAGIC) Population for Genetic Analysis and Improvement of Cowpea (Vigna unguiculata L. Walp.). Plant J. 2018, 93, 1129–1142. [Google Scholar] [CrossRef] [PubMed]
- Burridge, J.D.; Schneider, H.M.; Huynh, B.-L.; Roberts, P.A.; Bucksch, A.; Lynch, J.P. Genome-Wide Association Mapping and Agronomic Impact of Cowpea Root Architecture. Theor. Appl. Genet. 2017, 130, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Paudel, D.; Dareus, R.; Rosenwald, J.; Muñoz-Amatriaín, M.; Rios, E.F. Genome-Wide Association Study Reveals Candidate Genes for Flowering Time in Cowpea (Vigna unguiculata [L.] Walp.). Front. Genet. 2021, 12, 667038. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Sun, T.; Xu, W.; Sun, Y.; Wang, B.; Wang, Y.; Li, Y.; Wang, J.; Wu, X.; Lu, Z.; et al. Unraveling the Genetic Architecture of Two Complex, Stomata-Related Drought-Responsive Traits by High-Throughput Physiological Phenotyping and GWAS in Cowpea (Vigna unguiculata L. Walp). Front. Genet. 2021, 12, 743758. [Google Scholar] [CrossRef]
- Kpoviessi, A.D.; Agbahoungba, S.; Agoyi, E.E.; Nuwamanya, E.; Assogbadjo, A.E.; Chougourou, D.C.; Adoukonou-Sagbadja, H. Primary and Secondary Metabolite Compounds in Cowpea Seeds Resistant to the Cowpea Bruchid [Callosobruchus maculatus (F.)] in Postharvest Storage. J. Stored Prod. Res. 2021, 93, 101858. [Google Scholar] [CrossRef]
- Olatoye, M.O.; Hu, Z.; Aikpokpodion, P.O. Epistasis Detection and Modeling for Genomic Selection in Cowpea (Vigna unguiculata L. Walp.). Front. Genet. 2019, 10, 677. [Google Scholar] [CrossRef]
- Fernandes Santos, C.A.; Campos da Costa, D.C.; Roberto da Silva, W.; Boiteux, L.S. Genetic Analysis of Total Seed Protein Content in Two Cowpea Crosses. Crop Sci. 2012, 52, 2501–2506. [Google Scholar] [CrossRef]
- Ravelombola, W.S.; Shi, A.; Weng, Y.; Motes, D.; Chen, P.; Srivastava, V.; Wingfield, C.; Ravelombola, W.S.; Shi, A.; Weng, Y.; et al. Evaluation of Total Seed Protein Content in Eleven Arkansas Cowpea (Vigna unguiculata (L.) Walp.) Lines. Am. J. Plant Sci. 2016, 7, 2288–2296. [Google Scholar] [CrossRef]
- Xiong, H.; Shi, A.; Mou, B.; Qin, J.; Motes, D.; Lu, W.; Ma, J.; Weng, Y.; Yang, W.; Wu, D. Genetic Diversity and Population Structure of Cowpea (Vigna unguiculata L. Walp). PLoS ONE 2016, 11, e0160941. [Google Scholar] [CrossRef]
- Xiong, H.; Qin, J.; Shi, A.; Mou, B.; Wu, D.; Sun, J.; Shu, X.; Wang, Z.; Lu, W.; Ma, J. Genetic Differentiation and Diversity upon Genotype and Phenotype in Cowpea (Vigna unguiculata L. Walp.). Euphytica 2018, 214, 4. [Google Scholar] [CrossRef]
- Boukar, O.; Belko, N.; Chamarthi, S.; Togola, A.; Batieno, J.; Owusu, E.; Haruna, M.; Diallo, S.; Umar, M.L.; Olufajo, O.; et al. Cowpea (Vigna unguiculata): Genetics, Genomics and Breeding. Plant Breed. 2019, 138, 415–424. [Google Scholar] [CrossRef]
- Phillips, R.D.; McWatters, K.H.; Chinnan, M.S.; Hung, Y.C.; Beuchat, L.R.; Sefa-Dedeh, S.; Sakyi-Dawson, E.; Ngoddy, P.; Nnanyelugo, D.; Enwere, J.; et al. Utilization of Cowpeas for Human Food. Field Crops Res. 2003, 82, 193–213. [Google Scholar] [CrossRef]
- Gerrano, A.S.; Jansen van Rensburg, W.S.; Venter, S.L.; Shargie, N.G.; Amelework, B.A.; Shimelis, H.A.; Labuschagne, M.T. Selection of Cowpea Genotypes Based on Grain Mineral and Total Protein Content. Acta Agric. Scand. B Soil. Plant Sci. 2019, 69, 155–166. [Google Scholar] [CrossRef]
- Asante, I.; Adu-Dapaah, H.; Addison, P. Seed Weight and Protein and Tannin Contents of 32 Cowpea Accessions in Ghana. Trop. Sci. 2004, 44, 77–79. [Google Scholar] [CrossRef]
- Ajeigbe, H.A.; Ihedioha, D.; Chikoye, D. Variation in Physico-Chemical Properties of Seed of Selected Improved Varieties of Cowpea as It Relates to Industrial Utilization of the Crop. Afr. J. Biotechnol. 2008, 7, 3642–3647. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Brandt, W.E.; Singh, B.B. Genetic Variability for Nutritional Composition and Cooking Time of Improved Cowpea Lines. Crop Sci. 1993, 33, 469–472. [Google Scholar] [CrossRef]
- Jean Baptiste, N.T.; Joseph, M.B.; Antoine, M.N.; Nicolas, Y.N.; Emmanuel, Y. Genetic Analysis of Seed Proteins Contents in Cowpea (Vigna unguiculata L. Walp.). Afr. J. Biotechnol. 2011, 10, 3077–3086. [Google Scholar] [CrossRef]
- Emebiri, L.C. Inheritance of Protein Content in Seeds of Selected Crosses of Cowpea (Vigna unguiculata). J. Sci. Food Agric. 1991, 54, 1–7. [Google Scholar] [CrossRef]
- Pandey, M.K.; Roorkiwal, M.; Singh, V.K.; Ramalingam, A.; Kudapa, H.; Thudi, M.; Chitikineni, A.; Rathore, A.; Varshney, R.K. Emerging Genomic Tools for Legume Breeding: Current Status and Future Prospects. Front. Plant Sci. 2016, 7, 455. [Google Scholar] [CrossRef]
- Cantor, R.M.; Lange, K.; Sinsheimer, J.S. Prioritizing GWAS Results: A Review of Statistical Methods and Recommendations for Their Application. Am. J. Hum. Genet. 2010, 86, 6–22. [Google Scholar] [CrossRef]
- Korte, A.; Farlow, A. The Advantages and Limitations of Trait Analysis with GWAS: A Review. Plant Methods 2013, 9, 29. [Google Scholar] [CrossRef]
- Begum, F.; Ghosh, D.; Tseng, G.C.; Feingold, E. Comprehensive Literature Review and Statistical Considerations for GWAS Meta-Analysis. Nucleic Acids Res. 2012, 40, 3777–3784. [Google Scholar] [CrossRef]
- Tibbs Cortes, L.; Zhang, Z.; Yu, J. Status and Prospects of Genome-Wide Association Studies in Plants. Plant Genome 2021, 14, e20077. [Google Scholar] [CrossRef]
- Priyanatha, C.; Rajcan, I. Phenotypic Evaluation of Canadian × Chinese Elite Germplasm in a Diversity Panel for Seed Yield and Seed Quality Traits. Can. J. Plant Sci. 2022, 102, 1032–1039. [Google Scholar] [CrossRef]
- Hwang, E.Y.; Song, Q.; Jia, G.; Specht, J.E.; Hyten, D.L.; Costa, J.; Cregan, P.B. A Genome-Wide Association Study of Seed Protein and Oil Content in Soybean. BMC Genom. 2014, 15, 1. [Google Scholar] [CrossRef]
- Zhang, S.; Hao, D.; Zhang, S.; Zhang, D.; Wang, H.; Du, H.; Kan, G.; Yu, D. Genome-Wide Association Mapping for Protein, Oil and Water-Soluble Protein Contents in Soybean. Mol. Genet. Genom. 2021, 296, 91–102. [Google Scholar] [CrossRef]
- Lee, S.; Van, K.; Sung, M.; Nelson, R.; LaMantia, J.; McHale, L.K.; Mian, M.A.R. Genome-Wide Association Study of Seed Protein, Oil and Amino Acid Contents in Soybean from Maturity Groups I to IV. Theor. Appl. Genet. 2019, 132, 1639–1659. [Google Scholar] [CrossRef]
- Upadhyaya, H.D.; Bajaj, D.; Narnoliya, L.; Das, S.; Kumar, V.; Gowda, C.L.L.; Sharma, S.; Tyagi, A.K.; Parida, S.K. Genome-Wide Scans for Delineation of Candidate Genes Regulating Seed-Protein Content in Chickpea. Front. Plant Sci. 2016, 7, 302. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Mancuso, N.; Spendlove, S.; Pasaniuc, B. Local Genetic Correlation Gives Insights into the Shared Genetic Architecture of Complex Traits. Am. J. Hum. Genet. 2017, 101, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Schaid, D.J.; Chen, W.; Larson, N.B. From Genome-Wide Associations to Candidate Causal Variants by Statistical Fine-Mapping. Nat. Rev. Genet. 2018, 19, 491–504. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. The Thioredoxin Superfamily in Oxidative Protein Folding. Antioxid. Redox Signal 2014, 21, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Hägglund, P.; Finnie, C.; Yano, H.; Shahpiri, A.; Buchanan, B.B.; Henriksen, A.; Svensson, B. Seed Thioredoxin h. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2016, 1864, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Gelhaye, E.; Rouhier, N.; Jacquot, J.-P. The Thioredoxin h System of Higher Plants. Plant Physiol. Biochem. 2004, 42, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, M.; Mata-Cabana, A.; Kieselbach, T. The Disulfide Proteome and Other Reactive Cysteine Proteomes: Analysis and Functional Significance. Antioxid. Redox Signal 2011, 14, 2581–2642. [Google Scholar] [CrossRef] [PubMed]
- Colville, L.; Kranner, I. Desiccation Tolerant Plants as Model Systems to Study Redox Regulation of Protein Thiols. Plant Growth Regul. 2010, 62, 241–255. [Google Scholar] [CrossRef]
- Lockwood, T.D. Redox Control of Protein Degradation. Antioxid. Redox Signal 2000, 2, 851–878. [Google Scholar] [CrossRef]
- Kawakami, A.; Yoshida, M. Fructan:Fructan 1-Fructosyltransferase, a Key Enzyme for Biosynthesis of Graminan Oligomers in Hardened Wheat. Planta 2005, 223, 90–104. [Google Scholar] [CrossRef]
- Guo, M.; Liu, J.H.; Ma, X.; Luo, D.X.; Gong, Z.H.; Lu, M.H. The Plant Heat Stress Transcription Factors (HSFS): Structure, Regulation, and Function in Response to Abiotic Stresses. Front. Plant Sci. 2016, 7, 114. [Google Scholar] [CrossRef]
- Márquez-López, R.E.; Loyola-Vargas, V.M.; Santiago-García, P.A. Interaction between Fructan Metabolism and Plant Growth Regulators. Planta 2022, 255, 49. [Google Scholar] [CrossRef]
- Bolouri-Moghaddam, M.R.; Le Roy, K.; Xiang, L.; Rolland, F.; Van Den Ende, W. Sugar Signalling and Antioxidant Network Connections in Plant Cells. FEBS J. 2010, 277, 2022–2037. [Google Scholar] [CrossRef]
- Nishizawa, A.; Yabuta, Y.; Yoshida, E.; Maruta, T.; Yoshimura, K.; Shigeoka, S. Arabidopsis Heat Shock Transcription Factor A2 as a Key Regulator in Response to Several Types of Environmental Stress. Plant J. 2006, 48, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, X.; Zhang, H.; Wang, L.; Zhu, Z.; Gao, J.; Li, C.; Zhu, Y. High Temperature Inhibits the Accumulation of Storage Materials by Inducing Alternative Splicing of OsbZIP58 during Filling Stage in Rice. Plant Cell Environ. 2020, 43, 1879–1896. [Google Scholar] [CrossRef] [PubMed]
- Jannink, J.-L.; Lorenz, A.J.; Iwata, H. Genomic Selection in Plant Breeding: From Theory to Practice. Brief. Funct. Genom. 2010, 9, 166–177. [Google Scholar] [CrossRef]
- Crossa, J.; Pérez-Rodríguez, P.; Cuevas, J.; Montesinos-López, O.; Jarquín, D.; de los Campos, G.; Burgueño, J.; González-Camacho, J.M.; Pérez-Elizalde, S.; Beyene, Y.; et al. Genomic Selection in Plant Breeding: Methods, Models, and Perspectives. Trends Plant Sci. 2017, 22, 961–975. [Google Scholar] [CrossRef]
- Goddard, M.E.; Hayes, B.J. Genomic Selection. J. Anim. Breed. Genet. 2007, 124, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Ravelombola, W.; Shi, A.; Huynh, B.L. Loci Discovery, Network-Guided Approach, and Genomic Prediction for Drought Tolerance Index in a Multi-Parent Advanced Generation Intercross (MAGIC) Cowpea Population. Hortic. Res. 2021, 8, 24. [Google Scholar] [CrossRef]
- Kristensen, P.S.; Jahoor, A.; Andersen, J.R.; Cericola, F.; Orabi, J.; Janss, L.L.; Jensen, J. Genome-Wide Association Studies and Comparison of Models and Cross-Validation Strategies for Genomic Prediction of Quality Traits in Advanced Winter Wheat Breeding Lines. Front. Plant Sci. 2018, 9, 69. [Google Scholar] [CrossRef]
- Jarquin, D.; Specht, J.; Lorenz, A. Prospects of Genomic Prediction in the USDA Soybean Germplasm Collection: Historical Data Creates Robust Models for Enhancing Selection of Accessions. G3 Genes Genomes Genet. 2016, 6, 2329–2341. [Google Scholar] [CrossRef]
- Stewart-Brown, B.B.; Song, Q.; Vaughn, J.N.; Li, Z. Genomic Selection for Yield and Seed Composition Traits Within an Applied Soybean Breeding Program. G3 Genes Genomes Genet. 2019, 9, 2253–2265. [Google Scholar] [CrossRef]
- Lan, S.; Zheng, C.; Hauck, K.; McCausland, M.; Duguid, S.D.; Booker, H.M.; Cloutier, S.; You, F.M. Genomic Prediction Accuracy of Seven Breeding Selection Traits Improved by QTL Identification in Flax. Int. J. Mol. Sci. 2020, 21, 1577. [Google Scholar] [CrossRef]
- Weng, Y.; Qin, J.; Eaton, S.; Yang, Y.; Ravelombola, W.S.; Shi, A. Evaluation of Seed Protein Content in USDA Cowpea Germplasm. HortScience 2019, 54, 814–817. [Google Scholar] [CrossRef]
- Isaac, R.A.; Johnson, W.C. Determination of Total Nitrogen in Plant Tissue, Using a Block Digestor. J. AOAC Int. 1976, 59, 98–100. [Google Scholar] [CrossRef]
- Moore, J.C.; DeVries, J.W.; Lipp, M.; Griffiths, J.C.; Abernethy, D.R. Total Protein Methods and Their Potential Utility to Reduce the Risk of Food Protein Adulteration. Compr. Rev. Food Sci. Food Saf. 2010, 9, 330–357. [Google Scholar] [CrossRef]
- Rogers, S.O.; Bendich, A.J. Extraction of DNA from Milligram Amounts of Fresh, Herbarium and Mummified Plant Tissues. Plant Mol. Biol. 1985, 5, 69–76. [Google Scholar] [CrossRef]
- Lonardi, S.; Muñoz-Amatriaín, M.; Liang, Q.; Shu, S.; Wanamaker, S.I.; Lo, S.; Tanskanen, J.; Schulman, A.H.; Zhu, T.; Luo, M.C.; et al. The Genome of Cowpea (Vigna unguiculata [L.] Walp.). Plant J. 2019, 98, 767–782. [Google Scholar] [CrossRef]
- Frichot, E.; François, O. LEA: An R Package for Landscape and Ecological Association Studies. Methods Ecol. Evol. 2015, 6, 925–929. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z. GAPIT Version 3: Boosting Power and Accuracy for Genomic Association and Prediction. Genom. Proteom. Bioinform. 2021, 19, 629–640. [Google Scholar] [CrossRef]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for Association Mapping of Complex Traits in Diverse Samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef]
- Yu, J.; Pressoir, G.; Briggs, W.H.; Bi, I.V.; Yamasaki, M.; Doebley, J.F.; McMullen, M.D.; Gaut, B.S.; Nielsen, D.M.; Holland, J.B.; et al. A Unified Mixed-Model Method for Association Mapping That Accounts for Multiple Levels of Relatedness. Nat. Genet. 2005, 38, 203–208. [Google Scholar] [CrossRef]
- Liu, X.; Huang, M.; Fan, B.; Buckler, E.S.; Zhang, Z. Iterative Usage of Fixed and Random Effect Models for Powerful and Efficient Genome-Wide Association Studies. PLoS Genet. 2016, 12, e1005767. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; De Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Endelman, J.B. Ridge Regression and Other Kernels for Genomic Selection with R Package RrBLUP. Plant Genome 2011, 4, 250–255. [Google Scholar] [CrossRef]
- Heslot, N.; Yang, H.-P.; Sorrells, M.E.; Jannink, J.-L. Genomic Selection in Plant Breeding: A Comparison of Models. Crop Sci. 2012, 52, 146–160. [Google Scholar] [CrossRef]
- Shikha, M.; Kanika, A.; Rao, A.R.; Mallikarjuna, M.G.; Gupta, H.S.; Nepolean, T. Genomic Selection for Drought Tolerance Using Genome-Wide SNPs in Maize. Front. Plant Sci. 2017, 8, 550. [Google Scholar] [CrossRef] [PubMed]


| SNP | Chr | Position | −Log(p-Value) Using GAPIT 3 | −Log(p-Value) in Tassel | t-Test | Rsq in Tassel | High Protein Content Allele | Low Protein Content Allele | MAF (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blink | FarmCPU | MLM | GLM | SMR | GLM | MLM | −LOG(p) | SMR | GLM | MLM | ||||||
| Vu08_3838280 | 8 | 3,838,280 | 0.04 | 0.28 | 3.22 | 7.89 | 10.59 | 9.19 | 3.35 | 13.94 | 26.56 | 21.56 | 10.21 | T | A | 47.52 |
| Vu08_3838282 | 8 | 3,838,282 | 0.04 | 0.28 | 3.22 | 7.89 | 10.59 | 9.19 | 3.35 | 13.94 | 26.56 | 21.56 | 10.21 | T | A | 47.52 |
| Vu08_3838296 | 8 | 3,838,296 | 0.16 | 0.19 | 3.33 | 8.14 | 10.92 | 9.54 | 3.42 | 14.28 | 27.26 | 22.26 | 10.44 | C | G | 46.89 |
| Vu08_3839577 | 8 | 3,839,577 | 10.78 | 6.60 | 3.62 | 8.34 | 10.95 | 9.63 | 3.20 | 14.50 | 27.33 | 22.44 | 9.755 | G | A | 46.58 |
| Vu08_3839579 | 8 | 3,839,579 | 0.00 | 0.00 | 3.62 | 8.34 | 10.95 | 9.63 | 3.20 | 14.50 | 27.33 | 22.44 | 9.755 | T | C | 46.58 |
| Vu08_3840180 | 8 | 3,840,180 | 0.35 | 0.25 | 3.59 | 6.55 | 9.04 | 6.95 | 3.01 | 12.64 | 23.17 | 16.82 | 9.144 | A | G | 44.10 |
| Vu08_3840193 | 8 | 3,840,193 | 0.48 | 0.15 | 3.75 | 7.14 | 9.68 | 7.98 | 3.60 | 13.09 | 24.59 | 19.03 | 11.04 | C | A | 49.69 |
| Gene | Function | Chr | Gene Start Pos | Gene End Pos | SNP | Chr | Pos | Distance (Bp) from Gene Start and End | |
|---|---|---|---|---|---|---|---|---|---|
| Vigun08g038900 | Fructan fructosyltransferase | Vu08 | 3,789,846 | 3,793,119 | Vu08_3839577 | 8 | 3839577 | −49,731 | −46,458 |
| Vigun08g039000 | Fructan fructosyltransferase | Vu08 | 3,799,649 | 3,802,436 | −39,928 | −37,141 | |||
| Vigun08g039100 | Fructan fructosyltransferase | Vu08 | 3,814,161 | 3,817,053 | −25,416 | −22,524 | |||
| Vigun08g039200 | Thioredoxin superfamily protein, OsGrx_C15—Glutaredoxin subgroup III, expressed | Vu08 | 3,832,765 | 3,834,779 | −6812 | −4798 | |||
| Vigun08g039300 | Heat shock transcription factor A2 | Vu08 | 3,846,346 | 3,848,728 | 6769 | 9151 | |||
| Vigun08g039400 | Thromboxane-A synthase/Thromboxane synthetase | Vu08 | 3,869,695 | 3,873,523 | 30,118 | 33,946 | |||
| Vigun08g039500 | IQ calmodulin-binding motif domain containing protein, expressed | Vu08 | 3,873,436 | 3,876,766 | 33,859 | 37,189 | |||
| Vigun08g039600 | LOC_Os06g05730, expressed protein | Vu08 | 3,887,821 | 3,888,663 | 48,244 | 49,086 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Xiong, H.; Ravelombola, W.; Bhattarai, G.; Barickman, C.; Alatawi, I.; Phiri, T.M.; Chiwina, K.; Mou, B.; Tallury, S.; et al. A Genome-Wide Association Study Reveals Region Associated with Seed Protein Content in Cowpea. Plants 2023, 12, 2705. https://doi.org/10.3390/plants12142705
Chen Y, Xiong H, Ravelombola W, Bhattarai G, Barickman C, Alatawi I, Phiri TM, Chiwina K, Mou B, Tallury S, et al. A Genome-Wide Association Study Reveals Region Associated with Seed Protein Content in Cowpea. Plants. 2023; 12(14):2705. https://doi.org/10.3390/plants12142705
Chicago/Turabian StyleChen, Yilin, Haizheng Xiong, Waltram Ravelombola, Gehendra Bhattarai, Casey Barickman, Ibtisam Alatawi, Theresa Makawa Phiri, Kenani Chiwina, Beiquan Mou, Shyam Tallury, and et al. 2023. "A Genome-Wide Association Study Reveals Region Associated with Seed Protein Content in Cowpea" Plants 12, no. 14: 2705. https://doi.org/10.3390/plants12142705
APA StyleChen, Y., Xiong, H., Ravelombola, W., Bhattarai, G., Barickman, C., Alatawi, I., Phiri, T. M., Chiwina, K., Mou, B., Tallury, S., & Shi, A. (2023). A Genome-Wide Association Study Reveals Region Associated with Seed Protein Content in Cowpea. Plants, 12(14), 2705. https://doi.org/10.3390/plants12142705








