Improving Seed Morphology and Germination Potential in Australian Native Grasses Using Seed Enhancement Technologies
Abstract
1. Introduction
2. Results
2.1. Germination Biology (Experiment 1)
Temperature Regimes
2.2. Removal of Floret Structures
2.3. KAR1
| Species | Temp Regime | Treatment | Maximum Germination (d) | T50m (e) | Germination Rate (b) |
|---|---|---|---|---|---|
| Neurachne alopecuroidea | Winter (18/7 °C) | Floret | 87 ± 4.25 | 11.08 ± 0.32 | 3.79 ± 0.7 |
| Floret + KAR1 | 84 ± 3.17 | 11.09 ± 0.26 | 5.14 ± 0.98 | ||
| Seed | 79 ± 2.31 | 5.92 ± 0.24 | 3.72 ± 0.83 | ||
| Seed + KAR1 | 72 ± 1.93 | 6.54 ± 0.19 | 5.22 ± 1.06 | ||
| Spring (26/13 °C) | Floret | 87 ± 2.84 | 7.71 ± 0.24 | 3.46 ± 0.54 | |
| Floret + KAR1 | 92 ± 2.34 | 8.12 ± 0.21 | 4.77 ± 0.88 | ||
| Seed | 68 ± 1.89 | 4.69 ± 0.21 | 3.58 ± 0.65 | ||
| Seed + KAR1 | 56 ± 1.82 | 4.92 ± 0.27 | 3.94 ± 0.89 | ||
| Summer (33/18 °C) | Floret | 82 ± 2.85 | 7.87 ± 0.26 | 3.53 ± 0.6 | |
| Floret + KAR1 | 85 ± 2.63 | 7.6 ± 0.24 | 3.66 ± 0.57 | ||
| Seed | 57 ± 1.71 | 4.46 ± 0.21 | 4.47 ± 1.1 | ||
| Seed + KAR1 | 61 ± 1.76 | 4.44 ± 0.2 | 4.10 ± 0.93 | ||
| Rytidosperma caespitosum | Winter (18/7 °C) | Floret | 35 ± 2.29 | 7.62 ± 0.5 | 3.35 ± 1.12 |
| Floret + KAR1 | 33 ± 2.48 | 7.99 ± 0.55 | 3.17 ± 1.04 | ||
| Seed | 32 ± 1.9 | 6.58 ± 0.47 | 3.53 ± 1.21 | ||
| Seed + KAR1 | 31 ± 1.79 | 5.72 ± 0.47 | 3.22 ± 1.1 | ||
| Spring (26/13 °C) | Floret | 30 ± 3.33 | 6.54 ± 0.68 | 2.31 ± 0.96 | |
| Floret + KAR1 | 36 ± 2.12 | 7.01 ± 0.46 | 3.23 ± 0.94 | ||
| Seed | 27 ± 6.17 | 8.6 ± 1.54 | 1.78 ± 0.92 | ||
| Seed + KAR1 | 22 ± 4.21 | 9.27 ± 1.21 | 2.53 ± 1.48 | ||
| Summer (33/18 °C) | Floret | 26 ± 5.1 | 10.27 ± 1.23 | 2.52 ± 1.36 | |
| Floret + KAR1 | 32 ± 4.09 | 8.62 ± 0.82 | 2.34 ± 0.9 | ||
| Seed | 23 ± 5.19 | 8.04 ± 1.44 | 1.9 ± 1.11 | ||
| Seed + KAR1 | 35 ± 5.49 | 8.18 ± 1.02 | 1.87 ± 0.71 |
| Species | Temp Regime | Treatment | Maximum Germination (d) | T50m (e) | Germination Rate (b) |
|---|---|---|---|---|---|
| Aristida inaequiglumis | Autumn (32/17 °C) | Floret | 91 ± 2.39 | 4.49 ± 0.18 | 4.14 ± 1.37 |
| Floret + KAR1 | 94 ± 2.33 | 4.67 ± 0.13 | 2.81 ± 0.54 | ||
| Seed | - | - | - | ||
| Seed + KAR11 | - | - | - | ||
| Summer (39/25 °C) | Floret | 91 ± 2.58 | 2.88 ± 0.14 | 2.05 ± 0.23 | |
| Floret + KAR11 | 91 ± 2.18 | 3.22 ± 0.20 | 2.63 ± 0.38 | ||
| Seed | - | - | - | ||
| Seed + KAR11 | - | - | - | ||
| Chrysopogon fallax | Autumn (32/17 °C) | Floret | 34 ± 3.38 | 3.42 ± 0.94 | 2.79 ± 2.13 |
| Floret + KAR11 | 32 ± 4.10 | 3.73 ± 4.28 | 4.27 ± 17.66 | ||
| Seed | 87 ± 2.92 | 1.83 ± 0.11 | 2.79 ± 1.17 | ||
| Seed + KAR11 | 87 ± 2.61 | 1.92 ± 0.08 | 3.91 ± 2.74 | ||
| Summer (39/25 °C) | Floret | 36 ± 3.80 | 3.22 ± 0.62 | 2.17 ± 0.97 | |
| Floret + KAR11 | 30 ± 2.99 | 2.81 ± 0.60 | 2.86 ± 1.50 | ||
| Seed | 96 ± 13.10 | 0.70 ± 0.40 | 0.81 ± 0.63 | ||
| Seed + KAR11 | 85 ± 3.73 | 1.40 ± 0.27 | 1.97 ± 1.02 | ||
| Cymbopogon ambiguus | Autumn (32/17 °C) | Floret | 96 ± 2.29 | 4.56 ± 0.14 | 3.94 ± 0.53 |
| Floret + KAR11 | 100 ± 2.25 | 4.30 ± 0.11 | 4.40 ± 0.77 | ||
| Seed | 72 ± 2.10 | 2.67 ± 0.16 | 2.84 ± 0.49 | ||
| Seed + KAR11 | 43 ± 2.28 | 2.65 ± 0.26 | 2.75 ± 0.91 | ||
| Summer (39/25 °C) | Floret | 94 ± 3.14 | 3.71 ± 0.19 | 2.08 ± 0.32 | |
| Floret + KAR11 | 95 ± 3.38 | 3.97 ± 0.20 | 2.03 ± 0.33 | ||
| Seed | 47 ± 2.09 | 2.67 ± 0.25 | 2.80 ± 0.71 | ||
| Seed + KAR11 | 49 ± 1.95 | 2.26 ± 0.16 | 3.27 ± 1.04 | ||
| Cymbopogon obtectus | Autumn (32/17 °C) | Floret | 92 ± 1.58 | 3.92 ± 0.07 | 4.72 ± 1.01 |
| Floret + KAR11 | 95 ± 1.57 | 3.93 ± 0.07 | 5.05 ± 1.20 | ||
| Seed | 97 ± 2.21 | 0.82 ± 0.31 | 1.59 ± 0.68 | ||
| Seed + KAR11 | 99 ± 1.43 | 1.20 ± 0.32 | 2.89 ± 1.51 | ||
| Summer (39/25 °C) | Floret | 90 ± 1.62 | 3.55 ± 0.16 | 4.51 ± 1.47 | |
| Floret + KAR11 | 93 ± 1.69 | 3.66 ± 0.11 | 3.97 ± 0.90 | ||
| Seed | 99 ± 1.49 | 0.96 ± 0.71 | 2.83 ± 2.89 | ||
| Seed + KAR11 | 100 ± 3.14 | 0.32 ± 0.59 | 1.23 ± 1.30 | ||
| Eriachne obtusa | Autumn (32/17 °C) | Floret | 54 ± 1.95 | 4.04 ± 0.17 | 3.94 ± 1.22 |
| Floret + KAR11 | 77 ± 2.08 | 3.28 ± 0.55 | 4.19 ± 0.14 | ||
| Seed | 85 ± 1.63 | 5.53 ± 4.85 | 3.12 ± 0.68 | ||
| Seed + KAR11 | 84 ± 1.62 | 4.82 ± 3.04 | 2.94 ± 0.57 | ||
| Summer (39/25 °C) | Floret | 53 ± 2.41 | 2.56 ± 0.82 | 3.64 ± 0.28 | |
| Floret + KAR11 | 62 ± 2.88 | 1.95 ± 0.39 | 3.85 ± 0.26 | ||
| Seed | 74 ± 1.63 | 3.29 ± 0.58 | 2.25 ± 0.09 | ||
| Seed + KAR11 | 73 ± 1.67 | 3.33 ± 0.87 | 2.02 ± 0.07 | ||
| Eulalia aurea | Autumn (32/17 °C) | Floret | 91 ± 3.52 | 3.14 ± 1.48 | 3.42 ± 0.58 |
| Floret + KAR11 | 93 ± 2.95 | 6.76 ± 17.76 | 3.99 ± 2.34 | ||
| Seed | 76 ± 3.56 | 2.33 ± 0.79 | 2.06 ± 0.15 | ||
| Seed + KAR11 | 65 ± 8.40 | 2.48 ± 0.73 | 2.14 ± 0.14 | ||
| Summer (39/25 °C) | Floret | 95 ± 3.43 | 2.46 ± 0.40 | 2.91 ± 0.22 | |
| Floret + KAR11 | 95 ± 3.10 | 2.99 ± 0.62 | 3.06 ± 0.30 | ||
| Seed | 54 ± 4.06 | 1.96 ± 0.98 | 1.93 ± 0.24 | ||
| Seed + KAR11 | 65 ± 8.40 | 1.16 ± 0.79 | 1.37 ± 0.35 |
2.4. Seed Enhancement Technologies (Experiments 2 and 3)
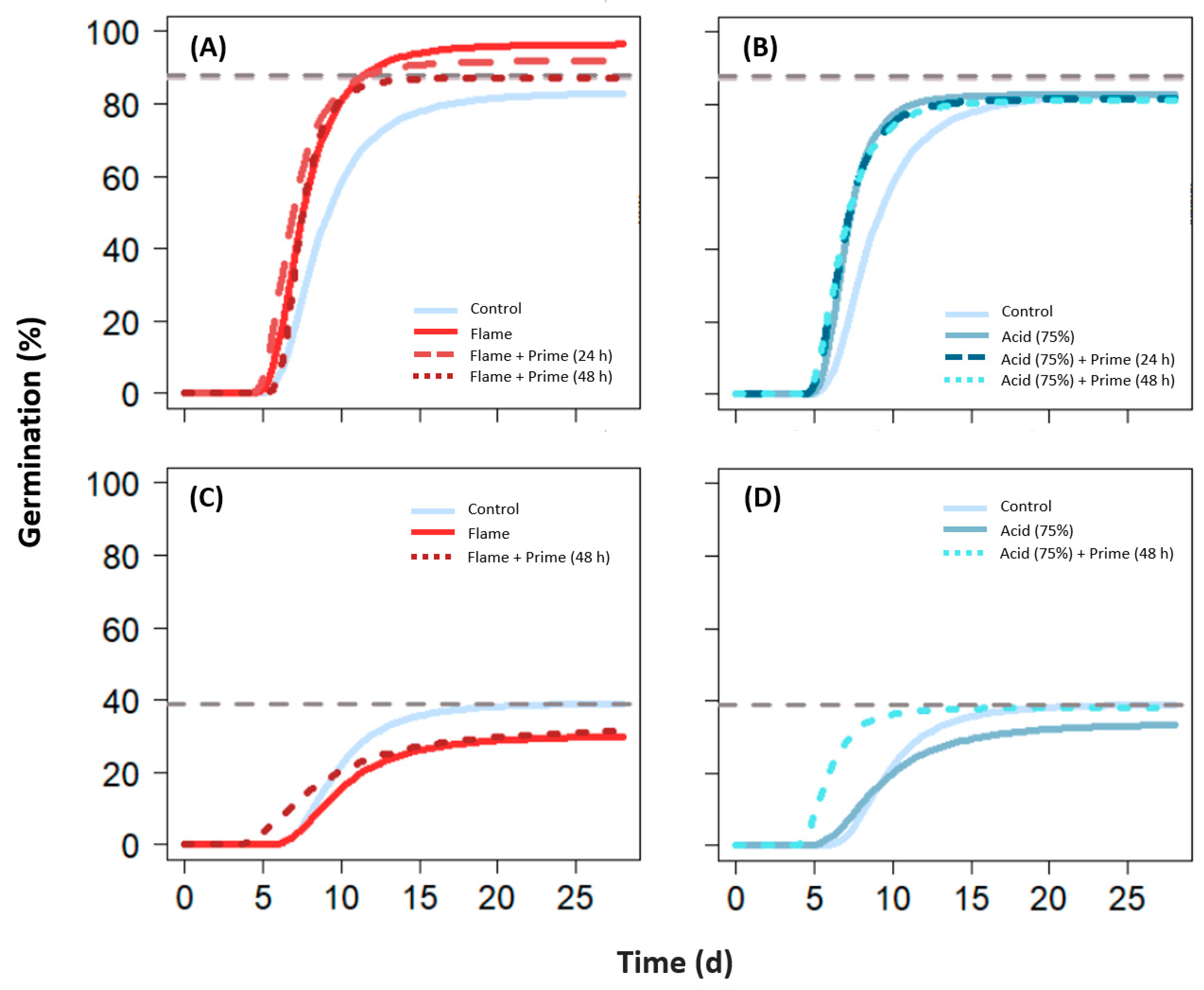
2.4.1. Flaming
2.4.2. Acid Digestion
2.4.3. Hydropriming
| Experiment 1: Germination Biology | |
| Treatment | Key findings |
| Temperature regimes | The majority of species demonstrated the capacity to germinate equally well under different temperature regimes. Cooler temperatures were favoured when exposed to KAR1 and/or cleaned to seed. T50m was generally shorter under warmer temperatures. |
| Removing floret structures | Decreased tolerance to higher temperatures (e.g., N. alopecuroidea, C. ambiguus, E. obtusa, and E. aurea). Alleviated seed dormancy (e.g., C. fallax and E. obtusa). Generally reduced T50m. |
| KAR1 | Neutral to inconsistent responses to exposure. |
| Experiments 2 and 3: SET Application | |
| Treatment | Key findings |
| Flash flaming | Fine hairs associated with floret successfully reduced with neutral effects on germination under the settings used (110 ± 10 °C). Including cooling periods (intermittent flaming) had no effect on germination. T50m often shorter (e.g., N. alopecuroidea, C. obtectus, E. aurea). |
| Acid digestion | Concentrations of 75–80% with exposure times of 1–2.5 min were generally effective for appendage reduction while maintaining (or enhancing) germination capacity. Using 50% concentration was less effective for appendage reduction and detrimental to germination in some species (e.g., N. alopecuroidea, C. ambiguus). |
| Hydropriming | Neutral effects on maximum germination when used alone, mixed effects when used in combination with other SETs. Overall faster germination. |
3. Discussion
3.1. Understanding Germination Biology
3.2. Seed Enhancement Application
3.2.1. Flash Flaming
3.2.2. Acid Digestion
3.2.3. Hydropriming
3.3. Scaled Application and Future Research of SETs
4. Conclusions
5. Materials and Methods
5.1. Study Species
5.2. Study Overview
5.3. Experiment 1: Germination Biology
5.4. Experiment 2: SET Applications to Improve Seed Handling
5.4.1. Flash Flaming
5.4.2. Acid Digestion
5.5. Experiment 3: SET Applications to Provide Additional Germination Benefits
Priming
5.6. Data Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pedrini, S.; Lewandrowski, W.; Stevens, J.C.; Dixon, K.W. Optimising seed processing techniques to improve germination and sowability of native grasses for ecological restoration. Plant Biol. 2019, 21, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Loch, D.S. Improved handling of chaffy grass seeds: Options, opportunities and value. Trop. Grassl. 1993, 27, 314–326. [Google Scholar]
- Cavanagh, A.M.; Godfree, R.C.; Morgan, J.W. An awn typology for Australian native grasses (Poaceae). Aust. J. Bot. 2019, 67, 309–334. [Google Scholar] [CrossRef]
- Loch, D.S.; Johnston, P.W.; Jensen, T.A.; Harvey, G.L. Harvesting, processing, and marketing Australian native grass seeds. N. Z. J. Agric. Res. 1996, 39, 591–599. [Google Scholar] [CrossRef]
- Berto, B.; Erickson, T.E.; Ritchie, A.L. Flash Flaming Improves Flow Properties of Mediterranean Grasses Used for Direct Seeding. Plants 2020, 9, 1699. [Google Scholar] [CrossRef]
- Berto, B.; Ritchie, A.L.; Erickson, T.E. Seed-enhancement combinations improve germination and handling in two dominant native grass species. Restor. Ecol. 2021, 29, e13275. [Google Scholar] [CrossRef]
- Brown, V.S.; Erickson, T.E.; Merritt, D.J.; Madsen, M.D.; Hobbs, R.J.; Ritchie, A.L. A global review of seed enhancement technology use to inform improved applications in restoration. Sci. Total. Environ. 2021, 798, 149096. [Google Scholar] [CrossRef]
- Erickson, T.; Kildisheva, O.; Baughman, O.; Breed, M.; Ruiz-Talonia, L.; Brown, V.; Madsen, M.; Merritt, D.; Ritchie, A. Florabank Guidelines Module 12—Seed Enhancement Technologies. In Florabank Guidelines—Best Practice Guidelines for Native Seed Collection and Use, 2nd ed.; Commander, L.E., Ed.; Florabank Consortium: Australia, 2021. [Google Scholar]
- Commander, L.E.; Golos, P.J.; Miller, B.; Merritt, D. Seed germination traits of desert perennials. Plant Ecol. 2017, 218, 1077–1091. [Google Scholar] [CrossRef]
- Kildisheva, O.A.; Dixon, K.W.; Silveira, F.A.O.; Chapman, T.; Di Sacco, A.; Mondoni, A.; Turner, S.R.; Cross, A.T. Dormancy and germination: Making every seed count in restoration. Restor. Ecol. 2020, 28, S256–S265. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Erickson, T.E.; Shackelford, N.; Dixon, K.W.; Turner, S.R.; Merritt, D.J. Overcoming physiological dormancy in seeds of Triodia (Poaceae) to improve restoration in the arid zone. Restor. Ecol. 2016, 24, S64–S76. [Google Scholar] [CrossRef]
- Erickson, T.; Munoz-Rojas, M.; Guzzomi, A.; Masarei, M.; Ling, E.; Bateman, A.; Kildisheva, O.; Ritchie, A.; Turner, S.; Parsons, B.; et al. A case study of seed-use technology development for Pilbara mine site rehabilitation. In Proceedings of the 13th International Conference on Mine Closure, Perth, Australia, 3–5 September 2019; Fourie, A.B., Tibbett, M., Eds.; Australian Centre for Geomechanics: Perth, Australia, 2019. [Google Scholar]
- Madsen, M.D.; Davies, K.W.; Boyd, C.S.; Kerby, J.D.; Svejcar, T.J. Emerging seed enhancement technologies for overcoming barriers to restoration. Restor. Ecol. 2016, 24, S77–S84. [Google Scholar] [CrossRef]
- Taylor, A.G.; Allen, P.S.; Bennett, M.A.; Bradford, K.J.; Burris, J.S.; Misra, M.K. Seed enhancements. Seed Sci. Res. 1998, 8, 245–256. [Google Scholar] [CrossRef]
- Halmer, P. Seed technology and seed enhancement. Acta Hortic. 2008, 771, 17–26. [Google Scholar] [CrossRef]
- Pedrini, S.; Balestrazzi, A.; Madsen, M.D.; Bhalsing, K.; Hardegree, S.P.; Dixon, K.W.; Kildisheva, O.A. Seed enhancement: Getting seeds restoration-ready. Restor. Ecol. 2020, 28, S266–S275. [Google Scholar] [CrossRef]
- Svejcar, L.N.; Brown, V.S.; Ritchie, A.L.; Davies, K.W.; Svejcar, T.J. A new perspective and approach to ecosystem restoration: A seed enhancement technology guide and case study. Restor. Ecol. 2021, 30, e13615. [Google Scholar] [CrossRef]
- Stevens, J.; Chivers, I.; Symons, D.; Dixon, K. Acid-digestion improves native grass seed handling and germination. Seed Sci. Technol. 2015, 43, 313–317. [Google Scholar] [CrossRef]
- Ling, E.; Masarei, M.; Guzzomi, A.L.; Merritt, D.J.; Renton, M.; Erickson, T.E. Flash flaming is a valid seed enhancement for a diverse range of species and seed morphologies. Seed Sci. Technol. 2022, 50, 387–405. [Google Scholar] [CrossRef]
- Paparella, S.; Araujo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef]
- Thacker, M.G.; Madsen, M.D.; Hoose, B.W.; Anderson, R.M.; Tryon, D.R.; Larsen, R.T.; Gunnell, K.L.; Summers, D.D.; Erickson, T.E. Use of Flash-Flaming Technology to Improve Seed Handling and Delivery of Winterfat (Krascheninnikovia lanata). Rangel. Ecol. Manag. 2023, 88, 22–27. [Google Scholar] [CrossRef]
- Guzzomi, A.L.; Erickson, T.E.; Ling, K.Y.; Dixon, K.W.; Merritt, D.J. Flash flaming effectively removes appendages and improves the seed coating potential of grass florets. Restor. Ecol. 2016, 24, S98–S105. [Google Scholar] [CrossRef]
- Nawaz, J.; Hussain, M.; Jabbar, A.; Nadeem, G.A.; Sajid, M.; Subtain, M.U.; Shabbir, I. Seed Priming A Technique. Int. J. Agric. Crop Sci. 2013, 6, 1373–1381. [Google Scholar]
- Goswami, A.; Banerjee, R.; Raha, S. Drought resistance in rice seedlings conferred by seed priming. Protoplasma 2013, 250, 1115–1129. [Google Scholar] [CrossRef]
- Hardegree, S.P.; Emmerich, W.E. Seed Germination Response of Four Southwestern Range Grasses to Equilibration at Subgermination Matric-Potentials. Agron. J. 1992, 84, 994–998. [Google Scholar] [CrossRef]
- Hardegree, S.P.; Van Vactor, S.S. Germination and emergence of primed grass seeds under field and simulated-field temperature regimes. Ann. Bot. 2000, 85, 379–390. [Google Scholar] [CrossRef]
- Berto, B.; Brown, V.S. 10 years to restore the planet: A seedy situation. Restor. Ecol. 2022, 31, e13755. [Google Scholar] [CrossRef]
- Ling, E.; Guzzomi, A.; Merritt, D.; Renton, M.; Erickson, T. Flash flaming technology shows promise to improve seed-based rehabilitation outcomes. In Proceedings of the 13th International Conference on Mine Closure, Perth, Australia, 3–5 September 2019; Fourie, A.B., Tibbett, M., Eds.; Australian Centre for Geomechanics: Perth, Australia, 2019. [Google Scholar]
- Madsen, M.D.; Hulet, A.; Phillips, K.; Staley, J.L.; Davies, K.W.; Svejcar, T.J. Extruded seed pellets: A novel approach for enhancing sagebrush seedling emergence. Nativ. Plants J. 2016, 17, 230–243. [Google Scholar] [CrossRef]
- Erickson, T.E.; Muñoz-Rojas, M.; Kildisheva, O.A.; Stokes, B.A.; White, S.A.; Heyes, J.L.; Dalziell, E.L.; Lewandrowski, W.; James, J.J.; Madsen, M.D.; et al. Benefits of adopting seed-based technologies for rehabilitation in the mining sector: A Pilbara perspective. Aust. J. Bot. 2017, 65, 646. [Google Scholar] [CrossRef]
- Vleeshouwers, L.M.; Bouwmeester, H.J.; Karssen, C.M. Redefining Seed Dormancy: An Attempt to Integrate Physiology and Ecology. J. Ecol. 1995, 83, 1031–1037. [Google Scholar] [CrossRef]
- Erickson, T.E. Seed Dormancy and Germination Traits of 89 Arid Zone Species Targeted for Mine-Site Restoration in the Pilbara Region of Western Australia; University of Western Australia: Perth, Australia, 2015. [Google Scholar]
- Prescott, A. Native Grasses: A Regional Guide; Natural Resources Adelaide and Mt Lofty Ranges; Government of South Australia: Eastwood, Australia, 2017.
- Gray, F.; Cochrane, A.; Poot, P. Provenance modulates sensitivity of stored seeds of the Australian native grass Neurachne alopecuroidea to temperature and moisture availability. Aust. J. Bot. 2019, 67, 106. [Google Scholar] [CrossRef]
- Lewandrowski, W.; Erickson, T.E.; Dalziell, E.L.; Stevens, J.C. Ecological niche and bet-hedging strategies for Triodia (R.Br.) seed germination. Ann. Bot. 2018, 121, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Mohamed-Yasseen, Y.; Barringer, S.A.; Splittstoesser, W.E.; Costanza, S. The role of seed coats in seed viability. Bot. Rev. 1994, 60, 426–439. [Google Scholar] [CrossRef]
- Farley, G.J.; Bellairs, S.M.; Adkins, S.W. Germination of selected Australian native grass species, with potential for minesite rehabilitation. Aust. J. Bot. 2013, 61, 283–290. [Google Scholar] [CrossRef]
- Turner, S.R.; Merritt, D.; Renton, M.; Dixon, K. Seed moisture content affects afterripening and smoke responsiveness in three sympatric Australian native species from fire-prone environments. Austral Ecol. 2009, 34, 866–877. [Google Scholar] [CrossRef]
- Keeley, J.; Bond, W.; Bradstock, R.; Pausas, J.G.; Rundel, P. Fire in Mediterranean Ecosystems: Ecology, Evolution and Management; Cambridge University Press: Cambridge, UK, 2011; pp. 20111–20515. [Google Scholar]
- Scotter, D.R. Scotter Soil temperatures under grass fires. Soil Res. 1970, 8, 273–279. [Google Scholar] [CrossRef]
- Tangney, R.; Merritt, D.J.; Callow, J.N.; Fontaine, J.B.; Miller, B.P. Seed traits determine species’ responses to fire under varying soil heating scenarios. Funct. Ecol. 2020, 34, 1967–1978. [Google Scholar] [CrossRef]
- Carrington, M.E. Effects of Soil Temperature during Fire on Seed Survival in Florida Sand Pine Scrub. Int. J. For. Res. 2010, 2010, 402346. [Google Scholar] [CrossRef]
- Ruckman, E.; Robinson, T.; Lyons, K.G.; Schwinning, S. Comparative Seed Heat Tolerances Among Native and Non-indigenous Invasive Grassland Species. Ecol. Restor. 2012, 30, 136–142. [Google Scholar] [CrossRef]
- Overbeck, G.E.; Müller, S.C.; Pillar, V.D.; Pfadenhauer, J. No heat-stimulated germination found in herbaceous species from burned subtropical grassland. Plant Ecol. 2006, 184, 237–243. [Google Scholar] [CrossRef]
- López-Mársico, L.; Farías-Moreira, L.; Lezama, F.; Altesor, A.; Rodríguez, C. Light intensity triggers different germination responses to fire-related cues in temperate grassland species. Folia Geobot. 2019, 54, 53–63. [Google Scholar] [CrossRef]
- Stanisavljevic, R.S.; Vuckovic, S.M.; Simic, A.S.; Marković, J.; Lakic, Z.P.; Terzic, D.V.; Dokic, D.J. Acid and Temperature Treatments Result in Increased Germination of Seeds of Three Fescue Species. Not. Bot. Horti Agrobot. Cluj Napoca 2012, 40, 220–226. [Google Scholar] [CrossRef]
- Figueiredo, M.A.; Baêta, H.E.; Kozovits, A.R. Germination of native grasses with potential application in the recovery of degraded areas in Quadrilátero Ferrífero, Brazil. Biota Neotrop. 2012, 12, 118–123. [Google Scholar] [CrossRef]
- Rubida, A.L.; Gutormson, T.J. Determining green needlegrass (Stipa viridula Trin.) seed viability. Newsl. Assoc. Off. Seed Anal. 1987, 61, 114–119. [Google Scholar]
- Sekutowski, T.R. Effect of chemical and physical factors on germination capacity of reed canary grass (Phalaris arundinacea L.) seed depending on storage time. Acta Agrobot. 2014, 67, 75–80. [Google Scholar] [CrossRef]
- Mehdadi, Z.; Benaouda, Z.; Latreche, A.; Benhassaini, H.; Bouchaour, I. Contribution to the study of the natural regeneration of Stipa tenacissima L. in the high steppe plains of Sidi Bel Abbes (Western Algeria). Secheresse 2004, 15, 167–171. [Google Scholar]
- Kildisheva, O.A. Improving the Outcomes of Seed-Based Restoration in Cold and Hot Deserts: An Investigation into Seed Dormancy, Germination, and Seed Enhancement. Ph.D. Thesis, University of Western Australia, Perth, Australia, 2019. [Google Scholar]
- Ling, E. Flash Flaming as a Scalable Seed Treatment Tool and Commercial Product; University of Western Australia: Perth, Australia, 2021. [Google Scholar]
- Flematti, G.R.; Dixon, K.W.; Smith, S.M. What are karrikins and how were they ‘discovered’ by plants? BMC Biol. 2015, 13, 108. [Google Scholar] [CrossRef]
- Merritt, D.J.; Turner, S.R.; Clarke, S.; Dixon, K.W. Seed dormancy and germination stimulation syndromes for Australian temperate species. Aust. J. Bot. 2007, 55, 336–344. [Google Scholar] [CrossRef]
- Erickson, T.; Barrett, R.; Merritt, D.; Dixon, K. Pilbara Seed Atlas and Field Guide: Plant Restoration in Australia’s Arid Northwest; CSIRO: Canberra, Australia, 2016.
- Turner, S.R.; Erickson, T.E.; Muñoz-Rojas, M.; Merritt, D.J. The Restoration Seed Bank initiative—A focus on biodiverse restoration in the semi-arid Pilbara of Western Australia. BGjournal 2016, 13, 20–23. [Google Scholar]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Australia, 2021. [Google Scholar]
- Ritz, C. Toward a unified approach to dose-response modeling in ecotoxicology. Environ. Toxicol. Chem. 2010, 29, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.F.; Mayer, D.G. Representing Cumulative Germination. 2. The Use of the Weibull Function and Other Empirically Derived Curves. Ann. Bot. 1988, 61, 127–138. [Google Scholar] [CrossRef]

| Species | Seed Treatment | Germination Temperatures | Growth Medium |
|---|---|---|---|
| Neurachne alopecuroidea | Florets Seeds | 15/7 °C (winter), 26/13 °C (spring), 33/18 °C (summer) | Water agar KAR1 agar |
| Rytidosperma caespitosum | |||
| Aristida inaequiglumis | Florets only | 39/25 °C (summer), 32/17 °C (autumn) | Water agar KAR1 agar |
| Chrysopogon fallax | Florets Seeds | ||
| Cymbopogon ambiguus | |||
| Cymbopogon obtectus | |||
| Eriachne obtusa | |||
| Eulalia aurea |
| Species | Flaming | Acid Digestion | Hydropriming | Combinations |
|---|---|---|---|---|
| Neurachne alopecuroidea | Continuous Intermittent | 50% (1 h) 75% (1 min 30 s) | 24 h 48 h | Flame (cont.) + Prime (24 h) Flame (cont.) + Prime (48 h) Acid (75%) + Prime (24 h) Acid (75%) + Prime (48 h) |
| Rytidosperma caespitosum | Continuous Intermittent | 50% (7 min) 75% (40 s) | 48 h | Flame (cont.) + Prime (48 h) Acid (75%) + Prime (48 h) |
| Aristida inaequiglumis | - | 75% (6 min) 80% (2 min 30 s) 90% (1 min 45 s) 100% (1 min) | - | - |
| Chrysopogon fallax | - | 75% (2 min 30 s) 100% (2 min 30 s) | - | - |
| Cymbopogon ambiguus | Continuous | 50% (8 min) 75% (1 min 30 s) | - | - |
| Cymbopogon obtectus | Continuous | 50% (7 min) 75% (1 min) | - | - |
| Eriachne obtusa | Continuous | 50% (2 min 30 s) 75% (30 s) | - | - |
| Eulalia aurea | Continuous | 50% (8 min) 75% (1 min 30 s) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berto, B.; Erickson, T.E.; Ritchie, A.L. Improving Seed Morphology and Germination Potential in Australian Native Grasses Using Seed Enhancement Technologies. Plants 2023, 12, 2432. https://doi.org/10.3390/plants12132432
Berto B, Erickson TE, Ritchie AL. Improving Seed Morphology and Germination Potential in Australian Native Grasses Using Seed Enhancement Technologies. Plants. 2023; 12(13):2432. https://doi.org/10.3390/plants12132432
Chicago/Turabian StyleBerto, Bianca, Todd E. Erickson, and Alison L. Ritchie. 2023. "Improving Seed Morphology and Germination Potential in Australian Native Grasses Using Seed Enhancement Technologies" Plants 12, no. 13: 2432. https://doi.org/10.3390/plants12132432
APA StyleBerto, B., Erickson, T. E., & Ritchie, A. L. (2023). Improving Seed Morphology and Germination Potential in Australian Native Grasses Using Seed Enhancement Technologies. Plants, 12(13), 2432. https://doi.org/10.3390/plants12132432





