Pimpinella anisum L. Essential Oil a Valuable Antibacterial and Antifungal Alternative
Abstract
1. Introduction
- a.
- Antibacterial, antifungal action
- b.
- Anticonvulsant action
- c.
- Action on the digestive system
- d.
- Analgesic and anti-inflammatory action
- e.
- Antioxidant action
- f.
- Acaricidal activity
- g.
- Antitumor activity
- h.
- Neural activity
2. Results
2.1. Phytochemical Compounds Identification
2.2. Antimicrobial Activity
2.2.1. Streptococcus Pyogenes
2.2.2. Pseudomonas Aeruginosa
2.2.3. Staphylococcus Aureus
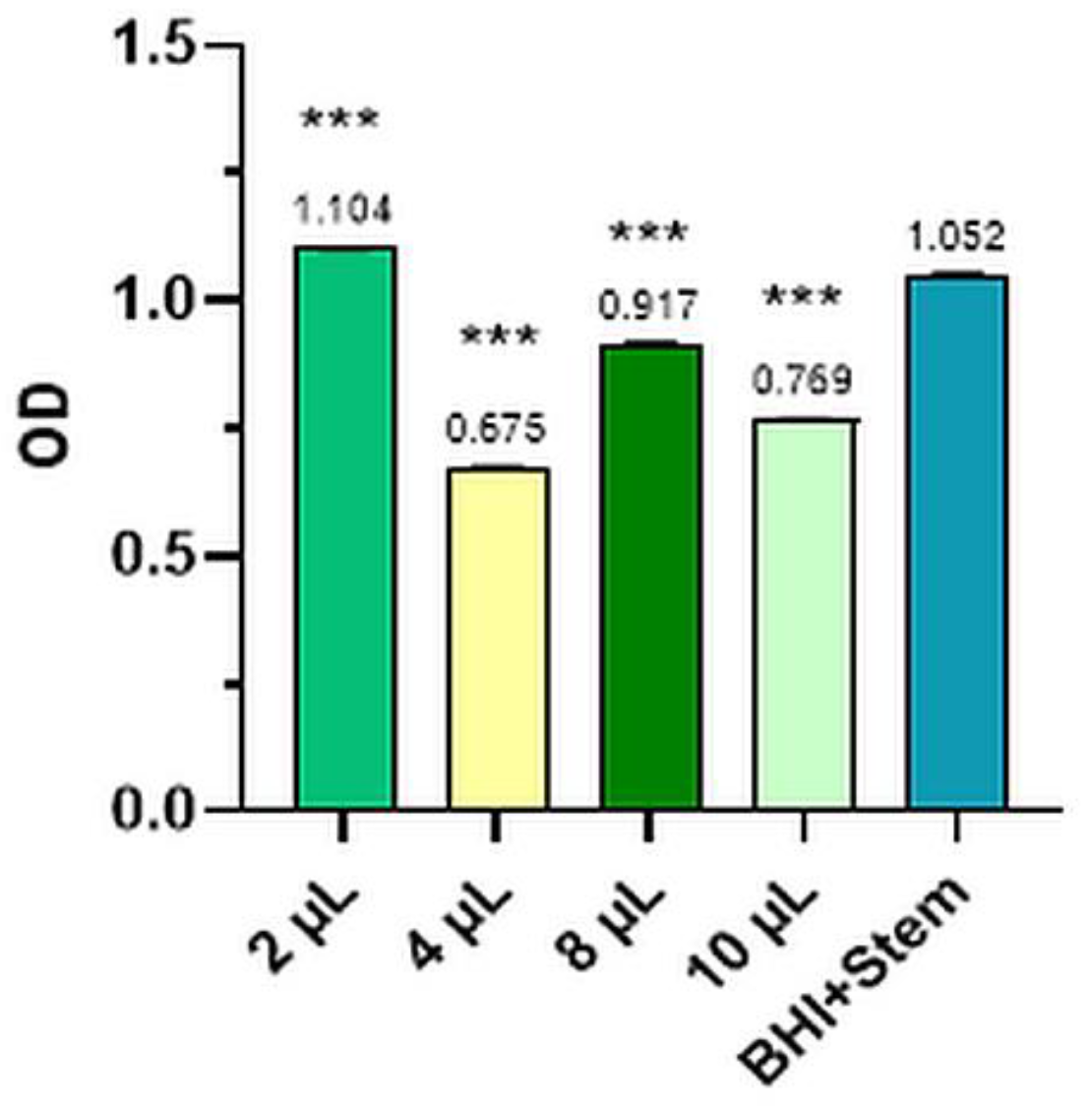
2.2.4. Escherichia Coli
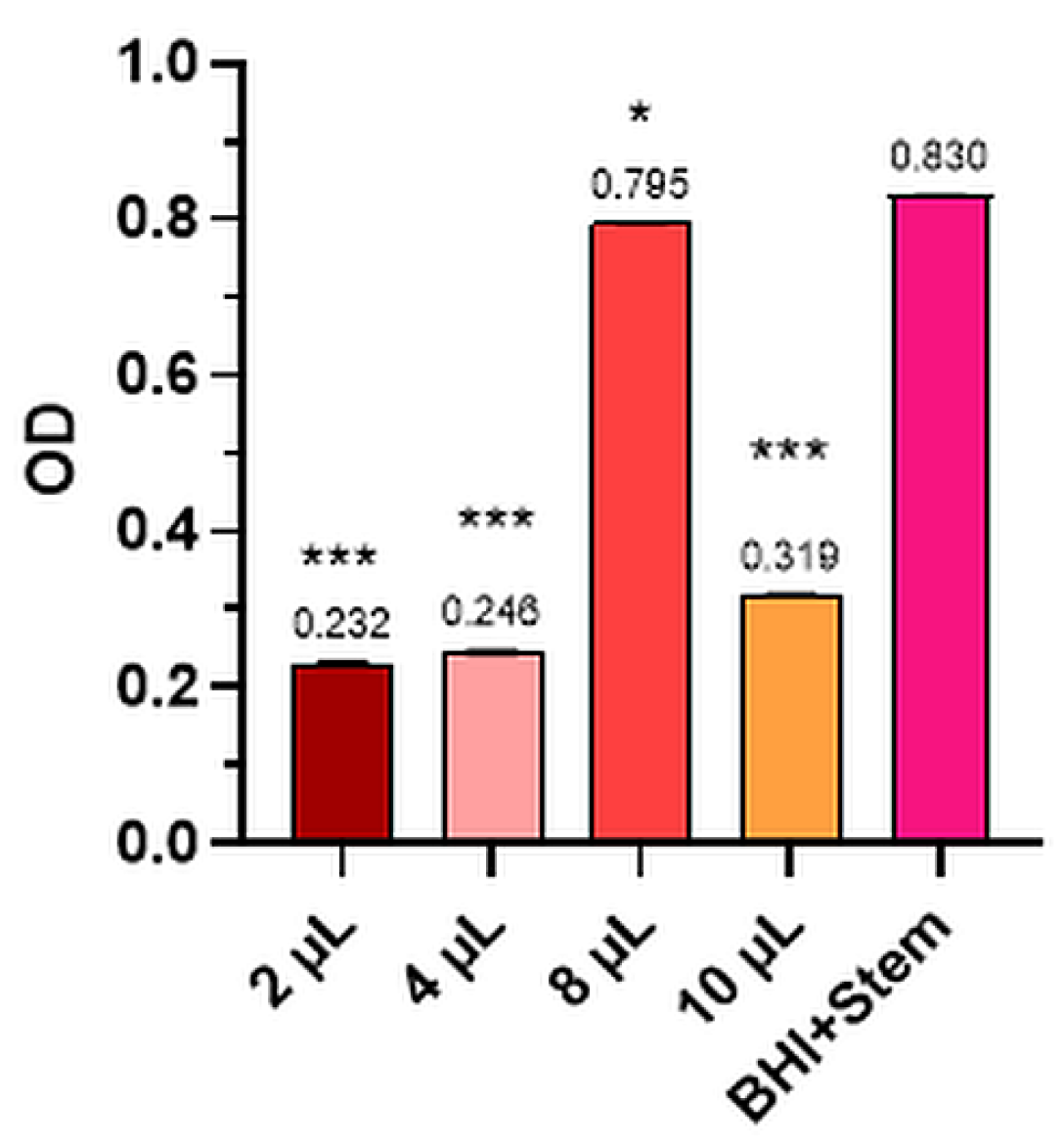
2.2.5. Candida Albicans
3. Discussion
- Volatile fraction: constituting 90–95% of the oil and containing monoterpene and sesquiterpene hydrocarbons with known derivatives (i.e., oxygenated, aliphatic aldehydes, alcohols, and esters fractions).
- The non-volatile residues: comprising only 1–10% of the oil, containing hydrocarbons, fatty acids, sterols, carotenoids, wax, and flavonoids [37].
4. Materials and Methods
4.1. The GC/MS Compounds Analysis in P. anisum Essential Oil
4.2. Determination of Antimicrobial Activity of P. anisum Essential Oils
Broth Micro Dilution Method
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saibi, S.; Belhadj, M.; Benyoussef, E.H. Essential oil composition of Pimpinella anisum from Algeria. Ann. Chem. Lett. 2013, 2, 401–404. [Google Scholar] [CrossRef]
- Muselin, F. Noţiuni de Biologia Plantelor Pentru uz Veterinar; Mirton Publisher: Timisoara, Romania, 2016; ISBN 978-973-52-1611-5. (In Romanian) [Google Scholar]
- Eugenia, D.; Cristina, R.T. Elemente de Terapie Alternativă şi Complementară în Medicina Veterinară; Solness Publisher: Timisoara, Romania, 2015; ISBN (13)978-973-729-451-7. (In Romanian) [Google Scholar]
- Sayed-Ahmad, B.; Talou, T.; Saad, Z.; Hijazi, A.; Merah, O. The Apiaceae: Ethnomedicinal family as source for industrial uses. Ind. Crops Prod. 2017, 109, 661–671. [Google Scholar] [CrossRef]
- Rebey, I.B.; Wannes, W.A.; BenKaab, S.; Bourgou, S.; Saidani Tounsi, M.; Ksouri, R.; Fauconnier, M.L. Bioactive compounds and antioxidant activity of Pimpinella anisum L. accessions at different ripening stages. Sci. Hort. 2019, 246, 453–461. [Google Scholar] [CrossRef]
- Omidbaigi, R.; Hadjiakhoondi, A.; Saharkhiz, M. Changes in content and chemical composition of Pimpinella anisum L. oil at various harvest time. J. Essent. Oil Bear. 2003, 6, 46–50. [Google Scholar] [CrossRef]
- Orav, A.; Raal, A.; Arak, E. Essential oil composition of Pimpinella anisum L. fruits from various European countries. Nat. Prod. Res. 2008, 22, 227–232. [Google Scholar] [CrossRef]
- Arslan, N.; Gürbüz, B.; Bayrak, A.; Gümüscü, A. Variation in essential oil content and composition in Turkish anise (Pimpinella anisum L.) populations. Turk. J. Agric. Forest. 2004, 28, 173–177. Available online: https://journals.tubitak.gov.tr/agriculture/vol28/iss3/4 (accessed on 27 April 2023).
- Ozcan, M.M.; Chalchat, J.C. Chemical composition and antifungal effect of anise (Pimpinella anisum L.) fruit oil at ripening stage. Ann. Microbiol. 2006, 56, 353–358. [Google Scholar] [CrossRef]
- Rodrigues, V.M.; Rosa, P.T.V.; Marques, M.O.M.; Petenate, A.J.; Meireles, M.A.A. Supercritical extraction of essential oil from aniseed (Pimpinella anisum L.) using CO2: Solubility, kinetics, and composition data. J. Agric. Food Chem. 2003, 51, 1518–1523. [Google Scholar] [CrossRef]
- Tabanca, N.; Demirci, B.; Özek, T.; Kirimer, N.; Baser, K.H.C.; Bedir, E.; Khan, I.K.; Wedge, D.E. Gas chromatographic-mass spectrometric analysis of essential oils from Pimpinella species gathered from Central and Northern Turkey. J. Chromat A 2006, 1117, 194–205. [Google Scholar] [CrossRef]
- Tort, N.; Honermeier, B. Investigation on the ratio of methylchavicol and transanethole components in essential oil of anis (Pimpinella anisum L.) from different regions of Turkey. Asian J. Chem. 2005, 17, 2365–2370. Available online: https://asianpubs.org/index.php/ajchem/article/view/13980/13954 (accessed on 27 April 2023).
- Hekmat AL-Hmadi, H.; El Mokni, R.; Joshi, R.K.; Ashour, M.L.; Hammami, S. The Impact of Geographical Location on the Chemical Compositions of Pimpinella lutea Desf. Growing in Tunisia. Appl. Sci. 2021, 11, 7739. [Google Scholar] [CrossRef]
- Koeduka, T.; Baiga, T.J.; Noel, J.P.; Pichersky, E. Biosynthesis of t-anethole in anise: Characterization of t-anol/isoeugenol synthase and an O-methyltransferase specific for a C7-C8 propenyl side chain. Plant. Physiol. 2009, 149, 384–394. [Google Scholar] [CrossRef]
- Lavaee, F.; Moqadas, A.; Modarresi, F.; Nowrouzi, M. The Effect of Pimpinella anisum and Origanum vulgare Extracts Against Streptococcus sanguinis, Streptococcus mutans, and Streptococcus salivarius. J. Dent. Shiraz 2022, 23, 113–120. [Google Scholar] [CrossRef]
- Al-Balawi, A.N.; Elmetwalli, A.; Baraka, D.M.; Alnagar, H.A.; Alamri, E.S.; Hassan, M.G. Chemical Constituents, Antioxidant Potential, and Antimicrobial Efficacy of Pimpinella anisum Extracts against Multidrug-Resistant Bacteria. Microorganisms 2023, 11, 1024. [Google Scholar] [CrossRef]
- Gulcin, I.; Oktay, M.; Kirecci, E.; Kufrevioglu, O.I. Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts. Food Chem. 2003, 83, 371–382. [Google Scholar] [CrossRef]
- Abdel-Reheem, M.A.T.; Oraby, M.M. Antimicrobial, cytotoxicity, and necrotic ripostes of Pimpinella anisum essential oil. Ann. Agric. Sci. 2015, 60, 335–340. [Google Scholar] [CrossRef]
- Al-Bayati, F.A. Synergistic antibacterial activity between Thymus vulgaris and Pimpinella anisum essential oils and methanol extracts. J. Ethnopharmacol. 2008, 116, 403–406. [Google Scholar] [CrossRef]
- Sukhdev, S.H.; Suman, P.S.K.; Gennaro, L.; Dev, D.R. Extraction Technologies for Medicinal and Aromatic Plants, International Centre for Science and High Technology. 2004, Italy. Available online: https://www.unido.org/sites/default/files/2009-10/Extraction_technologies_for_medicinal_and_aromatic_plants_0.pdf (accessed on 27 April 2023).
- Kosalec, I.; Pepeljnjak, S.; Kuatrak, D. Antifungal activity of fluid extract and essential oil from anise fruits (Pimpinella anisum L., Apiaceae). Acta Pharmaceutica 2005, 55, 377–385. [Google Scholar]
- Puvaca, N.; Milenkovic, J.; Galonja Coghill, T.; Bursic, V.; Petrovic, A.; Tanaskovic, S.; Pelic, M.; Ljubojevic-Pelic, D.; Miljkovic, T. Antimicrobial Activity of Selected Essential Oils against Selected Pathogenic Bacteria: In Vitro Study. Antibiotics 2021, 10, 546. [Google Scholar] [CrossRef]
- Dumitrescu, E.; Muselin, F.; Dumitrescu, C.S.; Orasan-Alic, S.A.; Moruzi, R.F.; Doma, A.O.; Mohamed, A.E.; Cristina, R.T. Juniper communis L. Essential Oils from Western Romanian Carpathians: Bio-Structure and Effective Antibacterial Activity. Appl. Sci. 2022, 12, 2949. [Google Scholar] [CrossRef]
- Pourgholami, M.H.; Majzoob, S.; Javadi, M.; Kamalinejad, M.; Fanaee, G.H.; Sayyah, M. The fruit essential oil of Pimpinella anisum exerts anticonvulsant effects in mice. J. Ethnopharmacol. 1999, 66, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Al-Mofleh, I.A.; Alhalder, A.A.; Mossa, J.S.; Al-Soohalbani, M.O.; Rafatullah, S. Aqueous suspension of anise Pimpinella anisum protects rats against chemically induced gastric ulcers. World J. Gastroenterol. 2007, 13, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Iannarelli, R.; Marinelli, O.; Morelli, M.B.; Santoni, G.; Amantini, C.; Nabissi, M.; Maggi, F. Aniseed (Pimpinella anisum L.) essential oil reduces pro-inflammatory cytokines and stimulates mucus secretion in primary airway bronchial and tracheal epithelial cell lines. Ind. Crops Prod. 2018, 114, 81–86. [Google Scholar] [CrossRef]
- Tas, A.; Özbe, H.; Atasoy, N.; Altug, M.E.; Ceylan, E. Evaluation of analgesic and antiinflammatory activity of Pimpinella anisum fixed oil extract. Indian. Vet. J. 2006, 83, 840–843. Available online: https://www.researchgate.net/publication/236232426_Evaluation_of_analgesic_and_antiinflammatory_activity_of_Pimpinella_anisum_fixed_oil_extract (accessed on 27 April 2023).
- Tas, A. Analgesic effect of Pimpinella anisum L. essential oil extract in mice. Indian. Vet. J. 2009, 86, 145–147. Available online: https://www.researchgate.net/publication/287854483_Analgesic_effect_of_Pimpinella_anisum_L_essential_oil_extract_in_mice (accessed on 27 April 2023).
- Lee, H.S. p-anisaldehyde: Acaricidal component of Pimpinella anisum seed oil against the house dust mites Dermatophagoides farinae and Dermatophagoides pteronyssinus. Planta Med. 2004, 70, 279–281. [Google Scholar] [CrossRef]
- Samaneh, R.H.; Malek, H.A. Anti-proliferative effect of the extracts and essential oil of Pimpinella anisum on gastric cancer cells. J. Herbmed Pharmacol. 2016, 5, 157–161. Available online: http://herbmedpharmacol.com/Article/JHP_1049_20160707042447 (accessed on 27 April 2023).
- Kahloula, K.; Slimani, M.; Houari Adli, D.E.; Rachdi, S.; Boumediene, D. Neuro beneficial effects of Pimpinella anisum against lead exposure. Int. J. Green. Pharm. 2013, 7, 18–24. [Google Scholar] [CrossRef]
- Acimovic, G.; Korac, J.; Jacimovic, G.; Oljaca, S.; Djukanovic, L.; Vuga-Janjatov, V. Influence of ecological conditions on seeds traits and essential oil contents in anise (Pimpinella anisum L.). Not. Bot. Horti Agrobot. Cluj. Napoca 2014, 42, 232–238. [Google Scholar] [CrossRef]
- Gende, L.B.; Maggi, M.D.; Fritz, R.; Eguaras, M.J.; Bailac, P.N.; Ponzi, M.I. Antimicrobial activity of Pimpinella anisum and Foeniculum vulgare essential oils against Paenibacillus larvae. J. Essential Oil Res. 2009, 21, 91–93. [Google Scholar] [CrossRef]
- Ullah, H.; Mahmood, A.; Ijaz, M.; Tadesse, B.; Honermeier, B. Evaluation of anise (Pimpinella anisum L.) accessions with regard to morphological characteristics, fruit yield, oil contents and composition. J. Med. Plants Res. 2013, 7, 2177–2186. [Google Scholar] [CrossRef]
- Hasimi, A.; Tolan, V.; Kizil, S.; Kilinc, E. Determination of essential oil composition, antimicrobial and antioxidant properties of anise (Pimpinella anisum L.) and cumin (Cuminum cyminum L.) seeds. J. Agric. Sci. 2014, 20, 19–26. [Google Scholar] [CrossRef]
- Mohammed, S.; Albulushi, A.; Al Saidi, H.; Amaresh, N.; Mullaicharam, A.R. Study of physicochemical properties, antibacterial and GC-MS analysis of essential oil of the aniseed (Pimpinella anisum Linn.) in Oman. J. Pharmacogn. Phytochem. 2014, 2, 24–33. [Google Scholar]
- Faleiro, M.L. The mode of antibacterial action of essential oils. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Méndez-Vilas, A., Ed.; Formatex: Badajoz, Spain, 2011; pp. 1143–1156. Available online: https://dsagrow.com/wp-content/uploads/2020/05/The-mode-of-antibacterial-action-of-essential-oils.pdf (accessed on 27 April 2023).
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Shukla, H.S.; Tripathi, S.C. Antifungal substance in the essential oil of anise (Pimpinella anisum L.). Agric. Biol. Chem. 1987, 51, 1991–1993. Available online: https://www.jstage.jst.go.jp/article/bbb1961/51/7/51_7_1991/_pdf (accessed on 27 April 2023). [CrossRef]
- Lis-Balchin, M.; Deans, S.G.; Eaglesham, E. Relationship between bioactivity and chemical composition of commercial essential oils. Flav Frag. J. 1998, 13, 98–104. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Shojaii, A.; Abdollahi Fard, M. Review of Pharmacological Properties and Chemical Constituents of Pimpinella anisum. Int. Sch. Res. Not. 2012, 2012, 510795. [Google Scholar] [CrossRef]



| Compound | R. Time | % |
|---|---|---|
| α-pinene | 6.382 | 3.263 |
| 3-carene | 9.847 | 1.327 |
| α-phellandrene | 10.309 | 1.822 |
| Limonene | 11.292 | 10.011 |
| β-terpinen | 11.548 | 2.511 |
| 1-pentanone, 1-(4-methylphenyl) | 13.259 | 0.179 |
| Linalool | 20.840 | 1.962 |
| Anisole | 23.697 | 5.003 |
| Propenyl-anisole | 27.360 | 72.499 |
| Anisaldehyde | 31.552 | 0.326 |
| p-(pentyloxy) acetophenone | 34.046 | 0.089 |
| Thymol | 34.583 | 0.580 |
| 1-(3-methyl-2-butenoxy)-4-(1-propenyl) benzene | 36.192 | 0.429 |
| Streptococcus pyogenes | Concentration /Replica | I | II | III | x¯ | SEM | Inhibition(%) |
| BHI+strain | 0.786 | 0.787 | 0.786 | 0.7864 | 0.0005 | 100.00 | |
| 10 µL | 0.465 | 0.466 | 0.464 | 0.465 | 0.0003 | 40.86% | |
| 8 µL | 0.593 | 0.591 | 0.594 | 0.5928 | 0.0005 | 24.61% | |
| 4 µL | 0.714 | 0.712 | 0.714 | 0.7132 | 0.0007 | 9.3% | |
| 2 µL | 0.722 | 0.724 | 0.721 | 0.7218 | 0.0006 | 8.21% | |
| Pseudomonas aeruginosa | BHI+strain | 0.368 | 0.370 | 0.371 | 0.3697 | 0.0008 | 100.00 |
| 10 µL | 0.274 | 0.268 | 0.276 | 0.2726 | 0.0019 | 26.28% | |
| 8 µL | 0.777 | 0.775 | 0.778 | 0.776 | 0.0008 | 109.84 | |
| 4 µL | 0.184 | 0.189 | 0.186 | 0.1864 | 0.0010 | 49.59 | |
| 2 µL | 0.331 | 0.332 | 0.334 | 0.3324 | 0.0008 | 10.11 | |
| Staphylococcus aureus | BHI+strain | 0.496 | 0.495 | 0.495 | 0.4954 | 0.0006 | 100.00 |
| 10 µL | 0.292 | 0.289 | 0.291 | 0.2906 | 0.0009 | 41.18 | |
| 8 µL | 0.459 | 0.458 | 0.461 | 0.4594 | 0.0006 | 7.26 | |
| 4 µL | 0.366 | 0.365 | 0.363 | 0.3646 | 0.0010 | 26.44 | |
| 2 µL | 0.362 | 0.360 | 0.366 | 0.3626 | 0.0013 | 26.71 | |
| Escherichia coli | BHI+strain | 1.053 | 1.050 | 1.052 | 1.052 | 0.0008 | 100.00 |
| 10 µL | 0.769 | 0.767 | 0.771 | 0.769 | 0.0008 | 26.90 | |
| 8 µL | 0.918 | 0.919 | 0.916 | 0.917 | 0.0010 | 12.77 | |
| 4 µL | 0.678 | 0.674 | 0.673 | 0.675 | 0.0009 | 35.80 | |
| 2 µL | 1.106 | 1.101 | 1.105 | 1.104 | 0.0009 | 4.92 | |
| Candida albicans | BHI+strain | 0.831 | 0.832 | 0.828 | 0.8304 | 0.0007 | 100.00 |
| 10 µL | 0.319 | 0.317 | 0.320 | 0.3186 | 0.0008 | 61.60 | |
| 8 µL | 0.796 | 0.795 | 0.795 | 0.7953 | 0.0007 | 4.23 | |
| 4 µL | 0.244 | 0.244 | 0.247 | 0.245 | 0.0027 | 70.42 | |
| 2 µL | 0.234 | 0.233 | 0.230 | 0.2323 | 0.0007 | 72.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumitrescu, E.; Muselin, F.; Tîrziu, E.; Folescu, M.; Dumitrescu, C.S.; Orboi, D.M.; Cristina, R.T. Pimpinella anisum L. Essential Oil a Valuable Antibacterial and Antifungal Alternative. Plants 2023, 12, 2428. https://doi.org/10.3390/plants12132428
Dumitrescu E, Muselin F, Tîrziu E, Folescu M, Dumitrescu CS, Orboi DM, Cristina RT. Pimpinella anisum L. Essential Oil a Valuable Antibacterial and Antifungal Alternative. Plants. 2023; 12(13):2428. https://doi.org/10.3390/plants12132428
Chicago/Turabian StyleDumitrescu, Eugenia, Florin Muselin, Emil Tîrziu, Mihai Folescu, Carmen S. Dumitrescu, Dora M. Orboi, and Romeo T. Cristina. 2023. "Pimpinella anisum L. Essential Oil a Valuable Antibacterial and Antifungal Alternative" Plants 12, no. 13: 2428. https://doi.org/10.3390/plants12132428
APA StyleDumitrescu, E., Muselin, F., Tîrziu, E., Folescu, M., Dumitrescu, C. S., Orboi, D. M., & Cristina, R. T. (2023). Pimpinella anisum L. Essential Oil a Valuable Antibacterial and Antifungal Alternative. Plants, 12(13), 2428. https://doi.org/10.3390/plants12132428









