Sage, Rosemary, and Bay Laurel Hydrodistillation By-Products as a Source of Bioactive Compounds
Abstract
1. Introduction
2. Results and Discussion
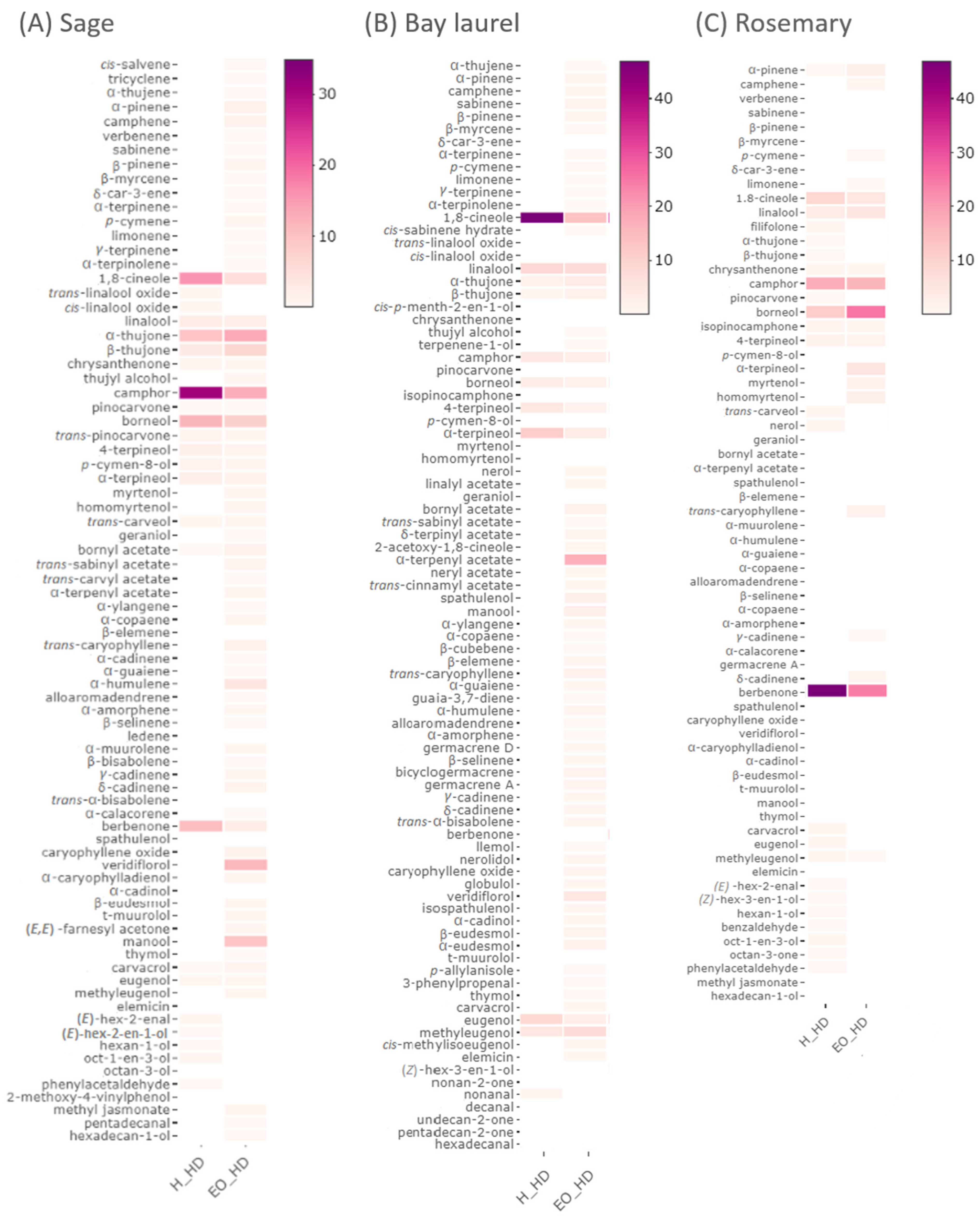
2.1. Impact of Different Pre-Treatments on the Chemical Composition of Hydrodistillation By-Products
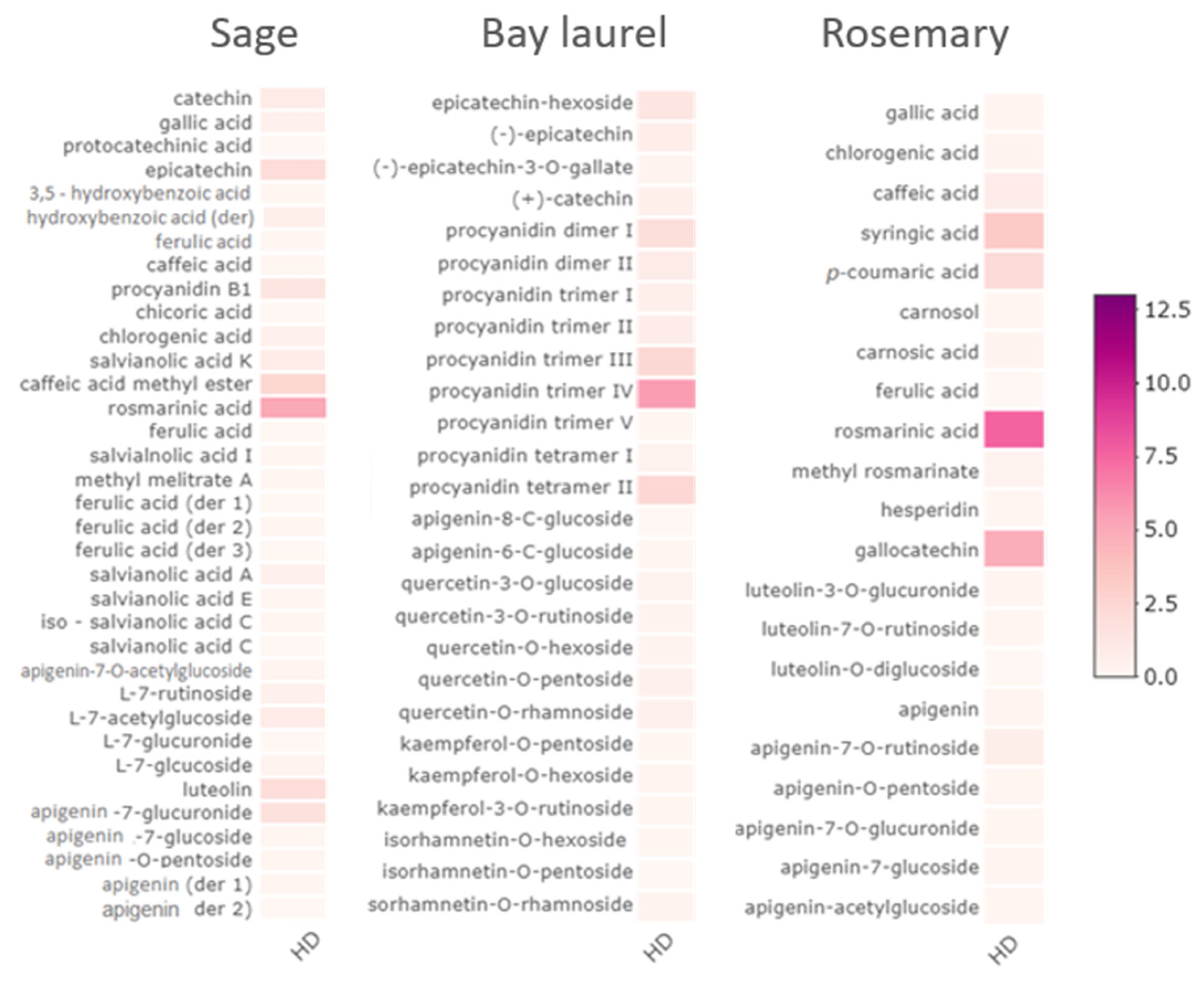
2.2. Composition of Sage, Bay Laurel, and Rosemary Hydrolates
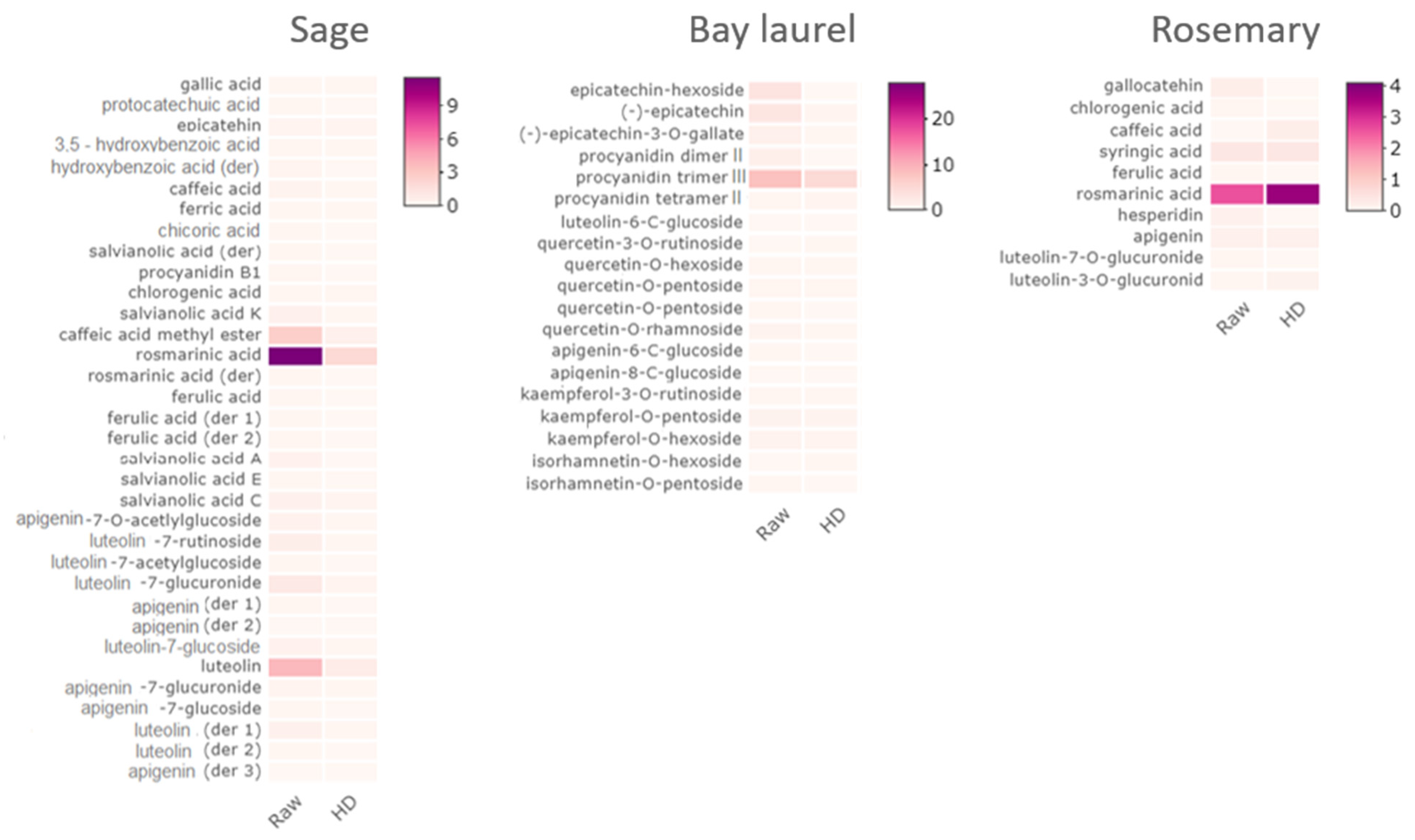
2.3. Composition of Water and Solid Residues of Mediterranean Wild Plants
3. Conclusions
4. Materials and Methods
4.1. Extraction Procedures
4.2. Gas Chromatography/Mass Spectrometry (GC-MS) Analysis of the Hydrolate Extracts
4.3. High-Performance Liquid Chromatography with Diode Array Detector (HPLC-DAD)
4.4. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prins, C.L.; Vieira, I.J.C.; Freitas, S.P. Growth regulators and essential oil production. Braz. J. Plant Physiol. 2010, 22, 91–102. [Google Scholar] [CrossRef]
- Rao, B.R.R. Hydrosols and water-soluble essential oils: Their medicinal and biological properties. In Recent Progress in Medicinal Plants: Essential Oils, 1st ed.; Govil, J.N., Bhattacharya, S., Eds.; Studium Press LLC: Houston, TX, USA, 2013; Volume 1, pp. 117–140. [Google Scholar]
- Sowbhagya, H.B.; Srinivas, P.; Krishnamurthy, N. Effect of enzymes on extraction of volatiles from celery seeds. Food Chem. 2010, 120, 230–234. [Google Scholar] [CrossRef]
- Sowbhagya, H.B.; Srinivas, P.; Purnima, K.T.; Krishnamurthy, N. Enzyme-assisted extraction of volatiles from cumin (Cuminum cyminum L.) seeds. Food Chem. 2011, 127, 1856–1861. [Google Scholar] [CrossRef]
- Chandran, J.; Amma, K.P.P.; Menon, N.; Purushothaman, J.; Nisha, P. Effect of enzyme assisted extraction on quality and yield of volatile oil from black pepper and cardamom. Food Sci. Biotechnol. 2012, 21, 1611–1617. [Google Scholar] [CrossRef]
- Śmigielski, K.B.; Majewska, M.; Kunicka-Styczyñska, A.; Gruska, R. The effect of ultrasound-assisted maceration on the bioactivity, chemical composition and yield of essential oil from waste carrot seeds (Daucus carota). J. Essent. Oil-Bear. Plants 2014, 17, 1075–1086. [Google Scholar] [CrossRef]
- Śmigielski, K.B.; Majewska, M.; Kunicka-Styczyńska, A.; Szczesna-Antczak, M.; Gruska, R.; Stańczyk, Ł. The effect of enzyme-assisted maceration on bioactivity, quality and yield of essential oil from waste carrot (Daucus carota) seeds. J. Food Qual. 2014, 37, 219–228. [Google Scholar] [CrossRef]
- Boulila, A.; Hassen, I.; Haouari, L.; Mejri, F.; Amor, I.B.; Casabianca, H.; Hosni, K. Enzyme-assisted extraction of bioactive compounds from bay leaves (Laurus nobilis L.). Ind. Crops Prod. 2015, 74, 485–493. [Google Scholar] [CrossRef]
- Baby, K.C.; Ranganathan, T.V. Effect of enzyme pre-treatment on extraction yield and quality of cardamom (Elettaria cardamomum maton.) volatile oil. Ind. Crops Prod. 2016, 89, 200–206. [Google Scholar] [CrossRef]
- Seidi Damyeh, M.; Niakousari, M.; Saharkhiz, M.J. Ultrasound pretreatment impact on Prangos ferulacea Lindl. and Satureja macrosiphonia Bornm. essential oil extraction and comparing their physicochemical and biological properties. Ind. Crops Prod. 2016, 87, 105–115. [Google Scholar] [CrossRef]
- Dimaki, V.D.; Iatrou, G.; Lamari, F.N. Effect of acidic and enzymatic pretreatment on the analysis of mountain tea (Sideritis spp.) volatiles via distillation and ultrasound-assisted extraction. J. Chromatogr. A 2017, 1524, 290–297. [Google Scholar] [CrossRef]
- Gavahian, M.; Farahnaky, A.; Javidnia, K.; Majzoobi, M. Comparison of ohmic-assisted hydrodistillation with traditional hydrodistillation for the extraction of essential oils from Thymus vulgaris L. Innov. Food Sci. Emerg. Technol. 2012, 14, 85–91. [Google Scholar] [CrossRef]
- Hosni, K.; Hassen, I.; Chaâbane, H.; Jemli, M.; Dallali, S.; Sebei, H.; Casabianca, H. Enzyme-assisted extraction of essential oils from thyme (Thymus capitatus L.) and rosemary (Rosmarinus officinalis L.): Impact on yield, chemical composition and antimicrobial activity. Ind. Crops Prod. 2013, 47, 291–299. [Google Scholar] [CrossRef]
- Sourmaghi, M.H.S.; Kiaee, G.; Golfakhrabadi, F.; Jamalifar, H.; Khanavi, M. Comparison of essential oil composition and antimicrobial activity of Coriandrum sativum L. extracted by hydrodistillation and microwave-assisted hydrodistillation. J. Food Sci. Technol. 2015, 52, 2452–2457. [Google Scholar] [CrossRef] [PubMed]
- Chávez-González, M.L.; López-López, L.I.; Rodríguez-Herrera, R.; Contreras-Esquivel, J.C.; Aguilar, C.N. Enzyme-assisted extraction of citrus essential oil. Chem. Pap. 2016, 70, 412–417. [Google Scholar] [CrossRef]
- Jeyaratnam, N.; Nour, A.H.; Kanthasamy, R.; Nour, A.H.; Yuvaraj, A.R.; Akindoyo, J.O. Essential oil from Cinnamomum cassia bark through hydrodistillation and advanced microwave assisted hydrodistillation. Ind. Crops Prod. 2016, 92, 57–66. [Google Scholar] [CrossRef]
- de Elguea-Culebras, G.O.; Bravo, E.M.; Sánchez-Vioque, R. Potential sources and methodologies for the recovery of phenolic compounds from distillation residues of Mediterranean aromatic plants. An approach to the valuation of by-products of the essential oil market—A review. Ind. Crops Prod. 2022, 175, 114261. [Google Scholar] [CrossRef]
- Rajeswara Rao, B.R.; Kaul, P.N.; Syamasundar, K.V.; Ramesh, S. Water soluble fractions of rose-scented geranium (Pelargonium species) essential oil. Bioresour. Technol. 2002, 84, 243–246. [Google Scholar] [CrossRef]
- Rajeswara Rao, B.R.; Kaul, P.N.; Syamasundar, K.V.; Ramesh, S. Comparative composition of decanted and recovered essential oils of Eucalyptus citriodora Hook. Flavour Fragr. J. 2003, 18, 133–135. [Google Scholar] [CrossRef]
- Aazza, S.; Lyoussi, B.; Miguel, M.G. Antioxidant activity of some Morrocan hydrosols. J. Med. Plants Res. 2011, 5, 6688–6696. [Google Scholar] [CrossRef]
- Maciąg, A.; Kalemba, D. Composition of rugosa rose (Rosa rugosa thunb.) hydrolate according to the time of distillation. Phytochem. Lett. 2015, 11, 373–377. [Google Scholar] [CrossRef]
- Bajer, T.; Šilha, D.; Ventura, K.; Bajerová, P. Composition and antimicrobial activity of the essential oil, distilled aromatic water and herbal infusion from Epilobium parviflorum Schreb. Ind. Crops Prod. 2017, 100, 95–105. [Google Scholar] [CrossRef]
- Veličković, D.T.; Milenović, D.M.; Ristić, M.S.; Veljković, V.B. Ultrasonic extraction of waste solid residues from the Salvia sp. essential oil hydrodistillation. Biochem. Eng. J. 2008, 42, 97–104. [Google Scholar] [CrossRef]
- Santana-Méridas, O.; Polissiou, M.; Izquierdo-Melero, M.E.; Astraka, K.; Tarantilis, P.A.; Herraiz-Peñalver, D.; Sánchez-Vioque, R. Polyphenol composition, antioxidant and bioplaguicide activities of the solid residue from hydrodistillation of Rosmarinus officinalis L. Ind. Crops Prod. 2014, 59, 125–134. [Google Scholar] [CrossRef]
- Abdel-Hameed, E.S.S.; Bazaid, S.A.; Sabra, A.N.A. Total phenolic, in vitro antioxidant activity and safety assessment (acute, sub-chronic and chronic toxicity) of industrial Taif rose water by-product in mice. Der. Pharm. Lett. 2015, 7, 251–259. [Google Scholar]
- Sánchez-Vioque, R.; Izquierdo-Melero, M.E.; Polissiou, M.; Astraka, K.; Tarantilis, P.A.; Herraiz-Peñalver, D.; Martín-Bejerano, M.; Santana-Méridas, O. Comparative chemistry and biological properties of the solid residues from hydrodistillation of Spanish populations of Rosmarinus officinalis L. Grasas Aceites 2015, 66, e079. [Google Scholar] [CrossRef]
- Wollinger, A.; Perrin, É.; Chahboun, J.; Jeannot, V.; Touraud, D.; Kunz, W. Antioxidant activity of hydro distillation water residues from Rosmarinus officinalis L. leaves determined by DPPH assays. C. R. Chim. 2016, 19, 754–765. [Google Scholar] [CrossRef]
- Christaki, S.; Bouloumpasi, E.; Lalidou, E.; Chatzopoulou, P.; Irakli, M. Bioactive Profile of Distilled Solid By-Products of Rosemary, Greek Sage and Spearmint as Affected by Distillation Methods. Molecules 2022, 27, 9058. [Google Scholar] [CrossRef]
- Jeannot, V.; Chahboun, J.; Russel, D.; Casabianca, H. Origanum compactum Bentham: Composition of the hydrolat aromatic fraction, comparison with the essential oil and its interest in arometherapy. Int. J. Aromather. 2003, 13, 90–94. [Google Scholar] [CrossRef]
- Moon, T.; Cavanagh, H.M.A.; Wilkinson, J.M. Antifungal activity of Australian grown Lavandula spp. essential oils against Aspergillus nidulans, Trichophyton mentagrophytes, Leptosphaeria maculans and Sclerotinia sclerotiorum. J. Essent. Oil Res. 2007, 19, 171–175. [Google Scholar] [CrossRef]
- Śmigielski, K.B.; Prusinowska, R.; Krosowiak, K.; Sikora, M. Comparison of qualitative and quantitative chemical composition of hydrolate and essential oils of lavender (Lavandula angustifolia). J. Essent. Oil Res. 2013, 25, 291–299. [Google Scholar] [CrossRef]
- Khan, M.; Khan, S.T.; Khan, N.A.; Mahmood, A.; Al-Kedhairy, A.A.; Alkhathlan, H.Z. The composition of the essential oil and aqueous distillate of Origanum vulgare L. growing in Saudi Arabia and evaluation of their antibacterial activity. Arab. J. Chem. 2018, 11, 1189–1200. [Google Scholar] [CrossRef]
- Chizzola, R.; Billiani, F.; Singer, S.; Novak, J. Diversity of essential oils and the respective hydrolates obtained from three Pinus cembra populations in the Austrian Alps. Appl. Sci. 2021, 11, 5686. [Google Scholar] [CrossRef]
- Ovidi, E.; Masci, V.L.; Zambelli, M.; Tiezzi, A.; Vitalini, S.; Garzoli, S. Laurus nobilis, Salvia sclarea and Salvia officinalis essential oils and hydrolates: Evaluation of liquid and vapor phase chemical composition and biological activities. Plants 2021, 10, 707. [Google Scholar] [CrossRef] [PubMed]
- Paolini, J.; Leandri, C.; Desjobert, J.M.; Barboni, T.; Costa, J. Comparison of liquid-liquid extraction with headspace methods for the characterization of volatile fractions of commercial hydrolats from typically Mediterranean species. J. Chromatogr. A 2008, 1193, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gil, A.M.; Pardo-García, A.I.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Lavandin hydrolat applications to Petit Verdot vineyards and their impact on their wine aroma compounds. Food Res. Int. 2013, 53, 391–402. [Google Scholar] [CrossRef]
- Mielnik, M.B.; Sem, S.; Egelandsdal, B.; Skrede, G. By-products from herbs essential oil production as ingredient in marinade for turkey thighs. LWT Food Sci. Technol. 2008, 41, 93–100. [Google Scholar] [CrossRef]
- Bashi, D.S.; Bazzaz, B.S.F.; Sahebkar, A.; Karimkhani, M.M.; Ahmadi, A. Investigation of optimal extraction, antioxidant, and antimicrobial activities of Achillea biebersteinii and A. wilhelmsii. Pharm. Biol. 2012, 50, 1168–1176. [Google Scholar] [CrossRef]
- Da Porto, C.; Porretto, E.; Decorti, D. Comparison of ultrasound-assisted extraction with conventional extraction methods of oil and polyphenols from grape (Vitis vinifera L.) seeds. Ultrason. Sonochem. 2013, 20, 1076–1080. [Google Scholar] [CrossRef]
- Foo, L.W.; Salleh, E.; Hana, S.N. Green extraction of antimicrobial bioactive compound from piper betle leaves: Probe type ultrasound-Assisted extraction Vs supercritical carbon dioxide extraction. Chem. Eng. Trans. 2017, 56, 109–114. [Google Scholar] [CrossRef]
- Kozłowska, M.; Laudy, A.E.; Przybył, J.; Ziarno, M.; Majewska, E. Chemical composition and antibacterial activity of some medicinal plants from Lamiaceae family. Acta Pol. Pharm. 2015, 72, 757–767. [Google Scholar]
- Vallverdú-Queralt, A.; Regueiro, J.; Martínez-Huélamo, M.; Rinaldi Alvarenga, J.F.; Leal, L.N.; Lamuela-Raventos, R.M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014, 154, 299–307. [Google Scholar] [CrossRef]
- Miljanović, A.; Bielen, A.; Grbin, D.; Marijanović, Z.; Andlar, M.; Rezić, T.; Roca, S.; Jerković, I.; Vikić-Topić, D.; Dent, M. Effect of enzymatic, ultrasound, and reflux extraction pretreatments on the yield and chemical composition of essential oils. Molecules 2020, 25, 4818. [Google Scholar] [CrossRef]
- Kowalski, R.; Wawrzykowski, J. Effect of ultrasound-assisted maceration on the quality of oil from the leaves of thyme Thymus vulgaris L. Flavour Fragr. J. 2009, 24, 69–74. [Google Scholar] [CrossRef]
- Kowalski, R.; Kowalska, G.; Jamroz, J.; Nawrocka, A.; Metyk, D. Effect of the ultrasound-assisted preliminary maceration on the efficiency of the essential oil distillation from selected herbal raw materials. Ultrason. Sonochem. 2015, 24, 214–220. [Google Scholar] [CrossRef]
- Assami, K.; Pingret, D.; Chemat, S.; Meklati, B.Y.; Chemat, F. Ultrasound induced intensification and selective extraction of essential oil from Carum carvi L. seeds. Chem. Eng. Process. Process Intensif. 2012, 62, 99–105. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Marzouki, M.; M’Rabet, Y.; Mezni, M.; Ait Ouazzou, A.; Hosni, K. Enzyme pretreatment improves the recovery of bioactive phytochemicals from sweet basil (Ocimum basilicum L.) leaves and their hydrodistilled residue by-products, and potentiates their biological activities. Arab. J. Chem. 2020, 13, 6451–6460. [Google Scholar] [CrossRef]
- Baydar, H.; Sangun, M.K.; Erbas, S.; Kara, N. Comparison of aroma compounds in distilled and extracted products of sage (Salvia officinalis L.). J. Essent. Oil-Bear. Plants 2013, 16, 39–44. [Google Scholar] [CrossRef]
- Šilha, D.; Švarcová, K.; Bajer, T.; Královec, K.; Tesařová, E.; Moučková, K.; Pejchalová, M.; Bajerová, P. Chemical composition of natural hydrolates and their antimicrobial activity on arcobacter-like cells in comparison with other microorganisms. Molecules 2020, 25, 5654. [Google Scholar] [CrossRef] [PubMed]
- Aćimović, M.; Tešević, V.; Smiljanić, K.; Cvetković, M.; Stanković, J.; Kiprovski, B.; Sikora, V. Hydrolates: By-products of essential oil distillation: Chemical composition, biological activity and potential uses. Adv. Technol. 2020, 9, 54–57. [Google Scholar] [CrossRef]
- Politi, M.; Menghini, L.; Conti, B.; Bedini, S.; Farina, P.; Cioni, P.L.; Braca, A.; De Leo, M. Reconsidering hydrosols as main products of aromatic plants manufactory: The lavandin (Lavandula × intermedia) case study in Tuscany. Molecules 2020, 25, 2225. [Google Scholar] [CrossRef]
- D’Amato, S.; Serio, A.; López, C.C.; Paparella, A. Hydrosols: Biological activity and potential as antimicrobials for food applications. Food Control 2018, 86, 126–137. [Google Scholar] [CrossRef]
- Perito, M.A.; Chiodo, E.; Serio, A.; Paparella, A.; Fantini, A. Factors influencing consumers’ attitude towards biopreservatives. Sustainability 2020, 12, 10338. [Google Scholar] [CrossRef]
- Tornuk, F.; Cankurt, H.; Ozturk, I.; Sagdic, O.; Bayram, O.; Yetim, H. Efficacy of various plant hydrosols as natural food sanitizers in reducing Escherichia coli O157:H7 and Salmonella typhimurium on fresh cut carrots and apples. Int. J. Food Microbiol. 2011, 148, 30–35. [Google Scholar] [CrossRef]
- Ozturk, I.; Tornuk, F.; Caliskan-Aydogan, O.; Durak, M.Z.; Sagdic, O. Decontamination of iceberg lettuce by some plant hydrosols. LWT Food Sci. Technol. 2016, 74, 48–54. [Google Scholar] [CrossRef]
- Chen, W.; Vermaak, I.; Viljoen, A. Camphor—A fumigant during the black death and a coveted fragrant wood in ancient egypt and babylon-A review. Molecules 2013, 18, 5434–5454. [Google Scholar] [CrossRef]
- Cai, Z.M.; Peng, J.Q.; Chen, Y.; Tao, L.; Zhang, Y.Y.; Fu, L.Y.; De Long, Q.; Shen, X.C. 1,8-Cineole: A review of source, biological activities, and application. J. Asian Nat. Prod. Res. 2020, 23, 938–954. [Google Scholar] [CrossRef]
- Méndez-Tovar, I.; Herrero, B.; Pérez-Magariño, S.; Pereira, J.A.; Asensio-S-Manzanera, M.C. By-product of Lavandula latifolia essential oil distillation as source of antioxidants. J. Food Drug Anal. 2015, 23, 225–233. [Google Scholar] [CrossRef]
- Berktas, S.; Cam, M. Peppermint leaves hydrodistillation by-products: Bioactive properties and incorporation into ice cream formulations. J. Food Sci. Technol. 2021, 58, 4282–4293. [Google Scholar] [CrossRef]
- Shanaida, M.; Hudz, N.; Jasicka-Misiak, I.; Wieczorek, P.P. Polyphenols and pharmacological screening of a Monarda fistulosa L. dry extract based on a hydrodistilled residue by-product. Front. Pharmacol. 2021, 12, 563436. [Google Scholar] [CrossRef] [PubMed]
- Durling, N.E.; Catchpole, O.J.; Grey, J.B.; Webby, R.F.; Mitchell, K.A.; Foo, L.Y.; Perry, N.B. Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol-water mixtures. Food Chem. 2007, 101, 1417–1424. [Google Scholar] [CrossRef]
- Dent, M.; Dragović-Uzelac, V.; Penić, M.; Brnčić, M.; Bosiljkov, T.; Levaj, B. The effect of extraction solvents, temperature and time on the composition and mass fraction of polyphenols in dalmatian wild sage (Salvia officinalis L.) extracts. Food Technol. Biotechnol. 2013, 50, 84–91. [Google Scholar]
- Vinha, A.F.; Guido, L.F.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P. Monomeric and oligomeric flavan-3-ols and antioxidant activity of leaves from different Laurus sp. Food Funct. 2015, 6, 1944–1949. [Google Scholar] [CrossRef] [PubMed]
- Lamien-Meda, A.; Nell, M.; Lohwasser, U.; Börner, A.; Franz, C.; Novak, J. Investigation of antioxidant and rosmarinic acid variation in the sage collection of the genebank in gatersleben. J. Agric. Food Chem. 2010, 58, 3813–3819. [Google Scholar] [CrossRef] [PubMed]
- Putnik, P.; Kovačević, D.B.; Penić, M.; Fegeš, M.; Dragović-Uzelac, V. Microwave-assisted extraction (MAE) of dalmatian sage leaves for the optimal yield of polyphenols: HPLC-DAD identification and quantification. Food Anal. Methods 2016, 9, 2385–2394. [Google Scholar] [CrossRef]
- Škerget, M.; Kotnik, P.; Hadolin, M.; Hraš, A.R.; Simonič, M.; Knez, Ž. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Dueñas, M.; Alves, R.C.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Nutritional and antioxidant contributions of Laurus nobilis L. leaves: Would be more suitable a wild or a cultivated sample? Food Chem. 2014, 156, 339–346. [Google Scholar] [CrossRef]
- Ouchikh, O.; Chahed, T.; Ksouri, R.; Taarit, M.B.; Faleh, H.; Abdelly, C.; Kchouk, M.E.; Marzouk, B. The effects of extraction method on the measured tocopherol level and antioxidant activity of L. nobilis vegetative organs. J. Food Compos. Anal. 2016, 24, 103–110. [Google Scholar] [CrossRef]
- Jacotet-Navarro, M.; Rombaut, N.; Fabiano-Tixier, A.S.; Danguien, M.; Bily, A.; Chemat, F. Ultrasound versus microwave as green processes for extraction of rosmarinic, carnosic and ursolic acids from rosemary. Ultrason. Sonochem. 2015, 27, 102–109. [Google Scholar] [CrossRef]
- Park, J.; Rho, S.J.; Kim, Y.R. Enhancing antioxidant and antimicrobial activity of carnosic acid in rosemary (Rosmarinus officinalis L.) extract by complexation with cyclic glucans. Food Chem. 2019, 299, 125119. [Google Scholar] [CrossRef]
- Ho, J.H.C.; Hong, C.Y. Salvianolic acids: Small compounds with multiple mechanisms for cardiovascular protection. J. Biomed. Sci. 2011, 18, 30. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Patel, I.S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Petersen, M.; Simmonds, M.S.J. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Albano, S.; Lima, A.S.; Miguel, M.G.; Pedro, L.G.; Barroso, J.G.; Figueiredo, A.C. Antioxidant, anti-5-lipoxygenase and antiacetylcholinesterase activities of essential oils and decoction waters of some aromatic plants. Rec. Nat. Prod. 2012, 6, 35–48. [Google Scholar]
- Dapkevicius, A.; Venskutonis, R.; van Beek, T.A.; Linssen, J.P. Antioxidant activity of extracts obtained by different isolation procedures from some aromatic herbs grown in Lithuania. J. Sci. Food Agric. 1998, 77, 140–146. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Peltoketo, A.; Hiltunen, R.; Tikkanen, M.J. Characterisation of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs. Food Chem. 2003, 83, 255–262. [Google Scholar] [CrossRef]
- Jordán, M.J.; Lax, V.; Rota, M.C.; Lorán, S.; Sotomayor, J.A. Relevance of carnosic acid, carnosol, and rosmarinic acid concentrations in the in vitro antioxidant and antimicrobial activities of Rosmarinus officinalis (L.) methanolic extracts. J. Agric. Food Chem. 2012, 60, 9603–9608. [Google Scholar] [CrossRef]
- Dragović, S.; Dragović-Uzelac, V.; Pedisić, S.; Čosić, Z.; Friščić, M.; Elez Garofulić, I.; Zorić, Z. The mastic tree (Pistacia lentiscus L.) leaves as source of BACs: Effect of growing location, phenological stage and extraction solvent on phenolic content. Food Technol. Biotechnol. 2020, 58, 303–313. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Astatkie, T.; Horgan, T.; Rogers, M.S. Effect of plant hormones and distillation water on mints. HortScience 2010, 45, 1338–1340. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Astatkie, T. Effect of residual distillation water of 15 plants and three plant hormones on Scotch spearmint (Mentha × gracilis Sole). Ind. Crops Prod. 2011, 33, 704–709. [Google Scholar] [CrossRef]
- Borrás-Linares, I.; Stojanović, Z.; Quirantes-Piné, R.; Arráez-Román, D.; Švarc-Gajić, J.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Rosmarinus Officinalis Leaves as a Natural Source of Bioactive Compounds. Int. J. Mol. Sci. 2014, 15, 20585–20606. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Q.; Wang, X.; Yang, J.; Wang, Q. Qualitative Analysis and Simultaneous Quantification of Phenolic Compounds in the Aerial Parts of Salvia miltiorrhiza by HPLC-DAD and ESI/MSn. Phytochem. Anal. 2011, 22, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Hcini, K.; Sotomayor, J.A.; Jordan, M.J.; Bouzid, S. Identification and Quantification of Phenolic Compounds of Tunisian Rosmarinus officinalis L. Asian J. Chem. 2013, 25, 9299–9301. [Google Scholar] [CrossRef]
- Sharma, Y.; Velamuri, R.; Fagan, J.; Schaefer, J. Full-Spectrum Analysis of Bioactive Compounds in Rosemary (Rosmarinus officinalis L.) as Influenced by Different Extraction Methods. Molecules 2020, 25, 4599. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. A Language and Environment for Statistical Compunting. Available online: https://www.r-project.org/ (accessed on 1 January 2020).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miljanović, A.; Dent, M.; Grbin, D.; Pedisić, S.; Zorić, Z.; Marijanović, Z.; Jerković, I.; Bielen, A. Sage, Rosemary, and Bay Laurel Hydrodistillation By-Products as a Source of Bioactive Compounds. Plants 2023, 12, 2394. https://doi.org/10.3390/plants12132394
Miljanović A, Dent M, Grbin D, Pedisić S, Zorić Z, Marijanović Z, Jerković I, Bielen A. Sage, Rosemary, and Bay Laurel Hydrodistillation By-Products as a Source of Bioactive Compounds. Plants. 2023; 12(13):2394. https://doi.org/10.3390/plants12132394
Chicago/Turabian StyleMiljanović, Anđela, Maja Dent, Dorotea Grbin, Sandra Pedisić, Zoran Zorić, Zvonimir Marijanović, Igor Jerković, and Ana Bielen. 2023. "Sage, Rosemary, and Bay Laurel Hydrodistillation By-Products as a Source of Bioactive Compounds" Plants 12, no. 13: 2394. https://doi.org/10.3390/plants12132394
APA StyleMiljanović, A., Dent, M., Grbin, D., Pedisić, S., Zorić, Z., Marijanović, Z., Jerković, I., & Bielen, A. (2023). Sage, Rosemary, and Bay Laurel Hydrodistillation By-Products as a Source of Bioactive Compounds. Plants, 12(13), 2394. https://doi.org/10.3390/plants12132394








