Silicon in Plants: Alleviation of Metal(loid) Toxicity and Consequential Perspectives for Phytoremediation
Abstract
1. Introduction
2. Silicon Uptake and Accumulation in Plants
3. Silicon-Mediated Metal(loid) Toxicity Alleviation in Plants
4. Metal(loid) Accumulation in Phytoliths
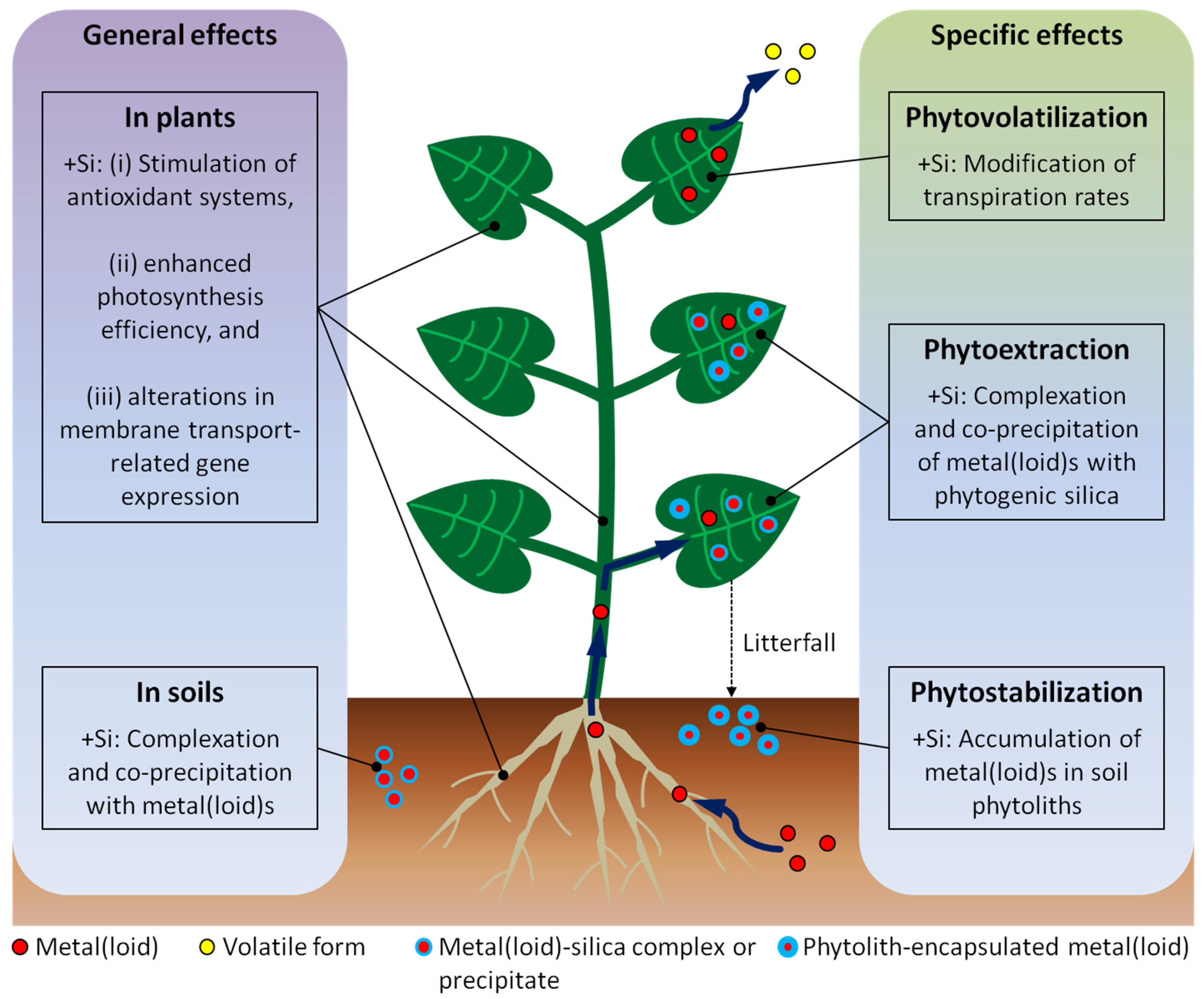
5. Consequential Perspectives for Phytoremediation
6. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ehrlich, H.; Demadis, K.D.; Pokrovsky, O.S.; Koutsoukos, P.G. Modern views on desilicification: Biosilica and abiotic silica dissolution in natural and artificial environments. Chem. Rev. 2010, 110, 4656–4689. [Google Scholar] [CrossRef] [PubMed]
- Puppe, D. Review on protozoic silica and its role in silicon cycling. Geoderma 2020, 365, 114224. [Google Scholar] [CrossRef]
- Hodson, M.J. The development of phytoliths in plants and its influence on their chemistry and isotopic composition. Implications for palaeoecology and archaeology. J. Archaeol. Sci. 2016, 68, 62–69. [Google Scholar] [CrossRef]
- Sangster, A.; Hodson, M.; Tubb, H. Silicon deposition in higher plants. In Studies in Plant Science; Elsevier: Amsterdam, The Netherlands, 2001; Volume 8, pp. 85–113. [Google Scholar]
- Kameník, J.; Mizera, J.; Řanda, Z. Chemical composition of plant silica phytoliths. Environ. Chem. Lett. 2013, 11, 189–195. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Wang, H.; Wang, C. The effects of chemical composition and distribution on the preservation of phytolith morphology. Appl. Phys. A 2014, 114, 503–507. [Google Scholar] [CrossRef]
- Hodson, M.J. The Relative Importance of Cell Wall and Lumen Phytoliths in Carbon Sequestration in Soil: A Hypothesis. Front. Earth Sci. 2019, 7, 167. [Google Scholar] [CrossRef]
- Epstein, E. Silicon. Annu. Rev. Plant Biol. 1999, 50, 641–664. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef]
- Puppe, D.; Sommer, M. Experiments, Uptake Mechanisms, and Functioning of Silicon Foliar Fertilization—A Review Focusing on Maize, Rice, and Wheat; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–49. [Google Scholar]
- Pourret, O.; Bollinger, J.-C.; Hursthouse, A. Heavy metal: A misused term? Acta Geochim. 2021, 40, 466–471. [Google Scholar] [CrossRef]
- Clemens, S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 2006, 88, 1707–1719. [Google Scholar] [CrossRef]
- Suman, J.; Uhlik, O.; Viktorova, J.; Macek, T. Phytoextraction of heavy metals: A promising tool for clean-up of polluted environment? Front. Plant Sci. 2018, 9, 1476. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Ali, Q.; Zahir, Z.A.; Ashraf, S.; Asghar, H.N. Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 714–727. [Google Scholar] [CrossRef]
- Berti, W.R.; Cunningham, S.D. Phytostabilization of metals. In Phytoremediation of Toxic Metals: Using Plants to Clean up the Environment; Wiley: New York, NY, USA, 2000; pp. 71–88. [Google Scholar]
- Mendez, M.O.; Maier, R.M. Phytoremediation of mine tailings in temperate and arid environments. Rev. Environ. Sci. Bio/Technol. 2007, 7, 47–59. [Google Scholar] [CrossRef]
- Wang, L.; Ji, B.; Hu, Y.; Liu, R.; Sun, W. A review on in situ phytoremediation of mine tailings. Chemosphere 2017, 184, 594–600. [Google Scholar] [CrossRef]
- Das, P.K.; Das, B.P.; Dash, P. Chromite mining pollution, environmental impact, toxicity and phytoremediation: A review. Environ. Chem. Lett. 2020, 19, 1369–1381. [Google Scholar] [CrossRef]
- Sharma, J.K.; Kumar, N.; Singh, N.P.; Santal, A.R. Phytoremediation technologies and their mechanism for removal of heavy metal from contaminated soil: An approach for a sustainable environment. Front. Plant Sci. 2023, 14, 1076876. [Google Scholar] [CrossRef]
- Koopmans, G.F.; Römkens, P.F.A.M.; Song, J.; Temminghoff, E.J.M.; Japenga, J. Predicting the Phytoextraction Duration to Remediate Heavy Metal Contaminated Soils. Water Air Soil Pollut. 2007, 181, 355–371. [Google Scholar] [CrossRef]
- Cunningham, S.D.; Berti, W.R.; Huang, J.W. Phytoremediation of contaminated soils. Trends Biotechnol. 1995, 13, 393–397. [Google Scholar] [CrossRef]
- Muszynska, E.; Hanus-Fajerska, E. Why are heavy metal hyperaccumulating plants so amazing? BioTechnol. J. Biotechnol. Comput. Biol. Bionanotechnol. 2015, 96, 265–271. [Google Scholar] [CrossRef]
- Reeves, R.D.; Baker, A.J.; Jaffré, T.; Erskine, P.D.; Echevarria, G.; van Der Ent, A. A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytol. 2018, 218, 407–411. [Google Scholar] [CrossRef]
- Krämer, U. Metal hyperaccumulation in plants. Annu. Rev. Plant Biol. 2010, 61, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Schaller, J.; Puppe, D.; Kaczorek, D.; Ellerbrock, R.; Sommer, M. Silicon Cycling in Soils Revisited. Plants 2021, 10, 295. [Google Scholar] [CrossRef] [PubMed]
- Hodson, M.J.; White, P.J.; Mead, A.; Broadley, M.R. Phylogenetic variation in the silicon composition of plants. Ann. Bot. 2005, 96, 1027–1046. [Google Scholar] [CrossRef]
- Ma, J.F.; Takahashi, E. Soil, Fertilizer, and Plant Silicon Research in Japan; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Ma, J.F.; Yamaji, N. A cooperative system of silicon transport in plants. Trends Plant Sci. 2015, 20, 435–442. [Google Scholar] [CrossRef]
- Yamaji, N.; Sakurai, G.; Mitani-Ueno, N.; Ma, J.F. Orchestration of three transporters and distinct vascular structures in node for intervascular transfer of silicon in rice. Proc. Natl. Acad. Sci. USA 2015, 112, 11401–11406. [Google Scholar] [CrossRef]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Belanger, R.R. The controversies of silicon’s role in plant biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Exley, C. A possible mechanism of biological silicification in plants. Front. Plant Sci. 2015, 6, 853. [Google Scholar] [CrossRef]
- Exley, C.; Guerriero, G.; Lopez, X. How is silicic acid transported in plants? Silicon 2020, 12, 2641–2645. [Google Scholar] [CrossRef]
- Zexer, N.; Kumar, S.; Elbaum, R. Silica deposition in plants: Scaffolding the mineralization. Ann. Bot. 2023, mcad056. [Google Scholar] [CrossRef]
- Piperno, D.R. The occurrence of phytoliths in the reproductive structures of selected tropical angiosperms and their significance in tropical paleoecology, paleoethnobotany and systematics. Rev. Palaeobot. Palynol. 1989, 61, 147–173. [Google Scholar] [CrossRef]
- Watteau, F.; Villemin, G. Ultrastructural study of the biogeochemical cycle of silicon in the soil and litter of a temperate forest. Eur. J. Soil Sci. 2001, 52, 385–396. [Google Scholar] [CrossRef]
- Puppe, D.; Kaczorek, D.; Schaller, J. Biological impacts on silicon availability and cycling in agricultural plant-soil systems. In Silicon and Nano-Silicon in Environmental Stress Management and Crop Quality Improvement; Elsevier: Amsterdam, The Netherlands, 2022; pp. 309–324. [Google Scholar]
- Lux, A.; Lukacova, Z.; Vaculik, M.; Svubova, R.; Kohanova, J.; Soukup, M.; Martinka, M.; Bokor, B. Silicification of Root Tissues. Plants 2020, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Soukup, M.; Elbaum, R. Silicification in Grasses: Variation between Different Cell Types. Front. Plant Sci. 2017, 8, 438. [Google Scholar] [CrossRef]
- International Committee for Phytolith Taxonomy. International Code for Phytolith Nomenclature (ICPN) 2.0. Ann. Bot. 2019, 124, 189–199. [Google Scholar] [CrossRef]
- Blecker, S.W.; McCulley, R.L.; Chadwick, O.A.; Kelly, E.F. Biologic cycling of silica across a grassland bioclimosequence. Glob. Biogeochem. Cycles 2006, 20. [Google Scholar] [CrossRef]
- Alexandre, A.; Bouvet, M.; Abbadie, L. The role of savannas in the terrestrial Si cycle: A case-study from Lamto, Ivory Coast. Glob. Planet. Change 2011, 78, 162–169. [Google Scholar] [CrossRef]
- Bartoli, F. The biogeochemical cycle of silicon in two temperate forest ecosystems. Ecol. Bull. 1983, 35, 469–476. [Google Scholar]
- Cornelis, J.-T.; Ranger, J.; Iserentant, A.; Delvaux, B. Tree species impact the terrestrial cycle of silicon through various uptakes. Biogeochemistry 2010, 97, 231–245. [Google Scholar] [CrossRef]
- Sommer, M.; Jochheim, H.; Höhn, A.; Breuer, J.; Zagorski, Z.; Busse, J.; Barkusky, D.; Meier, K.; Puppe, D.; Wanner, M.; et al. Si cycling in a forest biogeosystem—The importance of transient state biogenic Si pools. Biogeosciences 2013, 10, 4991–5007. [Google Scholar] [CrossRef]
- Keller, C.; Guntzer, F.; Barboni, D.; Labreuche, J.; Meunier, J.-D. Impact of agriculture on the Si biogeochemical cycle: Input from phytolith studies. Comptes Rendus Geosci. 2012, 344, 739–746. [Google Scholar] [CrossRef]
- Savant, N.K.; Korndörfer, G.H.; Datnoff, L.E.; Snyder, G.H. Silicon nutrition and sugarcane production: A review. J. Plant Nutr. 1999, 22, 1853–1903. [Google Scholar] [CrossRef]
- Savant, N.K.; Snyder, G.H.; Datnoff, L.E. Silicon Management and Sustainable Rice Production. Adv. Agron. 1996, 58, 151–199. [Google Scholar]
- Struyf, E.; Smis, A.; Van Damme, S.; Garnier, J.; Govers, G.; Van Wesemael, B.; Conley, D.J.; Batelaan, O.; Frot, E.; Clymans, W.; et al. Historical land use change has lowered terrestrial silica mobilization. Nat. Commun. 2010, 1, 129. [Google Scholar] [CrossRef]
- Vandevenne, F.; Struyf, E.; Clymans, W.; Meire, P. Agricultural silica harvest: Have humans created a new loop in the global silica cycle? Front. Ecol. Environ. 2012, 10, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Meena, V.; Dotaniya, M.; Coumar, V.; Rajendiran, S.; Kundu, S.; Subba Rao, A. A case for silicon fertilization to improve crop yields in tropical soils. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2014, 84, 505–518. [Google Scholar] [CrossRef]
- Das, S.; Galgo, S.J.; Alam, M.A.; Lee, J.G.; Hwang, H.Y.; Lee, C.H.; Kim, P.J. Recycling of ferrous slag in agriculture: Potentials and challenges. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1247–1281. [Google Scholar] [CrossRef]
- Puppe, D.; Kaczorek, D.; Schaller, J.; Barkusky, D.; Sommer, M. Crop straw recycling prevents anthropogenic desilication of agricultural soil–plant systems in the temperate zone—Results from a long-term field experiment in NE Germany. Geoderma 2021, 403, 115187. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Rizwan, M.; Zia-Ur-Rehman, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Qayyum, M.F.; Irshad, M.K. Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: A review. Ecotoxicol. Environ. Saf. 2015, 119, 186–197. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Rizwan, M.; Ali, S.; Hassan, M.J.; Brestic, M.; Zhang, X.; Huang, L. Effects of silicon on heavy metal uptake at the soil-plant interphase: A review. Ecotoxicol. Environ. Saf. 2021, 222, 112510. [Google Scholar] [CrossRef]
- Schindler, P.; Fürst, B.; Dick, R.; Wolf, P. Ligand properties of surface silanol groups. I. Surface complex formation with Fe3+, Cu2+, Cd2+, and Pb2+. J. Colloid Interface Sci. 1976, 55, 469–475. [Google Scholar] [CrossRef]
- Gu, H.-H.; Qiu, H.; Tian, T.; Zhan, S.-S.; Chaney, R.L.; Wang, S.-Z.; Tang, Y.-T.; Morel, J.-L.; Qiu, R.-L. Mitigation effects of silicon rich amendments on heavy metal accumulation in rice (Oryza sativa L.) planted on multi-metal contaminated acidic soil. Chemosphere 2011, 83, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Yates, D.M.; Joyce, K.J.; Heaney, P.J. Complexation of copper with polymeric silica in aqueous solution. Appl. Geochem. 1998, 13, 235–241. [Google Scholar] [CrossRef]
- Stein, M.; Georgiadis, A.; Gudat, D.; Rennert, T. Formation and properties of inorganic Si-contaminant compounds. Environ. Pollut. 2020, 265, 115032. [Google Scholar] [CrossRef]
- Stein, M.; Georgiadis, A.; Ingwersen, J.; Rennert, T. Does silica addition affect translocation and leaching of cadmium and copper in soil? Environ. Pollut. 2021, 288, 117738. [Google Scholar] [CrossRef]
- Da Cunha, K.P.V.; do Nascimento, C.W.A. Silicon Effects on Metal Tolerance and Structural Changes in Maize (Zea mays L.) Grown on a Cadmium and Zinc Enriched Soil. Water Air Soil Pollut. 2009, 197, 323–330. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Luo, C.; Liu, X.; Wang, S.; Liu, T.; Li, X. Foliar application of two silica sols reduced cadmium accumulation in rice grains. J. Hazard. Mater. 2009, 161, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Cai, H.; He, C.; Zhang, W.; Wang, L. A hemicellulose-bound form of silicon inhibits cadmium ion uptake in rice (Oryza sativa) cells. New Phytol. 2015, 206, 1063–1074. [Google Scholar] [CrossRef]
- Wang, S.; Wang, F.; Gao, S. Foliar application with nano-silicon alleviates Cd toxicity in rice seedlings. Environ. Sci. Pollut. Res. Int. 2015, 22, 2837–2845. [Google Scholar] [CrossRef]
- Wang, S.; Wang, F.; Gao, S.; Wang, X. Heavy Metal Accumulation in Different Rice Cultivars as Influenced by Foliar Application of Nano-silicon. Water Air Soil Pollut. 2016, 227, 228. [Google Scholar] [CrossRef]
- Wang, M.; Wang, R.; Mur, L.A.J.; Ruan, J.; Shen, Q.; Guo, S. Functions of silicon in plant drought stress responses. Hortic. Res. 2021, 8, 254. [Google Scholar] [CrossRef]
- Hussain, I.; Ashraf, M.A.; Rasheed, R.; Asghar, A.; Sajid, M.A.; Iqbal, M. Exogenous application of silicon at the boot stage decreases accumulation of cadmium in wheat (Triticum aestivum L.) grains. Braz. J. Bot. 2015, 38, 223–234. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.J. The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci. 2009, 14, 43–50. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, C.; Wang, H.; Zhang, F. Effect of Si on the distribution of Cd in rice seedlings. Plant Soil 2005, 272, 53–60. [Google Scholar] [CrossRef]
- Sytar, O.; Kumar, A.; Latowski, D.; Kuczynska, P.; Strzałka, K.; Prasad, M.N.V. Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanisms in plants. Acta Physiol. Plant. 2013, 35, 985–999. [Google Scholar] [CrossRef]
- Choudhury, S.; Panda, P.; Sahoo, L.; Panda, S.K. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal Behav. 2013, 8, e23681. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.M.; Nguyen, V.; Schroeder, J.I. The role of reactive oxygen species in hormonal responses. Plant Physiol. 2006, 141, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.; Leishman, M.R. Consistent alleviation of abiotic stress with silicon addition: A meta-analysis. Funct. Ecol. 2016, 30, 1340–1357. [Google Scholar] [CrossRef]
- Farooq, M.A.; Ali, S.; Hameed, A.; Ishaque, W.; Mahmood, K.; Iqbal, Z. Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes; suppressed cadmium uptake and oxidative stress in cotton. Ecotoxicol. Environ. Saf. 2013, 96, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Mihaličová Malčovská, S.; Dučaiová, Z.; Maslaňáková, I.; Bačkor, M. Effect of Silicon on Growth, Photosynthesis, Oxidative Status and Phenolic Compounds of Maize (Zea mays L.) Grown in Cadmium Excess. Water Air Soil Pollut. 2014, 225, 2056. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, V.P.; Prasad, S.M.; Dubey, N.K.; Chauhan, D.K.; Rai, A.K. LIB spectroscopic and biochemical analysis to characterize lead toxicity alleviative nature of silicon in wheat (Triticum aestivum L.) seedlings. J. Photochem. Photobiol. B 2016, 154, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-W.; Shi, Y.; Zhu, Y.-X.; Wang, Y.-C.; Gong, H.-J. Mechanisms of Enhanced Heavy Metal Tolerance in Plants by Silicon: A Review. Pedosphere 2013, 23, 815–825. [Google Scholar] [CrossRef]
- Nwugo, C.C.; Huerta, A.J. The effect of silicon on the leaf proteome of rice (Oryza sativa L.) plants under cadmium-stress. J. Proteome Res. 2011, 10, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Leisner, S.M.; Frantz, J. Alleviation of copper toxicity in Arabidopsis thaliana by silicon addition to hydroponic solutions. J. Am. Soc. Hortic. Sci. 2008, 133, 670–677. [Google Scholar] [CrossRef]
- Khandekar, S.; Leisner, S. Soluble silicon modulates expression of Arabidopsis thaliana genes involved in copper stress. J. Plant Physiol. 2011, 168, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Khan, A.L.; Kim, D.-H.; Lee, S.-Y.; Kim, K.-M.; Waqas, M.; Jung, H.-Y.; Shin, J.-H.; Kim, J.-G.; Lee, I.-J. Silicon mitigates heavy metal stress by regulating P-type heavy metal ATPases, Oryza sativalow silicon genes, and endogenous phytohormones. BMC Plant Biol. 2014, 14, 13. [Google Scholar] [CrossRef]
- Neumann, D.; zur Nieden, U.; Schwieger, W.; Leopold, I.; Lichtenberger, O. Heavy metal tolerance of Minuartia verna. J. Plant Physiol. 1997, 151, 101–108. [Google Scholar] [CrossRef]
- Bringezu, K.; Lichtenberger, O.; Leopold, I.; Neumann, D. Heavy metal tolerance of Silene vulgaris. J. Plant Physiol. 1999, 154, 536–546. [Google Scholar] [CrossRef]
- Buján, E. Elemental composition of phytoliths in modern plants (Ericaceae). Quat. Int. 2013, 287, 114–120. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Nguyen, M.N.; McNamara, M.; Dultz, S.; Meharg, A.; Nguyen, V.T. Encapsulation of lead in rice phytoliths as a possible pollutant source in paddy soils. Environ. Exp. Bot. 2019, 162, 58–66. [Google Scholar] [CrossRef]
- Tran, T.T.; Nguyen, T.T.; Nguyen, V.T.; Huynh, H.T.; Nguyen, T.T.; Nguyen, M.N. Copper encapsulated in grass-derived phytoliths: Characterization, dissolution properties and the relation of content to soil properties. J. Environ. Manag. 2019, 249, 109423. [Google Scholar] [CrossRef] [PubMed]
- Delplace, G.; Schreck, E.; Pokrovsky, O.S.; Zouiten, C.; Blondet, I.; Darrozes, J.; Viers, J. Accumulation of heavy metals in phytoliths from reeds growing on mining environments in Southern Europe. Sci. Total Environ. 2020, 712, 135595. [Google Scholar] [CrossRef] [PubMed]
- De Melo Farnezi, M.M.; de Barros Silva, E.; Lopes dos Santos, L.; Christofaro Silva, A.; Grazziotti, P.H.; Taline Prochnow, J.; Marinho Pereira, I.; Costa Ilhéu Fontan, I.d. Potential of grasses in phytolith production in soils contaminated with cadmium. Plants 2020, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Min, H.-G.; Kim, M.-S.; Kim, J.-G. Effect of soil characteristics on arsenic accumulation in phytolith of gramineae (Phragmites japonica) and fern (Thelypteris palustris) near the gilgok gold mine. Sustainability 2021, 13, 3421. [Google Scholar] [CrossRef]
- Liu, L.; Song, Z.; Li, Q.; Ellam, R.M.; Tang, J.; Wang, Y.; Sarkar, B.; Wang, H. Accumulation and partitioning of toxic trace metal (loid) s in phytoliths of wheat grown in a multi-element contaminated soil. Environ. Pollut. 2022, 294, 118645. [Google Scholar] [CrossRef]
- Liu, L.; Song, Z.; Tang, J.; Li, Q.; Sarkar, B.; Ellam, R.M.; Wang, Y.; Zhu, X.; Bolan, N.; Wang, H. New insight into the mechanisms of preferential encapsulation of metal (loid) s by wheat phytoliths under silicon nanoparticle amendment. Sci. Total Environ. 2023, 875, 162680. [Google Scholar] [CrossRef]
- Hodson, M.J.; Guppy, C.N. Some thoughts on silicon and carbon trade-offs in plants. Plant Soil 2022, 477, 233–239. [Google Scholar] [CrossRef]
- Hodson, M.; Sangster, A. X-ray microanalysis of the seminal root of Sorghum bicolor with particular reference to silicon. Ann. Bot. 1989, 64, 659–667. [Google Scholar] [CrossRef]
- Carey, J.C.; Fulweiler, R.W. Silica uptake by Spartina-evidence of multiple modes of accumulation from salt marshes around the world. Front. Plant Sci. 2014, 5, 186. [Google Scholar] [CrossRef]
- Lux, A.; Luxova, M.; Abe, J.; Tanimoto, E.; Hattori, T.; Inanaga, S. The dynamics of silicon deposition in the sorghum root endodermis. New Phytol. 2003, 158, 437–441. [Google Scholar] [CrossRef]
- Pilon-Smits, E. Phytoremediation. Annu. Rev. Plant Biol. 2005, 56, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.C.; Bajpai, O. Phytoremediation: From theory toward practice. In Phytomanagement of Polluted Sites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–49. [Google Scholar]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Katz, O.; Puppe, D.; Kaczorek, D.; Prakash, N.B.; Schaller, J. Silicon in the Soil-Plant Continuum: Intricate Feedback Mechanisms within Ecosystems. Plants 2021, 10, 652. [Google Scholar] [CrossRef] [PubMed]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. Int. Sch. Res. Not. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Jacob, J.M.; Karthik, C.; Saratale, R.G.; Kumar, S.S.; Prabakar, D.; Kadirvelu, K.; Pugazhendhi, A. Biological approaches to tackle heavy metal pollution: A survey of literature. J. Environ. Manag. 2018, 217, 56–70. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Maah, M.; Yusoff, I. Heavy metals accumulation in plants growing in ex tin mining catchment. Int. J. Environ. Sci. Technol. 2011, 8, 401–416. [Google Scholar] [CrossRef]
- Wehrhan, M.; Puppe, D.; Kaczorek, D.; Sommer, M. Spatial patterns of aboveground phytogenic Si stocks in a grass-dominated catchment—Results from UAS-based high-resolution remote sensing. Biogeosciences 2021, 18, 5163–5183. [Google Scholar] [CrossRef]
- Puppe, D.; Höhn, A.; Kaczorek, D.; Wanner, M.; Wehrhan, M.; Sommer, M. How big is the influence of biogenic silicon pools on short-term changes in water-soluble silicon in soils? Implications from a study of a 10-year-old soil–plant system. Biogeosciences 2017, 14, 5239–5252. [Google Scholar] [CrossRef]
- Yang, X.; Song, Z.; Liu, H.; Bolan, N.S.; Wang, H.; Li, Z. Plant silicon content in forests of north China and its implications for phytolith carbon sequestration. Ecol. Res. 2015, 30, 347–355. [Google Scholar] [CrossRef]
- An, X.; Xie, B. Phytoliths from Woody Plants: A Review. Diversity 2022, 14, 339. [Google Scholar] [CrossRef]
- Puppe, D.; Leue, M.; Sommer, M.; Schaller, J.; Kaczorek, D. Auto-Fluorescence in Phytoliths—A Mechanistic Understanding Derived From Microscopic and Spectroscopic Analyses. Front. Environ. Sci. 2022, 10, 677. [Google Scholar] [CrossRef]
- Clemens, S.; Ma, J.F. Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef] [PubMed]
| Year | Metal(loid) | Plant | Location of Metal(loid) Accumulation | Reference |
|---|---|---|---|---|
| 1997 | Zinc (Zn) | Minuartia verna (Caryophyllaceae) | Silicates in epidermal cell walls of leaves | Neumann et al. [82] |
| 1999 | Zinc (Zn), Tin (Sn) | Silene vulgaris (Caryophyllaceae) | Silicates in epidermal cell walls of leaves | Bringezu et al. [83] |
| 2013 | Diverse | Different species (Ericaceae) | Phytoliths in leaves | Buján [84] |
| 2013 | Diverse | Hordeum vulgare (Poaceae) | Phytoliths in awns, stems, and leaves | Kameník et al. [5] |
| 2019 | Lead (Pb) | Oryza sativa (Poaceae) | Phytoliths in rice straw and soils | Nguyen et al. [85] |
| 2019 | Copper (Cu) | Grasses dominated by Axonopus compressus (Poaceae) | Phytoliths in grass shoots and soils | Tran et al. [86] |
| 2020 | Diverse | Arundo donax and Phragmites australis (Poaceae) | Phytoliths in reed shoots | Delplace et al. [87] |
| 2020 | Cadmium (Cd) | Urochloa decumbens, Urochloa brizantha, and Megathyrsus maximus (Poaceae) | Phytoliths in grass shoots | de Melo Farnezi et al. [88] |
| 2021 | Arsenic (As) | Phragmites japonicus (Poaceae) and Thelypteris palustris (Thelypteridaceae) | Phytoliths in plant shoots | Min et al. [89] |
| 2022 | Diverse | Triticum aestivum (Poaceae) | Phytoliths in inflorescences, leaf sheaths, and stems | Liu et al. [90] |
| 2023 | Diverse | Triticum aestivum (Poaceae) | Phytoliths in leaf sheaths, stems, and panicles | Liu et al. [91] |
| Metal(loid) | Plant Species | Plant Family | Si Content 4 (%) |
|---|---|---|---|
| Arsenic (As) | Pteris vittata 1 | Pteridaceae | ns |
| Pteridium aquilinum 2 | Dennstaedtiaceae | 1.5 | |
| Corrigiola telephiifolia 2 | Caryophyllaceae | ns | |
| Sacciolepis cymbiandra 2 | Poaceae | ns | |
| Cadmium (Cd) | Arabidopsis halleri 1 | Brassicaceae | ns |
| Malva rotundifolia 2 | Malvaceae | ns | |
| Abelmoschus manihot 2 | Malvaceae | ns | |
| Pterocypsela laciniata 2 | Asteraceae | ns | |
| Lantana camara 2 | Verbenaceae | ns | |
| Chromium (Cr) | Brachiaria mutica 2 | Poaceae | ns |
| Leptochloa fusca 2 | Poaceae | ns | |
| Canna indica 2 | Cannaceae | 0.4 | |
| Cobalt (Co) | Haumaniastrum robertii 1 | Lamiaceae | ns |
| Copper (Cu) | Aeolanthus biformifolius 1 | Lamiaceae | ns |
| Brassica campestris 2 | Brassicaceae | ns | |
| Helianthus annuus 2 | Asteraceae | 0.03 | |
| Lead (Pb) | Noccaea rotondifolia subsp. cepaeifolia 1 | Brassicaceae | ns |
| Pinus sylvestris 2 | Pinaceae | 0.2 | |
| Quercus robur 2 | Fagaceae | 0.6 | |
| Mercury (Hg) | Plectranthus sp. 2 | Lamiaceae | 0.07 (P. japonicus) |
| Clidemia sp. 2 | Melastomataceae | ns | |
| Capsicum annuum 2 | Solanaceae | 0.05 | |
| Phyllanthus niruri 2 | Phyllanthaceae | ns | |
| Inga edulis 2 | Fabaceae | ns | |
| Nickel (Ni) | Berkheya coddii 1 | Asteraceae | ns |
| Brassica juncea 2 | Brassicaceae | ns | |
| Typha angustifolia 2 | Typhaceae | 0.02 | |
| Tin (Sn) | Cyperus rotundus 3 | Cyperaceae | ns |
| Imperata cylindrica 3 | Poaceae | 0.6 | |
| Zinc (Zn) | Noccaea caerulescens 1 | Brassicaceae | ns |
| Sinapis arvensis 2 | Brassicaceae | ns | |
| Tagetes erecta 2 | Asteraceae | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puppe, D.; Kaczorek, D.; Stein, M.; Schaller, J. Silicon in Plants: Alleviation of Metal(loid) Toxicity and Consequential Perspectives for Phytoremediation. Plants 2023, 12, 2407. https://doi.org/10.3390/plants12132407
Puppe D, Kaczorek D, Stein M, Schaller J. Silicon in Plants: Alleviation of Metal(loid) Toxicity and Consequential Perspectives for Phytoremediation. Plants. 2023; 12(13):2407. https://doi.org/10.3390/plants12132407
Chicago/Turabian StylePuppe, Daniel, Danuta Kaczorek, Mathias Stein, and Jörg Schaller. 2023. "Silicon in Plants: Alleviation of Metal(loid) Toxicity and Consequential Perspectives for Phytoremediation" Plants 12, no. 13: 2407. https://doi.org/10.3390/plants12132407
APA StylePuppe, D., Kaczorek, D., Stein, M., & Schaller, J. (2023). Silicon in Plants: Alleviation of Metal(loid) Toxicity and Consequential Perspectives for Phytoremediation. Plants, 12(13), 2407. https://doi.org/10.3390/plants12132407








