Foliar Spraying of Glycine Betaine Alleviated Growth Inhibition, Photoinhibition, and Oxidative Stress in Pepper (Capsicum annuum L.) Seedlings under Low Temperatures Combined with Low Light
Abstract
:1. Introduction
2. Results
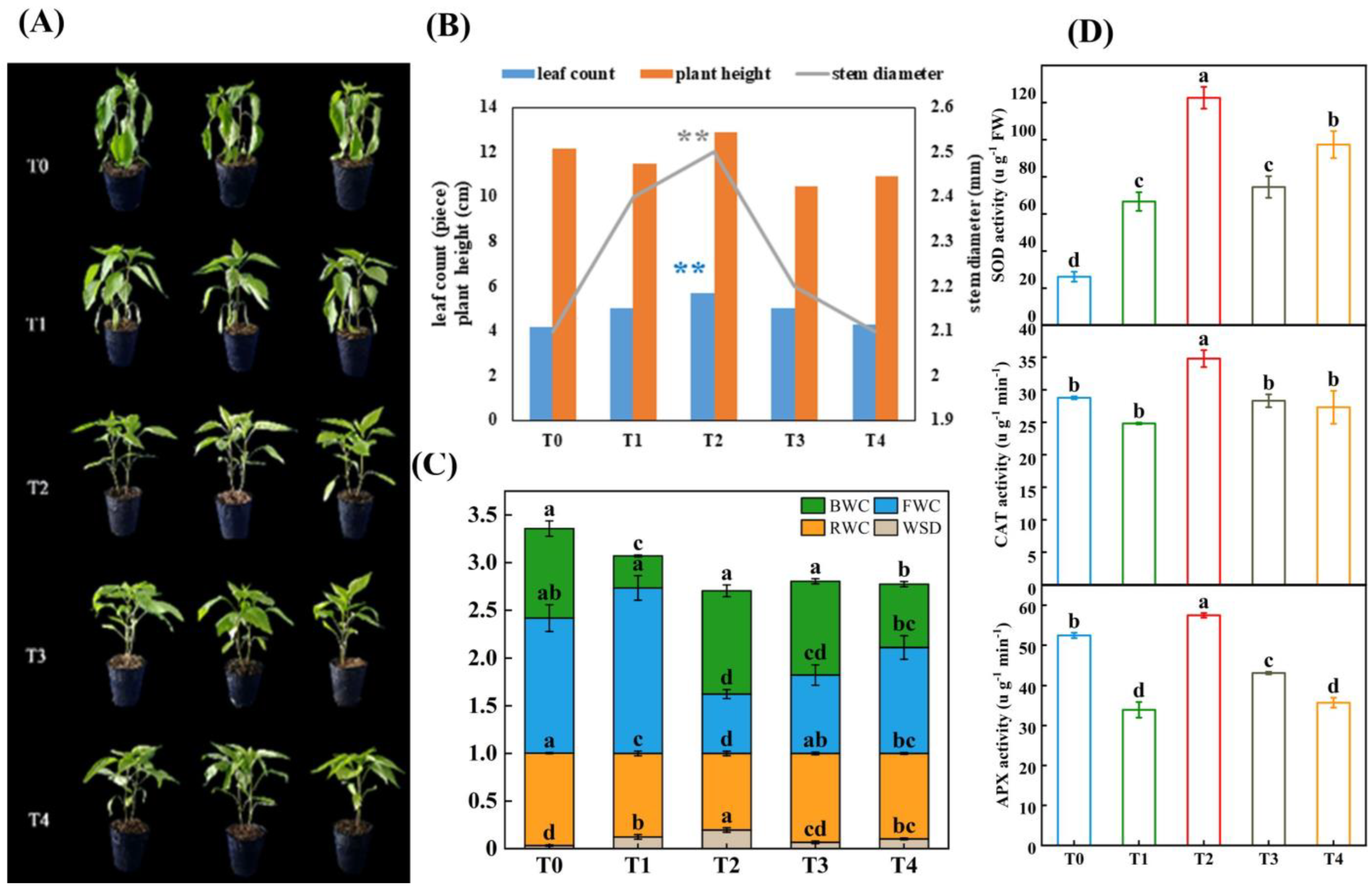
2.1. Changes in Growth, Water Content, and Antioxidant Enzyme Activity under LL Stress by GB at Different Concentrations
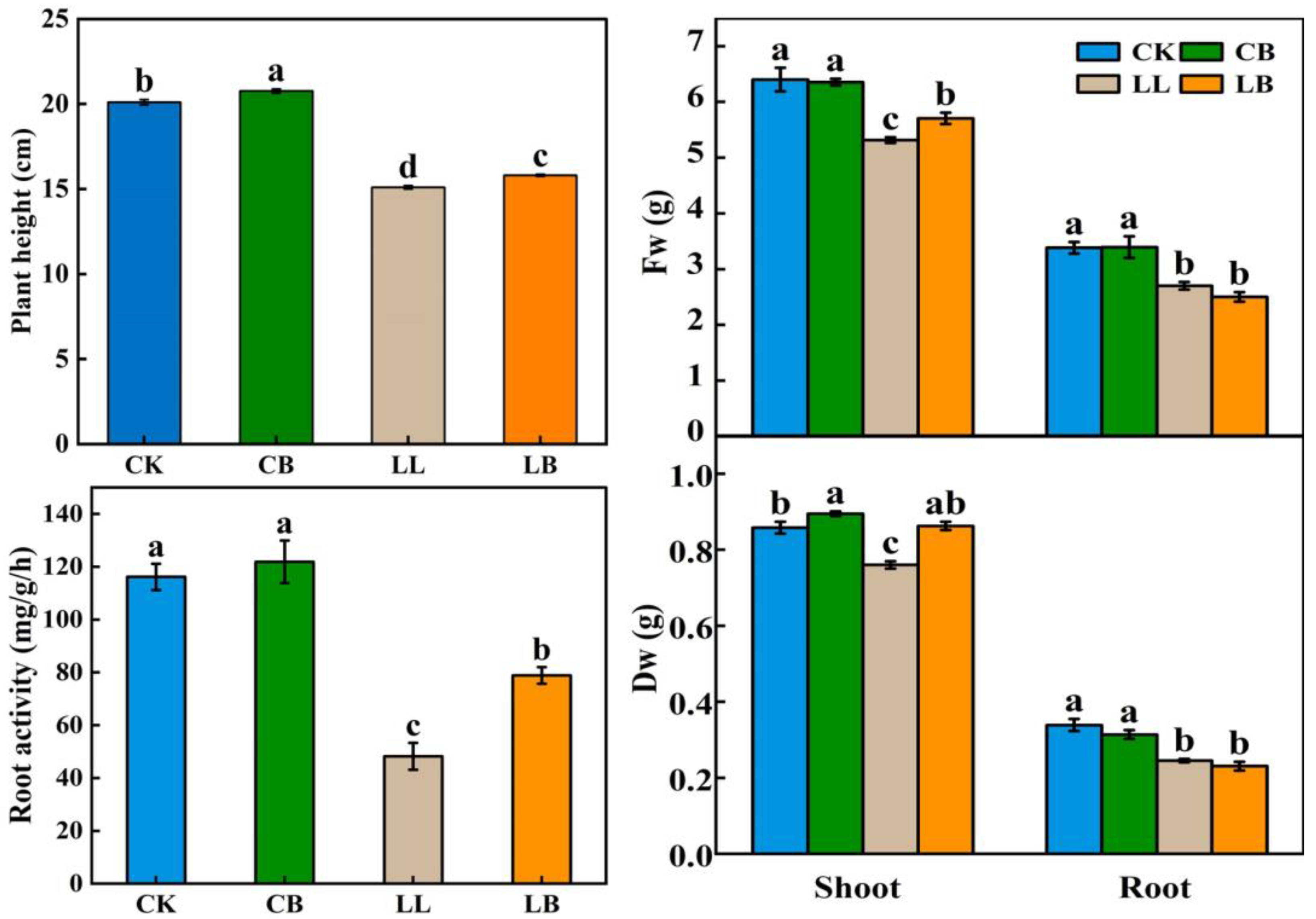
2.2. GB Affected the Growth, Dry Biomass, and Root Activity under LL Stress
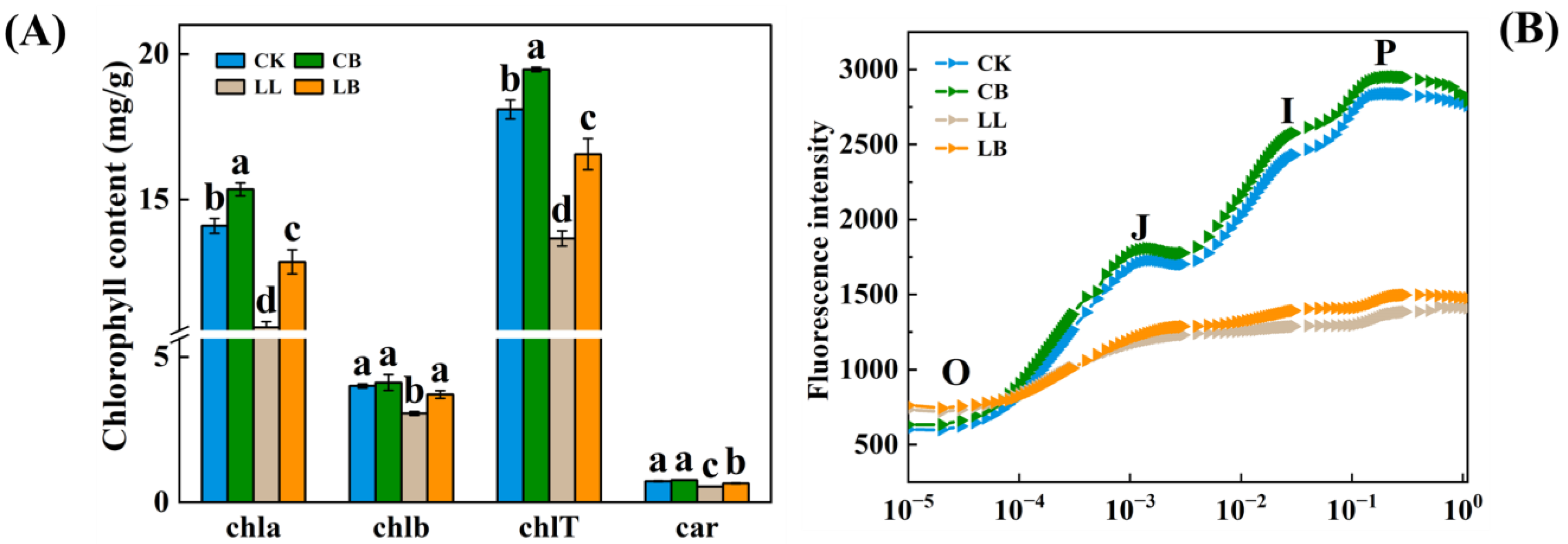
2.3. GB Affected the Photosynthetic Pigment Content and Analysis of the OJIP Curve under LL Stress
2.4. GB Affected Chlorophyll Fluorescence Parameters and Energy Distribution under LL Stress
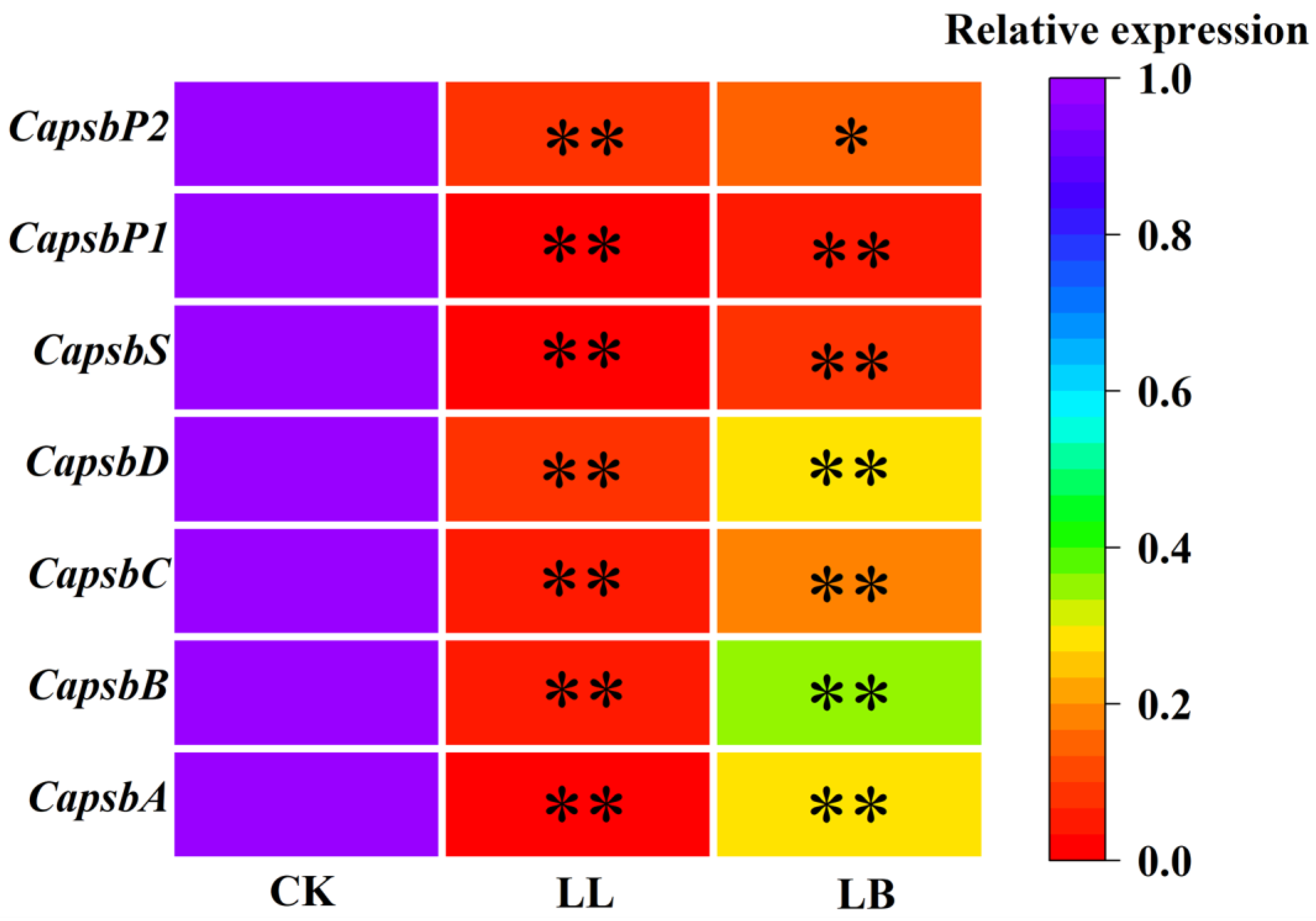
2.5. GB Affected the Expression of Genes Encoding the PSΠ Reaction Center Proteins under LL Stress
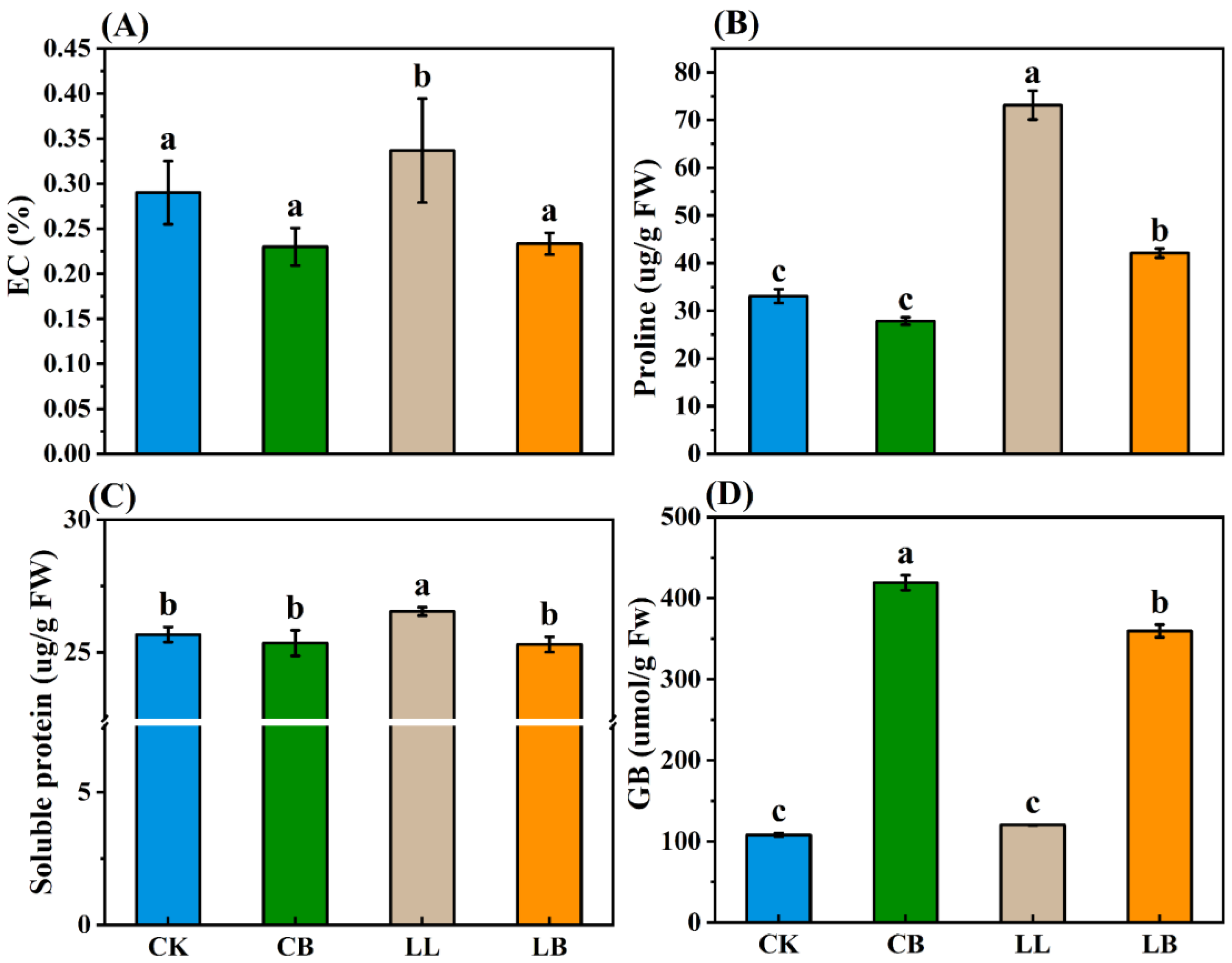
2.6. GB Affected the Osmotic Substances under LL Stress
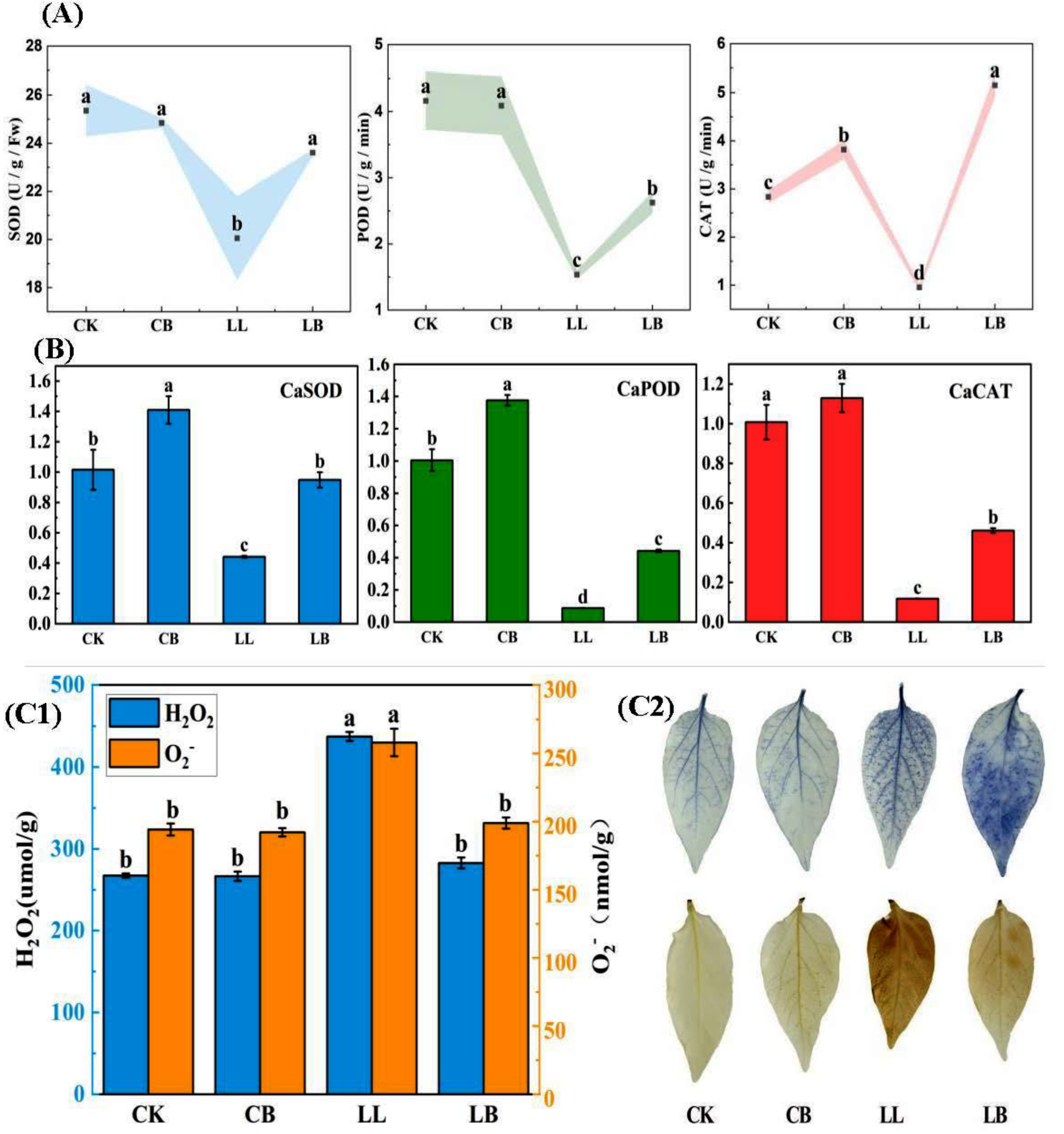
2.7. GB Affected the ROS Scavenging-Ability under LL Stress
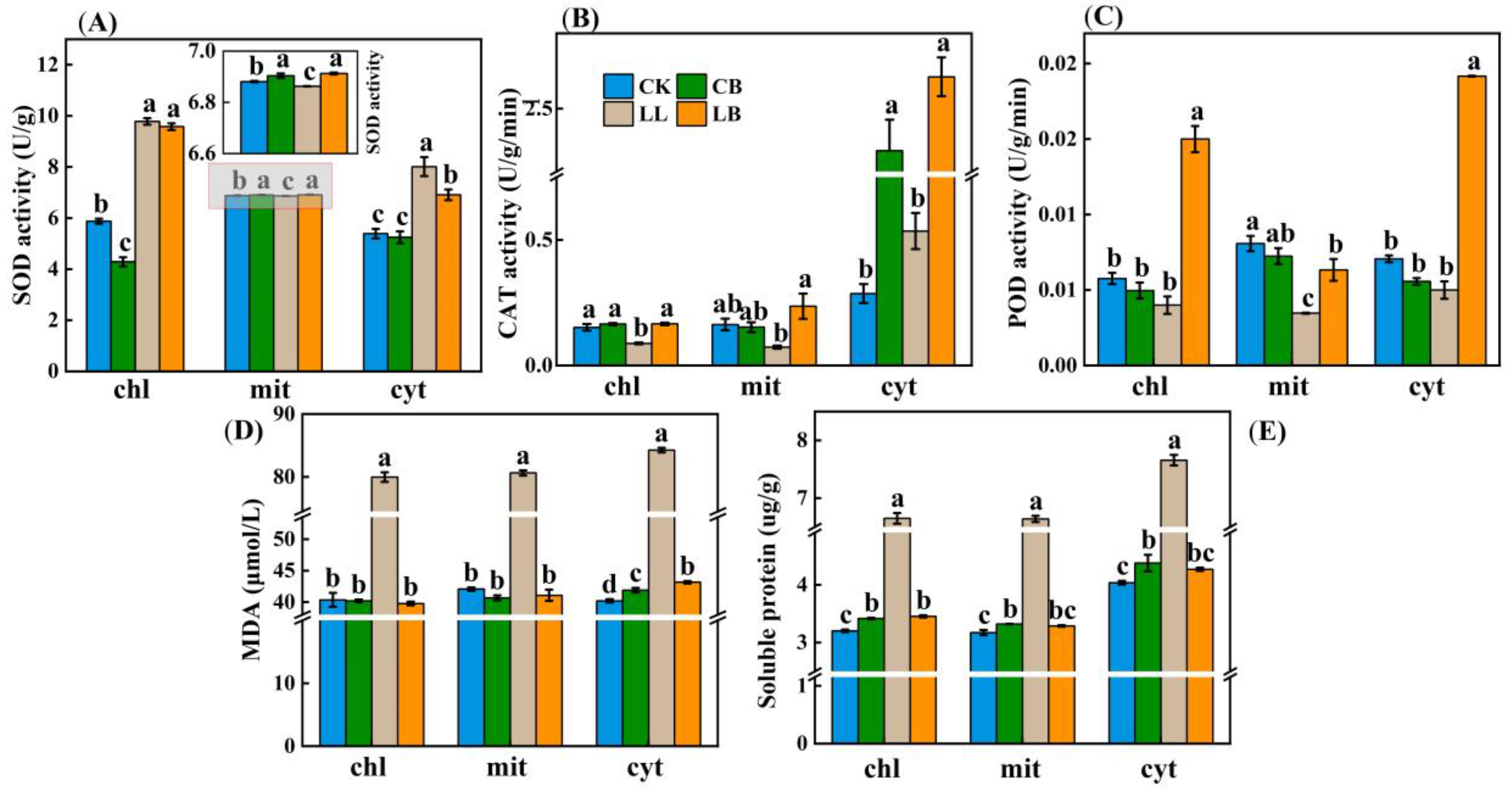
2.8. GB Affected the Subcellular Localization of Antioxidant Systems in Pepper Leaves under LL Stress
3. Discussion
4. Materials and Methods
4.1. Experimental Materials and Growth Conditions
4.2. Treatments
4.2.1. Different GB Concentration Treatments
4.2.2. Alleviating Effects of Exogenous GB on Pepper Plants under LL Stress
4.3. Determinations
4.3.1. Analysis of Morphology and Root Activity
4.3.2. Related Water Content of Pepper Leaf Cells
4.3.3. Determination of Photosynthetic Pigment Content and Chlorophyll Fluorescence Parameter Determination
Photosynthetic Pigment Contents of chla, chlb, chlT, and car
Chlorophyll Fluorescence Parameters
Measurement of the “OJIP” Curve and the JIP Test
4.3.4. Analyses of Biological Membrane Damage
4.3.5. Determination of the ROS-Scavenging Capability
4.3.6. Determination of the Subcellular Location of ROS-Scavenging Enzymes
4.3.7. Content of Glycine Betaine
4.3.8. Quantitative Polymerase Chain Reaction (PCR) Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.H.; Zhang, L.; Sun, Y.P.; Zheng, S.; Wang, J.; Zhang, T.G. Hydrogen peroxide is involved in strigolactone induced low temperature stress tolerance in rape seedlings (Brassica rapa L.). Plant Physiol. Biochem. 2020, 157, 402–415. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Wang, J.; Xiang, Z.; Xi, P.; Zhao, D. Physiological Changes and Differential Gene Expression of Tea Plants (Camellia sinensis (L.) Kuntze var. niaowangensis Q.H. Chen) Under Cold Stress. DNA Cell Biol. 2021, 40, 906–920. [Google Scholar] [CrossRef]
- Li, M.; Wang, C.; Shi, J.; Zhang, Y.; Liu, T.; Qi, H. Abscisic acid and putrescine synergistically regulate the cold tolerance of melon seedlings. Plant Physiol. Biochem. 2021, 166, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Xie, J.; Lv, J.; Li, J.; Zhang, J.; Wang, C.; Liang, G. Alleviating damage of photosystem and oxidative stress from chilling stress with exogenous zeaxanthin in pepper (Capsicum annuum L.) seedlings. Plant Physiol. Biochem. 2021, 162, 395–409. [Google Scholar] [CrossRef]
- Guo, W.L.; Chen, R.G.; Gong, Z.H.; Yin, Y.X.; Li, D.W. Suppression Subtractive Hybridization Analysis of Genes Regulated by Application of Exogenous Abscisic Acid in Pepper Plant (Capsicum annuum L.) Leaves under Chilling Stress. PLoS ONE 2013, 8, e66667. [Google Scholar] [CrossRef]
- Genzel, F.; Dicke, M.D.; Junker-Frohn, L.V.; Neuwohner, A.; Thiele, B.; Putz, A.; Usadel, B.; Wormit, A.; Wiese-Klinkenberg, A. Impact of Moderate Cold and Salt Stress on the Accumulation of Antioxidant Flavonoids in the Leaves of Two Capsicum Cultivars. J. Agric. Food Chem. 2021, 69, 6431–6443. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, L.Y.; Dai, J.X.; Wang, Y.; Lin, D. The NAC-type transcription factor CaNAC46 regulates the salt and drought tolerance of transgenic Arabidopsis thaliana. BMC Plant Biol. 2021, 21, 11. [Google Scholar] [CrossRef]
- Yadav, S.K. Cold stress tolerance mechanisms in plants. A review. Agron. Sustain. Dev. 2010, 30, 515–527. [Google Scholar] [CrossRef] [Green Version]
- Casal, J.J.; Questa, J.I. Light and temperature cues: Multitasking receptors and transcriptional integrators. New Phytol. 2018, 217, 1029–1034. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, P.; Abd Allah, E.F.; Alyemeni, M.N.; Wijaya, L.; Alam, P.; Bhardwaj, R.; Siddique, K.H.M. Exogenous application of calcium to 24-epibrassinosteroid pre-treated tomato seedlings mitigates NaCl toxicity by modifying ascorbate-glutathione cycle and secondary metabolites. Sci. Rep. 2018, 8, 13515. [Google Scholar] [CrossRef]
- Naus, J.; Smecko, S.; Spundova, M. Chloroplast avoidance movement as a sensitive indicator of relative water content during leaf desiccation in the dark. Photosynth. Res. 2016, 129, 217–225. [Google Scholar] [CrossRef]
- Snider, J.L.; Thangthong, N.; Pilon, C.; Virk, G.; Tishchenko, V. OJIP-fluorescence parameters as rapid indicators of cotton (Gossypium hirsutum L.) seedling vigor under contrasting growth temperature regimes. Plant Physiol. Biochem. 2018, 132, 249–257. [Google Scholar] [CrossRef]
- Heerden, P.D.R.v.; Villiers, O.T.d. Evaluation of the relative water content and the reduction of 2,3,5-triphenyltetrazoliumchloride as indicators of drought tolerance in spring wheat cultivars. S. Afr. J. Plant Soil 2013, 13, 131–135. [Google Scholar] [CrossRef] [Green Version]
- Shan, Z.Y.; Luo, X.L.; Wei, M.G.; Huang, T.W.; Khan, A.; Zhu, Y.M. Physiological and proteomic analysis on long-term drought resistance of cassava (Manihot esculenta Crantz). Sci. Rep. 2018, 8, 17982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufmann, H.; Blanke, M. Changes in carbohydrate levels and relative water content (RWC) to distinguish dormancy phases in sweet cherry. J. Plant Physiol. 2017, 218, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Syeed, S.; Sehar, Z.; Masood, A.; Anjum, N.A.; Khan, N.A. Control of Elevated Ion Accumulation, Oxidative Stress, and Lipid Peroxidation with Salicylic Acid-Induced Accumulation of Glycine Betaine in Salinity-Exposed Vigna radiata L. Appl. Biochem. Biotechnol. 2021, 193, 3301–3320. [Google Scholar] [CrossRef]
- El-Amier, Y.; Elhindi, K.; El-Hendawy, S.; Al-Rashed, S.; Abd-ElGawad, A. Antioxidant System and Biomolecules Alteration in Pisum sativum under Heavy Metal Stress and Possible Alleviation by 5-Aminolevulinic Acid. Molecules 2019, 24, 4194. [Google Scholar] [CrossRef] [Green Version]
- Iwaniuk, P.; Borusiewicz, A.; Lozowicka, B. Fluazinam and its mixtures induce diversified changes of crucial biochemical and antioxidant profile in leafy vegetable. Sci. Hortic. 2022, 298, 110988. [Google Scholar] [CrossRef]
- Saja-Garbarz, D.; Libik-Konieczny, M.; Fellner, M.; Jurczyk, B.; Janowiak, F. Silicon-induced alterations in the expression of aquaporins and antioxidant system activity in well-watered and drought-stressed oilseed rape. Plant Physiol. Biochem. 2022, 174, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Moradi, F. Effects of growth regulators on enzymatic and non-enzymatic antioxidants in leaves of two contrasting wheat cultivars under water stress. Braz. J. Bot. 2016, 39, 495–505. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Y.; Yang, Z.; Wang, P.; Li, D.; Zhang, J. Effects of low temperature stress on antioxidant enzymes activity in the subcellular of two Sabrina species. GuangXi Plants 2014, 34, 686–693. [Google Scholar]
- Geng, X.M.; Xiao, L.; Zhao, H.; Liu, P. Sub-cellular location of ROS-scavenging system in rhodoendron leaves under heat stress and H2O2 pretreatment. J. Northwest Bot. 2019, 39, 791–800. [Google Scholar]
- Pinero, M.C.; Lorenzo, P.; Sanchez-Guerrero, M.C.; Medrano, E.; Lopez-Marin, J.; del Amor, F.M. Reducing extreme weather impacts in greenhouses: The effect of a new passive climate control system on nutritional quality of pepper fruits. J. Sci. Food Agric. 2021, 102, 2723–2730. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Soto, C.G.; Valenzuela-Soto, E.M. Glycine betaine rather than acting only as an osmolyte also plays a role as regulator in cellular metabolism. Biochimie 2018, 147, 89–97. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Chong, K.; Xu, Y. Chilling tolerance in rice: Past and present. J. Plant Physiol. 2022, 268, 153576. [Google Scholar] [CrossRef]
- Wu, J.X.; Nadeem, M.; Galagedara, L.; Thomas, R.; Cheema, M. Effects of Chilling Stress on Morphological, Physiological, and Biochemical Attributes of Silage Corn Genotypes during Seedling Establishment. Plants 2022, 11, 1217. [Google Scholar] [CrossRef]
- Wei, D.D.; Zhang, T.P.; Wang, B.Q.; Zhang, H.L.; Ma, M.Y.; Li, S.F.; Chen, T.H.H.; Brestic, M.; Liu, Y.; Yang, X.H. Glycinebetaine mitigates tomato chilling stress by maintaining high-cyclic electron flow rate of photosystem I and stability of photosystem II. Plant Cell Rep. 2022, 41, 1087–1101. [Google Scholar] [CrossRef]
- Park, E.J.; Jeknic, Z.; Chen, T.H. Exogenous application of glycinebetaine increases chilling tolerance in tomato plants. Plant Cell Physiol. 2006, 47, 706–714. [Google Scholar] [CrossRef] [Green Version]
- Jia, W.; Ma, M.; Chen, J.; Wu, S. Plant Morphological, Physiological and Anatomical Adaption to Flooding Stress and the Underlying Molecular Mechanisms. Int. J. Mol. Sci. 2021, 22, 1088. [Google Scholar] [CrossRef]
- Yamori, N.; Levine, C.P.; Mattson, N.S.; Yamori, W. Optimum root zone temperature of photosynthesis and plant growth depends on air temperature in lettuce plants. Plant Mol. Biol. 2022, 110, 385–395. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Naz, S.; Altaf, M.M.; Khan, L.U.; Tiwari, R.K.; Lal, M.K.; Shahid, M.A.; Kumar, R.; et al. Melatonin Improves Drought Stress Tolerance of Tomato by Modulating Plant Growth, Root Architecture, Photosynthesis, and Antioxidant Defense System. Antioxidants 2022, 11, 309. [Google Scholar] [CrossRef]
- Giri, J. Glycinebetaine and abiotic stress tolerance in plants. Plant Signal. Behav. 2011, 6, 1746–1751. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.P.; Ran, C.; Zhang, Y.H.; Wang, X.L.; Lu, S.F.; Geng, Y.Q.; Guo, L.Y.; Shao, X.W. Effect of different concentrations of foliar iron fertilizer on chlorophyll fluorescence characteristics of iron-deficient rice seedlings under saline sodic conditions. Plant Physiol. Biochem. 2022, 185, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Chen, K.C.; Cheng, T.S.; Lee, C.; Lin, S.H.; Tung, C.W. Chlorophyll fluorescence analysis in diverse rice varieties reveals the positive correlation between the seedlings salt tolerance and photosynthetic efficiency. BMC Plant Biol. 2019, 19, 403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arikan, B.; Ozfidan-Konakci, C.; Alp, F.N.; Zengin, G.; Yildiztugay, E. Rosmarinic acid and hesperidin regulate gas exchange, chlorophyll fluorescence, antioxidant system and the fatty acid biosynthesis-related gene expression in Arabidopsis thaliana under heat stress. Phytochemistry 2022, 198, 113157. [Google Scholar] [CrossRef]
- Shin, Y.K.; Bhandari, S.R.; Lee, J.G. Monitoring of Salinity, Temperature, and Drought Stress in Grafted Watermelon Seedlings Using Chlorophyll Fluorescence. Front. Plant Sci. 2021, 12, 786309. [Google Scholar] [CrossRef]
- He, X.Y.; Richmond, M.E.A.; Williams, D.V.; Zheng, W.T.; Wu, F.B. Exogenous Glycinebetaine Reduces Cadmium Uptake and Mitigates Cadmium Toxicity in Two Tobacco Genotypes Differing in Cadmium Tolerance. Int. J. Mol. Sci. 2019, 20, 1612. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.Q.; Wang, W.; Li, Y.H.; Li, D.Q.; Zou, Q. Alleviation of photoinhibition in drought-stressed wheat (Triticum aestivum) by foliar-applied glycinebetaine. J. Plant Physiol. 2006, 163, 165–175. [Google Scholar] [CrossRef]
- Ran, X.; Wang, X.; Gao, X.; Liang, H.; Liu, B.; Huang, X. Effects of salt stress on the photosynthetic physiology and mineral ion absorption and distribution in white willow (Salix alba L.). PLoS ONE 2021, 16, e0260086. [Google Scholar] [CrossRef]
- Alexandrina Stirbet, G. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photoch Photobio B 2011, 104, 236–257. [Google Scholar] [CrossRef] [PubMed]
- Benkov, M.A.; Yatsenko, A.M.; Tikhonov, A.N. Light acclimation of shade-tolerant and sun-resistant Tradescantia species: Photochemical activity of PSII and its sensitivity to heat treatment. Photosynth. Res. 2019, 139, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Neelam, S.; Subramanyam, R. Alteration of photochemistry and protein degradation of photosystem II from Chlamydomonas reinhardtii under high salt grown cells. J. Photoch Photobio B 2013, 124, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Chen, L.; Fan, J.; Fu, J. Alleviation of heat damage to photosystem II by nitric oxide in tall fescue. Photosynth. Res. 2013, 116, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Hargett, S.R.; Liu, H.; Frankel, L.K.; Bricker, T.M. The PsbP protein is required for photosystem II complex assembly/stability and photoautotrophy in Arabidopsis thaliana. J. Biol. Chem. 2007, 282, 24833–24841. [Google Scholar] [CrossRef] [Green Version]
- Yi, X.; Hargett, S.R.; Frankel, L.K.; Bricker, T.M. The PsbP protein, but not the PsbQ protein, is required for normal thylakoid architecture in Arabidopsis thaliana. FEBS Lett. 2009, 583, 2142–2147. [Google Scholar] [CrossRef] [Green Version]
- Endo, T.; Uebayashi, N.; Ishida, S.; Ikeuchi, M.; Sato, F. Light energy allocation at PSII under field light conditions: How much energy is lost in NPQ-associated dissipation? Plant Physiol. Biochem. 2014, 81, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.Y.; Li, Y.P.; Wang, X.J.; Li, X.M.; Dong, S.K. Physiology and metabonomics reveal differences in drought resistance among soybean varieties. Bot. Stud. 2022, 63, 8. [Google Scholar] [CrossRef]
- Weretilnyk, E.A.; Bednarek, S.; McCue, K.F.; Rhodes, D.; Hanson, A.D. Comparative biochemical and immunological studies of the glycine betaine synthesis pathway in diverse families of dicotyledons. Planta 1989, 178, 342–352. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Akram, N.A.; Ashraf, M. Osmoprotection in plants under abiotic stresses: New insights into a classical phenomenon. Planta 2020, 251, 3. [Google Scholar] [CrossRef] [Green Version]
- Kaya, C.; Shabala, S. Melatonin improves drought stress tolerance of pepper (Capsicum annuum) plants via upregulating nitrogen metabolism. Funct. Plant Biol. 2023. [Google Scholar] [CrossRef]
- Nadarajah, K.K. ROS Homeostasis in Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P.; Kim, C.; Landgraf, F.; Apel, K. EXECUTER1- and EXECUTER2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2007, 104, 10270–10275. [Google Scholar] [CrossRef] [PubMed]
- Janku, M.; Luhova, L.; Petrivalsky, M. On the Origin and Fate of Reactive Oxygen Species in Plant Cell Compartments. Antioxidants 2019, 8, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alyemeni, M.N.; Ahanger, M.A.; Wijaya, L.; Alam, P.; Bhardwaj, R.; Ahmad, P. Correction to: Selenium mitigates cadmium-induced oxidative stress in tomato (Solanum lycopersicum L.) plants by modulating chlorophyll fluorescence, osmolyte accumulation, and antioxidant system. Protoplasma 2018, 255, 985–986. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Yao, Y.; Li, J.; Zhang, J.; Zhang, X.; Hu, L.; Ding, D.; Bakpa, E.P.; Xie, J. Trehalose Alleviated Salt Stress in Tomato by Regulating ROS Metabolism, Photosynthesis, Osmolyte Synthesis, and Trehalose Metabolic Pathways. Front. Plant Sci. 2022, 13, 772948. [Google Scholar] [CrossRef]
- Huang, X.M.; Song, S.Q.; Fu, J.R. Changes in water state and soluble protein contents of wampee axes during inducing desiccation tolerance by sucrose preculture. Zhi Wu Sheng Li Yu Fen Zi Sheng Wu Xue Xue Bao 2006, 32, 245–251. [Google Scholar]
- You, L.; Song, Q.; Wu, Y.; Li, S.; Jiang, C.; Chang, L.; Yang, X.; Zhang, J. Accumulation of glycine betaine in transplastomic potato plants expressing choline oxidase confers improved drought tolerance. Planta 2019, 249, 1963–1975. [Google Scholar] [CrossRef]

| Gene Name | Sequence (5′–3′) | GenBank Accession Number | Encoded Target | Amplicons Size (bp) |
|---|---|---|---|---|
| Actin | F: GTCCTTCCATCGTCCACAGG | XM_016722297.1 | Capsicum annuum actin | 1134 |
| R: GAAGGGCAAAGGTTCACAACA | ||||
| CaSOD | F: GTGAGCCTCCAAAGGGTTCTCTTG | AF036936.2 | Manganese superoxide dismutase | 687 |
| R: AAACCAAGCCACACCCAACCAG | ||||
| CaPOD | F: GCCAGGACAGCAAGCCAAGG | FJ596178.1 | Peroxidase | 975 |
| R: TGAGCACCTGATAAGGCAACCATG | ||||
| CaCAT | F: TTAACGCTCCCAAGTGTGCTCATC | NM_001324674.1 | Catalase | 1479 |
| R: GGCAGGACGACAAGGATCAAACC | ||||
| CaPsbA | F: GAATAGGGAGCCGCCGAATACAC | NC_018552.1:565–1626 | Turnover proteins D1 | 1062 |
| R: TATTCCAGGCTGAGCACAACATCC | ||||
| CaPsbB | F: TGGGTTTGCCTTGGTATCGTGTTC | NC_018552.1:75670–77196 | Internal light-harvesting complex proteins CP43 | 1527 |
| R: GCCCAACCAGCAACCAGAGC | ||||
| CaPsbC | F: GGATCTGCGTGCTCCATGGTTAG | NC_018552.1:35289–36674 | Internal light-harvesting complex proteins CP47 | 1386 |
| R: CCGTTCCTGCCAAGGTTGTATGTC | ||||
| CaPsbD | F: TGGTCACCGCTAACCGCTTTTG | NC_018552.1:34244–35305 | Turnover proteins D2 | 1062 |
| R: AGACCGACTACTCCAAGAGCACTC | ||||
| CaPsbS | F: AGGGAAAGGAGCATTGGCACAAC | XM_016719676.1 | Photosystem II 22 kDa protein | 834 |
| R: GCAGCAAAGAAGAAGAAGGCAACG | ||||
| CaPsbP1 | F: GCTGCTTCCACACAATGCTTCTTG | XM_016701483.1 | Oxygen-evolving enhancer protein 2 | 783 |
| R: TGGTTAGGCTTGAGGGTTGAAACG | ||||
| CaPsbP2 | F: CTCGGGCAGCATTTGCTACCATAG | XM_016690370.1 | Capsicum annuum photosynthetic NDH subunit | 699 |
| R: CCTGAAATGAGTCGGCCACCAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Pu, K.; Ding, D.; Yang, Y.; Niu, T.; Li, J.; Xie, J. Foliar Spraying of Glycine Betaine Alleviated Growth Inhibition, Photoinhibition, and Oxidative Stress in Pepper (Capsicum annuum L.) Seedlings under Low Temperatures Combined with Low Light. Plants 2023, 12, 2563. https://doi.org/10.3390/plants12132563
Li N, Pu K, Ding D, Yang Y, Niu T, Li J, Xie J. Foliar Spraying of Glycine Betaine Alleviated Growth Inhibition, Photoinhibition, and Oxidative Stress in Pepper (Capsicum annuum L.) Seedlings under Low Temperatures Combined with Low Light. Plants. 2023; 12(13):2563. https://doi.org/10.3390/plants12132563
Chicago/Turabian StyleLi, Nenghui, Kaiguo Pu, Dongxia Ding, Yan Yang, Tianhang Niu, Jing Li, and Jianming Xie. 2023. "Foliar Spraying of Glycine Betaine Alleviated Growth Inhibition, Photoinhibition, and Oxidative Stress in Pepper (Capsicum annuum L.) Seedlings under Low Temperatures Combined with Low Light" Plants 12, no. 13: 2563. https://doi.org/10.3390/plants12132563
APA StyleLi, N., Pu, K., Ding, D., Yang, Y., Niu, T., Li, J., & Xie, J. (2023). Foliar Spraying of Glycine Betaine Alleviated Growth Inhibition, Photoinhibition, and Oxidative Stress in Pepper (Capsicum annuum L.) Seedlings under Low Temperatures Combined with Low Light. Plants, 12(13), 2563. https://doi.org/10.3390/plants12132563





