Alleviation of Shade Stress in Chinese Yew (Taxus chinensis) Seedlings with 5-Aminolevulinic Acid (ALA)
Abstract
:1. Introduction
2. Results
2.1. Changes in Visual Quality Index in Response to Shade Stress
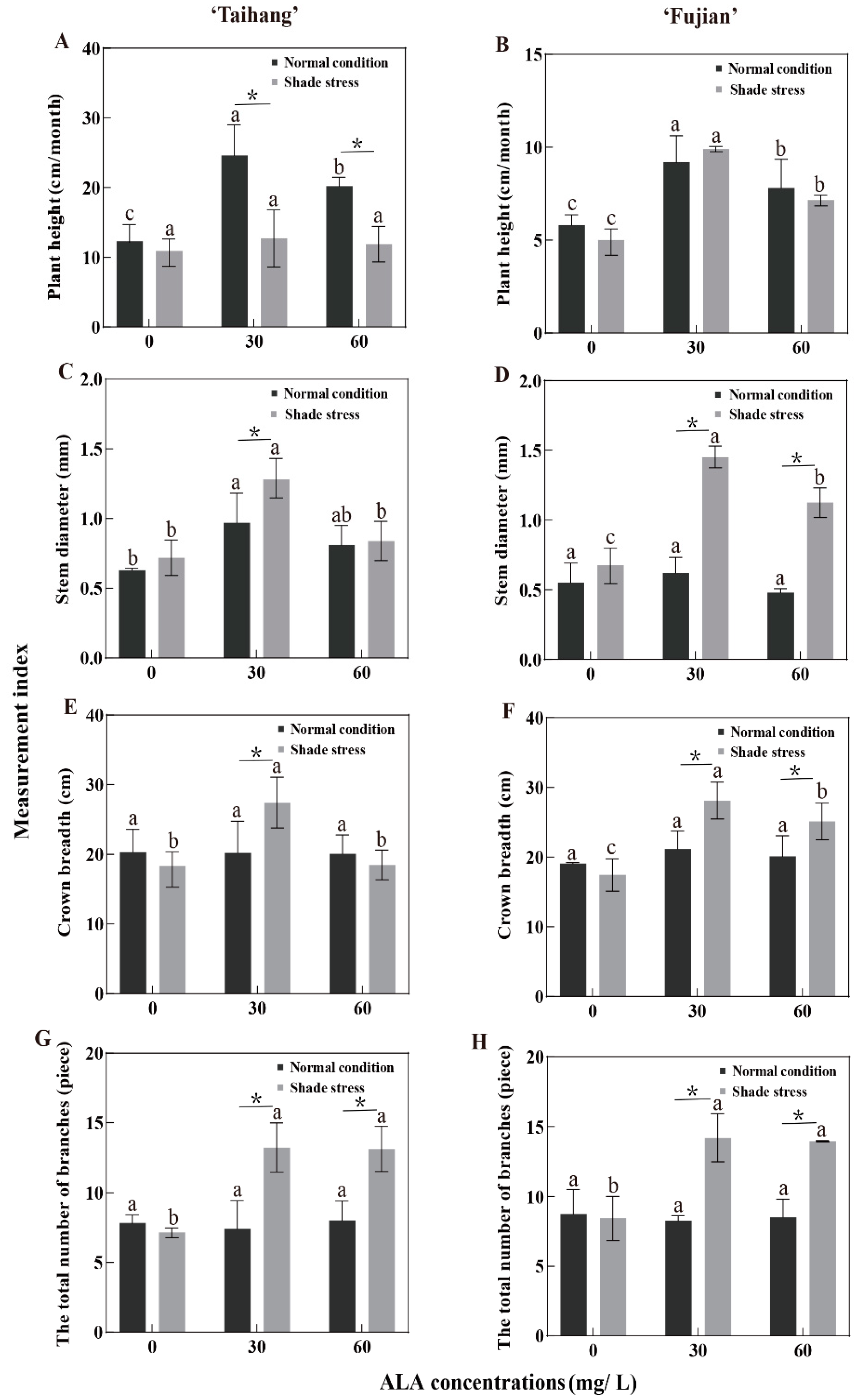
2.2. Changes in Growth Parameters in Response to Shade Stress
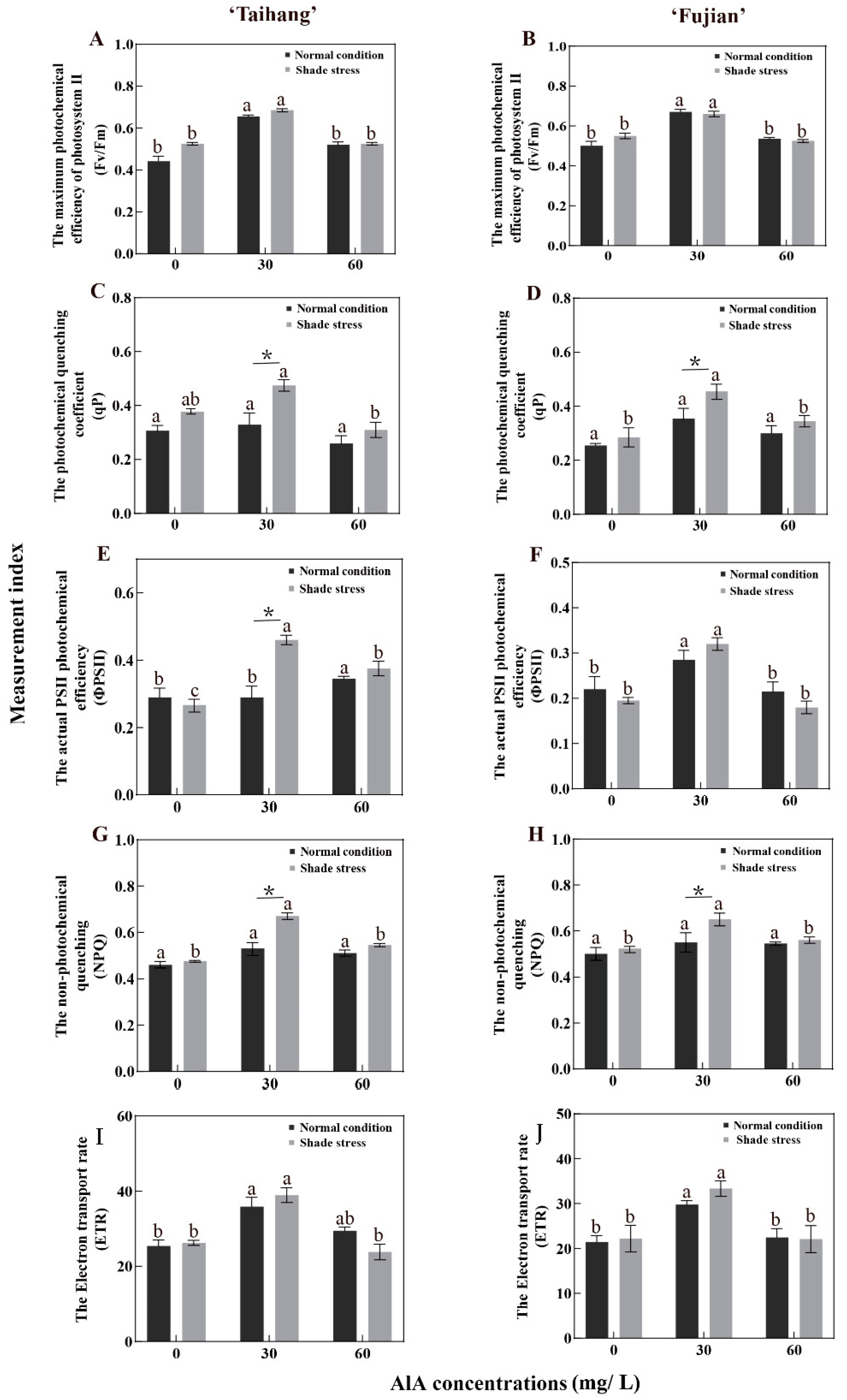
2.3. Changes in Gas Exchange and Photosynthetic Parameters in Response to Shade Stress
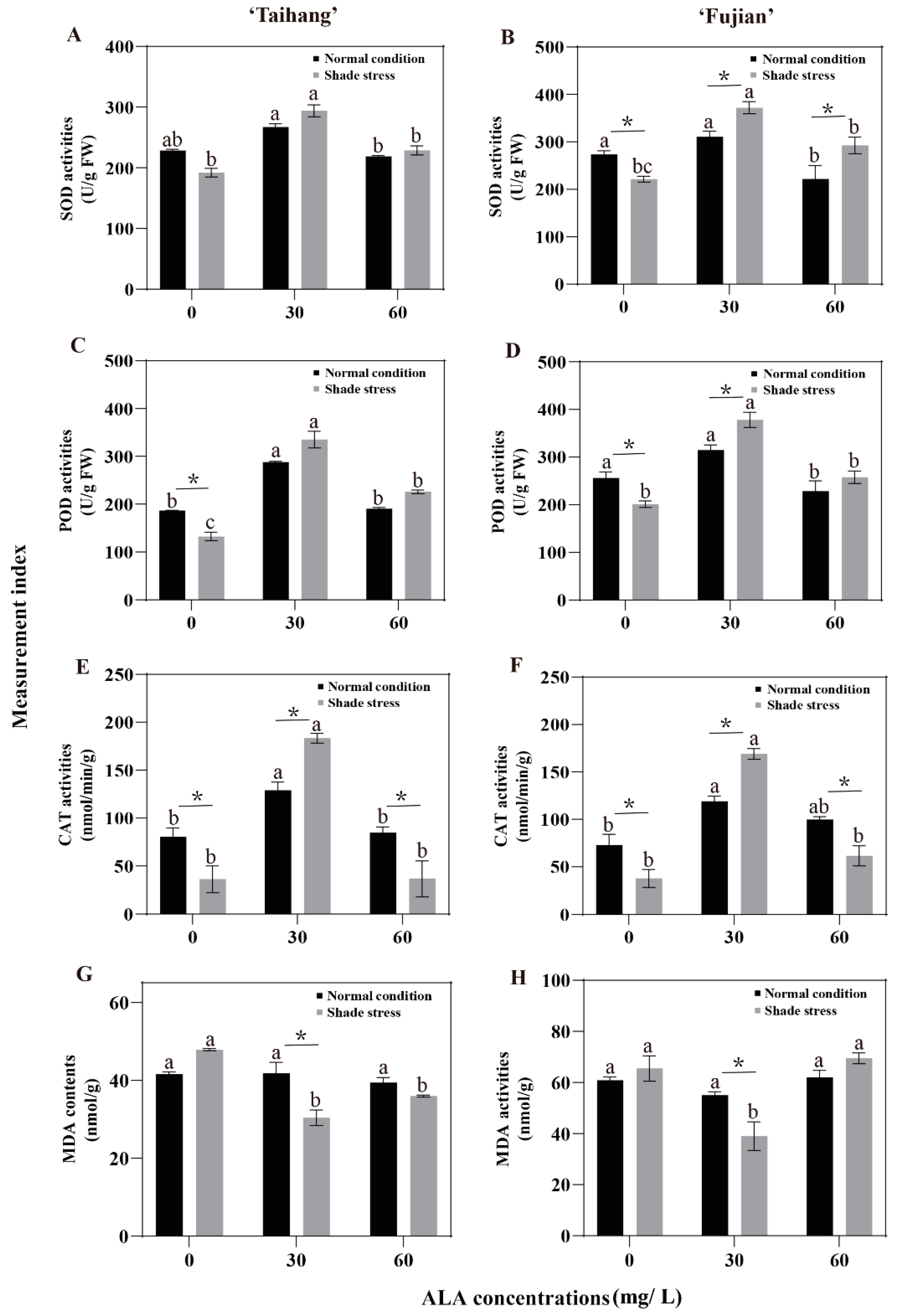
2.4. Changes in Antioxidant Enzyme Activities and MDA Content in Response to Shade Stress
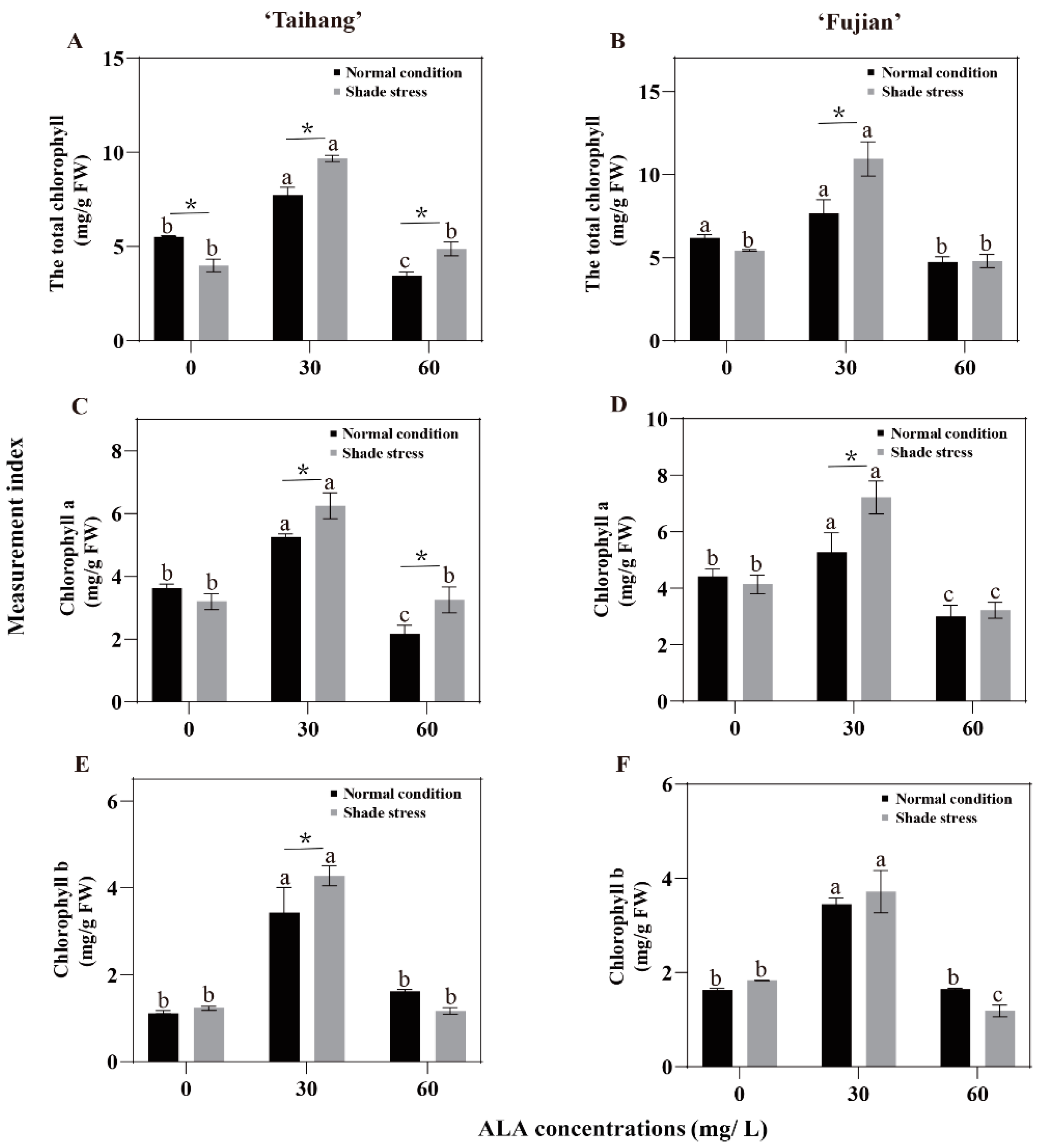
2.5. Changes in Chlorophyll Concentration in Response to Shade Stress
2.6. Changes in Secondary Metabolites in Response to Shade Stress
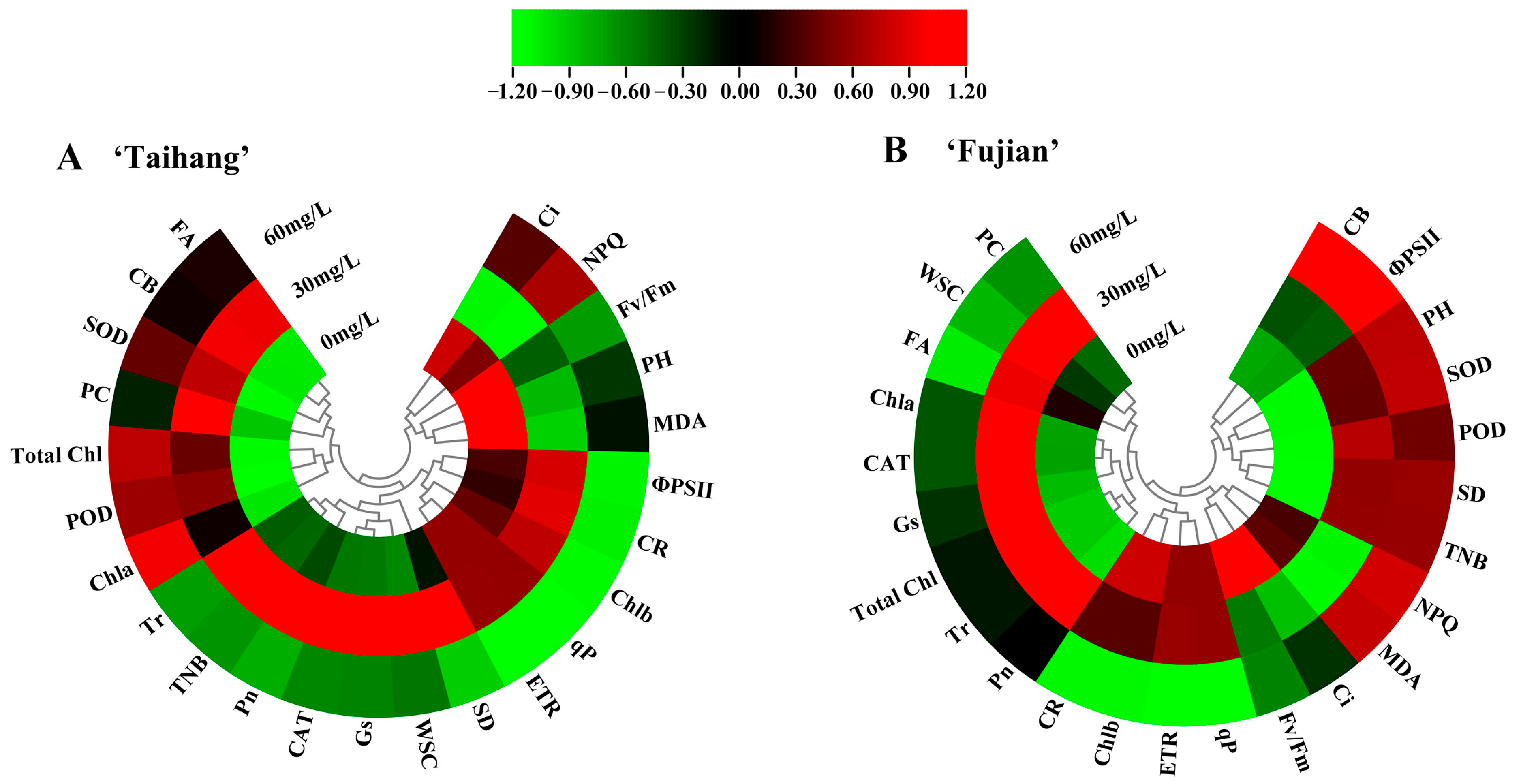
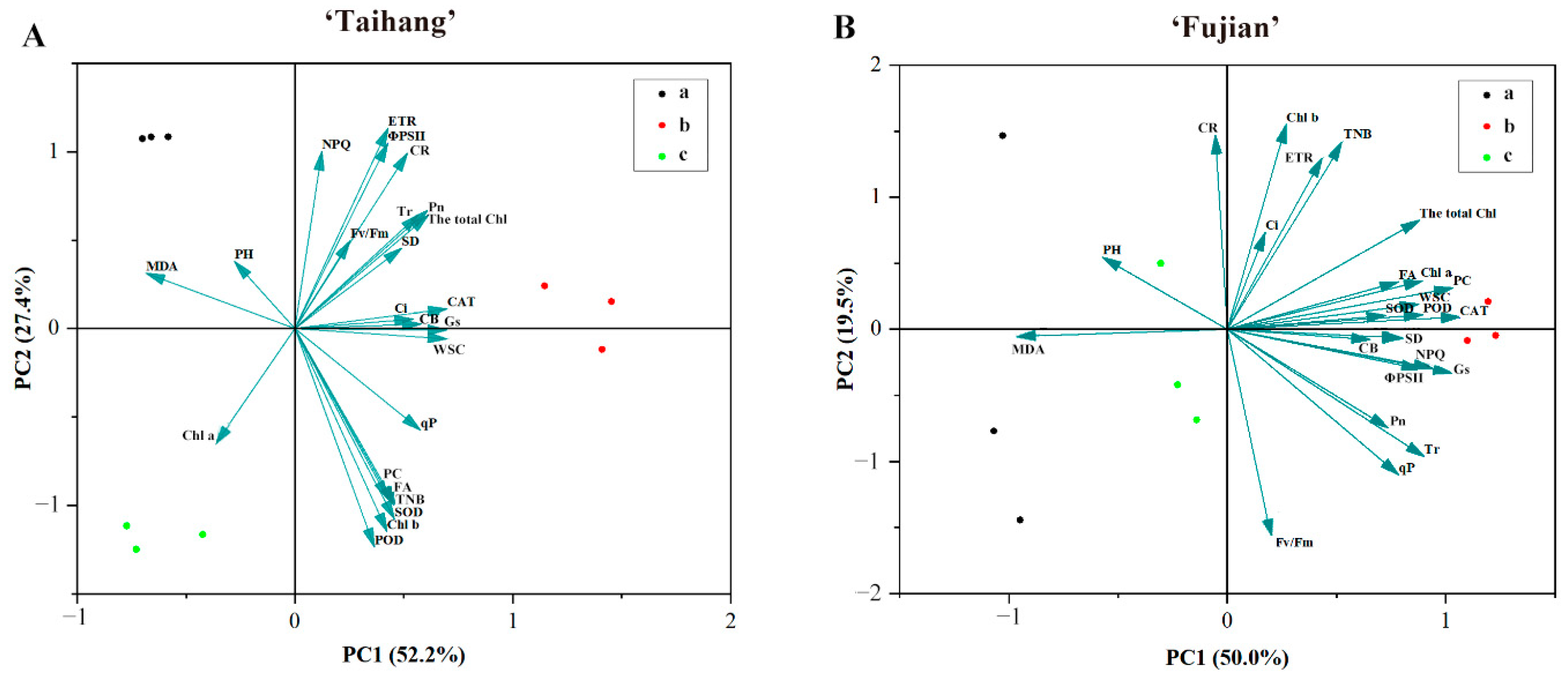
2.7. Stress Index, Hierarchical Clustering, and Principal Component Analysis in Response to Shade Stress
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant Material and Experimental Design
5.2. Determination of the Visual Quality Index
5.3. Determination of Plant Growth Indicators
5.4. Determination of Gas Exchange Parameters and Chlorophyll Fluorescence Parameters
5.5. Determination of Antioxidant Systems and MDA Content
5.6. Determination of Secondary Metabolites
5.7. Data Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Cope, E.A. Taxaceae: The genera and cultivated species. Bot. Rev. 1998, 64, 291–322. [Google Scholar] [CrossRef]
- Li, N.; Bai, B.; Wang, Z.; Luo, F.; Lu, X.Z.; Lu, C.H. Avian seed dispersal and seedling distribution of the endangered tree species, Taxus chinensis, in patchy habitats. Plant Ecol. Divers. 2015, 8, 407–414. [Google Scholar] [CrossRef]
- Li, N.; An, S.; Liu, Z.; Lu, C.H. Fruit consumption and seed dispersal by birds in native vs. ex situ individuals of the endangered Chinese yew, Taxus chinensis. Ecol. Res. 2014, 29, 917–923. [Google Scholar] [CrossRef]
- Perrin, P.M.; Kelly, D.L.; Mitchell, F.J. Long-term deer exclusion in yew-wood and oakwood habitats in southwest Ireland: Natural regeneration and stand dynamics. For. Ecol. Manag. 2006, 236, 356–367. [Google Scholar] [CrossRef]
- Vu, D.D.; Bui, T.T.X.; Nguyen, M.T.; Bui, V.T.; Huang, X.; Zhang, Y. Genetic diversity in two threatened species in Vietnam: Taxus chinensis and Taxus wallichiana. J. For. Res. 2017, 28, 265–272. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Feng, J.; Chen, C.; Chen, J.; Long, T.; Li, J.; Zang, R.; Li, J. Differential responses to climate and land-use changes in threatened chinese taxus species. Forests 2019, 10, 766. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Li, S.; Su, L.; Su, J. Variation and correlations of leaf traits of two Taxus species with different shade tolerance along the light gradient. Pol. J. Ecol. 2013, 61, 329–339. [Google Scholar]
- Liu, W.; Su, J. Effects of light acclimation on shoot morphology, structure, and biomass allocation of two Taxus species in southwestern China. Sci. Rep. 2016, 6, 35384. [Google Scholar] [CrossRef] [Green Version]
- Fankhauser, C.; Batschauer, A. Shadow on the plant: A strategy to exit. Cell 2016, 164, 15–17. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Jiang, S.; Liang, J.; Chen, M.; Shi, Y. Current knowledge of bermudagrass responses to abiotic stresses. Breed. Sci. 2019, 69, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Yu, D.J.; Kim, T.C.; Lee, H.J. Growth and photosynthetic characteristics of blueberry (Vaccinium corymbosum cv. bluecrop) under various shade levels. Sci. Hortic. 2011, 129, 486–492. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, K.; Liu, W.; Feng, G.; Peng, Y.; Li, Z. Adaptive responses of common and hybrid bermudagrasses to shade stress associated with changes in morphology, photosynthesis, and secondary metabolites. Front. Plant Sci. 2022, 13, 817105. [Google Scholar] [CrossRef] [PubMed]
- Esmailpourmoghadam, E.; Salehi, H. Tall fescue is a superturfgrass: Tolerance to shade conditions under deficit irrigation. J. Saudi Soc. Agric. Sci. 2021, 20, 290–301. [Google Scholar] [CrossRef]
- Begum, T.; Gogoi, R.; Sarma, N.; Pandey, S.K.; Lal, M. Direct sunlight and partial shading alter the quality, quantity, biochemical activities of Kaempferia parviflora Wall., ex Baker rhizome essential oil: A high industrially important species. Ind. Crop. Prod. 2022, 180, 114765. [Google Scholar] [CrossRef]
- Sanchez, F.; Bassil, E.; Crane, J.H.; Shahid, M.A.; Vincent, C.I.; Schaffer, B. Spectral light distribution affects photosynthesis, leaf reflective indices, antioxidant activity and growth of Vanilla planifolia. Plant Physiol. Biochem. 2022, 182, 145–153. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Mao, Z.; Liu, W.; Ding, L.; Zhang, X.; Yang, Y.; Wu, S.; Chen, X.; Wang, Y. Transcription factor McWRKY71 induced by ozone stress regulates anthocyanin and proanthocyanidin biosynthesis in Malus crabapple. Ecotoxicol. Environ. Saf. 2022, 232, 113274. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.P.; Burgess, S.J.; Doran, L.; Hansen, J.; Manukyan, L.; Maryn, N.; Gotarkar, D.; Leonelli, L.; Niyogi, K.K.; Long, S.P. Soybean photosynthesis and crop yield are improved by accelerating recovery from photoprotection. Science 2022, 377, 851–854. [Google Scholar] [CrossRef]
- Zakar, T.; Laczko Dobos, H.; Toth, T.N.; Zoltan, G. Carotenoids Assist in Cyanobacterial Photosystem II Assembly and Function. Front. Plant Sci. 2016, 7, 295. [Google Scholar] [CrossRef] [Green Version]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Chen, J.; Wu, S.; Dong, F.; Li, J.; Zeng, L.; Tang, J.; Gu, D. Mechanism underlying the shading-induced chlorophyll accumulation in tea leaves. Front. Plant Sci. 2021, 12, 779819. [Google Scholar] [CrossRef]
- Huang, D.; Wu, L.; Chen, J.R.; Dong, L. Morphological plasticity, photosynthesis and chlorophyll fluorescence of Athyrium pachyphlebium at different shade levels. Photosynthetica 2011, 49, 611–618. [Google Scholar] [CrossRef]
- Akram, N.A.; Ashraf, M. Regulation in plant stress tolerance by a potential plant growth regulator, 5-aminolevulinic acid. J. Plant Growth Regul. 2013, 32, 663–679. [Google Scholar] [CrossRef]
- Feng, T.; Chen, S.S.; Gao, D.Q.; Liu, G.Q.; Bai, H.X.; Li, A.; Peng, L.X.; Ren, Z.Y. Selenium improves photosynthesis and protects photosystem II in pear (Pyrus bretschneideri), grape (Vitis vinifera), and peach (Prunus persica). Photosynthetica 2015, 53, 609–612. [Google Scholar] [CrossRef]
- Shu, S.; Tang, Y.; Yuan, Y.; Sun, J.; Zhong, M.; Guo, S. The role of 24-epibrassinolide in the regulation of photosynthetic characteristics and nitrogen metabolism of tomato seedlings under a combined low temperature and weak light stress. Plant Physiol. Biochem. 2016, 107, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, R.; Proietti, S. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- An, Y.; Cheng, D.; Rao, Z.; Sun, Y.; Tang, Q.; Wang, L. 5-Aminolevulinic acid (ALA) promotes primary root elongation through modulation of auxin transport in Arabidopsis. Acta Physiol. Plant 2019, 41, 85. [Google Scholar] [CrossRef] [Green Version]
- Aksakal, O.; Omer, F.A.; Feyza, I.A.; Ferhunde, A. Exogenous 5-aminolevulinic acid alleviates the detrimental effects of UV-B stress on lettuce (Lactuca sativa L.) seedlings. Acta Physiol. Plant 2017, 39, 55. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, X.; You, X.; Li, Y.; Long, S.; Wen, S.; Liu, Q.; Liu, T.; Guo, H.; Xu, Y. Hydrogen sulfide improves tall fescue photosynthesis response to low-light stress by regulating chlorophyll and carotenoid metabolisms. Plant Physiol. Biochem. 2022, 170, 133–145. [Google Scholar] [CrossRef]
- Melis, A.; Harvey, G.W. Regulation of photosystem stoichiometry, chlorophyll a and chlorophyll b content and relation to chloroplast ultrastructure. Biochim. Biophys. Acta 1981, 637, 138–145. [Google Scholar] [CrossRef]
- Pires, M.V.; Almeida, A.A.; Figueiredo, A.L.; Gomes, F.P.; Souza, M.M. Photosynthetic characteristics of ornamental passion flowers grown under different light intensities. Photosynthetica 2011, 49, 593–602. [Google Scholar] [CrossRef]
- Van Huylenbroeck, J.M.; Van Bockstaele, E. Effects of shading on photosynthetic capacity and growth of turfgrass species. Int. Turfgrass Soc. Res. J. 2001, 9, 353–359. [Google Scholar]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic acid-induced stomatal closure: An important component of plant defense against abiotic and biotic stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef] [PubMed]
- Borel, C.; Frey, A.; Marion-Poll, A.; Tardieu, F.; Simonneau, T. Does engineering abscisic acid biosynthesis in Nicotiana plumbaginifolia modify stomatal response to drought? Plant Cell. Environ. 2001, 24, 477–489. [Google Scholar] [CrossRef] [Green Version]
- Geng, W.; Qiu, Y.; Peng, Y.; Zhang, Y.; Li, Z. Water and oxidative homeostasis, Na+/K+ transport, and stress-defensive proteins associated with spermine-induced salt tolerance in creeping bentgrass. Environ. Exp. Bot. 2021, 192, 104659. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, J.; Zhang, H.; Xu, Y.; An, Y.; Wang, L. Effect of 5-aminolevulinic acid (ALA) on leaf chlorophyll fast fluorescence characteristics and mineral element content of Buxus megistophylla grown along urban roadsides. Horticulturae 2021, 7, 95. [Google Scholar] [CrossRef]
- Suliman, M.S.E.; Elradi, S.B.M.; Nimir, N.E.A.; Zhou, G.; Zhu, G.; Ibrahim, M.E.H.; Ali, A.Y.A. Foliar application of 5-aminolevulinic acid alleviated high temperature and drought stresses on wheat plants at seedling stage. Chil. J. Agric. Res. 2021, 81, 291–299. [Google Scholar] [CrossRef]
- Yan, K.; Mei, H.; Dong, X.; Zhou, S.; Cui, J.; Sun, Y. Dissecting photosynthetic electron transport and photosystems performance in Jerusalem artichoke (Helianthus tuberosus L.) under salt stress. Front. Plant Sci. 2022, 13, 905100. [Google Scholar] [CrossRef]
- Kosar, F.; Akram, N.A.; Ashraf, M. Exogenously-applied 5-aminolevulinic acid modulates some key physiological characteristics and antioxidative defense system in spring wheat (Triticum aestivum L.) seedlings under water stress. S. Afr. J. Bot. 2015, 96, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Niu, K.; Ma, X.; Liang, G.; Ma, H.; Jia, Z.; Liu, W.; Yu, Q. 5-Aminolevulinic acid modulates antioxidant defense systems and mitigates drought-induced damage in Kentucky bluegrass seedlings. Protoplasma 2017, 254, 2083–2094. [Google Scholar] [CrossRef]
- Niu, K.; Ma, H. The positive effects of exogenous 5-aminolevulinic acid on the chlorophyll biosynthesis, photosystem and calvin cycle of Kentucky bluegrass seedlings in response to osmotic stress. Environ. Exp. Bot. 2018, 155, 260–271. [Google Scholar] [CrossRef]
- Sun, H.; Hou, Y.; Mei, Y.; Hao, P.; Wang, X.; Lyu, D. Role of NADPH oxidase-mediated hydrogen peroxide in 5-aminolevulinic acid induced photooxidative stress tolerance in pear leaves. Sci. Hortic. 2022, 294, 110771. [Google Scholar] [CrossRef]
- Sher, A.; Tahira, A.S.; Sattar, A.; Nawaz, A.; Qayyum, A.; Hussain, S.; Manaf, A. Foliage application of 5-aminolevulinic acid alleviates drought stress in sunflower (Helianthus annuus L.) through improving stay green and antioxidant enzymes activities. Acta Physiol. Plant 2021, 43, 22. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, L.; Liao, W.; Dawuda, M.M.; Lyu, J.; Xie, J.; Feng, Z.; Calderón-Urrea, A.; Yu, J. Foliar application of 5-aminolevulinic acid (ALA) alleviates NaCl stressin cucumber (Cucumis sativus L.) seedlings through the enhancement of ascorbate-glutathione cycle. Sci. Hortic. 2019, 257, 108761. [Google Scholar] [CrossRef]
- Xu, J.B.; Bartley, J.P.; Johnson, R.A. Preparation and characterization of alginate-carrageenan hydrogel films crosslinked using a water-soluble carbodiimide (WSC). J. Membr. Sci. 2003, 218, 131–146. [Google Scholar] [CrossRef]
- Wang, C.C.; Chang, S.C.; Inbaraj, B.S.; Chen, B.H. Isolation of carotenoids, flavonoids and polysaccharides from Lycium barbarum L. and evaluation of antioxidant activity. Food Chem. 2010, 120, 184–192. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Anwar, A.; Yan, Y.; Liu, Y.; Li, Y.; Yu, X. 5-Aminolevulinic acid improves nutrient uptake and endogenous hormone accumulation, enhancing low-temperature stress tolerance in cucumbers. Int. J. Mol. Sci. 2018, 19, 3379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korkmaz, A.; Uzunlu, M.; Demirkiran, A.R. Acetyl salicylic acid alleviates chilling-induced damage in muskmelon seedlings. Can. J. Plant Sci. 2007, 87, 581–585. [Google Scholar] [CrossRef] [Green Version]
- Laviola, B.G.; Alves, A.A.; Gurgel, F.D.L.; Rosado, T.B.; Rocha, R.B.; Albrecht, J.C. Estimates of genetic parameters for physic nut traits based in the germplasm two years evaluation. Ciência Rural 2012, 42, 429–435. [Google Scholar] [CrossRef] [Green Version]
- White, A.J.; Critchley, C. Rapid light curves: A new fluorescence method to assess the state of the photosynthetic apparatus. Photosynth. Res. 1999, 59, 63–72. [Google Scholar] [CrossRef]
- Wu, Y.; Jin, X.; Liao, W.; Hu, L.; Dawuda, M.M.; Zhao, X.; Tang, Z.; Gong, T.; Yu, J. 5-Aminolevulinic acid (ALA) alleviated salinity stress in cucumber seedlings by enhancing chlorophyll synthesis pathway. Front. Plant Sci. 2018, 9, 635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, G.; Mouithys-Mickalad, A.; Sluse, F.E. Generation of superoxide anion by mitochondria and impairment of their functions during anoxia and reoxygenation in vitro. Free Radic. Biol. Med. 1998, 25, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jing, W.; Peng, Y.; Zhang, X.Q.; Ma, X.; Huang, L.K.; Yan, Y.-H. Spermine alleviates drought stress in white clover with different resistance by influencing carbohydrate metabolism and Dehydrins Synthesis. PLoS ONE 2015, 10, e0120708. [Google Scholar] [CrossRef] [Green Version]
- Bayar, N.; Kriaa, M.; Kammoun, R. Extraction and characterization of three polysaccharides extracted from Opuntia ficus indica cladodes. Int. J. Biol. Macromol. 2016, 92, 441–450. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [Green Version]
- Midway, S.; Robertson, M.; Flinn, S.; Kaller, M. Comparing multiple comparisons: Practical guidance for choosing the best multiple comparisons test. PeerJ 2020, 8, e10387. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Jiang, Y.; Li, H.; Zhang, Z.; Xu, Z.; Xu, B.; Huang, B. Natural variation of physiological traits, molecular markers, and chlorophyll catabolic genes associated with heat tolerance in perennial ryegrass accessions. BMC Plant Biol. 2020, 20, 520. [Google Scholar] [CrossRef]


| Treatments | Visual Quality Index (1–5) |
|---|---|
| App. Method | |
| Normal conditions | 2.68 ± 0.13 a |
| Shade stress | 1.57 ± 0.21 c |
| Cultivar × 5-ALA concentrations | 2.13 ± 0.15 b |
| ‘Taihang’ (mg/L) | |
| 0 | 0.55 ± 0.09 c |
| 30 | 1.82 ± 0.21 a |
| 60 | 1.14 ± 0.15 b |
| ‘Fujian’ (mg/L) | |
| 0 | 1.32 ± 0.13 c |
| 30 | 2.98 ± 0.21 a |
| 60 | 2.18 ± 0.15 b |
| ANOVA | |
| App. method (M) | ** |
| ALA concentrations (C) | *** |
| M × C | NS |
| Parameter | Normal Conditions | Shade Stress | Stress Index (%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taihang | Fujian | Taihang | Fujian | Taihang | Fujian | |||||||||||||
| 0 | 30 | 60 | 0 | 30 | 60 | 0 | 30 | 60 | 0 | 30 | 60 | 0 | 30 | 60 | 0 | 30 | 60 | |
| PH | 12.48 | 24.67 | 20.20 | 5.82 | 9.24 | 7.83 | 10.21 | 12.72 | 11.96 | 4.94 | 9.93 | 8.84 | 82 | 52 | 59 | 85 | 107 | 113 |
| SD | 0.63 | 0.97 | 0.81 | 0.55 | 0.62 | 0.48 | 0.72 | 1.28 | 0.84 | 0.69 | 1.45 | 1.13 | 114 | 132 | 104 | 125 | 234 | 235 |
| CB | 20.31 | 20.22 | 20.10 | 19.22 | 27.42 | 18.49 | 19.09 | 21.18 | 20.11 | 18.38 | 28.13 | 25.14 | 94 | 105 | 100 | 96 | 103 | 136 |
| TNB | 8.30 | 7.43 | 8.03 | 8.76 | 8.28 | 7.90 | 7.90 | 14.24 | 7.04 | 7.22 | 14.70 | 14.10 | 95 | 192 | 88 | 82 | 178 | 178 |
| Pn | 3.87 | 2.71 | 3.32 | 2.61 | 3.31 | 2.44 | 3.54 | 4.22 | 2.63 | 2.18 | 3.71 | 2.35 | 91 | 156 | 79 | 84 | 112 | 96 |
| Tr | 0.48 | 0.39 | 0.59 | 0.46 | 0.40 | 0.47 | 0.38 | 0.67 | 0.41 | 0.39 | 0.58 | 0.49 | 79 | 172 | 69 | 85 | 145 | 104 |
| Ci | 173.45 | 245.54 | 207.53 | 148.58 | 251.44 | 203.56 | 197.51 | 192.00 | 201.33 | 185.34 | 195.57 | 180.50 | 114 | 78 | 97 | 125 | 78 | 89 |
| Gs | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.03 | 0.02 | 0.02 | 0.03 | 0.02 | 90 | 173 | 89 | 88 | 159 | 105 |
| Fv/Fm | 0.41 | 0.66 | 0.52 | 0.50 | 0.67 | 0.54 | 0.51 | 0.69 | 0.53 | 0.55 | 0.66 | 0.53 | 124 | 105 | 102 | 110 | 99 | 98 |
| qP | 0.33 | 0.33 | 0.26 | 0.39 | 0.39 | 0.30 | 0.48 | 0.48 | 0.31 | 0.50 | 0.50 | 0.35 | 145 | 145 | 119 | 128 | 128 | 115 |
| ΦPSII | 0.23 | 0.33 | 0.35 | 0.19 | 0.29 | 0.21 | 0.30 | 0.46 | 0.38 | 0.22 | 0.32 | 0.18 | 130 | 139 | 109 | 116 | 110 | 86 |
| NPQ | 0.54 | 0.67 | 0.57 | 0.56 | 0.63 | 0.58 | 0.53 | 0.58 | 0.56 | 0.54 | 0.57 | 0.57 | 98 | 87 | 98 | 96 | 90 | 98 |
| ETR | 35.89 | 35.89 | 29.45 | 29.83 | 29.83 | 22.49 | 38.91 | 38.95 | 23.83 | 33.36 | 33.40 | 22.11 | 108 | 109 | 81 | 112 | 112 | 98 |
| SOD | 228.54 | 267.33 | 218.95 | 273.61 | 310.75 | 222.27 | 191.27 | 294.01 | 228.74 | 221.82 | 372.17 | 292.71 | 84 | 110 | 104 | 81 | 120 | 132 |
| POD | 186.78 | 288.09 | 190.75 | 255.92 | 314.76 | 228.67 | 132.44 | 335.37 | 226.02 | 201.33 | 378.09 | 257.78 | 71 | 116 | 118 | 79 | 120 | 113 |
| CAT | 80.46 | 129.04 | 84.75 | 73.33 | 119.11 | 99.95 | 36.32 | 183.33 | 36.69 | 37.86 | 169.30 | 61.72 | 45 | 142 | 43 | 52 | 142 | 62 |
| MDA | 41.96 | 41.76 | 39.42 | 64.54 | 55.00 | 62.12 | 52.94 | 30.39 | 35.96 | 65.57 | 39.00 | 69.54 | 126 | 73 | 91 | 102 | 71 | 112 |
| Total Chl | 5.35 | 7.73 | 3.44 | 6.18 | 8.73 | 4.74 | 3.79 | 9.68 | 4.87 | 5.45 | 10.94 | 4.80 | 71 | 125 | 142 | 88 | 125 | 101 |
| Chla | 3.57 | 5.25 | 2.17 | 3.86 | 5.28 | 3.19 | 3.18 | 6.25 | 3.25 | 3.65 | 7.22 | 3.22 | 89 | 119 | 150 | 95 | 137 | 101 |
| Chlb | 1.26 | 3.43 | 1.62 | 1.63 | 3.45 | 1.65 | 1.42 | 4.28 | 1.17 | 2.00 | 3.72 | 1.19 | 113 | 125 | 72 | 123 | 108 | 72 |
| WSC | 22.84 | 34.50 | 34.50 | 22.77 | 34.11 | 31.81 | 21.33 | 41.50 | 32.54 | 26.00 | 49.81 | 32.97 | 93 | 120 | 94 | 114 | 146 | 104 |
| PC | 57.59 | 80.99 | 60.75 | 63.59 | 60.47 | 65.41 | 60.50 | 123.89 | 72.89 | 60.50 | 80.75 | 59.89 | 105 | 153 | 120 | 95 | 134 | 92 |
| CR | 7.42 | 12.28 | 5.62 | 1.63 | 3.45 | 1.65 | 6.26 | 13.93 | 2.67 | 1.99 | 3.72 | 1.19 | 84 | 113 | 48 | 122 | 108 | 72 |
| FA | 57.84 | 89.50 | 69.50 | 72.77 | 84.11 | 89.31 | 46.32 | 111.50 | 72.54 | 71.00 | 114.81 | 52.97 | 80 | 125 | 104 | 98 | 136 | 59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Song, L.; Cao, L.; Meng, L. Alleviation of Shade Stress in Chinese Yew (Taxus chinensis) Seedlings with 5-Aminolevulinic Acid (ALA). Plants 2023, 12, 2333. https://doi.org/10.3390/plants12122333
Wu L, Song L, Cao L, Meng L. Alleviation of Shade Stress in Chinese Yew (Taxus chinensis) Seedlings with 5-Aminolevulinic Acid (ALA). Plants. 2023; 12(12):2333. https://doi.org/10.3390/plants12122333
Chicago/Turabian StyleWu, Liuliu, Linlin Song, Lifan Cao, and Li Meng. 2023. "Alleviation of Shade Stress in Chinese Yew (Taxus chinensis) Seedlings with 5-Aminolevulinic Acid (ALA)" Plants 12, no. 12: 2333. https://doi.org/10.3390/plants12122333
APA StyleWu, L., Song, L., Cao, L., & Meng, L. (2023). Alleviation of Shade Stress in Chinese Yew (Taxus chinensis) Seedlings with 5-Aminolevulinic Acid (ALA). Plants, 12(12), 2333. https://doi.org/10.3390/plants12122333





