Do Antimonite and Silicon Share the Same Root Uptake Pathway by Lsi1 in Sorghum bicolor L. Moench?
Abstract
:1. Introduction
2. Results
2.1. Plant Growth
2.2. Lipid Peroxidation
2.3. Ascorbate Content
2.4. Concentration of Sb and Si in Roots and Shoots
2.5. Expression of SbLsi1 Gene in Roots
3. Discussion
4. Materials and Methods
4.1. Experimental Set Up
- Control (C): Hoagland solution without Sb and Si.
- Antimony (Sb): Hoagland solution with 10 mg Sb L−1.
- Silicon (Si): Hoagland solution with 1 mM Si.
- Antimony + silicon (Sb + Si): Hoagland solution with 10 mg Sb L−1 and 1 mM Si.
4.2. Determination of Growth Parameters
4.3. Determination of Sb and Si Concentrations in Roots and Shoots
4.4. Determination of Lipid Peroxidation
4.5. Ascorbate Assay
4.6. RNA Extraction and Real-Time PCR Analyses
4.7. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gregori, I.D.; Fuentes, E.; Rojas, M.; Pinochet, H.; Potin-Gautier, M. Monitoring of copper, arsenic and antimony levels in agricultural soils impacted and non-impacted by mining activities, from three regions in Chile. J. Environ. Monit. 2003, 5, 287–295. [Google Scholar] [CrossRef]
- Wilson, S.C.; Lockwood, P.V.; Ashley, P.M.; Tighe, M. The chemistry and behaviour of antimony in the soil environment with comparisons to arsenic: A critical review. Environ. Pollut. 2010, 158, 1169–1181. [Google Scholar] [CrossRef]
- Vidya, C.S.-N.; Shetty, R.; Vaculíková, M.; Vaculík, M. Antimony toxicity in soils and plants, and mechanisms of its alleviation. Environ. Exp. Bot. 2022, 202, 104996. [Google Scholar] [CrossRef]
- Guéguen, F.; Stille, P.; Geagea, M.L.; Boutin, R. Atmospheric pollution in an urban environment by tree bark biomonitoring—Part I: Trace element analysis. Chemosphere 2012, 86, 1013–1019. [Google Scholar] [CrossRef]
- Pan, X.; Zhang, D.; Chen, X.; Bao, A.; Li, L. Antimony accumulation, growth performance, antioxidant defense system and photosynthesis of Zea mays in response to antimony pollution in soil. Water Air Soil Pollut. 2011, 215, 517–523. [Google Scholar] [CrossRef]
- Vaculíková, M.; Vaculík, M.; Šimková, L.; Fialová, I.; Kochanová, Z.; Sedláková, B.; Luxová, M. Influence of silicon on maize roots exposed to antimony—Growth and antioxidative response. Plant Physiol. Biochem. 2014, 83, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Ortega, A.; Garrido, I.; Casimiro, I.; Espinosa, F. Effects of antimony on redox activities and antioxidant defence systems in sunflower (Helianthus annuus L.) plants. PLoS ONE 2017, 12, e0183991. [Google Scholar] [CrossRef] [Green Version]
- Filella, M.; Williams, P.A.; Belzile, N. Antimony in the environment: Knowns and unknowns. Environ. Chem. 2009, 6, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Reimann, C.; Matschullat, J.; Birke, M.; Salminen, R. Antimony in the environment: Lessons from geochemical mapping. Appl. Geochem. 2010, 25, 175–198. [Google Scholar] [CrossRef]
- Multani, R.S.; Feldmann, T.; Demopoulos, G.P. Antimony in the metallurgical industry: A review of its chemistry and environmental stabilization options. Hydrometallurgy 2016, 164, 141–153. [Google Scholar] [CrossRef]
- Tschan, M.; Robinson, B.H.; Schulin, R. Antimony in the soil—plant system—A review. Environ. Chem. 2009, 6, 106–115. [Google Scholar] [CrossRef] [Green Version]
- Porquet, A.; Filella, M. Structural evidence of the similarity of Sb(OH)3 and As(OH)3 with glycerol: Implications for their uptake. Chem. Res. Toxicol. 2007, 20, 1269–1276. [Google Scholar] [CrossRef]
- Bhattacharjee, H.; Mukhopadhyay, R.; Thiyagarajan, S.; Rosen, B.P. Aquaglyceroporins: Ancient channels for metalloids. J. Biol. 2008, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Bienert, G.P.; Thorsen, M.; Schüssler, M.D.; Nilsson, H.R.; Wagner, A.; Tamás, M.J.; Jahn, T.P. A subgroup of plant aquaporins facilitate the bi-directional diffusion of As(OH)3 and Sb(OH)3 across membranes. BMC Biol. 2008, 6, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Takano, J.; Wada, M.; Ludewig, U.; Schaaf, G.; von Wirén, N.; Fujiwara, T. The Arabidopsis Major Intrinsic Protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell 2006, 18, 1498–1509. [Google Scholar] [CrossRef] [Green Version]
- Azad, A.K.; Ahmed, J.; Alum, A.; Hasan, M.; Ishikawa, T.; Sawa, Y. Prediction of arsenic and antimony transporter major intrinsic proteins from the genomes of crop plants. Int. J. Biol. Macromol. 2018, 107, 2630–2642. [Google Scholar] [CrossRef]
- Lux, A.; Luxová, M.; Hattori, T.; Inanaga, S.; Sugimoto, Y. Silicification in sorghum (Sorghum bicolor) cultivars with different drought tolerance. Physiol. Plant. 2002, 115, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Vaculíková, M.; Vaculík, M.; Tandy, S.; Luxová, M.; Schulin, R. Alleviation of antimonate (SbV) toxicity in maize by silicon (Si). Environ. Exp. Bot. 2016, 128, 11–17. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. A cooperative system of silicon transport in plants. Trends Plant Sci. 2015, 20, 435–442. [Google Scholar] [CrossRef]
- Gaur, S.; Kumar, J.; Kumar, D.; Chauhan, D.K.; Prasad, S.M.; Srivastava, P.K. Fascinating impact of silicon and silicon transporters in plants: A review. Ecotoxicol. Environ. Saf. 2020, 202, 110885. [Google Scholar] [CrossRef]
- Hodson, M.; White, P.; Mead, A.; Broadley, M.R. Phylogenetic variation in the silicon composition of plants. Ann. Bot. 2005, 96, 1027–1046. [Google Scholar] [CrossRef] [Green Version]
- S Soukup, M.; Martinka, M.; Bosnić, D.; Čaplovičová, M.; Elbaum, R.; Lux, A. Formation of silica aggregates in sorghum root endodermis is predetermined by cell wall architecture and development. Ann. Bot. 2017, 120, 739–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soukup, M.; Zancajo, V.M.R.; Kneipp, J.; Elbaum, R. Formation of root silica aggregates in sorghum is an active process of the endodermis. J. Exp. Bot. 2020, 71, 6807–6817. [Google Scholar] [CrossRef] [Green Version]
- Markovich, O.; Kumar, S.; Cohen, D.; Addadi, S.; Fridman, E.; Elbaum, R. Silicification in leaves of sorghum mutant with low silicon accumulation. Silicon 2019, 11, 2385–2391. [Google Scholar] [CrossRef]
- Markovich, O.; Zexer, N.; Negin, B.; Zait, Y.; Blum, S.; Ben-Gal, A.; RivkaElbaum, R. Low Si combined with drought causes reduced transpiration in sorghum Lsi1 mutant. Plant Soil 2022, 477, 57–67. [Google Scholar] [CrossRef]
- Herath, I.; Vithanage, M.; Bundschuh, J. Antimony as a global dilemma: Geochemistry, mobility, fate and transport. Environ. Pollut. 2017, 223, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.-X.; Gao, N.; Li, X.; Xi, X. Toxic effects of antimony on the seed germination and seedlings accumulation in Raphanus sativus L. radish and Brassica napus L. Mol. Biol. Rep. 2018, 45, 2609–2614. [Google Scholar] [CrossRef] [PubMed]
- Tschan, M.; Robinson, B.; Johnson, C.A.; Bürgi, A.; Schulin, R. Antimony uptake and toxicity in sunflower and maize growing in SbIII and SbV contaminated soil. Plant Soil 2010, 334, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Shtangeeva, I.; Bali, R.; Harris, A. Bioavailability and toxicity of antimony. J. Geochem. Explor. 2011, 110, 40–45. [Google Scholar] [CrossRef]
- Vaculík, M.; Mrázová, A.; Lux, A. Antimony (SbIII) reduces growth, declines photosynthesis, and modifies leaf tissue anatomy in sunflower (Helianthus annuus L.). Environ. Sci. Pollut. Res. 2015, 22, 18699–18706. [Google Scholar] [CrossRef] [PubMed]
- Vaculík, M.; Lux, A.; Luxová, M.; Tanimoto, E.; Lichtscheidl, I. Silicon mitigates cadmium inhibitory effects in young maize plants. Environ. Exp. Bot. 2009, 67, 52–58. [Google Scholar] [CrossRef]
- Vaculík, M.; Pavlovič, A.; Lux, A. Silicon alleviates cadmium toxicity by enhanced photosynthetic rate and modified bundle sheath’s cell chloroplasts ultrastructure in maize. Ecotoxicol. Environ. Saf. 2015, 120, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Ali, S.; Ibrahim, M.; Rizwan, M.; Hannan, F.; Adrees, M.; Khan, M.D. Chapter 12 Silicon and chromium toxicity in plants: An Overview. In Silicon in Plants; Tripathi, D.K., Singh, V.P., Ahmad, P., Chauhan, D.K., Prasad, S.M., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 213–226. [Google Scholar] [CrossRef]
- Pandey, C.; Khan, E.; Panthri, M.; Tripathi, R.D.; Gupta, M. Impact of silicon on Indian mustard (Brassica juncea L.) root traits by regulating growth parameters, cellular antioxidants and stress modulators under arsenic stress. Plant Physiol. Biochem. 2016, 104, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Masarovič, D.; Slováková, L.; Bokor, B.; Bujdoš, M.; Lux, A. Effect of silicon application on Sorghum bicolor exposed to toxic concentration of zinc. Biologia 2012, 67, 706–712. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Mitani, N.; Ma, J.F. Uptake system of silicon in different plant species. J. Exp. Bot. 2005, 56, 1255–1261. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Sun, Y.; Xiao, H.; Ma, Q.; Si, L.; Ni, L.; Wu, L. Silicon uptake and translocation in low-silica rice mutants investigated by isotope fractionation. Agron. J. 2021, 113, 2732–2741. [Google Scholar] [CrossRef]
- Ma, J.F.; Tamai, K.; Ichii, M.; Wu, G.F. A rice mutant defective in Si uptake. Plant Physiol. 2002, 130, 2111–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shetty, R.; Vidya, C.S.-N.; Weidinger, M.; Vaculík, M. Silicon alleviates antimony phytotoxicity in giant reed (Arundo donax L.). Planta 2021, 254, 100. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Xu, X.-Y.; Su, Y.-H.; McGrath, S.P.; Zhao, F.-J. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc. Natl. Acad. Sci. USA 2008, 105, 9931–9935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Yang, W.; Zhou, F.; Du, Z.; Xue, M.; Chen, T.; Liang, D. Effect of phosphate and silicate on selenite uptake and phloem-mediated transport in tomato (Solanum lycopersicum L.). Environ. Sci. Pollut. Res. 2019, 26, 20475–20484. [Google Scholar] [CrossRef]
- Ma, J.F.; Tamai, K.; Yamaji, N.; Mitani, N.; Konishi, S.; Katsuhara, M.; Ishiguro, M.; Murata, Y.; Yano, M. A silicon transporter in rice. Nature 2006, 440, 688–691. [Google Scholar] [CrossRef]
- Lin, H.; Fang, C.; Li, Y.; Lin, W.; He, J.; Lin, R.; Lin, W. Cadmium-stress mitigation through gene expression of rice and silicon addition. Plant Growth Regul. 2017, 81, 91–101. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C.; Schat, H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytol. 2009, 181, 759–776. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. The Water-culture method for growing plants without soil. In California Agricultural Experiment Station; College of Agriculture, University of California: Berkeley, CA, USA, 1950. [Google Scholar]
- Cakmak, I.; Horst, W.J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Nakagawara, S.; Sagisaka, S. Increase in enzyme activities related to ascorbate metabolism during cold acclimation in poplar twigs1. Plant Cell Physiol. 1984, 25, 899–906. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]

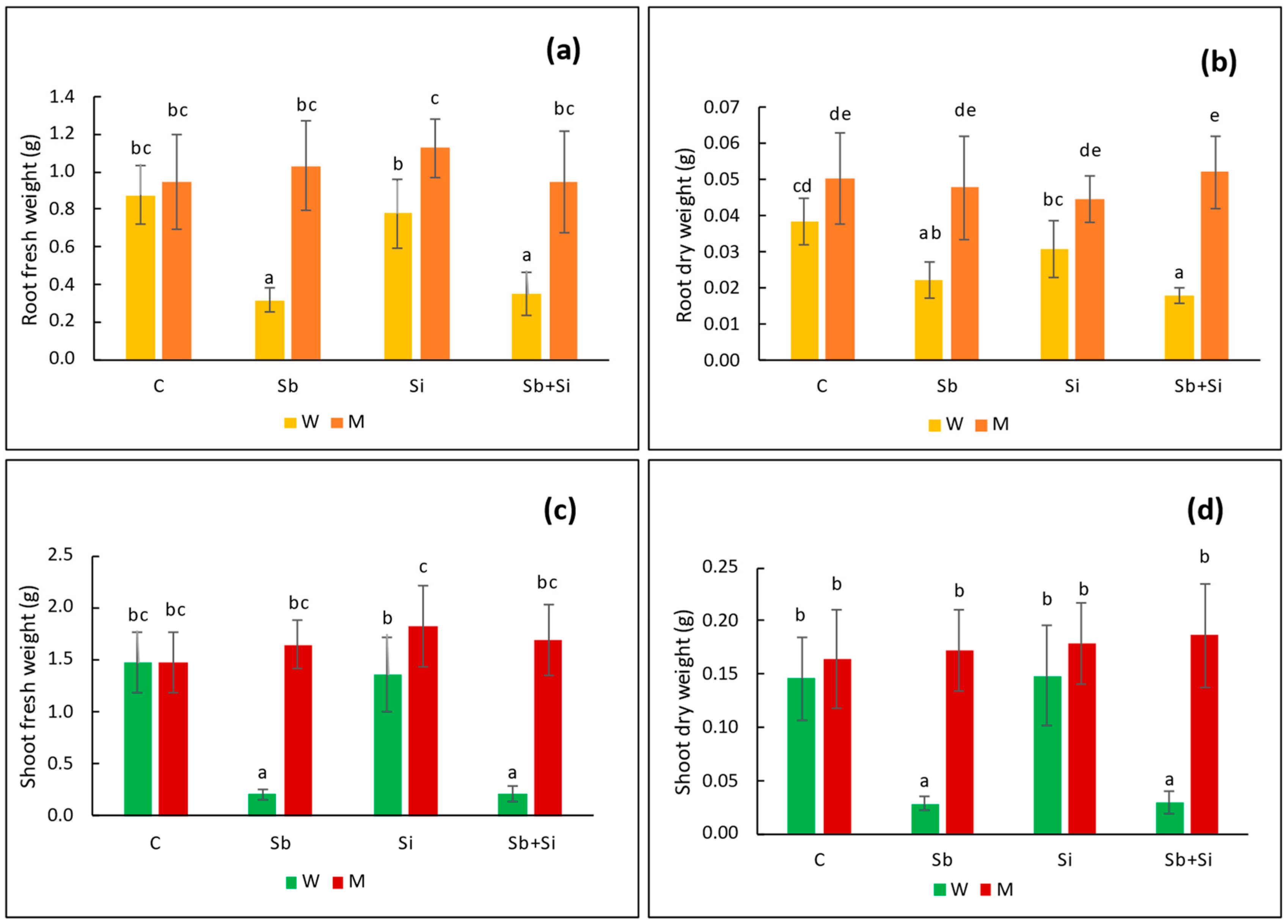
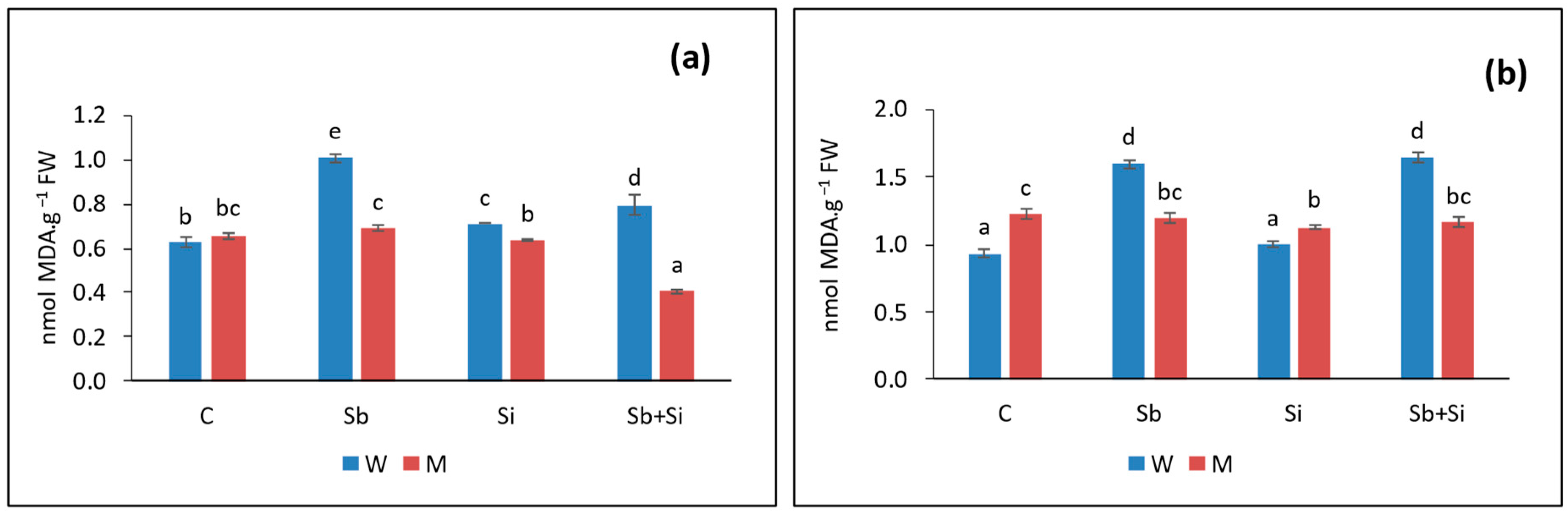

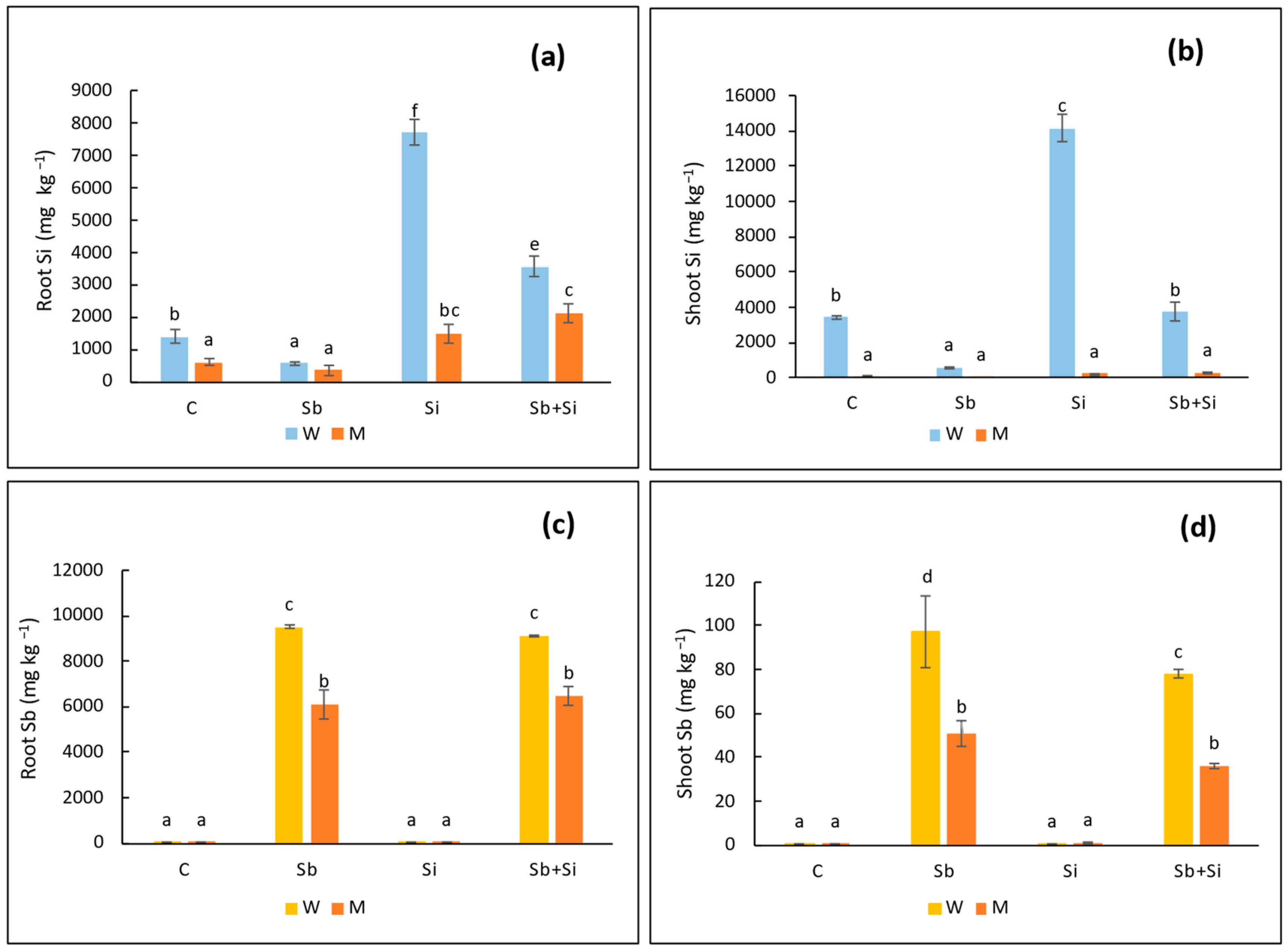
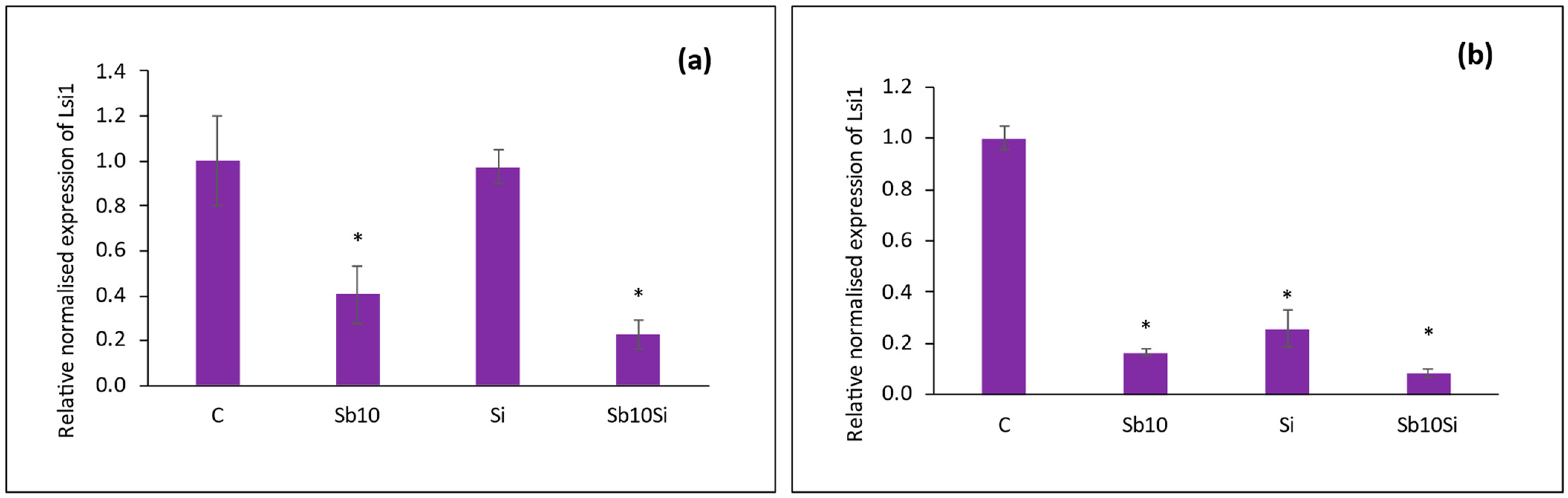
| Treatments | Root Length (cm) | Shoot Length (cm) | Leaf Length (cm) | Leaf Width (cm) | |
|---|---|---|---|---|---|
| C | W M | 23.5 ± 4.4 abc 28.95 ± 3.5 cd | 39.03 ± 1.1 bc 40.95 ± 2.2 bc | 12.37 ± 0.8 c 12.4 ± 0.46 c | 0.9 ± 0.06 b 0.91 ± 0.08 b |
| Sb | W M | 19.81 ± 3.9 a 27.17 ± 2.8 cd | 19.92 ± 2.6 a 41.91 ± 1.8 bc | 4.8 ± 0.26 a 12.52 ± 0.57 c | 0.65 ± 0.05 a 0.93 ± 0.08 b |
| Si | W M | 26.1 ± 5.6 bc 32.56 ± 5.8 d | 38.3 ± 3.5 b 42.7 ± 2.5 c | 12.13 ± 0.55 c 12.02 ± 0.50 c | 0.94 ± 0.1 b 0.91 ± 0.07 b |
| Sb + Si | W M | 20.53 ± 2.4 ab 25.2 ± 4 abc | 21.01 ± 2.9 a 42.06 ± 4.1 bc | 5.78 ± 0.48 b 12.73 ± 0.61 c | 0.63 ± 0.04 a 0.99 ± 0.07 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidya, C.S.N.; Shetty, R.; Bokor, B.; Fialová, I.; Luxová, M.; Jašková, K.; Vaculík, M. Do Antimonite and Silicon Share the Same Root Uptake Pathway by Lsi1 in Sorghum bicolor L. Moench? Plants 2023, 12, 2368. https://doi.org/10.3390/plants12122368
Vidya CSN, Shetty R, Bokor B, Fialová I, Luxová M, Jašková K, Vaculík M. Do Antimonite and Silicon Share the Same Root Uptake Pathway by Lsi1 in Sorghum bicolor L. Moench? Plants. 2023; 12(12):2368. https://doi.org/10.3390/plants12122368
Chicago/Turabian StyleVidya, Chirappurathu Sukumaran Nair, Rajpal Shetty, Boris Bokor, Ivana Fialová, Miroslava Luxová, Katarína Jašková, and Marek Vaculík. 2023. "Do Antimonite and Silicon Share the Same Root Uptake Pathway by Lsi1 in Sorghum bicolor L. Moench?" Plants 12, no. 12: 2368. https://doi.org/10.3390/plants12122368
APA StyleVidya, C. S. N., Shetty, R., Bokor, B., Fialová, I., Luxová, M., Jašková, K., & Vaculík, M. (2023). Do Antimonite and Silicon Share the Same Root Uptake Pathway by Lsi1 in Sorghum bicolor L. Moench? Plants, 12(12), 2368. https://doi.org/10.3390/plants12122368






