Response of Maize Seedlings to Silicon Dioxide Nanoparticles (SiO2NPs) under Drought Stress
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of SiO2
2.1.1. XRD Patterns
2.1.2. Surface Morphology by TEM
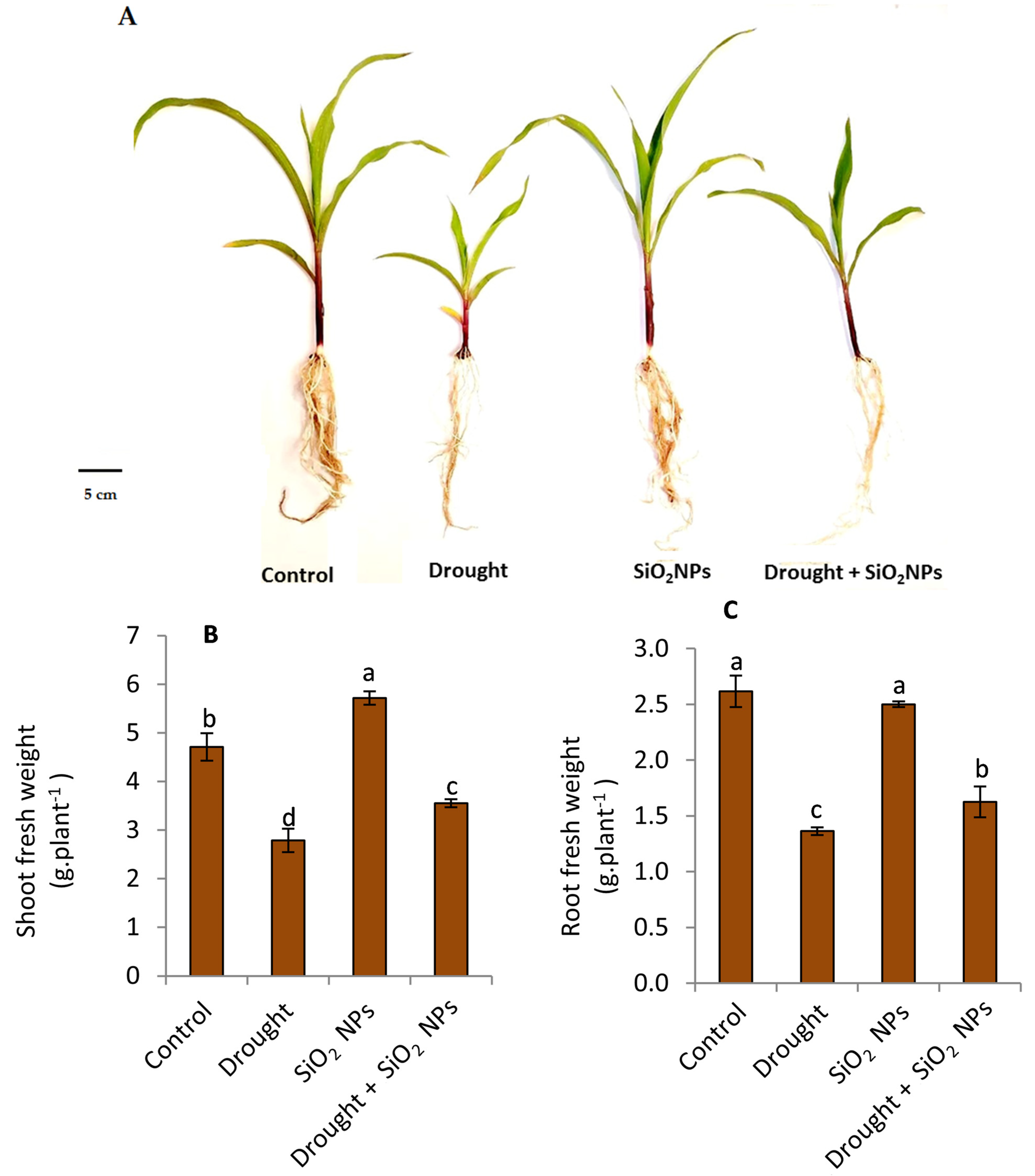
2.2. Effect of SiO2NPs Application on Growth
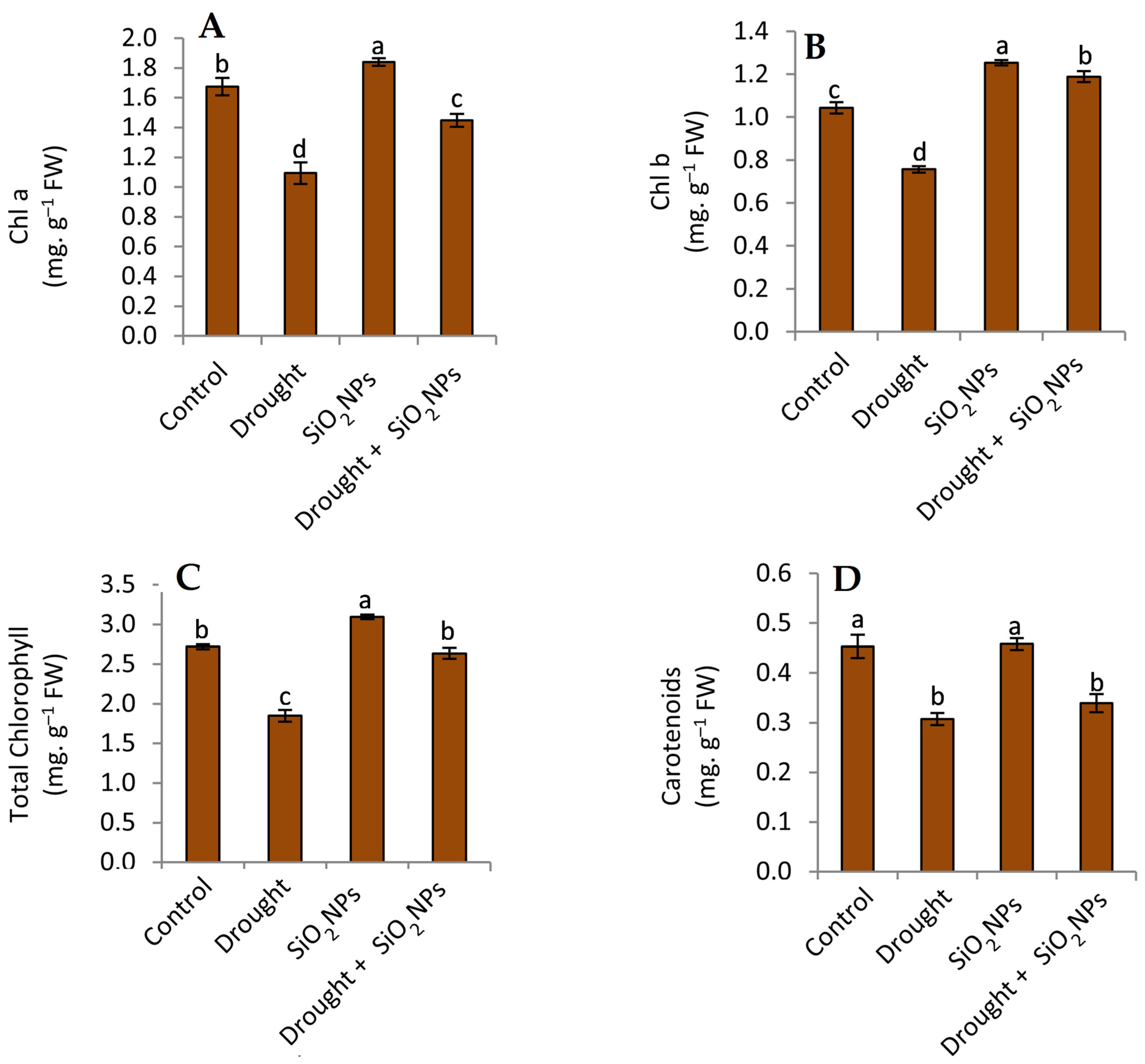
2.3. Effect of SiO2NPs Application on Photosynthesis Pigments
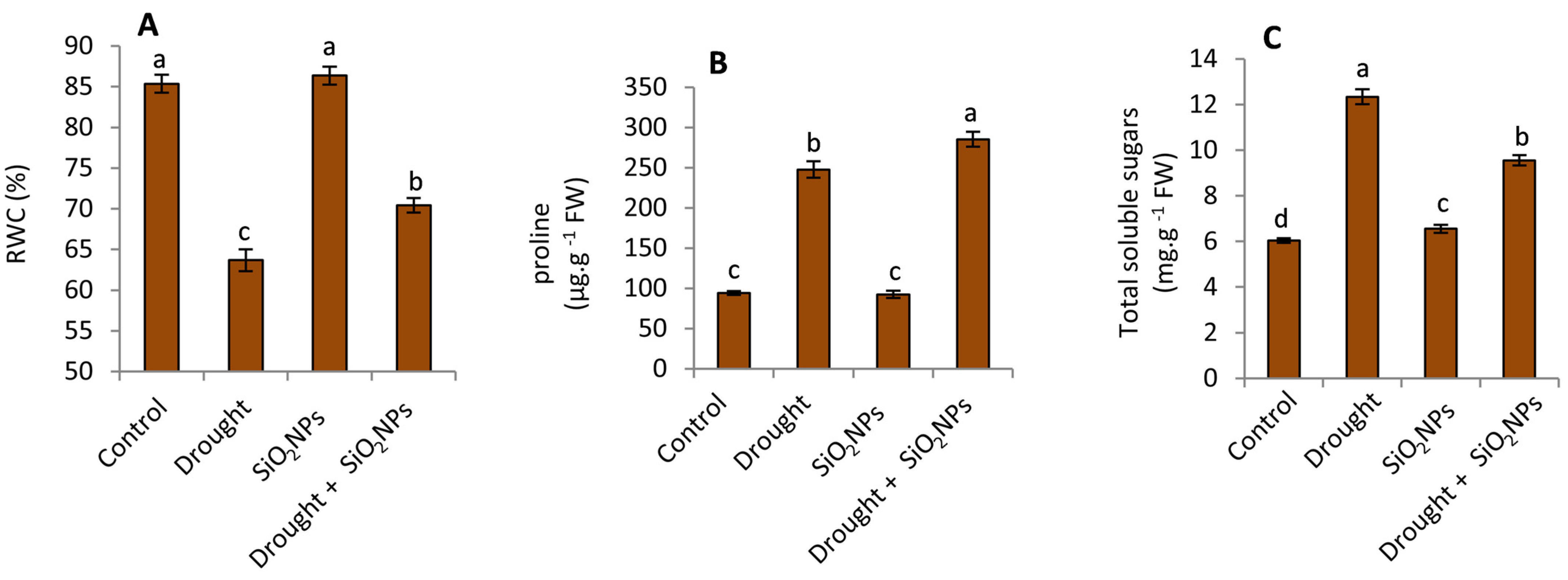
2.4. Effect of SiO2NPs Application on RWC, Proline and Total Soluble Sugars
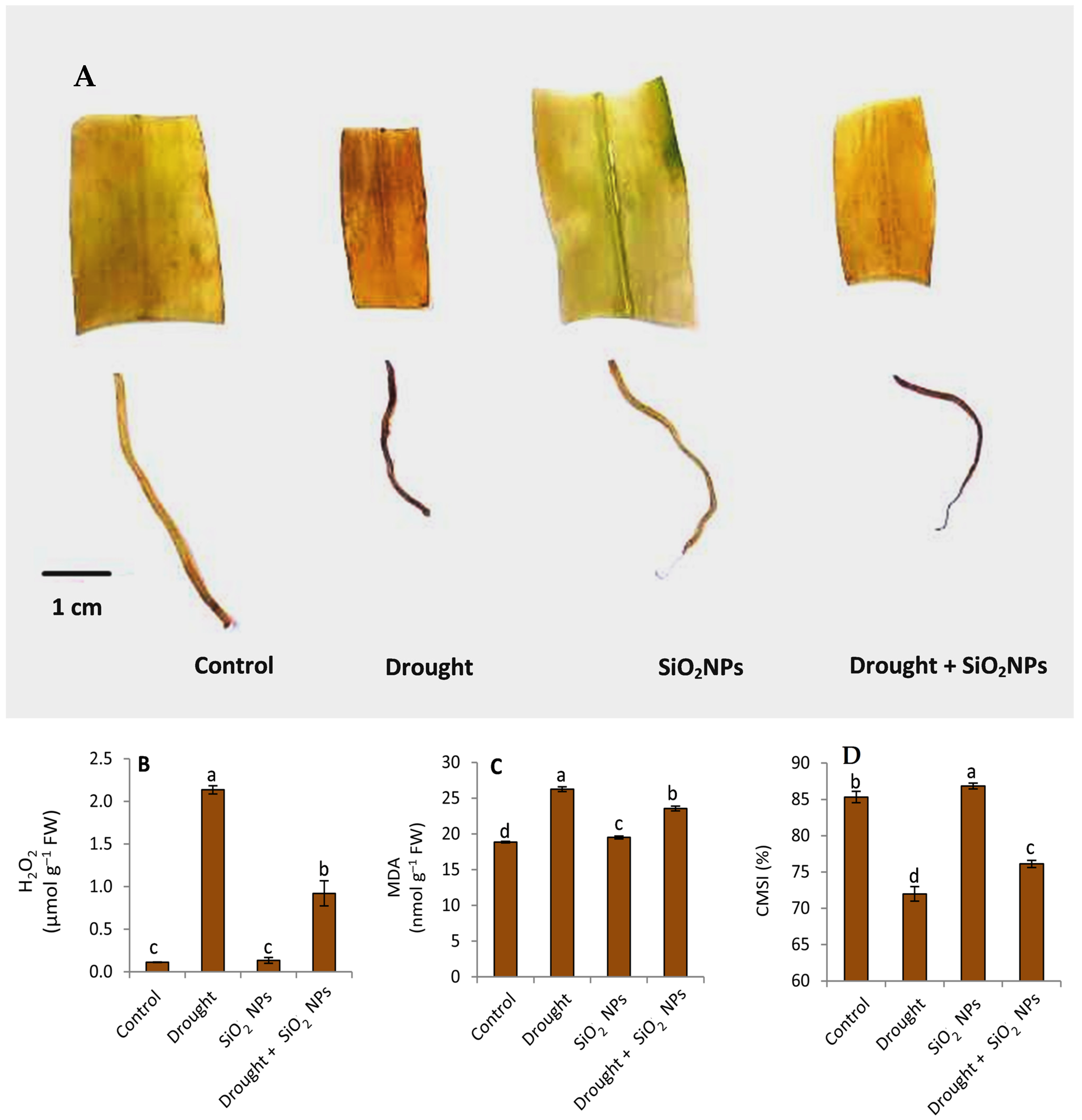
2.5. Effect of SiO2NPs Application on H2O2, MDA, and CMSI
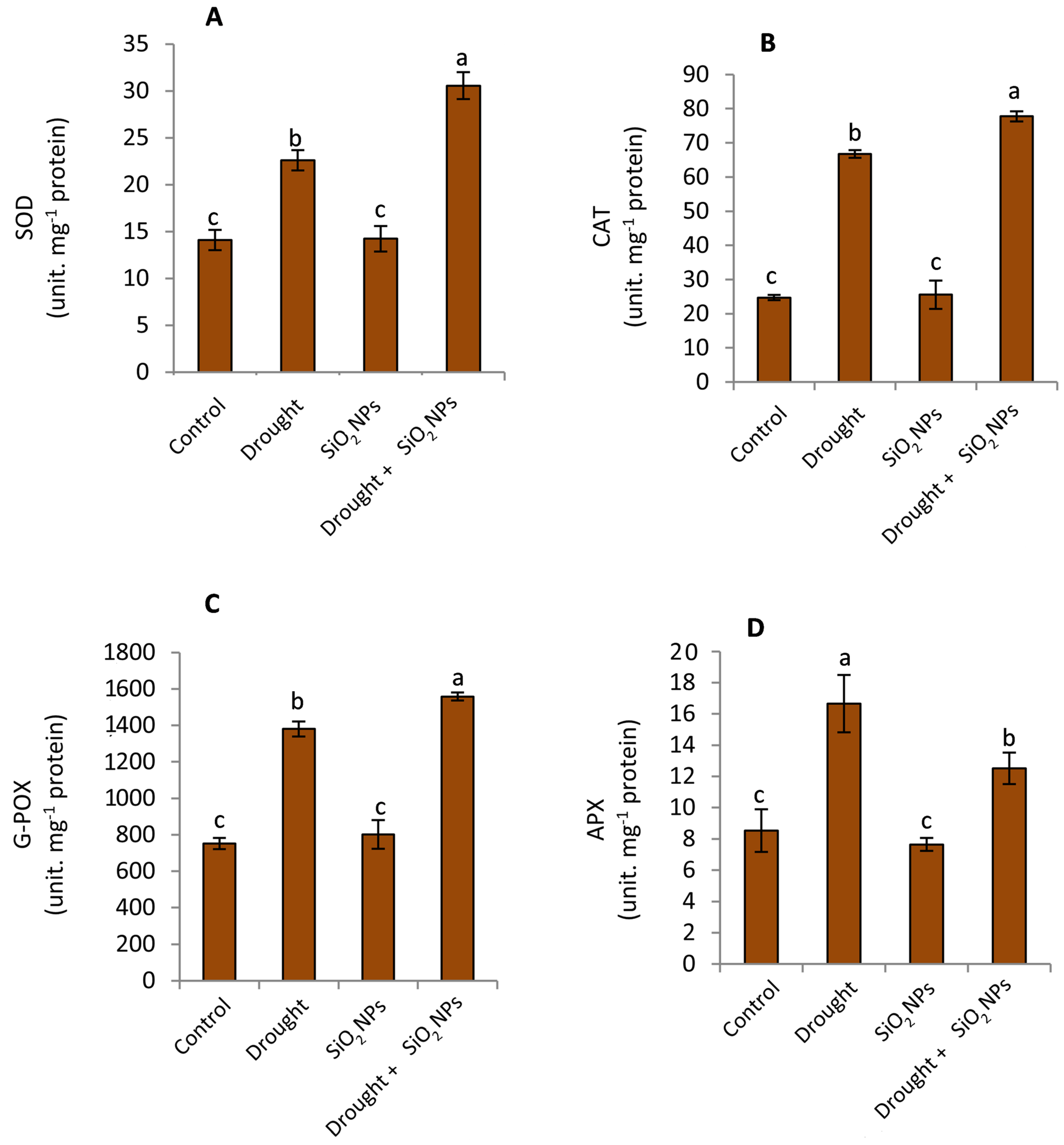
2.6. Impact of SiO2NPs Administration on Antioxidant Enzymes
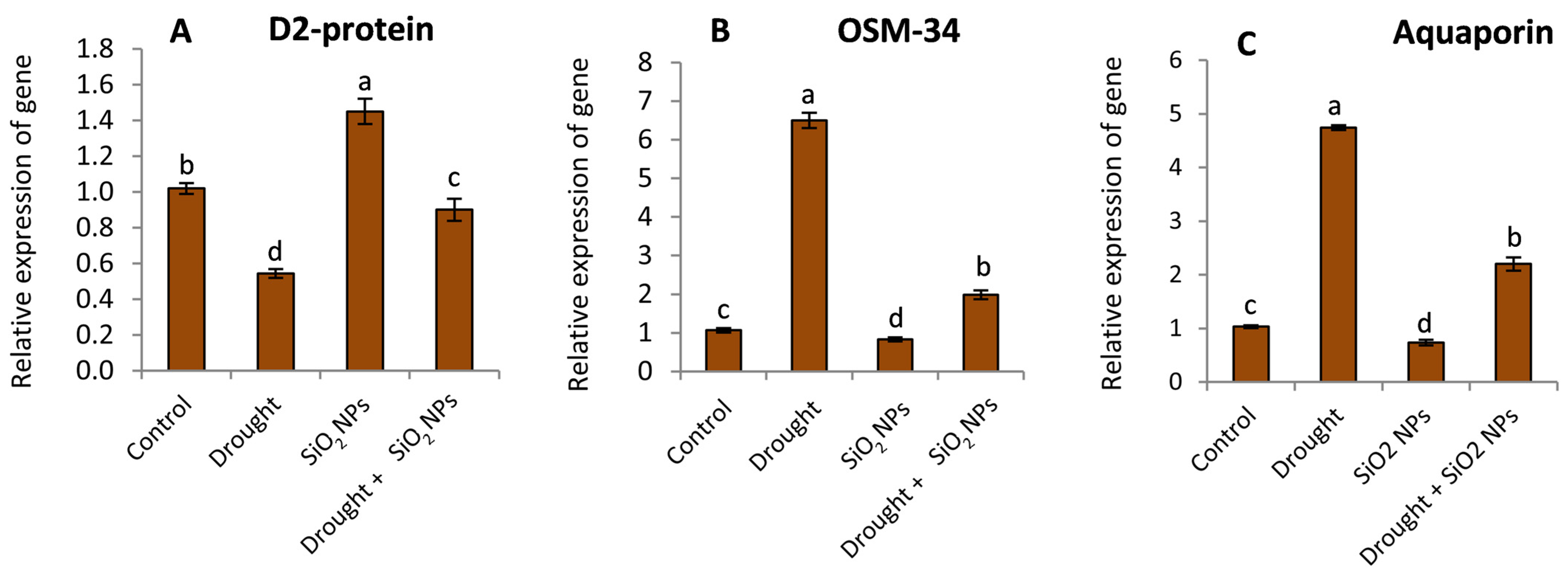
2.7. Effect of SiO2NPs Application on Gene Expression
3. Materials and Methods
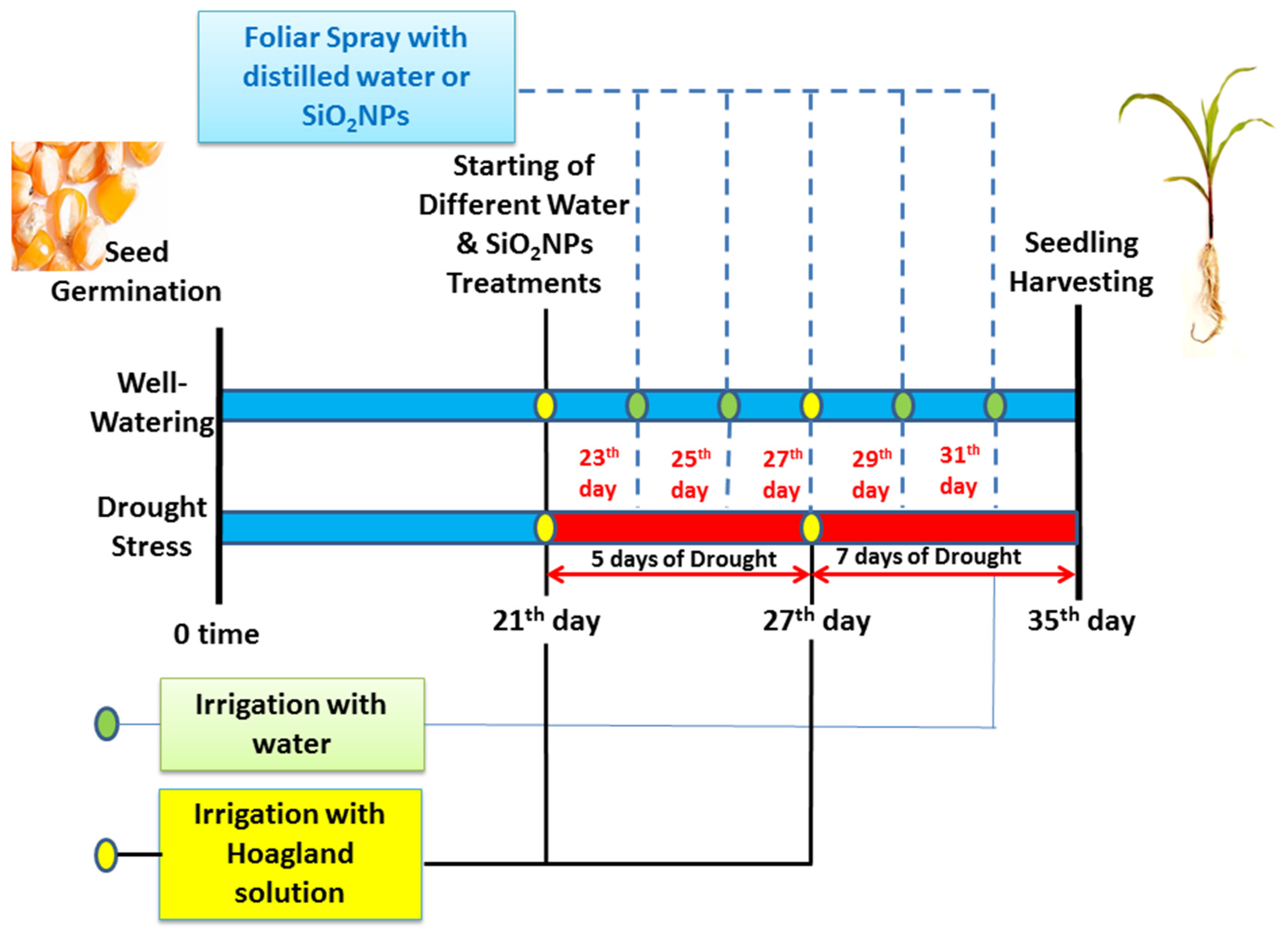
3.1. Plant and Experimental Details
3.2. Experimental Design and Treatments Organization
3.3. Experimental Approach and SiO2 Preparation
3.4. Characterization of SiO2
3.4.1. Phase Development Determination
3.4.2. Transmission Electron Microscopy (TEM)
3.5. Determination of Growth Parameters
3.6. Determination of Photosynthetic Pigments
3.7. Histochemical Detection of H2O2
3.8. Quantification of H2O2 and Lipid Peroxidation
3.9. Determination of Cell Membranes Stability Index (CMSI)
3.10. Determination the Activities of Antioxidant Enzymes
3.11. Relative Water Content (RWC), Total Soluble Sugars, and Proline
3.12. Gene Expression
3.13. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El Bilali, H.; Bassole, I.H.N.; Dambo, L.; Berjan, S. Climate change and food security. Agric. For. Poljopr. Sumar. 2020, 66, 197–210. [Google Scholar] [CrossRef]
- Lin, H.-I.; Yu, Y.-Y.; Wen, F.-I.; Liu, P.-T. Status of food security in East and Southeast Asia and challenges of climate change. Climate 2022, 10, 40. [Google Scholar] [CrossRef]
- Morote, Á.-F.; Olcina, J.; Hernández, M. The use of non-conventional water resources as a means of adaptation to drought and climate change in semi-arid regions: South-Eastern Spain. Water 2019, 11, 93. [Google Scholar] [CrossRef]
- Pulido-Velazquez, D.; Collados-Lara, A.-J.; Fernandez-Chacon, F. The impact of climate change scenarios on droughts and their propagation in an arid Mediterranean basin. A useful approach for planning adaptation strategies. Sci. Total Environ. 2022, 820, 153128. [Google Scholar]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Sheoran, S.; Kaur, Y.; Kumar, S.; Shukla, S.; Rakshit, S.; Kumar, R. Recent Advances for Drought Stress Tolerance in Maize (Zea mays L.): Present Status and Future Prospects. Front. Plant Sci. 2022, 13, 872566. [Google Scholar] [CrossRef]
- Schuppler, U.; He, P.H.; John, P.C.; Munns, R. Effect of water stress on cell division and cell-division-cycle 2-like cell-cycle kinase activity in wheat leaves. Plant Physiol. 1998, 117, 667–678. [Google Scholar] [CrossRef]
- Huang, B.; Chen, Y.-E.; Zhao, Y.-Q.; Ding, C.-B.; Liao, J.-Q.; Hu, C.; Zhou, L.-J.; Zhang, Z.-W.; Yuan, S.; Yuan, M. Exogenous melatonin alleviates oxidative damages and protects photosystem II in maize seedlings under drought stress. Front. Plant Sci. 2019, 10, 677. [Google Scholar] [CrossRef]
- Sakoda, K.; Taniyoshi, K.; Yamori, W.; Tanaka, Y. Drought stress reduces crop carbon gain due to delayed photosynthetic induction under fluctuating light conditions. Physiol. Plant. 2022, 174, e13603. [Google Scholar] [CrossRef]
- Turner, N.C. Turgor maintenance by osmotic adjustment: 40 years of progress. J. Exp. Bot. 2018, 69, 3223–3233. [Google Scholar] [CrossRef]
- Razi, K.; Muneer, S. Drought stress-induced physiological mechanisms, signaling pathways and molecular response of chloroplasts in common vegetable crops. Crit. Rev. Biotechnol. 2021, 41, 669–691. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.A.; Silvestri, C.; Ahmad, T.; Hafiz, I.A.; Abbasi, N.A.; Manzoor, A.; Cristofori, V.; Rugini, E. Osmotin: A cationic protein leads to improve biotic and abiotic stress tolerance in plants. Plants 2020, 9, 992. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, H.; Zou, Q.; Nie, Y. Divergent root water uptake depth and coordinated hydraulic traits among typical karst plantations of subtropical China: Implication for plant water adaptation under precipitation changes. Agric. Water Manag. 2021, 249, 106798. [Google Scholar] [CrossRef]
- Patel, J.; Mishra, A. Plant aquaporins alleviate drought tolerance in plants by modulating cellular biochemistry, root-architecture, and photosynthesis. Physiol. Plant. 2021, 172, 1030–1044. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmad, B.; Boote, K.; Hoogenboom, G. Adaptation strategies for maize production under climate change for semi-arid environments. Eur. J. Agron. 2020, 115, 126040. [Google Scholar] [CrossRef]
- Ranum, P.; Peña-Rosas, J.P.; Garcia-Casal, M.N. Global maize production, utilization, and consumption. Ann. N. Y. Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef]
- Dhugga, K.S. Maize biomass yield and composition for biofuels. Crop Sci. 2007, 47, 2211–2227. [Google Scholar] [CrossRef]
- Kleinmans, J.; Densley, R.; Hurley, T.; Williams, I. Brief Communication: Feed value of maize silage in New Zealand-a review. Proc. N. Z. Soc. Anim. Prod. 2016, 76, 100–102. [Google Scholar]
- Ostrander, B.M. Maize Starch for Industrial Applications. In Industrial Crops: Breeding for BioEnergy and Bioproducts; Cruz, V.M.V., Dierig, D.A., Eds.; Springer: New York, NY, USA, 2015; pp. 171–189. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Desoky, E.-S.M.; Babalghith, A.O.; El-Tahan, A.M.; Ibrahim, O.M.; Ebrahim, A.A.M.; Abd El-Mageed, T.A.; et al. Role of Nanoparticles in Enhancing Crop Tolerance to Abiotic Stress: A Comprehensive Review. Front. Plant Sci. 2022, 13, 946717. [Google Scholar] [CrossRef]
- Cao, Z.; Stowers, C.; Rossi, L.; Zhang, W.; Lombardini, L.; Ma, X. Physiological effects of cerium oxide nanoparticles on the photosynthesis and water use efficiency of soybean (Glycine max (L.) Merr.). Environ. Sci. Nano. 2017, 4, 1086–1094. [Google Scholar] [CrossRef]
- Elmer, W.; Ma, C.; White, J. Nanoparticles for plant disease management. Curr. Opin. Environ. Sci. Health 2018, 6, 66–70. [Google Scholar] [CrossRef]
- Singh, A.; Tiwari, S.; Pandey, J.; Lata, C.; Singh, I.K. Role of nanoparticles in crop improvement and abiotic stress management. J. Biotechnol. 2021, 337, 57–70. [Google Scholar] [CrossRef]
- Kumari, P.; Alam, M.; Siddiqi, W.A. Usage of nanoparticles as adsorbents for waste water treatment: An emerging trend. Sustain. Mater. Technol. 2019, 22, e00128. [Google Scholar] [CrossRef]
- Zhou, P.; Adeel, M.; Shakoor, N.; Guo, M.; Hao, Y.; Azeem, I.; Li, M.; Liu, M.; Rui, Y. Application of nanoparticles alleviates heavy metals stress and promotes plant growth: An overview. Nanomaterials 2020, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.j.; Chen, K.m.; Chen, G.c.; Wang, S.m.; Zhang, C.l. Effects of silicon on growth of wheat under drought. J. Plant Nutr. 2003, 26, 1055–1063. [Google Scholar] [CrossRef]
- Savant, N.K.; Korndörfer, G.H.; Datnoff, L.E.; Snyder, G.H. Silicon nutrition and sugarcane production: A review. J. Plant Nutr. 1999, 22, 1853–1903. [Google Scholar] [CrossRef]
- Ali, S.; Farooq, M.A.; Yasmeen, T.; Hussain, S.; Arif, M.S.; Abbas, F.; Bharwana, S.A.; Zhang, G. The influence of silicon on barley growth, photosynthesis and ultra-structure under chromium stress. Ecotoxicol. Environ. Saf. 2013, 89, 66–72. [Google Scholar] [CrossRef]
- Gao, X.; Zou, C.; Wang, L.; Zhang, F. Silicon improves water use efficiency in maize plants. J. Plant Nutr. 2005, 27, 1457–1470. [Google Scholar] [CrossRef]
- Ibrahim, M.F.; Abd El-Samad, G.; Ashour, H.; El-Sawy, A.M.; Hikal, M.; Elkelish, A.; El-Gawad, H.A.; El-Yazied, A.A.; Hozzein, W.N.; Farag, R. Regulation of agronomic traits, nutrient uptake, osmolytes and antioxidants of maize as influenced by exogenous potassium silicate under deficit irrigation and semiarid conditions. Agronomy 2020, 10, 1212. [Google Scholar] [CrossRef]
- Epstein, E. Silicon in plants: Facts vs. concepts. In Studies in Plant Science; Elsevier: Amsterdam, The Netherlands, 2001; Volume 8, pp. 1–15. [Google Scholar]
- Jeelani, P.G.; Mulay, P.; Venkat, R.; Ramalingam, C. Multifaceted application of silica nanoparticles. A review. Silicon 2020, 12, 1337–1354. [Google Scholar] [CrossRef]
- Mathur, P.; Roy, S. Nanosilica facilitates silica uptake, growth and stress tolerance in plants. Plant Physiol. Biochem. 2020, 157, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Rajwade, J.M.; Chikte, R.; Paknikar, K. Nanomaterials: New weapons in a crusade against phytopathogens. Appl. Microbiol. Biotechnol. 2020, 104, 1437–1461. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanpour, M.; Mohammadi, H.; Kariman, K. Nanosilicon-based recovery of barley (Hordeum vulgare) plants subjected to drought stress. Environ. Sci. Nano 2020, 7, 443–461. [Google Scholar] [CrossRef]
- Aqaei, P.; Weisany, W.; Diyanat, M.; Razmi, J.; Struik, P.C. Response of maize (Zea mays L.) to potassium nano-silica application under drought stress. J. Plant Nutr. 2020, 43, 1205–1216. [Google Scholar] [CrossRef]
- Ashkavand, P.; Tabari, M.; Zarafshar, M.; Tomásková, I.; Struve, D. Effect of SiO2 nanoparticles on drought resistance in hawthorn seedlings. Leśne Pract. Badaw. 2015, 76, 350–359. [Google Scholar] [CrossRef]
- Yuvakkumar, R.; Elango, V.; Rajendran, V.; Kannan, N.S.; Prabu, P. Influence of nanosilica powder on the growth of maize crop (Zea mays L.). Int. J. Green Nanotechnol. 2011, 3, 180–190. [Google Scholar] [CrossRef]
- Sun, J.; Xu, Z.; Li, W.; Shen, X. Effect of nano-SiO2 on the early hydration of alite-sulphoaluminate cement. Nanomaterials 2017, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Huseynov, E.; Garibov, A.; Mehdiyeva, R. TEM and SEM study of nano SiO2 particles exposed to influence of neutron flux. J. Mater. Res. Technol. 2016, 5, 213–218. [Google Scholar] [CrossRef]
- Flores, I.; Sobolev, K.; Torres-Martinez, L.; Cuellar, E.; Valdez, P.; Zarazua, E. Performance of cement systems with nano-SiO2 particles produced by using the sol–gel method. Transp. Res. Rec. 2010, 2141, 10–14. [Google Scholar] [CrossRef]
- Gong, H.; Zhu, X.; Chen, K.; Wang, S.; Zhang, C. Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci. 2005, 169, 313–321. [Google Scholar] [CrossRef]
- Shen, X.; Zhou, Y.; Duan, L.; Li, Z.; Eneji, A.E.; Li, J. Silicon effects on photosynthesis and antioxidant parameters of soybean seedlings under drought and ultraviolet-B radiation. J. Plant Physiol. 2010, 167, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.T.; Haddad, R. Study of silicon effects on antioxidant enzyme activities and osmotic adjustment of wheat under drought stress. Czech J. Genet. Plant Breed. 2011, 47, 17–27. [Google Scholar] [CrossRef]
- Sonobe, K.; Hattori, T.; An, P.; Tsuji, W.; Eneji, A.E.; Kobayashi, S.; Kawamura, Y.; Tanaka, K.; Inanaga, S. Effect of silicon application on sorghum root responses to water stress. J. Plant Nutr. 2010, 34, 71–82. [Google Scholar] [CrossRef]
- Steudle, E.; Peterson, C.A. How does water get through roots? J. Exp. Bot. 1998, 49, 775–788. [Google Scholar] [CrossRef]
- Perez, C.E.A.; Rodrigues, F.Á.; Moreira, W.R.; DaMatta, F.M. Leaf gas exchange and chlorophyll a fluorescence in wheat plants supplied with silicon and infected with Pyricularia oryzae. Phytopathology 2014, 104, 143–149. [Google Scholar] [CrossRef]
- Hattori, T.; Inanaga, S.; Araki, H.; An, P.; Morita, S.; Luxová, M.; Lux, A. Application of silicon enhanced drought tolerance in Sorghum bicolor. Physiol. Plant. 2005, 123, 459–466. [Google Scholar] [CrossRef]
- Gao, X.; Zou, C.; Wang, L.; Zhang, F. Silicon decreases transpiration rate and conductance from stomata of maize plants. J. Plant Nutr. 2006, 29, 1637–1647. [Google Scholar] [CrossRef]
- Malik, M.A.; Wani, A.H.; Mir, S.H.; Rehman, I.U.; Tahir, I.; Ahmad, P.; Rashid, I. Elucidating the role of silicon in drought stress tolerance in plants. Plant Physiol. Biochem. 2021, 165, 187–195. [Google Scholar] [CrossRef]
- Tezara, W.; Mitchell, V.; Driscoll, S.; Lawlor, D. Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature 1999, 401, 914–917. [Google Scholar] [CrossRef]
- Chaves, M.M.; Pereira, J.S.; Maroco, J.; Rodrigues, M.L.; Ricardo, C.P.P.; Osório, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How plants cope with water stress in the field? Photosynthesis and growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef]
- Mafakheri, A.; Siosemardeh, A.; Bahramnejad, B.; Struik, P.; Sohrabi, Y. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010, 4, 580–585. [Google Scholar]
- Wang, D.; Poss, J.; Donovan, T.; Shannon, M.; Lesch, S. Biophysical properties and biomass production of elephant grass under saline conditions. J. Arid Environ. 2002, 52, 447–456. [Google Scholar] [CrossRef]
- Sattar, A.; Cheema, M.A.; Sher, A.; Ijaz, M.; Ul-Allah, S.; Nawaz, A.; Abbas, T.; Ali, Q. Physiological and biochemical attributes of bread wheat (Triticum aestivum L.) seedlings are influenced by foliar application of silicon and selenium under water deficit. Acta Physiol. Plant. 2019, 41, 146. [Google Scholar] [CrossRef]
- Liu, P.; Yin, L.; Deng, X.; Wang, S.; Tanaka, K.; Zhang, S. Aquaporin-mediated increase in root hydraulic conductance is involved in silicon-induced improved root water uptake under osmotic stress in Sorghum bicolor L. J. Exp. Bot. 2014, 65, 4747–4756. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Ilyas, N.; Mashwani, Z.-u.-R.; Hayat, R.; Yasmin, H.; Noureldeen, A.; Ahmad, P. Synergistic effects of plant growth promoting rhizobacteria and silicon dioxide nano-particles for amelioration of drought stress in wheat. Plant Physiol. Biochem. 2021, 166, 160–176. [Google Scholar] [CrossRef]
- Amin, M.; Ahmad, R.; Ali, A.; Hussain, I.; Mahmood, R.; Aslam, M.; Lee, D.J. Influence of silicon fertilization on maize performance under limited water supply. Silicon 2018, 10, 177–183. [Google Scholar] [CrossRef]
- Snyder, G.H.; Matichenkov, V.V.; Datnoff, L.E. Silicon. In Handbook of Plant Nutrition; CRC Press: Boca Raton, FL, USA, 2007; pp. 551–568. [Google Scholar]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.-M.; Qian, P.; Xin, W.; Li, H.-Y.; Burritt, D.J.; Fujita, M.; Tran, L.-S.P. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Chen, K.; Zhao, Z.; Chen, G.; Zhou, W. Effects of silicon on defense of wheat against oxidative stress under drought at different developmental stages. Biol. Plant. 2008, 52, 592–596. [Google Scholar] [CrossRef]
- Pei, Z.; Ming, D.; Liu, D.; Wan, G.; Geng, X.; Gong, H.; Zhou, W. Silicon improves the tolerance to water-deficit stress induced by polyethylene glycol in wheat (Triticum aestivum L.) seedlings. J. Plant Growth Regul. 2010, 29, 106–115. [Google Scholar] [CrossRef]
- Ma, D.; Sun, D.; Wang, C.; Qin, H.; Ding, H.; Li, Y.; Guo, T. Silicon application alleviates drought stress in wheat through transcriptional regulation of multiple antioxidant defense pathways. J. Plant Growth Regul. 2016, 35, 1–10. [Google Scholar] [CrossRef]
- Premachandra, G.; Saneoka, H.; Kanaya, M.; Ogata, S. Cell membrane stability and leaf surface wax content as affected by increasing water deficits in maize. J. Exp. Bot. 1991, 42, 167–171. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Emam, Y.; Pessarakli, M. Effect of silicon on photosynthetic gas exchange, photosynthetic pigments, cell membrane stability and relative water content of different wheat cultivars under drought stress conditions. J. Plant Nutr. 2016, 39, 1001–1015. [Google Scholar] [CrossRef]
- Egert, M.; Tevini, M. Influence of drought on some physiological parameters symptomatic for oxidative stress in leaves of chives (Allium schoenoprasum). Environ. Exp. Bot. 2002, 48, 43–49. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Shah, S.; Ullah, S.; Mansour, A.T.; Shalaby, T.A. Impacts of ascorbic acid and alpha-tocopherol on Chickpea (Cicer arietinum L.) grown in water deficit regimes for sustainable production. Sustainability 2022, 14, 8861. [Google Scholar] [CrossRef]
- Ramadan, K.M.A.; Alharbi, M.M.; Alenzi, A.M.; El-Beltagi, H.S.; Darwish, D.B.; Aldaej, M.I.; Shalaby, T.A.; Mansour, A.T.; El-Gabry, Y.A.; Ibrahim, M.F.M. Alpha Lipoic Acid as a Protective Mediator for Regulating the Defensive Responses of Wheat Plants against Sodic Alkaline Stress: Physiological, Biochemical and Molecular Aspects. Plants 2022, 11, 787. [Google Scholar] [CrossRef]
- Youssef, M.H.M.; Raafat, A.; El-Yazied, A.A.; Selim, S.; Azab, E.; Khojah, E.; El Nahhas, N.; Ibrahim, M.F.M. Exogenous Application of Alpha-Lipoic Acid Mitigates Salt-Induced Oxidative Damage in Sorghum Plants through Regulation Growth, Leaf Pigments, Ionic Homeostasis, Antioxidant Enzymes, and Expression of Salt Stress Responsive Genes. Plants 2021, 10, 2519. [Google Scholar] [CrossRef]
- Zhu, Y.; Gong, H. Beneficial effects of silicon on salt and drought tolerance in plants. Agron. Sustain. Dev. 2014, 34, 455–472. [Google Scholar] [CrossRef]
- Al-aghabary, K.; Zhu, Z.; Shi, Q. Influence of silicon supply on chlorophyll content, chlorophyll fluorescence, and antioxidative enzyme activities in tomato plants under salt stress. J. Plant Nutr. 2005, 27, 2101–2115. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Ashraf, M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv. 2009, 27, 84–93. [Google Scholar] [CrossRef]
- Che, Y.; Zhang, N.; Zhu, X.; Li, S.; Wang, S.; Si, H. Enhanced tolerance of the transgenic potato plants overexpressing Cu/Zn superoxide dismutase to low temperature. Sci. Hortic. 2020, 261, 108949. [Google Scholar] [CrossRef]
- Asada, K. The water–water cycle as alternative photon and electron sinks. Philos. Trans. R. Soc. B 2000, 355, 1419–1431. [Google Scholar] [CrossRef]
- Gunes, A.; Pilbeam, D.J.; Inal, A.; Coban, S. Influence of silicon on sunflower cultivars under drought stress, I: Growth, antioxidant mechanisms, and lipid peroxidation. Commun. Soil Sci. Plant Anal. 2008, 39, 1885–1903. [Google Scholar] [CrossRef]
- Shekari, F.; Abbasi, A.; Mustafavi, S.H. Effect of silicon and selenium on enzymatic changes and productivity of dill in saline condition. J. Saudi Soc. Agric. Sci. 2017, 16, 367–374. [Google Scholar] [CrossRef]
- Zhu, Z.; Wei, G.; Li, J.; Qian, Q.; Yu, J. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci. 2004, 167, 527–533. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Rohman, M.; Anee, T.; Huang, Y.; Fujita, M. Exogenous silicon protects Brassica napus plants from salinity-induced oxidative stress through the modulation of AsA-GSH pathway, thiol-dependent antioxidant enzymes and glyoxalase systems. Gesunde Pflanz. 2018, 70, 185–194. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Khan, A.L.; Waqas, M.; Jeong, H.-J.; Kim, D.-H.; Shin, J.S.; Kim, J.-G.; Yeon, M.-H.; Lee, I.-J. Regulation of jasmonic acid biosynthesis by silicon application during physical injury to Oryza sativa L. J. Plant Res. 2014, 127, 525–532. [Google Scholar] [CrossRef]
- Manivannan, A.; Ahn, Y.-K. Silicon regulates potential genes involved in major physiological processes in plants to combat stress. Front. Plant Sci. 2017, 8, 1346. [Google Scholar] [CrossRef]
- Jansen, M.A.; Greenberg, B.M.; Edelman, M.; Mattoo, A.K.; Gaba, V. Accelerated degradation of the D2 protein of photosystem II under ultraviolet radiation. Photochem. Photobiol. 1996, 63, 814–817. [Google Scholar] [CrossRef]
- Kale, R.; Hebert, A.E.; Frankel, L.K.; Sallans, L.; Bricker, T.M.; Pospíšil, P. Amino acid oxidation of the D1 and D2 proteins by oxygen radicals during photoinhibition of Photosystem II. Proc. Natl. Acad. Sci. USA 2017, 114, 2988–2993. [Google Scholar] [CrossRef]
- Afzal, Z.; Howton, T.; Sun, Y.; Mukhtar, M.S. The roles of aquaporins in plant stress responses. J. Dev. Biol. 2016, 4, 9. [Google Scholar] [CrossRef]
- Ullah, A.; Hussain, A.; Shaban, M.; Khan, A.H.; Alariqi, M.; Gul, S.; Jun, Z.; Lin, S.; Li, J.; Jin, S. Osmotin: A plant defense tool against biotic and abiotic stresses. Plant Physiol. Biochem. 2018, 123, 149–159. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Saravanan, S.; Dubey, R. Synthesis of SiO2 nanoparticles by sol-gel method and their optical and structural properties. Rom. J. Inf. Sci. Technol. 2020, 23, 105–112. [Google Scholar]
- Yang, C.-M.; Chang, K.-W.; Yin, M.-H.; Huang, H.-M. Methods for the determination of the chlorophylls and their derivatives. Taiwania 1998, 43, 116–122. [Google Scholar]
- Ibrahim, M.F.; Elbar, O.H.A.; Farag, R.; Hikal, M.; El-Kelish, A.; El-Yazied, A.A.; Alkahtani, J.; El-Gawad, H.G.A. Melatonin counteracts drought induced oxidative damage and stimulates growth, productivity and fruit quality properties of tomato plants. Plants 2020, 9, 1276. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- González, L.; González-Vilar, M. Determination of relative water content. In Handbook of Plant Ecophysiology Techniques; Springer: New York, NY, USA, 2001; pp. 207–212. [Google Scholar]
- Beyer, W.F., Jr.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Dias, M.A.; Costa, M.M. Effect of low salt concentrations on nitrate reductase and peroxidase of sugar beet leaves. J. Exp. Bot. 1983, 34, 537–543. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; El-Yazied, A.A.; El-Gawad, H.G.A.; Kandeel, M.; Shalaby, T.A.; Mansour, A.T.; Al-Harbi, N.A.; Al-Qahtani, S.M.; Alkhateeb, A.A.; Ibrahim, M.F. Synergistic Impact of Melatonin and Putrescine Interaction in Mitigating Salinity Stress in Snap Bean Seedlings: Reduction of Oxidative Damage and Inhibition of Polyamine Catabolism. Horticulturae 2023, 9, 285. [Google Scholar] [CrossRef]
- Chow, P.S.; Landhäusser, S.M. A method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiol. 2004, 24, 1129–1136. [Google Scholar] [CrossRef]
- Bates, L.; Waldren, R.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- SAS. SAS/STAT User’s Guide: Release 6.03 Edition; SAS Institute Inc.: Cary, NC, USA, 1988. [Google Scholar]


| Gene Name | Sequence | Accession Number | |
|---|---|---|---|
| Osmotin-like protein (Osmotin-34) | F | 5′-GAACGGAGGGTGTCACAAAATC-3′ | NM_001153626.2 |
| R | 5′-CGTAGTGGGTCCACAAGTTCCT-3′ | ||
| D2 protein (psbD) | F | 5′- GGAAGATCAATCGACCGAAA-3′ | S46931.1 |
| R | 5′- CCTTATGCACCCATTTCACA-3′ | ||
| Aquaporin (AQPs) | F | 5′- GTTCCTATCCTTGCCCCACT-3′ | AY243801.1 |
| R | 5′- AGGCGTGATCCCTGTTGTAG-3′ | ||
| GAPDH (The housekeeping gene) | F | 5′- TTGGTTTCCACTGACTTCGTT-3′ | X15596.1 |
| R | 5′-CTGTAGCCCCACTCGTTGT-3′ | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharf-Eldin, A.A.; Alwutayd, K.M.; El-Yazied, A.A.; El-Beltagi, H.S.; Alharbi, B.M.; Eisa, M.A.M.; Alqurashi, M.; Sharaf, M.; Al-Harbi, N.A.; Al-Qahtani, S.M.; et al. Response of Maize Seedlings to Silicon Dioxide Nanoparticles (SiO2NPs) under Drought Stress. Plants 2023, 12, 2592. https://doi.org/10.3390/plants12142592
Sharf-Eldin AA, Alwutayd KM, El-Yazied AA, El-Beltagi HS, Alharbi BM, Eisa MAM, Alqurashi M, Sharaf M, Al-Harbi NA, Al-Qahtani SM, et al. Response of Maize Seedlings to Silicon Dioxide Nanoparticles (SiO2NPs) under Drought Stress. Plants. 2023; 12(14):2592. https://doi.org/10.3390/plants12142592
Chicago/Turabian StyleSharf-Eldin, Asmaa A., Khairiah Mubarak Alwutayd, Ahmed Abou El-Yazied, Hossam S. El-Beltagi, Basmah M. Alharbi, Mohammad A. M. Eisa, Mohammed Alqurashi, Mohamed Sharaf, Nadi Awad Al-Harbi, Salem Mesfir Al-Qahtani, and et al. 2023. "Response of Maize Seedlings to Silicon Dioxide Nanoparticles (SiO2NPs) under Drought Stress" Plants 12, no. 14: 2592. https://doi.org/10.3390/plants12142592
APA StyleSharf-Eldin, A. A., Alwutayd, K. M., El-Yazied, A. A., El-Beltagi, H. S., Alharbi, B. M., Eisa, M. A. M., Alqurashi, M., Sharaf, M., Al-Harbi, N. A., Al-Qahtani, S. M., & Ibrahim, M. F. M. (2023). Response of Maize Seedlings to Silicon Dioxide Nanoparticles (SiO2NPs) under Drought Stress. Plants, 12(14), 2592. https://doi.org/10.3390/plants12142592










